Development of Cerium Oxide/Corncob Nanocomposite: A Cost-Effective and Eco-Friendly Adsorbent for the Removal of Heavy Metals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.1.1. Preparation of Corncob
2.1.2. Activation of the Corncob
2.1.3. CeO2 Nanoparticle
2.1.4. Preparation of Nanocomposites of Cerium Oxide and Activated Corncob
2.1.5. Calcination
2.2. Characterization
Batch Adsorption Experiment
3. Results and Discussion
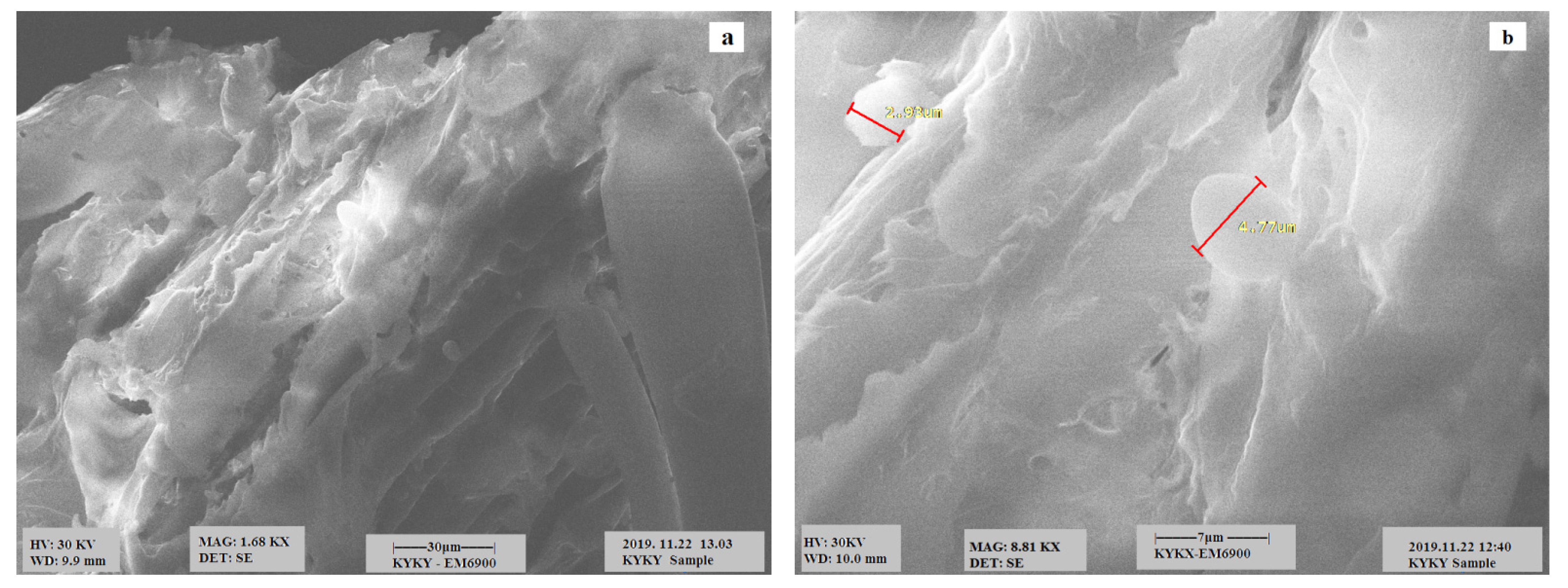
3.1. Scanning Electron Microscopy (SEM) Analysis
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3. X-ray Diffraction (XRD) Analysis
3.4. Batch Adsorption Experiment
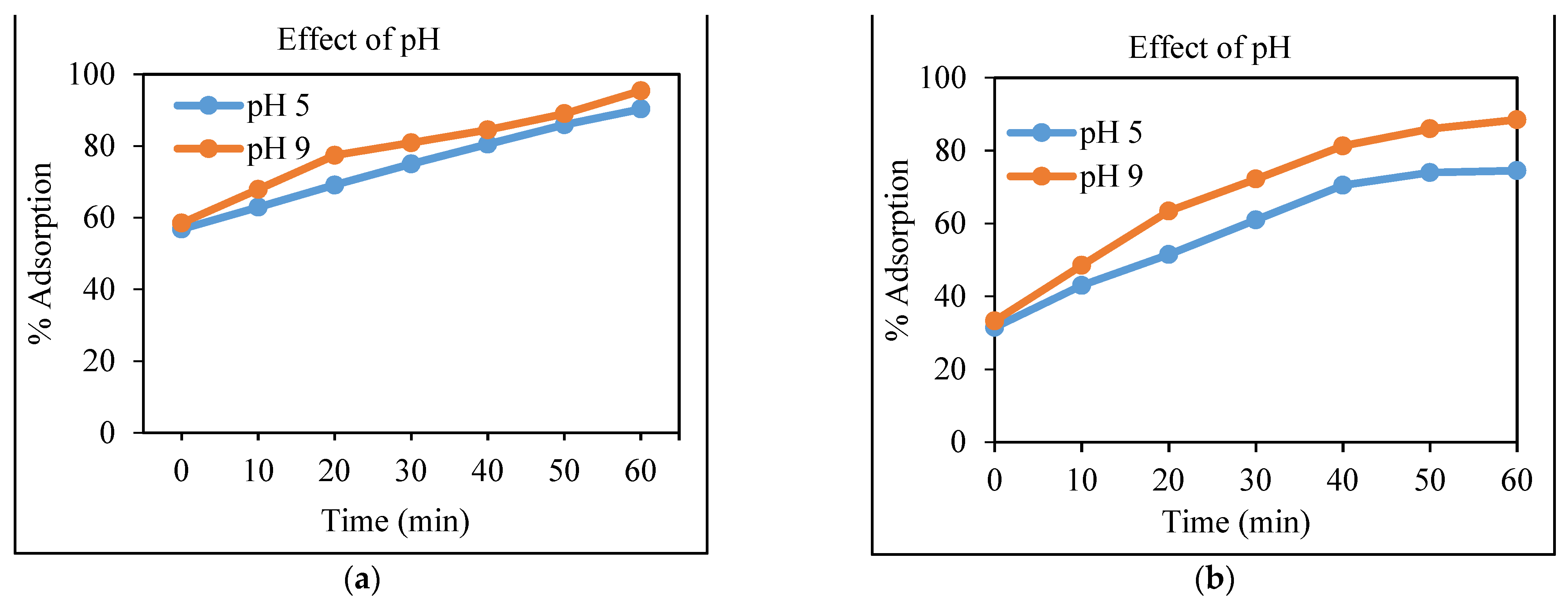
3.4.1. Effect of the pH on the Removal of Cd and Cr
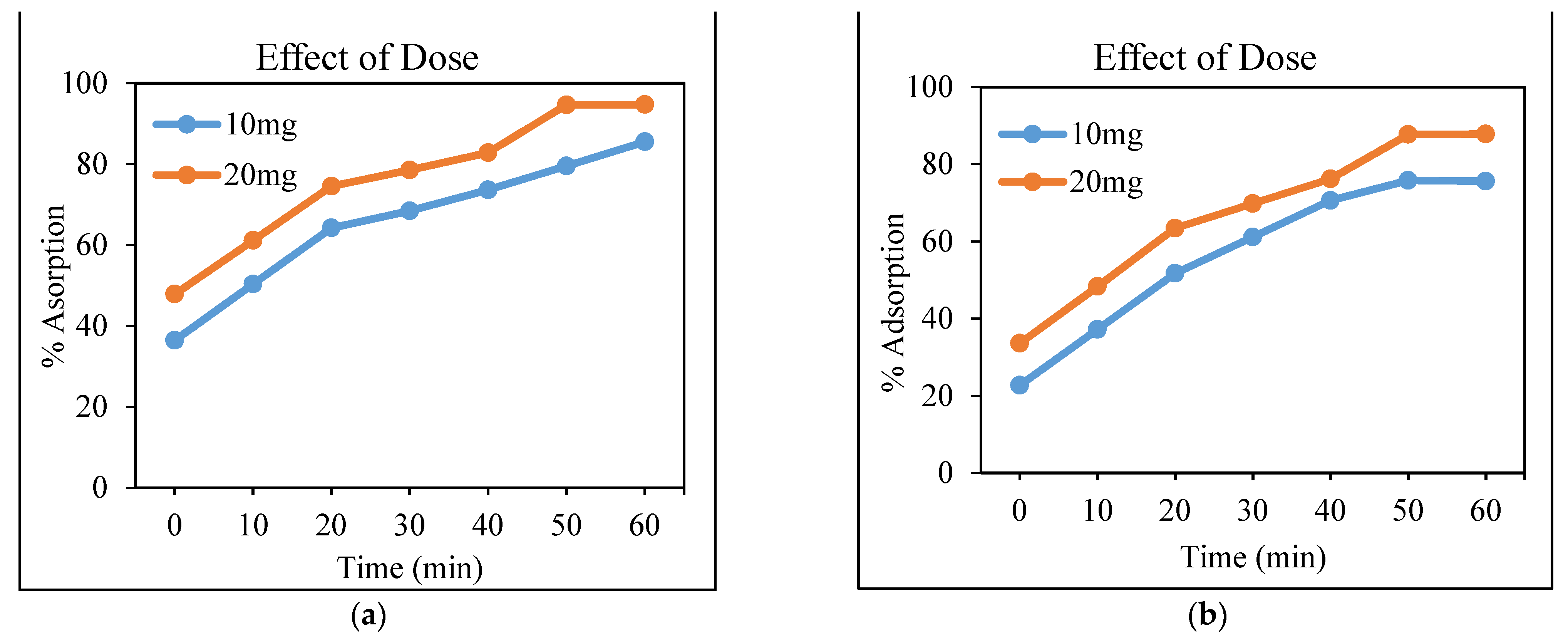
3.4.2. Effect of the Adsorbent Dose
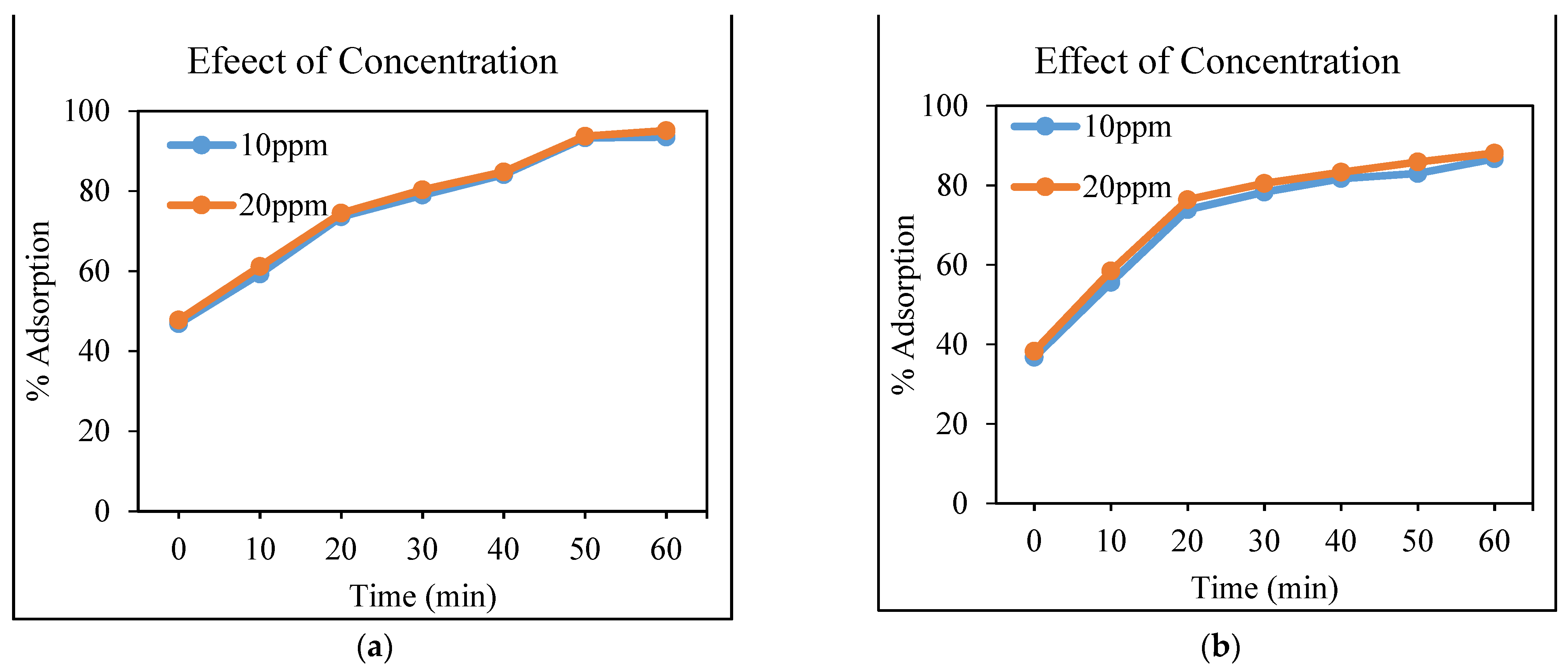
3.4.3. Effect of Concentration
3.4.4. Effect of Contact Time on the Removal of Cd and Cr
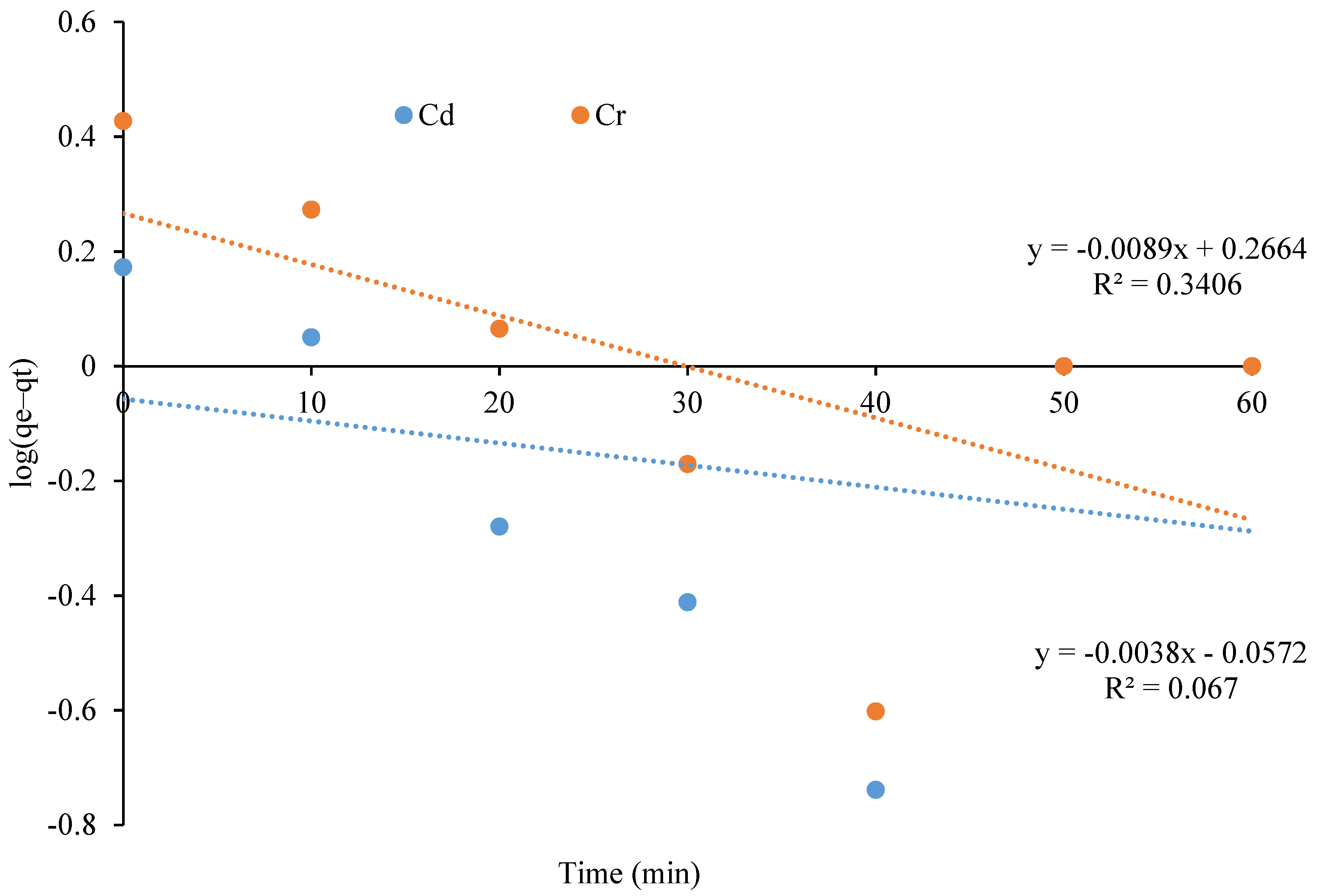
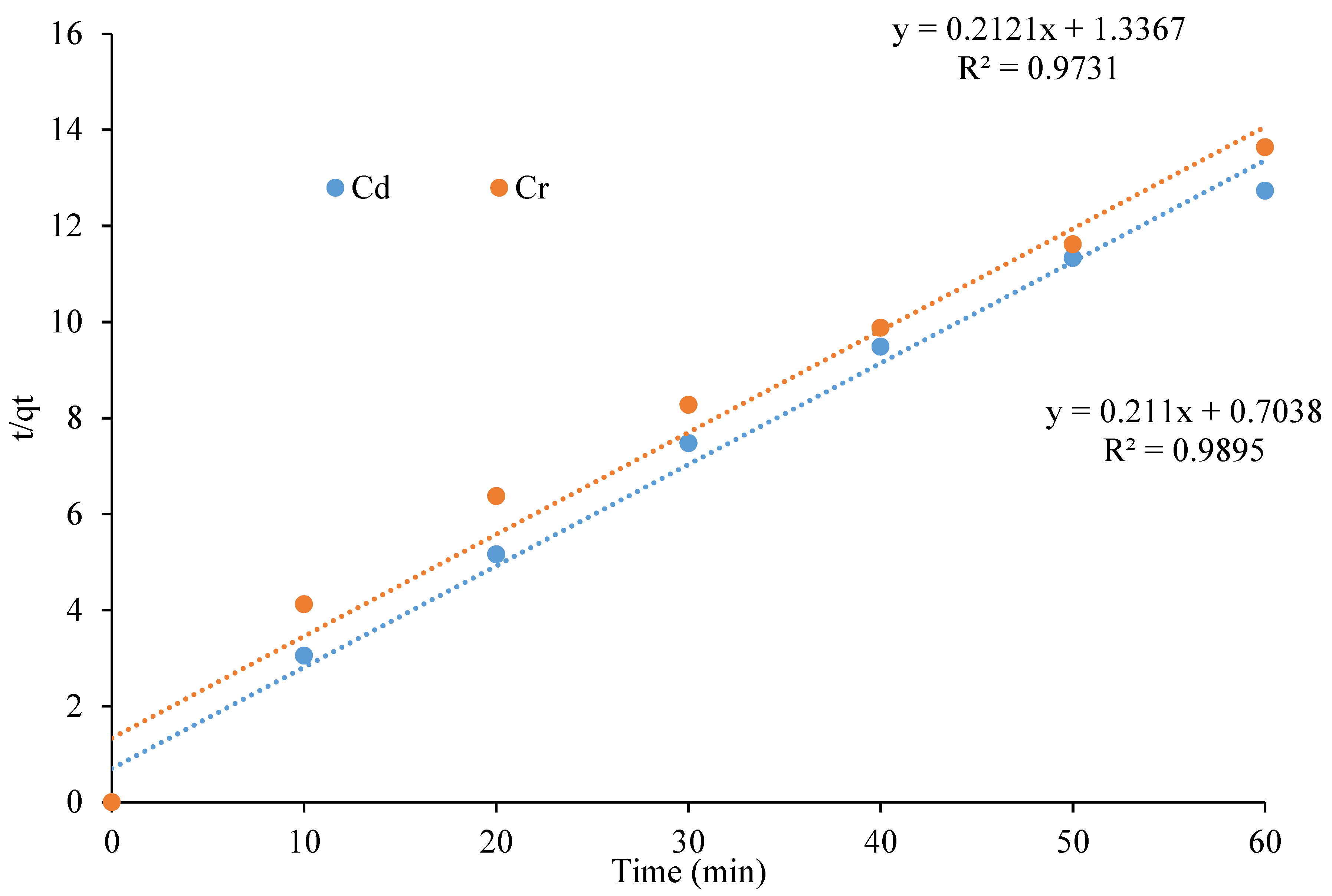
3.5. Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jabeen, A.; Huang, X.; Aamir, M. The Challenges of Water Pollution, Threat to Public Health, Flaws of Water Laws and Policies in Pakistan. J. Water Resour. Prot. 2015, 7, 1516–1526. [Google Scholar] [CrossRef] [Green Version]
- Gakwisiri, C.; Raut, N.; Al-Saadi, A.; Al-Aisri, S.; Al-Ajmi, A. critical review of removal of zinc from wastewater. Proc. Int. Conf. Eng. 2012, 1, 4–6. [Google Scholar]
- Bordoloi, N.; Goswami, R.; Kumar, M.; Kataki, R. Biosorption of Co (II) from aqueous solution using algal biochar: Kinetics and isotherm studies. Bioresour. Technol. 2017, 244, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Ostovar, F.; Ansari, R.; Moafi, H.F. Preparation and application of silver oxide/sawdust nanocomposite for Chromium (VI) ion removal from aqueous solutions using column system. Glob. Nest J. 2017, 19, 412–422. [Google Scholar] [CrossRef]
- Swarnabala, J.; Sahoo, K.R. Removal of Pb(II) from Aqueous Solution Using Fruits Peel as a Low Cost Adsorbent. Int. J. Eng. Sci. Technol. 2017, 5, 2348–4098. [Google Scholar]
- Nuhoglu, A.; Pekdemir, T.; Yildiz, E.; Keskinler, B.; Akay, G. Drinking water denitrification by a membrane bio-reactor. Water Resour. 2002, 36, 1155–1166. [Google Scholar] [CrossRef]
- Igwe, J.C.; Abia, A.A. Adsorption isotherm studies of Cd (II), Pb (II) and Zn (II) ions bioremediation from aqueous solution using unmodified and EDTA-modified maize cob. Eclética Química 2007, 32, 32–47. [Google Scholar] [CrossRef] [Green Version]
- Ognibene, G.; Cristaldi, A.D.; Fiorenza, R.; Blanco, R.; Cicala, G.; Scirè, S.; Fragalà, M.E. Photoactivity of hierarchically nanostructured ZnO–PES fibre mats for water treatments. RSC Adv. 2016, 6, 42778–42785. [Google Scholar] [CrossRef]
- Siracusa, V.; Blanco, I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio Poly(ethylene terephthalate) (Bio-PET): Recent Developments in Bio-Based Polymers Analogous to Petroleum-Derived Ones for Packaging and Engineering Applications. Polymers 2020, 12, 81641. [Google Scholar] [CrossRef]
- Wolowiec, M.; Komorowska-Kaufman, M.; Pruss, A.; Rzepa, G.; Bajda, T. Removal of Heavy Metals and Metalloids from Water Using Drinking Water Treatment Residuals as Adsorbents: A Review. Minerals 2019, 9, 487. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, A.; Sillanpää, M.; Krowiak, W.A. Agricultural waste peels as versatile biomass for water purification—A review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Shi, Y.; Chengzhang, W.; Zirong, X. The application and prospect of nanotechnology in animal husbandry. J. Northwest Sci-Tech Univ. Agric. For. 2006, 34, 49–52. [Google Scholar]
- Younis, A.; Chu, D.; Li, S. Cerium Oxide Nanostructures and their Applications. Funct. Nanomater. 2016, 3, 53–68. [Google Scholar] [CrossRef] [Green Version]
- Kannan, S.K.; Sundrarajan, M. A Green Approach for the synthesis of a Cerium Oxide Nanoparticle: Characterization and Antibacterial Activity. Int. J. Nanosci. 2014, 13, 1450018. [Google Scholar] [CrossRef]
- Montanher, F.S.; Oliveira, A.E.; Rollenberg, C.M. Removal of metal ions from aqueous solutions by sorption onto rice bran. J. Hazard. Mater. 2005, 117, 207–211. [Google Scholar] [CrossRef]
- Nasernejad, B.; Zalah, E.T.; Pou, B.B.; Bygi, E.M.; Zamani, A. Comparison for biosorption modeling of heavy metals [Cr(III), Cu(II), Zn(II)] adsorption from wastewater by carrot residues. Process Biochem. 2005, 40, 1319–1322. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Minocha, K.A. Utilization of industrial waste for cadmium removal from water and immobilization in cement. Chem. Eng. J. 2009, 150, 145–151. [Google Scholar] [CrossRef]
- Buah, W.; MacCarthy, J.; Ndur, S. Conversion of Corn Cobs Waste into Activated Carbons for Adsorption of Heavy Metals from Minerals Processing Wastewater. Int. J. Environ. Prot. Policy 2016, 4, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Garg, K.U.; Kaur, P.M.; Garg, K.V.; Sud, D. Removal of hexavalent chromium from aqueous solution by agricultural waste biomass. J. Hazard. Mater. 2007, 140, 60–68. [Google Scholar] [CrossRef]
- Isa, I.; Setiawati, E.; Mohammad, E.; Kunusa, W. Utilization of Corncob Cellulose Isolate (Zea mays) as Adsorbent of Heavy Metal Copper and Cadmium. Environ. Earth Sci. 2020, 589, 012035. [Google Scholar] [CrossRef]
- Kaushik, P.C.; Tuteja, R.; Kaushik, N.; Sharma, K.J. Minimization of organic chemical load in direct dyes effluent using low cost adsorbents. Chem. Eng. J. 2009, 155, 234–240. [Google Scholar] [CrossRef]
- Hoyos-Sánchez, C.M.; Córdoba-Pacheco, C.A.; Rodríguez-Herrera, F.L.; Uribe-Kaffure, R. Removal of Cd (II) from Aqueous Media by Adsorption onto Chemically and Thermally Treated Rice Husk. J. Chem. 2017, 8, 5763832. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.; Bandyopadhyay, M. Sorption of cadmium from aqueous solution using pretreated rice husk. Bioresour. Technol. 2006, 97, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Bayahia, H. Cerium Oxide Nanoparticles as Catalyst for the Oxidation of Methanol. Orient. J. Chem. 2019, 35, 1539–1545. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Nakkeeran, E.; Anu, N.; Selvaraju, N. Biosorption potentials of a novel Ficus auriculata leaves powder for the sequestration of hexavalent chromium from aqueous solutions. Res. Chem. Intermed. 2015, 41, 8405–8424. [Google Scholar] [CrossRef]
- Badmus, M.A.O.; Audu, T.O.K.; Anyata, B.U. Removal of lead ion from industrial wastewaters by activated carbon prepared from periwinkle shells. Turk. J. Eng. Environ. Sci. 2007, 31, 251–263. [Google Scholar]
- Akhtar, M.; Iqbal, S.; Kausar, A.; Bhanger, I.M.; Shaheen, M.A. An economically viable method for the removal of selected divalent metal ions from aqueous solutions using activated rice husk. Colloids Surf. B Biointerfaces 2010, 75, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Okorie, O.H.; Ekemezie, N.P.; Akpomie, G.K.; Olikagu, S.C. Calcined Corncob-Kaolinite Combo as New Sorbent for Sequestration of Toxic Metal Ions from Polluted Aqua Media and Desorption. Front. Chem. 2018, 6, 273. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, R.; Parvaz, S.; Rabbani, M. Preparation of corn cob based bioadsorbent as an effective adsorbent. In Proceedings of the 21st International Electronic Conference on Synthetic Organic Chemistry, Online Conference, 1–30 November 2017. [Google Scholar] [CrossRef]
- Khan, S.; Farooqi, A.; Danish, I.A.; Zeb, A. Biosorption of CU(II) from Aqueous Solution Using Citrus Sinensis Peel and Wood Sawdust: Utilization in Purification of Drinking and Waste Water. Ijrras 2013, 16, 297–306. [Google Scholar]
- Batzias, A.F.; Sidiras, K.D. Simulation of dye adsorption by beech sawdust as affected by pH. J. Hazard. Mater. 2007, 2, 273–281. [Google Scholar] [CrossRef]
- Idhn, J.I.; Abdullah, C.L.; Mahdi, S.D.; Obaid, K.M. Adsorption of Anionic Dye Using Cationic Surfactant-Modified Kenaf Core Fibers. Open Access Libr. J. 2017, 4, 1–18. [Google Scholar] [CrossRef]
- Bakka, A.; Mamouni, R.; Saffaj, N.; Laknifli, A.; Benlhachemi, A.; Bakiz, B.; Haddad, E.M.; Taleb, A.M.; Roudani, A.; Faouzi, A. The Treated Eggshells as a New Biosorbent for Elimination of Carbaryl Pesticide from Aqueous Solutions: Kinetics, Thermodynamics and Isotherms. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2016, 17, 271–284. [Google Scholar]
- Jain, M.; Yadav, M.; Kohout, T.; Lahtinen, M.; Vinod, K.G.; Sillanpää, M. Development of iron oxide/activated carbon nanoparticle composite for the removal of Cr(VI), Cu(II) and Cd(II) ions from aqueous solution. Water Resour. Ind. 2018, 20, 54–74. [Google Scholar] [CrossRef]
- Wannahari, R.; Sannasi, P.; Nordin, M.F.M.; Mukhtar, H. Sugarcane Bagass derived Nano Magnetic Adsorbent Composite (SCB-NMAC) for removal of CU2+ from Aqueous Solution. J. Eng. Appl. Sci. 2018, 13, 1819–6608. [Google Scholar]
- Panda, L.; Das, B.; Rao, D.S.; Mishra, K.B. Application of dolochar in the removal of cadmium and hexavalent chromium ions from aqueous solutions. J. Hazard. Mater. 2011, 192, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Bastami, R.T.; Entezari, H.M. Activated carbon from carrot dross combined with magnetite nanoparticles for the efficient removal of p-nitrophenol from aqueous solution. Chem. Eng. Sci. 2012, 210, 510–519. [Google Scholar] [CrossRef]
- Singh, K.S.; Townsend, G.T.; Mazyck, D.; Boyer, H.T. Equilibrium and intra-particle diffusion of stabilized landfill leachate onto micro- and meso-porous activated carbon. Water Res. 2012, 146, 491–499. [Google Scholar] [CrossRef]
- Jacob, J.J.; Varalakshmi, R.; Gargi, S.; Jayasri, M.A.; Suthindhiran, K. Removal of Cr (III) and Ni (II) from tannery effluent using calcium carbonate coated bacterial magnetosomes. NPJ Clean Water 2018, 1, 1. [Google Scholar] [CrossRef]



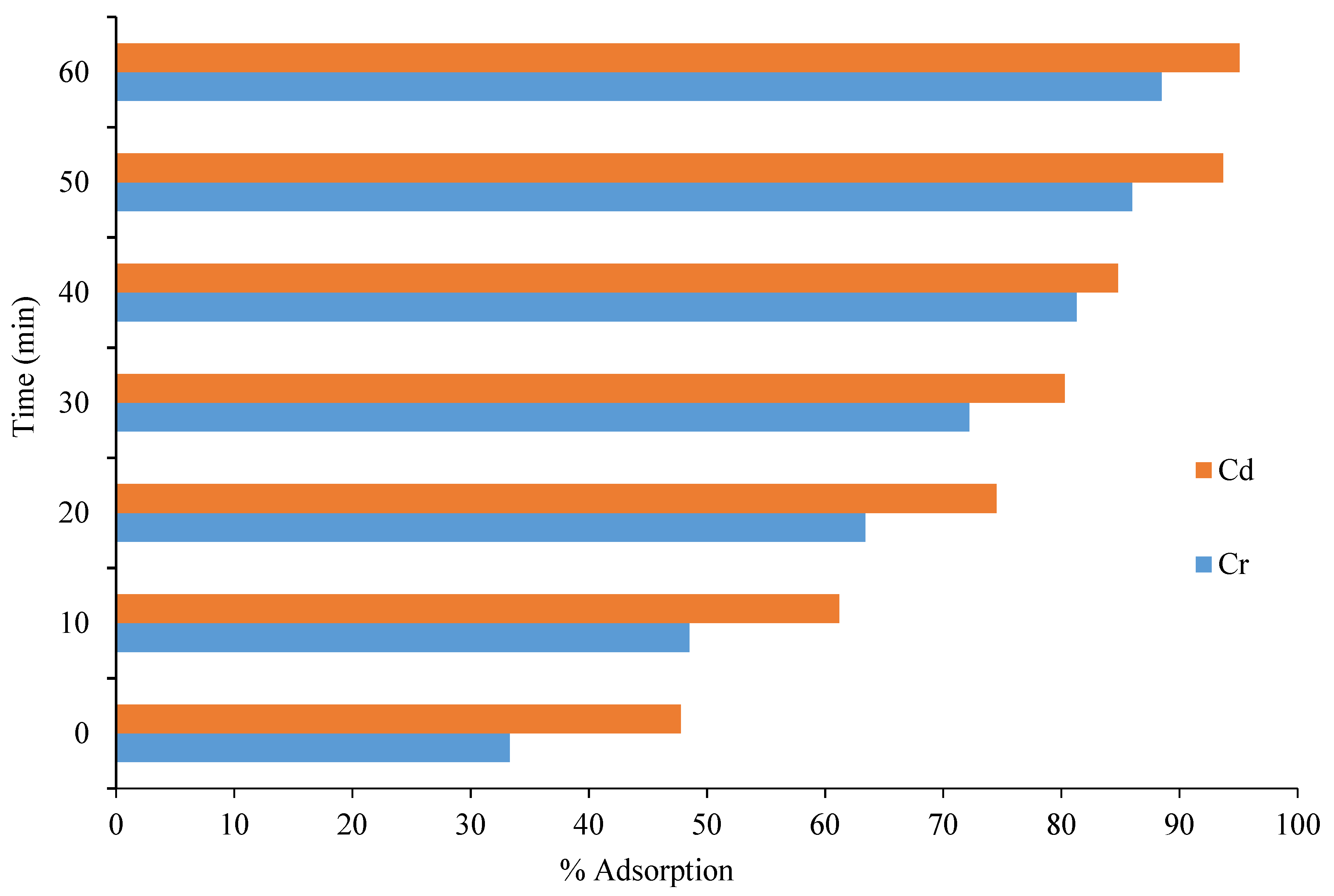
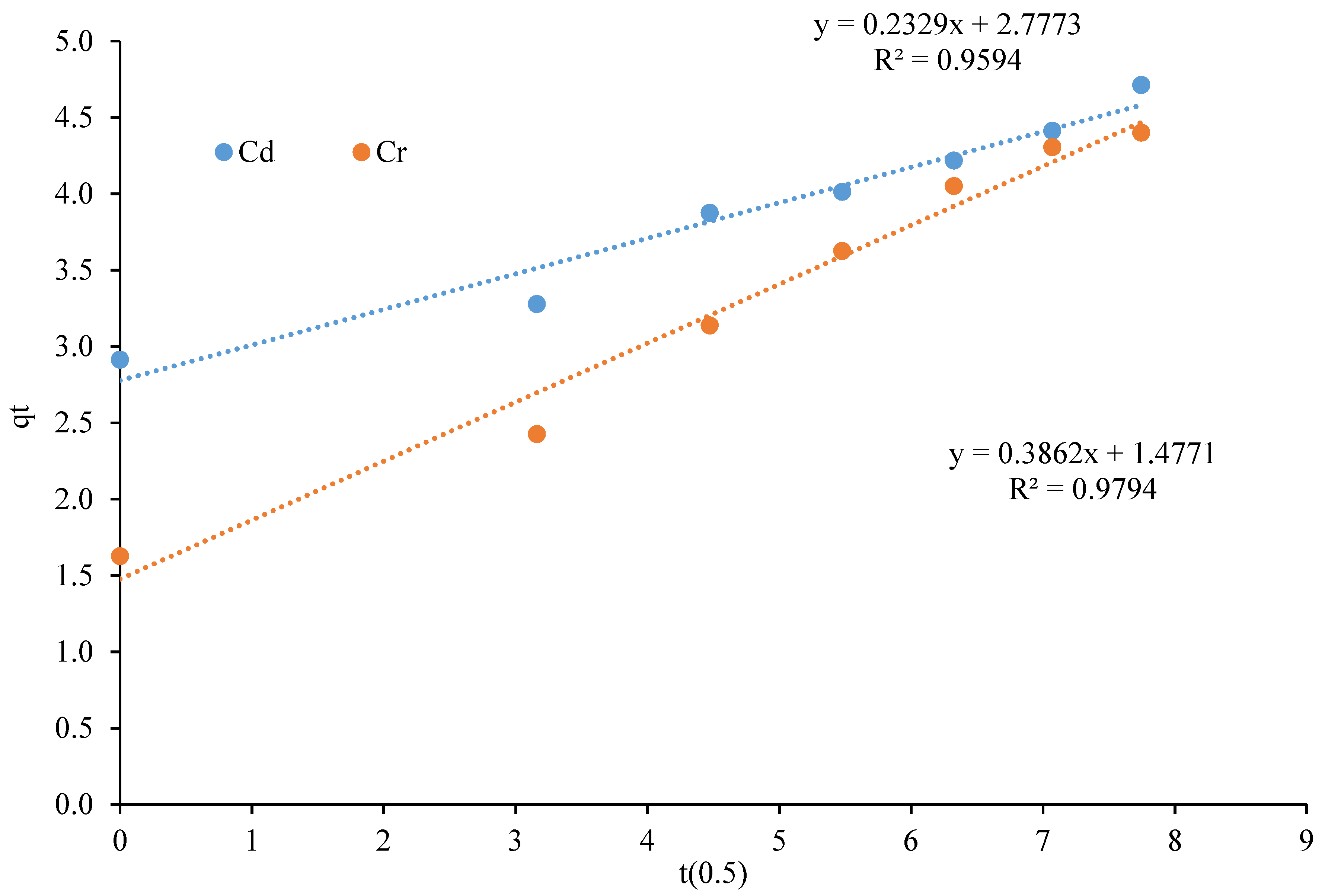
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gran, S.; Aziz, R.; Rafiq, M.T.; Abbasi, M.; Qayyum, A.; Elnaggar, A.Y.; Elganzory, H.H.; El-Bahy, Z.M.; Hussein, E.E. Development of Cerium Oxide/Corncob Nanocomposite: A Cost-Effective and Eco-Friendly Adsorbent for the Removal of Heavy Metals. Polymers 2021, 13, 4464. https://doi.org/10.3390/polym13244464
Gran S, Aziz R, Rafiq MT, Abbasi M, Qayyum A, Elnaggar AY, Elganzory HH, El-Bahy ZM, Hussein EE. Development of Cerium Oxide/Corncob Nanocomposite: A Cost-Effective and Eco-Friendly Adsorbent for the Removal of Heavy Metals. Polymers. 2021; 13(24):4464. https://doi.org/10.3390/polym13244464
Chicago/Turabian StyleGran, Sidra, Rukhsanda Aziz, Muhammad Tariq Rafiq, Maryam Abbasi, Abdul Qayyum, Ashraf Y. Elnaggar, Hussein H. Elganzory, Zeinhom M. El-Bahy, and Enas E. Hussein. 2021. "Development of Cerium Oxide/Corncob Nanocomposite: A Cost-Effective and Eco-Friendly Adsorbent for the Removal of Heavy Metals" Polymers 13, no. 24: 4464. https://doi.org/10.3390/polym13244464
APA StyleGran, S., Aziz, R., Rafiq, M. T., Abbasi, M., Qayyum, A., Elnaggar, A. Y., Elganzory, H. H., El-Bahy, Z. M., & Hussein, E. E. (2021). Development of Cerium Oxide/Corncob Nanocomposite: A Cost-Effective and Eco-Friendly Adsorbent for the Removal of Heavy Metals. Polymers, 13(24), 4464. https://doi.org/10.3390/polym13244464





