The Use of Bioactive Polymers for Intervention and Tissue Engineering: The New Frontier for Cardiovascular Therapy
Abstract
:1. The Clinical Problem
2. From Bare-Metal Stents to Absorbable Stents: The Evolutionary Phase of Percutaneous Coronary Intervention
2.1. First Generation of Drug-Eluting Stents
2.2. Second Generation of Drug-Eluting Stents
3. New Frontiers of Stenting
4. Bioresorbable Vascular Scaffolds
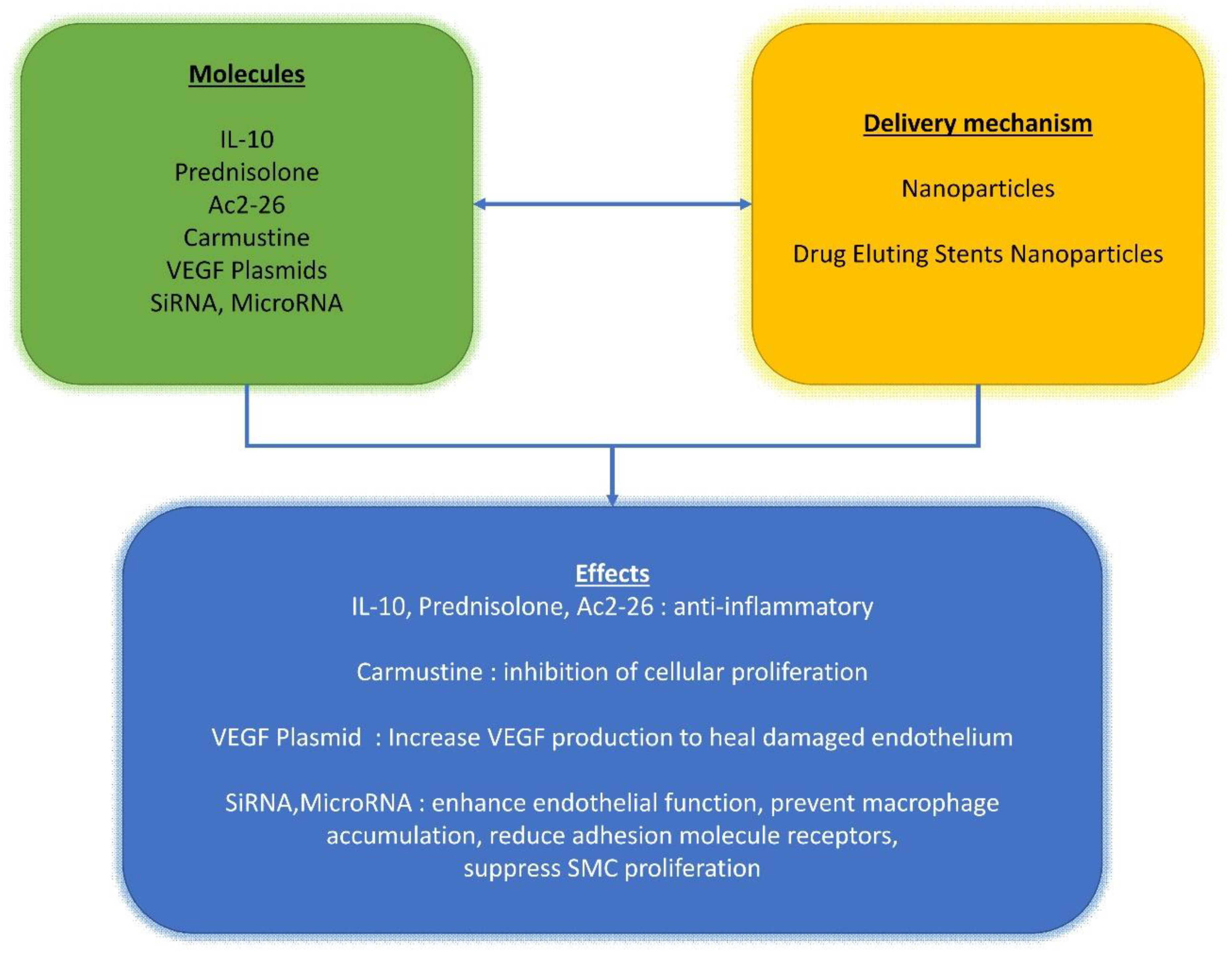
5. Drug Delivery Options for Cardiovascular Interventions: How and When
6. Future Direction for the Stent Design
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gaye, B.; Tajeu, G.S.; Vasan, R.S.; Lassale, C.; Allen, N.B.; Singh-Manoux, A.; Jouven, X. Association of Changes in Cardiovascular Health Metrics and Risk of Subsequent Cardiovascular Disease and Mortality. J. Am. Hear. Assoc. 2020, 9, e017458. [Google Scholar] [CrossRef] [PubMed]
- Boland, L.L.; Folsom, A.R.; Sorlie, P.D.; Taylor, H.A.; Rosamond, W.D.; Chambless, L.E.; Cooper, L.S. Occurrence of unrecognized myocardial infarction in subjects aged 45 to 65 years (the ARIC study). Am. J. Cardiol. 2002, 90, 927–931. [Google Scholar] [CrossRef]
- Fuster, V.; O’Rourke, R.A.; Walsh, R.; Poole-Wilson, P. Hurst’s the Heart; McGraw Hill Professional: New York, NY, USA, 2007. [Google Scholar]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Executive Summary: Heart Disease and Stroke Statistics—2014 Update: A Report from the American Heart Association. Circulation 2014, 129, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Community Surveillance Event Rates. Atherosclerosis Risk in Communities (ARIC) Study Website. Available online: https://sites.cscc.unc.edu/aric/ (accessed on 30 September 2020).
- Thom, T.; Kannel, W.B.; Silbershatz, H.; D’Agostino, R.B., Sr. Cardiovascular Diseases in the United States and Prevention Approaches. In Hurst’s the Heart; Fuster, V., Alexander, R.W., O’Rourke, R.A., Roberts, R., King, S.B., 3rd, Wellens, H.J.J., Eds.; McGraw-Hill: New York, NY, USA, 2001; pp. 3–18. [Google Scholar]
- Spadaccio, C.; Antoniades, C.; Nenna, A.; Chung, C.; Will, R.; Chello, M.; Gaudino, M.F.L. Preventing treatment failures in coronary artery disease: What can we learn from the biology of in-stent restenosis, vein graft failure, and internal thoracic arteries? Cardiovasc. Res. 2019, 116, 505–519. [Google Scholar] [CrossRef]
- Räber, L.; Brugaletta, S.; Yamaji, K.; O’Sullivan, C.J.; Otsuki, S.; Koppara, T.; Taniwaki, M.; Onuma, Y.; Freixa, X.; Eberli, F.R.; et al. Very Late Scaffold Thrombosis: Intracoronary Imaging and Histopathological and Spectroscopic Findings. J. Am. Coll. Cardiol. 2015, 66, 1901–1914. [Google Scholar] [CrossRef] [Green Version]
- Finn, A.V.; Nakazawa, G.; Joner, M.; Kolodgie, F.D.; Mont, E.K.; Gold, H.K.; Virmani, R. Vascular Responses to Drug Eluting Stents: Importance of Delayed Healing. Arter. Thromb. Vasc. Biol. 2007, 27, 1500–1510. [Google Scholar] [CrossRef]
- Joner, M.; Finn, A.V.; Farb, A.; Mont, E.K.; Kolodgie, F.D.; Ladich, E.; Kutys, R.; Skorija, K.; Gold, H.K.; Virmani, R. Pathology of Drug-Eluting Stents in Humans: Delayed Healing and Late Thrombotic Risk. J. Am. Coll. Cardiol. 2006, 48, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, G.; Finn, A.V.; Joner, M.; Ladich, E.; Kutys, R.; Mont, E.K.; Gold, H.K.; Burke, A.P.; Kolodgie, F.D.; Virmani, R. Delayed Arterial Healing and Increased Late Stent Thrombosis at Culprit Sites After Drug-Eluting Stent Placement for Acute Myocardial Infarction Patients. Circulation 2008, 118, 1138–1145. [Google Scholar] [CrossRef] [Green Version]
- Tada, T.; Byrne, R.A.; Simunovic, I.; King, L.A.; Cassese, S.; Joner, M.; Fusaro, M.; Schneider, S.; Schulz, S.; Ibrahim, T.; et al. Risk of Stent Thrombosis Among Bare-Metal Stents, First-Generation Drug-Eluting Stents, and Second-Generation Drug-Eluting Stents: Results from a registry of 18,334 patients. JACC Cardiovasc. Interv. 2013, 6, 1267–1274. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.S.; John, J.M.; Chew, D.P.; Lee, D.S.; Ellis, S.G.; Bhatt, D.L. Bare metal stent restenosis is not a benign clinical entity. Am. Hear. J. 2006, 151, 1260–1264. [Google Scholar] [CrossRef]
- Holmes, D.R.; Firth, B.G.; Wood, D.L. Paradigm shifts in cardiovascular medicine. J. Am. Coll. Cardiol. 2004, 43, 507–512. [Google Scholar] [CrossRef] [Green Version]
- Bozsak, F.; Gonzalez-Rodríguez, D.; Sternberger, Z.; Belitz, P.; Bewley, T.; Chomaz, J.-M.; Barakat, A.I. Optimization of Drug Delivery by Drug-Eluting Stents. PLoS ONE 2015, 10, e0130182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharya, G.; Park, K. Mechanisms of controlled drug release from drug-eluting stents. Adv. Drug Deliv. Rev. 2006, 58, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Seo, T.; Lafont, A.; Choi, S.-Y.; Barakat, A.I. Drug-Eluting Stent Design is a Determinant of Drug Concentration at the Endothelial Cell Surface. Ann. Biomed. Eng. 2016, 44, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.-W.; Levin, A.D.; Jonas, M.; Li, P.H.; Edelman, E.R. Thrombosis Modulates Arterial Drug Distribution for Drug-Eluting Stents. Circulation 2005, 111, 1619–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balakrishnan, B.; Dooley, J.F.; Kopia, G.; Edelman, E.R. Intravascular drug release kinetics dictate arterial drug deposition, retention, and distribution. J. Control. Release 2007, 123, 100–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, J.E.; Costa, M.A.; Abizaid, A.; Rensing, B.J.; Abizaid, A.S.; Tanajura, L.F.; Kozuma, K.; Van Langenhove, G.; Sousa, A.G.; Falotico, R.; et al. Sustained Suppression of Neointimal Proliferation by Sirolimus-Eluting Stents: One-year angiographic and intravascular ultrasound follow-up. Circulation 2001, 104, 2007–2011. [Google Scholar] [CrossRef] [Green Version]
- Grube, E.; Silber, S.; Hauptmann, K.E.; Mueller, R.; Buellesfeld, L.; Gerckens, U.; Russell, M.E. TAXUS I: Six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation 2003, 107, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Morice, M.-C.; Serruys, P.W.; Sousa, J.E.; Fajadet, J.; Hayashi, E.B.; Perin, M.; Colombo, A.; Schuler, G.; Barragan, P.; Guagliumi, G.; et al. A Randomized Comparison of a Sirolimus-Eluting Stent with a Standard Stent for Coronary Revascularization. N. Engl. J. Med. 2002, 346, 1773–1780. [Google Scholar] [CrossRef] [Green Version]
- Colombo, A.; Drzewiecki, J.; Banning, A.; Grube, E.; Hauptmann, K.; Silber, S.; Dudek, D.; Fort, S.; Schiele, F.; Zmudka, K.; et al. Randomized Study to Assess the Effectiveness of Slow- and Moderate-Release Polymer-Based Paclitaxel-Eluting Stents for Coronary Artery Lesions. Circulation 2003, 108, 788–794. [Google Scholar] [CrossRef] [Green Version]
- Moses, J.W.; Leon, M.B.; Popma, J.J.; Fitzgerald, P.J.; Holmes, D.R.; O’Shaughnessy, C.; Caputo, R.; Kereiakes, D.J.; Williams, D.O.; Teirstein, P.S.; et al. Sirolimus-Eluting Stents versus Standard Stents in Patients with Stenosis in a Native Coronary Artery. N. Engl. J. Med. 2003, 349, 1315–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, G.W.; Ellis, S.G.; Cox, D.A.; Hermiller, J.; O’Shaughnessy, C.; Mann, J.T.; Turco, M.; Caputo, R.; Bergin, P.; Greenberg, J.; et al. A Polymer-Based, Paclitaxel-Eluting Stent in Patients with Coronary Artery Disease. N. Engl. J. Med. 2004, 350, 221–231. [Google Scholar] [CrossRef]
- Marx, S.O.; Jayaraman, T.; Go, L.O.; Marks, A.R. Rapamycin-FKBP Inhibits Cell Cycle Regulators of Proliferation in Vascular Smooth Muscle Cells. Circ. Res. 1995, 76, 412–417. [Google Scholar] [CrossRef]
- Axel, D.I.; Kunert, W.; Göggelmann, C.; Oberhoff, M.; Herdeg, C.; Küttner, A.; Wild, D.H.; Brehm, B.R.; Riessen, R.; Köveker, G.; et al. Paclitaxel Inhibits Arterial Smooth Muscle Cell Proliferation and Migration In Vitro and In Vivo Using Local Drug Delivery. Circulation 1997, 96, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Farb, A.; Heller, P.F.; Shroff, S.; Cheng, L.; Kolodgie, F.D.; Carter, A.J.; Scott, D.S.; Froehlich, J.; Virmani, R. Pathological analysis of local delivery of paclitaxel via a polymer-coated stent. Circulation 2001, 104, 473–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfisterer, M.; Rocca, H.P.B.-L.; Buser, P.T.; Rickenbacher, P.; Hunziker, P.; Mueller, C.; Jeger, R.; Bader, F.; Osswald, S.; Kaiser, C. Late Clinical Events After Clopidogrel Discontinuation May Limit the Benefit of Drug-Eluting Stents: An observational study of drug-eluting versus bare-metal stents. J. Am. Coll. Cardiol. 2006, 48, 2584–2591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finn, A.V.; Joner, M.; Nakazawa, G.; Kolodgie, F.; Newell, J.; John, M.C.; Gold, H.K.; Virmani, R. Pathological Correlates of Late Drug-Eluting Stent Thrombosis: Strut Coverage as a Marker of Endothelialization. Circulation 2007, 115, 2435–2441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Kopia, G.; Hayashi, S.-I.; Bailey, L.R.; Llanos, G.; Wilensky, R.; Klugherz, B.D.; Papandreou, G.; Narayan, P.; Leon, M.B.; et al. Stent-Based Delivery of Sirolimus Reduces Neointimal Formation in a Porcine Coronary Model. Circulation 2001, 104, 1188–1193. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, G.; Otsuka, F.; Nakano, M.; Vorpahl, M.; Yazdani, S.K.; Ladich, E.; Kolodgie, F.D.; Finn, A.V.; Virmani, R. The Pathology of Neoatherosclerosis in Human Coronary Implants Bare-Metal and Drug-Eluting Stents. J. Am. Coll. Cardiol. 2011, 57, 1314–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaulding, C.; Teiger, E.; Commeau, P.; Varenne, O.; Bramucci, E.; Slama, M.; Beatt, K.; Tirouvanziam, A.; Polonski, L.; Stella, P.R.; et al. Four-Year Follow-Up of TYPHOON (Trial to Assess the Use of the CYPHer Sirolimus-Eluting Coronary Stent in Acute Myocardial Infarction Treated With BallOON Angioplasty). JACC Cardiovasc. Interv. 2011, 4, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Mehilli, J.; Byrne, R.A.; Tiroch, K.; Pinieck, S.; Schulz, S.; Kufner, S.; Massberg, S.; Laugwitz, K.-L.; Schömig, A.; Kastrati, A. Randomized Trial of Paclitaxel- Versus Sirolimus-Eluting Stents for Treatment of Coronary Restenosis in Sirolimus-Eluting Stents: The ISAR-DESIRE 2 (Intracoronary Stenting and Angiographic Results: Drug Eluting Stents for In-Stent Restenosis 2) study. J. Am. Coll. Cardiol. 2010, 55, 2710–2716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, R.A.; Neumann, F.-J.; Mehilli, J.; Pinieck, S.; Wolff, B.; Tiroch, K.; Schulz, S.; Fusaro, M.; Ott, I.; Ibrahim, T.; et al. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): A randomised, open-label trial. Lancet 2013, 381, 461–467. [Google Scholar] [CrossRef]
- Colleran, R.; Joner, M.; Kufner, S.; Altevogt, F.; Neumann, F.-J.; Abdel-Wahab, M.; Bohner, J.; Valina, C.; Richardt, G.; Zrenner, B.; et al. Comparative efficacy of two paclitaxel-coated balloons with different excipient coatings in patients with coronary in-stent restenosis: A pooled analysis of the Intracoronary Stenting and Angiographic Results: Optimizing Treatment of Drug Eluting Stent In-Stent Restenosis 3 and 4 (ISAR-DESIRE 3 and ISAR-DESIRE 4) trials. Int. J. Cardiol. 2018, 252, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Radeleff, B.; Lopez-Benitez, R.; Stampfl, U.; Stampfl, S.; Sommer, C.; Thierjung, H.; Berger, I.; Kauffmann, G.; Richter, G.M. Paclitaxel-induced Arterial Wall Toxicity and Inflammation: Tissue Uptake in Various Dose Densities in a Minipig Model. J. Vasc. Interv. Radiol. 2010, 21, 1262–1270. [Google Scholar] [CrossRef]
- Dibra, A.; Kastrati, A.; Mehilli, J.; Pache, J.; Schühlen, H.; Von Beckerath, N.; Ulm, K.; Wessely, R.; Dirschinger, J.; Schömig, A. Paclitaxel-Eluting or Sirolimus-Eluting Stents to Prevent Restenosis in Diabetic Patients. N. Engl. J. Med. 2005, 353, 663–670. [Google Scholar] [CrossRef] [Green Version]
- Kastrati, A.; Dibra, A.; Eberle, S.; Mehilli, J.; De Lezo, J.S.; Goy, J.-J.; Ulm, K.; Schömig, A. Sirolimus-Eluting Stents vs Paclitaxel-Eluting Stents in Patients With Coronary Artery Disease: Meta-Analysis of Randomized Trials. JAMA 2005, 294, 819–825. [Google Scholar] [CrossRef]
- Bozsak, F.; Chomaz, J.-M.; Barakat, A.I. Modeling the transport of drugs eluted from stents: Physical phenomena driving drug distribution in the arterial wall. Biomech. Model. Mechanobiol. 2014, 13, 327–347. [Google Scholar] [CrossRef]
- Rittger, H.; Brachmann, J.; Sinha, A.-M.; Waliszewski, M.; Ohlow, M.; Brugger, A.; Thiele, H.; Birkemeyer, R.; Kurowski, V.; Breithardt, O.-A.; et al. A Randomized, Multicenter, Single-Blinded Trial Comparing Paclitaxel-Coated Balloon Angioplasty with Plain Balloon Angioplasty in Drug-Eluting Stent Restenosis: The PEPCAD-DES Study. J. Am. Coll. Cardiol. 2012, 59, 1377–1382. [Google Scholar] [CrossRef] [Green Version]
- Miura, K.; Kadota, K.; Habara, S.; Shimada, T.; Ohya, M.; Amano, H.; Kubo, S.; Hyodo, Y.; Otsuru, S.; Tada, T.; et al. Five-Year Outcomes After Paclitaxel-Coated Balloon Angioplasty for Drug-Eluting Stent Restenosis. Am. J. Cardiol. 2017, 119, 365–371. [Google Scholar] [CrossRef]
- Chevalier, B.; Serruys, P.W.; Silber, S.; Garcia, E.; Suryapranata, H.; Hauptmann, K.; Wijns, W.; Schuler, G.; Fath-Ordoubadi, F.; Worthley, S.; et al. Randomised comparison of Nobori, biolimus A9-eluting coronary stent with a Taxus(R), paclitaxel-eluting coronary stent in patients with stenosis in native coronary arteries: The Nobori 1 trial. EuroIntervention 2007, 2, 426–434. [Google Scholar]
- Chevalier, B.; Silber, S.; Park, S.-J.; Garcia, E.; Schuler, G.; Suryapranata, H.; Koolen, J.; Hauptmann, K.E.; Wijns, W.; Morice, M.-C.; et al. Randomized Comparison of the Nobori Biolimus A9-Eluting Coronary Stent With the Taxus Liberté Paclitaxel-Eluting Coronary Stent in Patients With Stenosis in Native Coronary Arteries: The NOBORI 1 Trial—Phase 2. Circ. Cardiovasc. Interv. 2009, 2, 188–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christiansen, E.H.; Jensen, L.O.; Thayssen, P.; Tilsted, H.-H.; Krusell, L.R.; Hansen, K.N.; Kaltoft, A.; Maeng, M.; Kristensen, S.D.; Bøtker, H.E.; et al. Biolimus-eluting biodegradable polymer-coated stent versus durable polymer-coated sirolimus-eluting stent in unselected patients receiving percutaneous coronary intervention (SORT OUT V): A randomised non-inferiority trial. Lancet 2013, 381, 661–669. [Google Scholar] [CrossRef] [Green Version]
- Smits, P.C.; Hofma, S.; Togni, M.; Vázquez, N.; Valdés, M.; Voudris, V.; Slagboom, T.; Goy, J.-J.; Vuillomenet, A.; Serra, A.; et al. Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (COMPARE II): A randomised, controlled, non-inferiority trial. Lancet 2013, 381, 651–660. [Google Scholar] [CrossRef]
- Windecker, S.; Serruys, P.W.; Wandel, S.; Buszman, P.; Trznadel, S.; Linke, A.; Lenk, K.; Ischinger, T.; Klauss, V.; Eberli, F.; et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): A randomised non-inferiority trial. Lancet 2008, 372, 1163–1173. [Google Scholar] [CrossRef]
- Navarese, E.P.; Tandjung, K.; Claessen, B.; Andreotti, F.; Kowalewski, M.; Kandzari, D.E.; Kereiakes, D.J.; Waksman, R.; Mauri, L.; Meredith, I.T.; et al. Safety and efficacy outcomes of first and second generation durable polymer drug eluting stents and biodegradable polymer biolimus eluting stents in clinical practice: Comprehensive network meta-analysis. BMJ 2013, 347, f6530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valgimigli, M.; Tebaldi, M.; Borghesi, M.; Vranckx, P.; Campo, G.; Tumscitz, C.; Cangiano, E.; Minarelli, M.; Scalone, A.; Cavazza, C.; et al. Two-Year Outcomes After First- or Second-Generation Drug-Eluting or Bare-Metal Stent Implantation in All-Comer Patients Undergoing Percutaneous Coronary Intervention: A Pre-Specified Analysis from the PRODIGY Study (Prolonging Dual Antiplatelet Treatment after Grading Stent-Induced Intimal Hyperplasia Study). JACC Cardiovasc. Interv. 2014, 7, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, N.; Ito, Y.; Hirano, K.; Yamawaki, M.; Araki, M.; Sakai, T.; Takimura, H.; Sakamoto, Y.; Mori, S.; Tsutsumi, M.; et al. Comparison of first- and second-generation drug-eluting stent efficacies for treating left main and/or three-vessel disease: A propensity matched study. Hear. Vessel. 2016, 31, 1930–1942. [Google Scholar] [CrossRef]
- Palmerini, T.; Benedetto, U.; Biondi-Zoccai, G.; Della Riva, D.; Bacchi-Reggiani, L.; Smits, P.C.; Vlachojannis, G.J.; Jensen, L.O.; Christiansen, E.H.; Berencsi, K.; et al. Long-Term Safety of Drug-Eluting and Bare-Metal Stents: Evidence From a Comprehensive Network Meta-Analysis. J. Am. Coll. Cardiol. 2015, 65, 2496–2507. [Google Scholar] [CrossRef] [Green Version]
- Bangalore, S.; Kumar, S.; Fusaro, M.; Amoroso, N.; Attubato, M.J.; Feit, F.; Bhatt, D.L.; Slater, J. Short- and Long-Term Outcomes With Drug-Eluting and Bare-Metal Coronary Stents: A mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation 2012, 125, 2873–2891. [Google Scholar] [CrossRef] [Green Version]
- Hou, D.; Huibregtse, B.; Dawkins, K.; Donnelly, J.; Roy, K.; Chen, J.P.; Akinapelli, A. Current State of Bioabsorbable Polymer-Coated Drug-Eluting Stents. Curr. Cardiol. Rev. 2017, 13, 139–154. [Google Scholar] [CrossRef] [Green Version]
- Camenzind, E.; Wijns, W.; Mauri, L.; Kurowski, V.; Parikh, K.; Gao, R.; Bode, C.; Greenwood, J.P.; Boersma, E.; Vranckx, P.; et al. Stent thrombosis and major clinical events at 3 years after zotarolimus-eluting or sirolimus-eluting coronary stent implantation: A randomised, multicentre, open-label, controlled trial. Lancet 2012, 380, 1396–1405. [Google Scholar] [CrossRef]
- Palmerini, T.; Biondi-Zoccai, G.; Della Riva, D.; Mariani, A.; Sabaté, M.; Smits, P.C.; Kaiser, C.; D’Ascenzo, F.; Frati, G.; Mancone, M.; et al. Clinical Outcomes with Bioabsorbable Polymer- Versus Durable Polymer-Based Drug-Eluting and Bare-Metal Stents: Evidence from a Comprehensive Network Meta-Analysis. J. Am. Coll. Cardiol. 2014, 63, 299–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lammer, J.; Zeller, T.; Hausegger, K.A.; Schaefer, P.J.; Gschwendtner, M.; Mueller-Huelsbeck, S.; Rand, T.; Funovics, M.; Wolf, F.; Rastan, A.; et al. Heparin-Bonded Covered Stents Versus Bare-Metal Stents for Complex Femoropopliteal Artery Lesions: The Randomized VIASTAR Trial (Viabahn endoprosthesis with PROPATEN Bioactive Surface [VIA] Versus Bare Nitinol Stent in the Treatment of Long Lesions in Superficial Femoral Artery Occlusive Disease). J. Am. Coll. Cardiol. 2013, 62, 1320–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxon, R.R.; Chervu, A.; Jones, P.A.; Bajwa, T.K.; Gable, D.R.; Soukas, P.A.; Begg, R.J.; Adams, J.G.; Ansel, G.M.; Schneider, D.B.; et al. Heparin-bonded, Expanded Polytetrafluoroethylene-lined Stent Graft in the Treatment of Femoropopliteal Artery Disease: 1-Year Results of the VIPER (Viabahn Endoprosthesis with Heparin Bioactive Surface in the Treatment of Superficial Femoral Artery Obstructive Disease) Trial. J. Vasc. Interv. Radiol. 2013, 24, 165–173. [Google Scholar] [CrossRef]
- Carrié, D.; Berland, J.; Verheye, S.; Hauptmann, K.E.; Vrolix, M.; Violini, R.; Dibie, A.; Berti, S.; Maupas, E.; Antoniucci, D.; et al. A Multicenter Randomized Trial Comparing Amphilimus- With Paclitaxel-Eluting Stents in De Novo Native Coronary Artery Lesions. J. Am. Coll. Cardiol. 2012, 59, 1371–1376. [Google Scholar] [CrossRef] [Green Version]
- Urban, P.; Meredith, I.T.; Abizaid, A.; Pocock, S.J.; Carrié, D.; Naber, C.; Lipiecki, J.; Richardt, G.; Iñiguez, A.; Brunel, P.; et al. Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. N. Engl. J. Med. 2015, 373, 2038–2047. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.R.; Abizaid, A.; Costa, R.; Feres, F.; Tanajura, L.F.; Abizaid, A.; Maldonado, G.; Staico, R.; Siqueira, D.; Sousa, A.G.; et al. 1-Year Results of the Hydroxyapatite Polymer-Free Sirolimus-Eluting Stent for the Treatment of Single De Novo Coronary Lesions. JACC Cardiovasc. Interv. 2009, 2, 422–427. [Google Scholar] [CrossRef] [Green Version]
- Van Der Giessen, W.J.; Sorop, O.; Serruys, P.W.; Peters-Krabbendam, I.; Van Beusekom, H.M. Lowering the Dose of Sirolimus, Released From a Nonpolymeric Hydroxyapatite Coated Coronary Stent, Reduces Signs of Delayed Healing. JACC Cardiovasc. Interv. 2009, 2, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein, A.; McClean, D.; Kar, S.; Takizawa, K.; Varghese, K.; Baek, N.; Park, K.; Fishbein, M.C.; Makkar, R.; Litvack, F.; et al. Local Drug Delivery via a Coronary Stent With Programmable Release Pharmacokinetics. Circulation 2003, 107, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Krucoff, M.W.; Kereiakes, D.J.; Petersen, J.L.; Mehran, R.; Hasselblad, V.; Lansky, A.J.; Fitzgerald, P.J.; Garg, J.; Turco, M.A.; Simonton, C.A.; et al. A Novel Bioresorbable Polymer Paclitaxel-Eluting Stent for the Treatment of Single and Multivessel Coronary Disease: Primary results of the COSTAR (Cobalt Chromium Stent with Antiproliferative for Restenosis) II study. J. Am. Coll. Cardiol. 2008, 51, 1543–1552. [Google Scholar] [CrossRef]
- Spadaccio, C.; Nappi, F.; De Marco, F.; Sedati, P.; Sutherland, F.W.; Chello, M.; Trombetta, M.; Rainer, A. Preliminary in Vivo Evaluation of a Hybrid Armored Vascular Graft Combining Electrospinning and Additive Manufacturing Techniques. Drug Target. Insights 2016, 10, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spadaccio, C.; Rainer, A.; Centola, M.; Trombetta, M.; Chello, M.; Lusini, M.; Covino, E.; Toyoda, Y.; Genovese, J.A. Heparin-releasing scaffold for stem cells: A differentiating device for vascular aims. Regen. Med. 2010, 5, 645–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, L.-C.; Chen, Y.-H.; Chen, M.-C. Dual Drug-Eluting Stents Coated with Multilayers of Hydrophobic Heparin and Sirolimus. ACS Appl. Mater. Interfaces 2013, 5, 12944–12953. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Liu, Z.; Shen, L.; Guo, Z.; Chou, L.L.; Zhong, W.; Du, Q.; Ge, J. The effect of a layer-by-layer chitosan–heparin coating on the endothelialization and coagulation properties of a coronary stent system. Biomaterials 2009, 30, 2276–2283. [Google Scholar] [CrossRef] [PubMed]
- Hossfeld, S.; Nolte, A.; Hartmann, H.; Recke, M.; Schaller, M.; Walker, T.; Kjems, J.; Schlosshauer, B.; Stoll, D.; Wendel, H.P.; et al. Bioactive coronary stent coating based on layer-by-layer technology for siRNA release. Acta Biomater. 2013, 9, 6741–6752. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, T.; Chen, J.; Maitz, M.; Chen, C.; Huang, N. Influence of a layer-by-layer-assembled multilayer of anti-CD34 antibody, vascular endothelial growth factor, and heparin on the endothelialization and anticoagulation of titanium surface. J. Biomed. Mater. Res. Part A 2012, 101, 1144–1157. [Google Scholar] [CrossRef]
- Spadaccio, C.; Nappi, F.; De Marco, F.; Sedati, P.; Taffon, C.; Nenna, A.; Crescenzi, A.; Chello, M.; Trombetta, M.; Gambardella, I.; et al. Implantation of a Poly-l-Lactide GCSF-Functionalized Scaffold in a Model of Chronic Myocardial Infarction. J. Cardiovasc. Transl. Res. 2017, 10, 47–65. [Google Scholar] [CrossRef] [Green Version]
- Spadaccio, C.; Rainer, A.; De Porcellinis, S.; Centola, M.; De Marco, F.; Chello, M.; Trombetta, M.; Genovese, J.A. A G-CSF functionalized PLLA scaffold for wound repair: An in vitro preliminary study. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2010, 2010, 843–846. [Google Scholar] [CrossRef]
- Haude, M.; Ince, H.; Abizaid, A.; Toelg, R.; Lemos, P.A.; Von Birgelen, C.; Christiansen, E.H.; Wijns, W.; Neumann, F.-J.; Kaiser, C.; et al. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month results of a prospective, multicentre, non-randomised, first-in-man trial. Lancet 2016, 387, 31–39. [Google Scholar] [CrossRef]
- Serruys, P.W.; Ormiston, J.; Van Geuns, R.-J.; De Bruyne, B.; Dudek, D.; Christiansen, E.; Chevalier, B.; Smits, P.; McClean, D.; Koolen, J.; et al. A Polylactide Bioresorbable Scaffold Eluting Everolimus for Treatment of Coronary Stenosis. J. Am. Coll. Cardiol. 2016, 67, 766–776. [Google Scholar] [CrossRef]
- Farhan, S.; Hemetsberger, R.; Matiasek, J.; Strehblow, C.; Pavo, N.; Khorsand, A.; Petnehazy, O.; Petrasi, Z.; Kaider, A.; Glogar, D.; et al. Implantation of paclitaxel-eluting stent impairs the vascular compliance of arteries in porcine coronary stenting model. Atherosclerosis 2009, 202, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Selvarasu, N.K.C.; Tafti, D.; Vlachos, P. Hydrodynamic Effects of Compliance Mismatch in Stented Arteries. J. Biomech. Eng. 2011, 133, 021008. [Google Scholar] [CrossRef] [PubMed]
- Abizaid, A.; Costa, R.A.; Schofer, J.; Ormiston, J.; Maeng, M.; Witzenbichler, B.; Botelho, R.V.; Costa, J.R.; Chamié, D.; Abizaid, A.S.; et al. Serial Multimodality Imaging and 2-Year Clinical Outcomes of the Novel DESolve Novolimus-Eluting Bioresorbable Coronary Scaffold System for the Treatment of Single De Novo Coronary Lesions. JACC Cardiovasc. Interv. 2016, 9, 565–574. [Google Scholar] [CrossRef] [PubMed]
- El-Hayek, G.; Bangalore, S.; Dominguez, A.C.; Devireddy, C.; Jaber, W.; Kumar, G.; Mavromatis, K.; Tamis-Holland, J.; Samady, H. Meta-Analysis of Randomized Clinical Trials Comparing Biodegradable Polymer Drug-Eluting Stent to Second-Generation Durable Polymer Drug-Eluting Stents. JACC Cardiovasc. Interv. 2017, 10, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.A.; Serruys, P.W.; Kimura, T.; Gao, R.; Ellis, S.G.; Kereiakes, D.J.; Onuma, Y.; Simonton, C.; Zhang, Z.; Stone, G.W. 2-year outcomes with the Absorb bioresorbable scaffold for treatment of coronary artery disease: A systematic review and meta-analysis of seven randomised trials with an individual patient data substudy. Lancet 2017, 390, 760–772. [Google Scholar] [CrossRef]
- Stone, G.W.; Gao, R.; Kimura, T.; Kereiakes, D.J.; Ellis, S.G.; Onuma, Y.; Cheong, W.-F.; Jones-McMeans, J.; Su, X.; Zhang, Z.; et al. 1-year outcomes with the Absorb bioresorbable scaffold in patients with coronary artery disease: A patient-level, pooled meta-analysis. Lancet 2016, 387, 1277–1289. [Google Scholar] [CrossRef]
- Kereiakes, D.J.; Ellis, S.G.; Metzger, D.C.; Caputo, R.P.; Rizik, D.G.; Teirstein, P.S.; Litt, M.R.; Kini, A.; Kabour, A.; Marx, S.O.; et al. Clinical Outcomes Before and After Complete Everolimus-Eluting Bioresorbable Scaffold Resorption: Five-Year Follow-Up From the ABSORB III Trial. Circulation 2019, 140, 1895–1903. [Google Scholar] [CrossRef]
- Stone, G.W.; Kimura, T.; Gao, R.; Kereiakes, D.J.; Ellis, S.G.; Onuma, Y.; Chevalier, B.; Simonton, C.; Dressler, O.; Crowley, A.; et al. Time-Varying Outcomes with the Absorb Bioresorbable Vascular Scaffold During 5-Year Follow-up: A Systematic Meta-analysis and Individual Patient Data Pooled Study. JAMA Cardiol. 2019, 4, 1261–1269. [Google Scholar] [CrossRef]
- Toyota, T.; Morimoto, T.; Shiomi, H.; Yoshikawa, Y.; Yaku, H.; Yamashita, Y.; Kimura, T. Very Late Scaffold Thrombosis of Bioresorbable Vascular Scaffold: Systematic Review and a Meta-Analysis. JACC Cardiovasc. Interv. 2017, 10, 27–37. [Google Scholar] [CrossRef]
- Nappi, F.; Fraldi, M.; Spadaccio, C.; Carotenuto, A.R.; Arcucci, A.; Castaldo, C.; Chachques, J.C.; Acar, C. Biomechanics drive histological wall remodeling of neoaortic root: A mathematical model to study the expression levels of ki 67, metalloprotease, and apoptosis transition. J. Biomed. Mater. Res. Part A 2016, 104, 2785–2793. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C.; Fouret, P.; Hammoudi, N.; Chachques, J.C.; Chello, M.; Acar, C. An experimental model of the Ross operation: Development of resorbable reinforcements for pulmonary autografts. J. Thorac. Cardiovasc. Surg. 2015, 149, 1134–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nappi, F.; Carotenuto, A.R.; Cutolo, A.; Fouret, P.; Acar, C.; Chachques, J.C.; Fraldi, M. Compliance mismatch and compressive wall stresses drive anomalous remodelling of pulmonary trunks reinforced with Dacron grafts. J. Mech. Behav. Biomed. Mater. 2016, 63, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Carotenuto, A.; Di Vito, D.; Spadaccio, C.; Acar, C.; Fraldi, M. Stress-shielding, growth and remodeling of pulmonary artery reinforced with copolymer scaffold and transposed into aortic position. Biomech. Model. Mechanobiol. 2015, 15, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Kedhi, E.; Suryapranata, H.; Galasso, G.; Dudek, D.; De Luca, G. Polymer-Free vs. Polymer-Coated Drug-Eluting Stents for the Treatment of Coronary Artery Disease: A Meta-Analysis of 16 Randomized Trials. Cardiovasc. Revasc. Med. 2020, 21, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Sanchez, J.; Chiarito, M.; Cortese, B.; Moretti, A.; Pagnotta, P.; Reimers, B.; Stefanini, G.G.; Ferrante, G. Drug-Coated balloons vs drug-eluting stents for the treatment of small coronary artery disease: A meta-analysis of randomized trials. Catheter. Cardiovasc. Interv. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Sun, X.; Liu, H.; Pang, X.; Dong, S. Drug-coated balloon versus drug-eluting stent for treating de novo coronary lesions in large vessels: A meta-analysis of clinical trials. Herz 2020. [Google Scholar] [CrossRef]
- Spadaccio, C.; Rainer, A.; Trombetta, M.; Centola, M.; Lusini, M.; Chello, M.; Covino, E.; De Marco, F.; Coccia, R.; Toyoda, Y.; et al. A G-CSF functionalized scaffold for stem cells seeding: A differentiating device for cardiac purposes. J. Cell. Mol. Med. 2010, 15, 1096–1108. [Google Scholar] [CrossRef]
- Biran, R.; Pond, D. Heparin coatings for improving blood compatibility of medical devices. Adv. Drug Deliv. Rev. 2017, 112, 12–23. [Google Scholar] [CrossRef]
- Jeon, O.; Song, S.J.; Kang, S.-W.; Putnam, A.J.; Kim, B.-S. Enhancement of ectopic bone formation by bone morphogenetic protein-2 released from a heparin-conjugated poly(l-lactic-co-glycolic acid) scaffold. Biomaterials 2007, 28, 2763–2771. [Google Scholar] [CrossRef]
- Kang, H.-J.; Kim, H.-S.; Zhang, S.-Y.; Park, K.W.; Cho, H.-J.; Koo, B.-K.; Kim, Y.-J.; Lee, D.S.; Sohn, D.-W.; Han, K.-S.; et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: The MAGIC cell randomised clinical trial. Lancet 2004, 363, 751–756. [Google Scholar] [CrossRef]
- Rainer, A.; Spadaccio, C.; Sedati, P.; De Marco, F.; Carotti, S.; Lusini, M.; Vadalà, G.; Di Martino, A.; Morini, S.; Chello, M.; et al. Electrospun Hydroxyapatite-Functionalized PLLA Scaffold: Potential Applications in Sternal Bone Healing. Ann. Biomed. Eng. 2011, 39, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Spadaccio, C.; Rainer, A.; Trombetta, M.; Vadala, G.; Chello, M.; Covino, E.; Denaro, V.; Toyoda, Y.; Genovese, J.A. Poly-l-Lactic Acid/Hydroxyapatite Electrospun Nanocomposites Induce Chondrogenic Differentiation of Human MSC. Ann. Biomed. Eng. 2009, 37, 1376–1389. [Google Scholar] [CrossRef] [PubMed]
- Centola, M.; Rainer, A.; Spadaccio, C.; De Porcellinis, S.; Genovese, J.A.; Trombetta, M. Combining electrospinning and fused deposition modeling for the fabrication of a hybrid vascular graft. Biofabrication 2010, 2, 014102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannitelli, S.; Accoto, D.; Trombetta, M.; Rainer, A. Current trends in the design of scaffolds for computer-aided tissue engineering. Acta Biomater. 2014, 10, 580–594. [Google Scholar] [CrossRef]
- Giannitelli, S.M.; Mozetic, P.; Trombetta, M.; Rainer, A. Combined additive manufacturing approaches in tissue engineering. Acta Biomater. 2015, 24, 1–11. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, X.; Han, F.; Zhao, J.; Zhao, Y.; Fan, Y.; Yuan, X. Dual-delivery of VEGF and PDGF by double-layered electrospun membranes for blood vessel regeneration. Biomaterials 2013, 34, 2202–2212. [Google Scholar] [CrossRef]
- Ismail, H.M.; Ali-Adib, S.; Younes, H.M. Reactive and functionalized electrospun polymeric nanofibers for drug delivery and tissue engineering applications. Ther. Deliv. 2019, 10, 397–399. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Kim, M.; Lee, H. Recent Advances on Nanofiber Fabrications: Unconventional State-of-the-Art Spinning Techniques. Polymers 2020, 12, 1386. [Google Scholar] [CrossRef]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2010, 8, 153–170. [Google Scholar] [CrossRef] [Green Version]
- Tabata, Y. Protein release from gelatin matrices. Adv. Drug Deliv. Rev. 1998, 31, 287–301. [Google Scholar] [CrossRef]
- Ismail, H.; Zamani, S.; Elrayess, M.A.; Kafienah, W.; Younes, H.M. New Three-Dimensional Poly(decanediol-co-tricarballylate) Elastomeric Fibrous Mesh Fabricated by Photoreactive Electrospinning for Cardiac Tissue Engineering Applications. Polymers 2018, 10, 455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nappi, F.; Spadaccio, C.; Chello, M.; Acar, C. The Ross procedure: Underuse or under-comprehension? J. Thorac. Cardiovasc. Surg. 2015, 149, 1463–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spadaccio, C.; Montagnani, S.; Acar, C.; Nappi, F. Introducing bioresorbable scaffolds into the show. A potential adjunct to resuscitate Ross procedure. Int. J. Cardiol. 2015, 190, 50–52. [Google Scholar] [CrossRef]
- Cho, I.-H.; Park, S. Preparation of a Microporous Polyurethane Film with Negative Surface Charge for siRNA Delivery via Stent. Int. J. Polym. Sci. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Che, H.-L.; Bae, I.-H.; Lim, K.S.; Song, I.T.; Lee, H.; Lee, D.; Kim, W.J.; Jeong, M.-H.; Park, I.-K.; Ahn, Y.; et al. Therapeutic Effect of Akt1 siRNA Nanoparticle Eluting Coronary Stent on Suppression of Post-Angioplasty Restenosis. J. Biomed. Nanotechnol. 2016, 12, 1211–1222. [Google Scholar] [CrossRef]
- Spadaccio, C.; Mozetic, P.; Nappi, F.; Nenna, A.; Sutherland, F.; Trombetta, M.; Chello, M.; Rainer, A. Cells and extracellular matrix interplay in cardiac valve disease: Because age matters. Basic Res Cardiol. 2016, 111, 16. [Google Scholar] [CrossRef]
- Spadaccio, C.; Rainer, A.; Mozetic, P.; Trombetta, M.; Dion, R.A.; Barbato, R.; Nappi, F.; Chello, M. The role of extracellular matrix in age-related conduction disorders: A forgotten player? J. Geriatr. Cardiol. 2015, 12, 76–82. [Google Scholar] [CrossRef]
- Adeel, M.Y.; Sharif, F. Advances in stent-mediated gene delivery. Expert Opin. Drug Deliv. 2016, 13, 465–468. [Google Scholar] [CrossRef] [Green Version]
- Yin, R.-X.; Yang, D.-Z.; Wu, J.-Z. Nanoparticle Drug- and Gene-eluting Stents for the Prevention and Treatment of Coronary Restenosis. Theranostics 2014, 4, 175–200. [Google Scholar] [CrossRef] [Green Version]


| Author/Year † Ref | Type of Study/Randomization | Treatment Total Number | Maximum Follow-Up (yrs) | Stent Compared /n Implanted | Main Finding | ||
|---|---|---|---|---|---|---|---|
| Valgimigli, 2013 [1] Int. J. Cardiol. | RCT 1:1 Multi-center | 744 | 3 | BMS 372 | SES 372 | Higher TVR failure based on death, MI, and clinically for BMS. SES was superior to BMS, | |
| Sinning, 2012 [2] Am. Heart J. | RCT 1:1 Multi-center | 200 | 5 | BMS 102 | SES 98 | Higher late luminal loss for BMS. SES was superior to BMS, | |
| Spaulding, 2011 [3] JACC Cardiovasc. Interv. | RCT 1:1 Multi-center | 712 | 4 | BMS 355 | SES 357 | Higher TVF for BMS. SES was superior to BMS. | |
| Mehilli, 2010 [4] J. Am. Coll. Cardiol. | RCT 1:1 Two centers | 450 | 5 | SES 250 | PES 250 | Higher late luminal loss for PES. SES not proved superior. | |
| Atary, 2010 [5] AJC | RCT 1:1 Single-center | 310 | 5 | BMS 152 | SES 158 | Higher late luminal loss in the coronary segment for BMS. SES was superior to BMS. | |
| Di Lorenzo, 2009 [6] JACC Cardiovasc. Interv. | RCT 1:1:1 Single-center | 270 | 4 | BMS 90 | PES 90 | SES 90 | Higher TLR for BMS. PES and SES were superior to BMS. |
| Mehran, 2008 [7] Am. Heart J. | RCT 3:1 Multi-center | 3006 | 3 | BMS 2257 | PES 749 | Higher TLR for BMS. No difference for death, MI, stroke, or ST. PES was superior to BMS for TLR and not inferior for clinical outcomes. | |
| Lee, 2008 [8] Catheter Cardiovasc Interv. | RCT 1:1 Multi-center | 308 | 3 | SES 154 | PES 154 | No difference between SES and PES for death, MI, ST, and *TLF, defined as cardiac death or target vessel MI. SES not proved superior. | |
| Menichelli, 2007 [9] JACC | RCT 1:1 Single-center | 320 | 5 | BMS 160 | SES 160 | Higher binary restenosis for BMS. SES was superior to BMS. | |
| Mehilli, 2006 [10] Eur. Heart J. | RCT 1:1 Two centers | 360 | 5 | SES 180 | PES 180 | Higher in-stent late luminal loss for PES. PES was inferior to SES. | |
| Suttorp, 2006 [11] Circulation | RCT 1:1 Two centers | 200 | 3 | BMS 100 | SES 100 | Higher grade of angiographic in-segment restenosis for BMS. SES was superior to BMS. | |
| Thuesen, 2006 [12] Am. Heart J. | RCT 1:1 Multi-center | 322 | 3 | BMS 159 | SES 163 | Inferior minimal lumen diameter for BMS. SES was superior to BMS. | |
| Valgimigli, 2005 [13] JAMA | RCT 1:1 Two centers | 175 | 5 | BMS 87 | SES 88 | Higher death, MI, stroke, and binary restenosis for BMS. SES was superior to BMS. | |
| Windecker, 2005 [14] NEJM | RCT 1:1 Single-center | 1012 | 5 | SES 503 | PES 509 | No difference between SES and PES for cardiac death, MI, TLR. SES not proved superior. | |
| Dibra, 2005 [15] NEJM | RCT 1:1 Two centers | 250 | 5 | SES 125 | PES 125 | Higher late luminal loss for PES. SES was superior to PES. | |
| Goy, 2005 [16] J. Am. Coll. Cardiol. | RCT 1:1 Single-center | 202 | 3 | SES 102 | PES 100 | No difference between SES and PES for cardiac death, MI, and TLR. SES not proved superior. | |
| Holmes, 2004 [17] Circulation | RCT 1:1 Multi-center | 1058 | 4 | BMS 533 | SES 525 | Higher *TVF or *TVR for BMS. SES was superior to BMS. | |
| Stone, 2004 [18] Circulation | RCT 1:1 Multi-center | 1314 | 5 | BMS 662 | PES 652 | Higher TVR failure based on ischemia for BMS. PES was superior to BMS. | |
| Morice, 2002 [19] NEJM | RCT 1:1 Multi-center | 238 | 4 | BMS 120 | SES 118 | Higher in-stent late luminal loss for BMS. SES was superior to BMS. | |
| Author/Year † Ref | Type of Study/Randomization | Treatment Total Number | Maximum Follow-Up (yrs) | Stent Compared/n Implanted | Main Finding | |
|---|---|---|---|---|---|---|
| Jakobsen, 2017 [1] EuroIntervention | RCT 1:1 Multi-center | 2468 | 3 | BP-BES 1229 | SES 1239 | No difference for cardiac death, MI, definite ST, and clinically based on TVR. Non-inferiority for BP-BES has not been demonstrated, |
| Raungaard, 2015 [2] Lancet | RCT 1:1 Multi-center | 2999 | 5 | BP-BES 1497 | PC-ZES 1502 | No difference for cardiac death and MI. PC-ZES was not inferior to BP-BES. |
| Smits, 2015 [3] JACC Cardiovasc. Interv. | RCT 1:1 Single-center | 1800 | 5 | CoCr-EES 897 | PES 903 | Higher death, MI, and TVR for PES. CoCr-EES was superior to PES. |
| Iqbal, 2015 [4] Circ Cardiovasc Interv. | RCT 1:1 Multi-center | 2292 | 4 | CoCr-EE 1152 | Re-ZES 1140 | No difference for TLF. Re-ZES was not inferior to CoCr-EES. |
| Natsuaki, 2015 [5] Catheter Cardiovasc Interv. | RCT 3:2 Multi-center | 326 | 3 | BP-BES 194 | SES 132 | No difference for TVF. BP-BES was not inferior to SES. |
| Maeng, 2014 [6] Lancet | RCT 1:1 Multi-center | 2332 | 5 | SES 1170 | PC-ZES 1162 | Higher cardiac death, MI, and TVR for PC-ZES. SES was superior to PC-ZES. |
| Di Lorenzo, 2014 [7] JACC Cardiovasc. Interv. | RCT 1:1 Single-center | 500 | 3 | EES 250 | SES 250 | No difference for cardiac death and reinfarction. EES similar efficacy as SES. EES proved significant reduction in ST. |
| Serruys, 2013 [8] JACC Cardiovasc. Interv. | RCT 1:1 Multi-center | 1707 | 4 | BP-BES 875 | SES 875 | No difference for cardiac death, MI, and TVR. BP-BES was not inferior to SES. |
| Jensen, 2012 [9] Circulation | RCT 1:1 Multi-center | 2774 | 5 | CoCr-EES 1390 | SES 1384 | No difference for cardiac death, MI, definite ST, and TVR. CoCr-EES was not inferior to SES. |
| Kandzari, 2011 [10] JACC Cardiovasc. Interv. | RCT 1:3 Multi-center | 436 | 5 | SES 113 | PC-ZES 323 | Higher grade of late lumen loss for PC-ZES. PC-ZES was inferior to SES. |
| Stone, 2011 [11] J. Am. Coll. Cardiol. | RCT 1:1 Multi-center | 1530 | 3 | PtCr-EES 768 | CoCr-EES 762 | No difference for TLF. PtCr-EES was not inferior to CoCr-EES. |
| Leon, 2010 [12] J. Am. Coll. Cardiol. | RCT 1:1 Multi-center | 1548 | 3 | PES 775 | PC-ZES 773 | No difference for TVF. PES was not inferior to PC-ZES. |
| Kereiakes, 2010 [13] JACC Cardiovasc. Interv. | RCT 2:1 Multi-center | 1002 | 5 | CoCr-EES 699 | PSE 333 | Higher-grade in-segment late luminal loss and higher TVR for PES. CoCr-EES was superior to PES. |
| Byrne, 2009 [14] Eur. Heart J. | RCT 1:1 Two centers | 1304 | 3 | CoCr-EES 652 | SES 652 | No difference for cardiac death, MI, and TLR. CoCr-EES was not inferior to SES. |
| Nicolsky, 2009 [15] Am. Heart J. | RCT 2:1 Multi-center | 3687 | 3 | CoCr-EES 2458 | PES 1229 | Higher TLF or TLR defined as cardiac death or target vessel MI for PES. CoCr EES was superior to PES. |
| Camenzind, 2009 [16] Am. Heart J. | RCT 1:1 Multi-center | 8791 | 4 | C-SES 4352 | E-ZES 4357 | No difference for ST. E-ZES was not superior to C-SES. |
| Garg, 2009 [17] JACC Cardiovasc. Interv. | RCT 3:1 Multi-center | 300 | 3 | CoCr-EES 233 | PSE 77 | No difference for in-stent late luminal loss. CoCr-EES was not inferior to PES. |
| Fajadet, 2006 [18] Circulation | RCT 1:1 Multi-center | 1197 | 5 | PC-ZES 598 | BMS 599 | Higher TVF for BMS. PC-ZES was superior to BMS. |
| Chevalier, 2006 [19] EuroIntervention | RCT 1:2 Multi-center | 120 | 5 | BP-BES 35 | PES 85 | No difference in-stent late luminal loss. BP-BES was not inferior to PES. |
| Smits, 2005 [20] Lancet | RCT 1:2 Multi-center | 2707 | 3 | CoCr-EES 912 | BP-BES 1795 | No difference for cardiac death, non-fatal MI, and TVR. BES was not inferior to CoCr-EES. |
| Commercial Name | Compound |
|---|---|
| PES | Paclitaxel |
| BES | Biolimus |
| BP-BES | Biodegradable polymer biolimus |
| SES | Sirolimus |
| C-SES | Cypher sirolimus |
| EES | Everolimus |
| CoCr-EES | Cobalt-chromium everolimus |
| PtCr-EES | Platinum-chromium everolimus |
| Re-ZES | Resolute zotarolimus |
| E-ZES | Endeavor zotarolimus |
| PC-ZES | Phosphorylcholine zotarolimus |
| SPC-ZES | Phosphorylcholine polymer-based zotarolimus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nappi, F.; Nenna, A.; Larobina, D.; Martuscelli, G.; Singh, S.S.A.; Chello, M.; Ambrosio, L. The Use of Bioactive Polymers for Intervention and Tissue Engineering: The New Frontier for Cardiovascular Therapy. Polymers 2021, 13, 446. https://doi.org/10.3390/polym13030446
Nappi F, Nenna A, Larobina D, Martuscelli G, Singh SSA, Chello M, Ambrosio L. The Use of Bioactive Polymers for Intervention and Tissue Engineering: The New Frontier for Cardiovascular Therapy. Polymers. 2021; 13(3):446. https://doi.org/10.3390/polym13030446
Chicago/Turabian StyleNappi, Francesco, Antonio Nenna, Domenico Larobina, Giorgia Martuscelli, Sanjeet Singh Avtaar Singh, Massimo Chello, and Luigi Ambrosio. 2021. "The Use of Bioactive Polymers for Intervention and Tissue Engineering: The New Frontier for Cardiovascular Therapy" Polymers 13, no. 3: 446. https://doi.org/10.3390/polym13030446
APA StyleNappi, F., Nenna, A., Larobina, D., Martuscelli, G., Singh, S. S. A., Chello, M., & Ambrosio, L. (2021). The Use of Bioactive Polymers for Intervention and Tissue Engineering: The New Frontier for Cardiovascular Therapy. Polymers, 13(3), 446. https://doi.org/10.3390/polym13030446