Abstract
Antibacterial packaging materials can reduce the microbial contamination of food surfaces. In this study, magnesium oxide (MgO) nanoparticles were synthesized and then coated with cetrimonium bromide (CTAB). CTAB-modified MgO (MgO@CTAB) was characterized by Fourier-transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), and thermogravimetric analysis. Then, different loadings of MgO@CTAB were mixed with poly(butylene adipate-co-terephthalate) (PBAT) by melt compounding. The results showed that the addition of MgO@CTAB deteriorated the thermal stability of PBAT due to MgO serving as a catalyst to promote the thermal degradation of PBAT. In addition, MgO@CTAB could serve as a nucleating agent to improve the crystallinity of PBAT. With the optimal 3 wt% of MgO@CTAB, the tensile strength of PBAT/MgO@CTAB increased from 26.66 to 29.90 MPa, with a slight enhancement in elongation at break. SEM observations and dynamical rheological measurements revealed that aggregation occurred when the content of MgO@CTAB exceeded 5 wt%. The presence of MgO@CTAB endowed PBAT with antibacterial properties. The bacterial inhibition zone increased with the increasing content of MgO@CTAB. In addition, MgO@CTAB had a better antibacterial efficiency against Gram-positive bacterial S. aureus than Gram-negative bacterial E. coli.
1. Introduction
Biodegradable food packaging polymers have gained considerable interest from both academia and industry due to the depletion of petroleum resources and the pressure of environmental protection. Poly(butylene adipate-co-terephthalate) (PBAT) is a type of aliphatic aromatic copolyester thermoplastic, which can be synthesized by the copolymerization of the monomers 1,4-butanediol, adipic acid, and terephthalic acid [1]. PBAT exhibits good biodegradability, high flexibility, excellent tear resistance, and good processability [2,3]. It has been widely utilized for different packaging applications, such as garbage bags, solid food containers, and film wraps. To fulfill requirements for the freshness, safety, and quality of foods, there is growing demand for antibacterial packaging materials in food packaging sectors [4,5,6]. Various types of metal oxides, such as zinc oxide (ZnO), silver oxide (Ag2O), magnesium oxide (MgO), and titanium oxide (TiO2), have been developed and exhibited different degrees of antibacterial effects on PBAT composites [7,8,9].
Magnesium oxide (MgO) can be easily synthesized from magnesium-rich minerals. It has been considered a promising alternative antibacterial agent due to its environmental friendliness, non-toxicity, low cost, and illumination independence [10,11]. Moreover, MgO has been listed as a safe material by the U.S. Food and Drug Administration (US-FDA). It has been proposed that the presence of abundant defects or oxygen vacancy on the surface of MgO nanoparticles will contribute to the generation of reactive oxygen species (ROS), which can destroy bacterial cells and protein peptides [12]. Wang et al. prepared nano–MgO/carboxymethyl chitosan (CMCS) films using a solution method [13]. The results revealed that the introduction of MgO contributed to the improvement of thermal stability, UV shielding performance, and water resistance of CMCS films. The CMCS films increased elasticity and ductility simultaneously with the addition of 1 wt% of MgO nanoparticles. Swaroop and his colleague demonstrated that the tensile strength and oxygen barrier properties of polylactide (PLA) composite films containing 2 wt% of MgO increased by 29% and 25%, respectively, as compared with those of pure PLA films [14]. In addition, PLA/MgO films also exhibited pronounced inhibition of E. coli bacterial [15].
Cetyltrimethylammonium bromide (CTAB) is a quaternary ammonium surfactant that has strong inhibitory effects on a variety of bacteria through electrostatic interactions that disrupt or disturb the cell membrane [16,17]. This inexpensive cationic surfactant is safe at low concentrations. Chang et al. utilized CTAB to modify Janus silver mesoporous silica nanoparticles using a one-pot method [17]. The minimum inhibitory concentrations (MIC) for S. aureus and E. coli were determined to be 20 and 10 μg/mL, respectively. Sasikumar et al. found that the presence of CTAB could not assist the synthesis of BaTiO3 nanoparticles via a hydrothermal method, but it endowed BaTiO3 nanoparticles with antimicrobial properties [18]. The CTAB-functionalized BaTiO3 exhibited profound antibacterial behaviors against C. albicans bacteria. To date, to the best of our knowledge, the application of MgO nanoparticles in combination with CTAB as a bactericide for PBAT has not yet been explored.
In this work, MgO nanoparticles were synthesized using a sol–gel method. Then, CTAB-functionalized MgO nanoparticles (MgO@CTAB) were incorporated into the PBAT matrix to prepare antibacterial nanocomposites. The effects of MgO@CTAB loading on the thermal stability, crystallization behavior, rheological and mechanical properties, as well as the antibacterial performance of PBAT nanocomposites were investigated.
2. Materials and Methods
2.1. Materials
Poly(butylene adipate-co-terephthalate) (PBAT) with a melt index of 5 (190 °C, 2.16 kg) was supplied by BASF (Shanghai, China). Magnesium chloride hexahydrate (MgCl2·6H2O), sodium hydroxide (NaOH), and cetyltrimethylammonium bromide (CTAB) were obtained from J&K (Shanghai, China).
2.2. Preparation of MgO@CTAB
Magnesium oxide (MgO) nanoparticles were prepared using a modified sol–gel method [19]. First, 0.5 g of CTAB and 1.6 g of MgCl2·6H2O were added to 40 mL of DI water with vigorous stirring for 2 h to form clear gel. A 20 mL solution of 0.4 M NaOH was slowly added to the as-obtained gel. Then, the mixture was transformed into a 100 mL PPL-lined stainless steel autoclave and kept at 120 °C overnight. The obtained precipitate was centrifuged with DI water and ethanol twice and dried at 60 °C for 6 h. The resulting powders were calcinated in a tubular furnace at 400 °C for 3 h. Two grams of the as-prepared MgO nanoparticles and 1.0 g of CTAB were dispersed in 100 mL of ethanol with ultrasonication for 30 min. Then, the CTAB-modified MgO nanoparticles (MgO@CTAB) were centrifuged and rinsed by ethanol twice to remove the excess CTAB.
2.3. Preparation of PBAT/MgO@CTAB Nanocomposites
PBAT nanocomposites containing MgO@CTAB nanoparticles were prepared using the melt compounding method. The desired amount of MgO@CTAB was mixed with PBAT in a Brabender internal mixer (Brabender GmbH & Co. KG, Duisburg, Germany) at a fixed speed of 60 rpm at 160 °C for 8 min. Then, the samples were hot-compressed into specimens with different dimensions for characterization. The as-prepared PBAT/MgO@CTAB nanocomposites were abbreviated as MgO@CTAB-X, where X stands for the weight percentage of MgO@CTAB in the nanocomposite. For comparison, pristine MgO nanoparticles were also mixed with PBAT using the same procedures.
2.4. Characterization
The morphology and structure of MgO@CTAB were investigated by transmission electron microscopy (TEM, JEOL JEM-2100F, Tokyo, Japan) and scanning electron microscopy (SEM, FEI 250, FEI Company, Hillsboro, OR, USA) with energy-dispersive X-ray (EDX) spectroscopy. The MgO@CTAB and cryo-fracture surfaces of PBAT/MgO@CTAB nanocomposites were sputtered with a thin layer of gold before SEM observation. Fourier-transform infrared spectroscopy (FT-IR) spectra were collected on a Nicolet NEXUS670 spectrometer (Thermo Fisher Scientific Inc., Dreieich, Germany). The samples were scanned 16 times in the range of 400–4000 cm−1. The thermal stability was measured by a Netzsch TGA-209F1 thermogravimetric analyzer (TGA, NETZSCH GmbH & Co.Holding KG, Selb, Germany) under a nitrogen atmosphere. The specimens were heated from 30 to 700 °C at a ramping rate of 10 °C min−1. The X-ray diffraction (XRD) patterns were collected from 10 to 60° by using a Bruker D8 diffractometer (Bruker Daltonik GmbH, Bremen, Germany) with a fixed step of 0.02°. The crystallization and melting behavior of the PBAT composites were investigated using a Netzsch DSC-214 differential scanning calorimeter (DSC, NETZSCH-Gerätebau GmbH, Selb, Germany) in the range of 30–160 °C. The dynamical rheological performance was analyzed on an Anton Paar MCR302 rheometer (Anton Paar Australia Pty. Ltd., Sydney, Australia). The frequency sweep was conducted at 150 °C at a fixed strain of 1%. The tensile properties were measured on an Instron 5566 electron universal testing machine (Instron, Norwood, MA, USA). The tensile speed was fixed at 50 mm min−1. The reported results are the mean values of five successful specimens.
2.5. Antibacterial Properties
The antibacterial activity of the PBAT nanocomposites was investigated using the zone inhibition method [20]. In brief, a specific amount of bacterial culture (i.e., 0.1 mL) was transferred to nutrient agar plates. Then, 5 mm diameter circular films were put over the bacteria colonies and then incubated at 37 °C for 24 h to measure the bacterial inhibition zone.
3. Results and Discussions
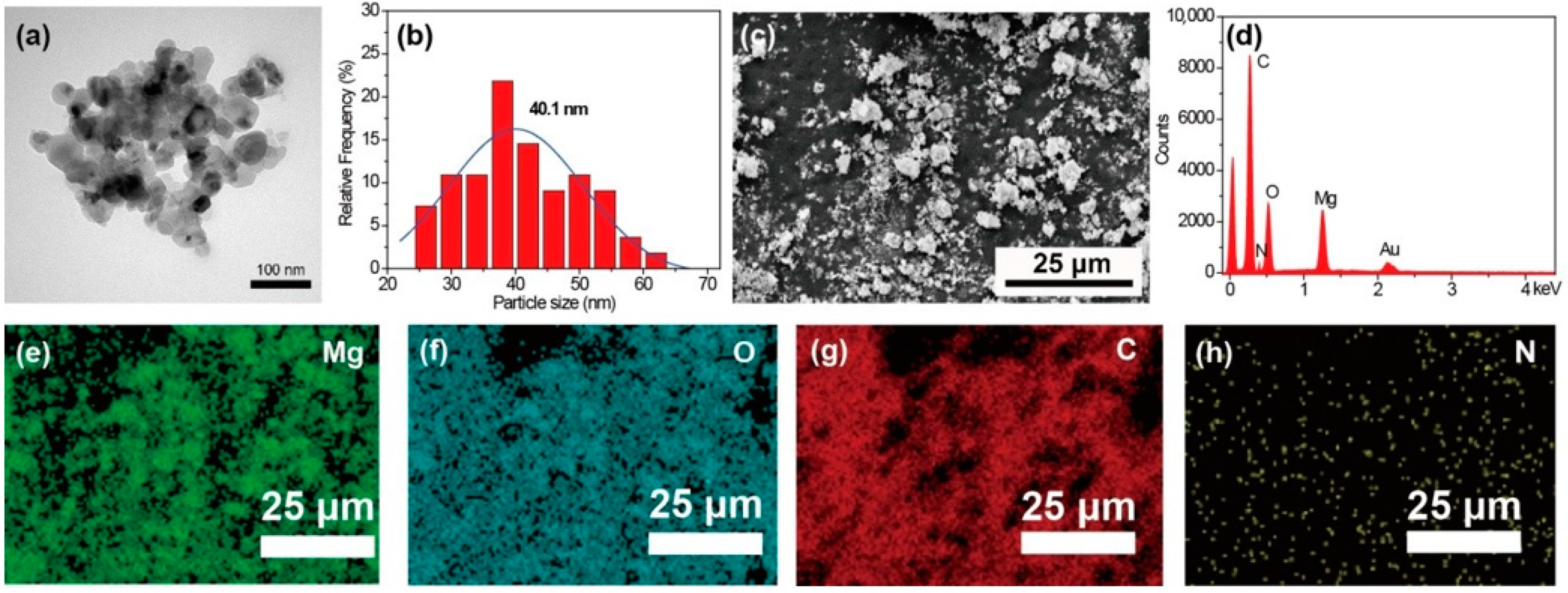
The morphology of the MgO nanoparticles is shown in Figure 1a. The obtained MgO exhibits a roughly spherical structure. The diameter distribution calculated from TEM images is shown in Figure 1b. The average diameter of the MgO calculated using ImageJ software is 40.1 nm. The SEM image of MgO@CTAB is shown in Figure 1c, and the corresponding EDX result in Figure 1d confirms the chemical composition of MgO@CTAB, which has two sharp signal peaks that are attributed to magnesium and oxygen elements. The strong carbon signal is mainly due to carbon conductive paste. In addition, the signal peaks for nitrogen are observed, which may result from the presence of grafted CTAB. The EDX mapping images in Figure 1e–h indicate that the CTAB was coated on the surface of MgO@CTAB uniformly.
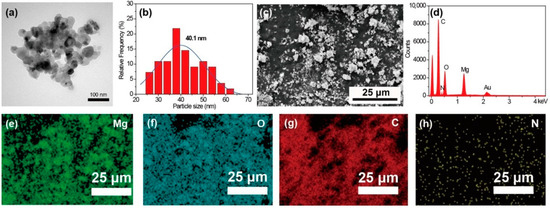
Figure 1.
(a) Transmission electron microscopy (TEM) image; (b) particle size distribution of MgO; (c) scanning electron microscopy (SEM) image of MgO@CTAB; (d) energy-dispersive X-ray (EDX) result; EDX mapping element of (e) Mg, (f) O, (g) C, and (h) N.
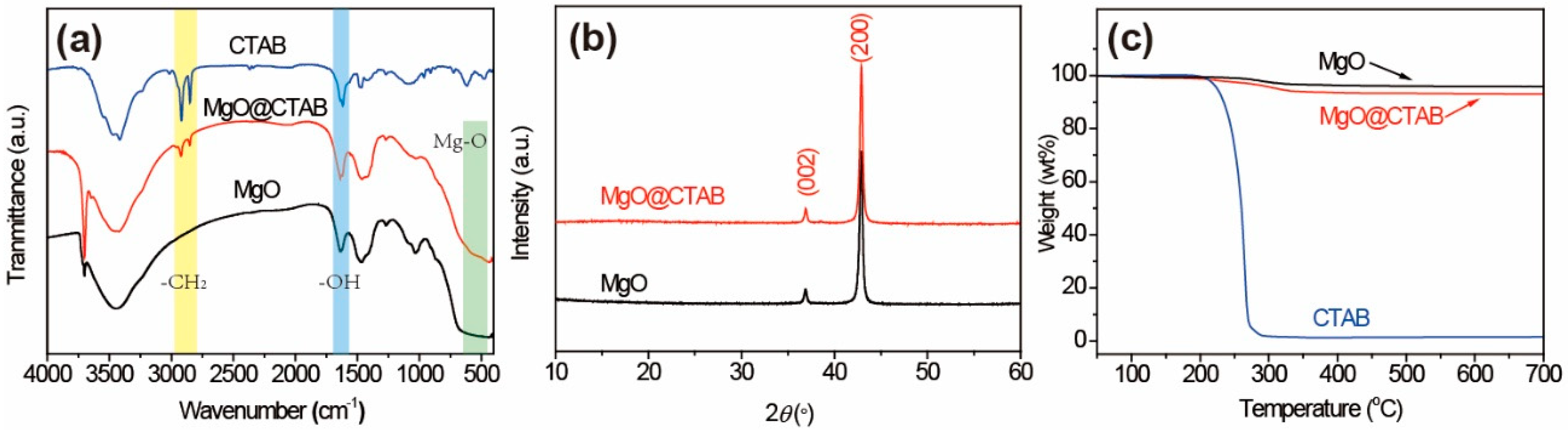
The FT-IR spectra of MgO and MgO@CTAB are shown in Figure 2a. The broad peaks at 3000–3700 cm−1 correspond to the stretching vibration of –OH groups, while the peaks at 1636 cm−1 represent the bending vibration of –OH groups due to the absorbed water molecules on the surfaces of the nanoparticles [21]. The detected peaks located at around 500 cm−1 are attributed to the stretching vibration of Mg-O bonds [22]. In addition, it is observed that two new peaks appear in MgO@CTAB at 2850 and 2922 cm−1, which are related to the asymmetric and symmetric stretching vibration of -CH2 bonds of the coated CTAB chains, respectively [23,24]. The above results indicate that the CTAB was coated on the surface of MgO successfully. The structures of MgO and MgO@CTAB are further confirmed by XRD patterns, as shown in Figure 2b. It is observed that the pure MgO nanoparticles have strong peaks located at 37.0° and 42.9°, which correspond to the (002) and (200) planes of JCPDS PDF-87-0652, respectively. After the decoration of CTAB, the MgO@CTAB exhibits similar absorption peaks as pure MgO, indicating that the addition of CTAB has no effect on the crystal structure of MgO. Figure 2c shows the thermal degradation behaviors of MgO and MgO@CTAB. It is observed that the pure MgO nanoparticles show 2.4 wt% loss in the temperature range of 250–340 °C, while a significant weight loss for MgO@CTAB occurs at temperatures from 200 to 350 °C. In addition, the char yields at 700 °C for MgO and MgO@CTAB are 95.8 wt% and 93.1 wt%, respectively, indicating that the coated CTAB in MgO@CTAB is around 2.7 wt%.
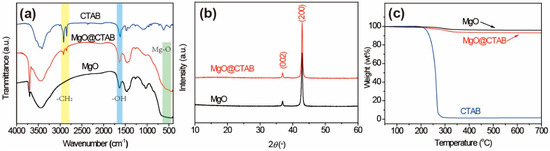
Figure 2.
(a) Fourier-transform infrared (FT-IR) spectra, (b) XRD patterns, and (c) thermogravimetric analysis (TGA) curves of MgO and MgO@CTAB.
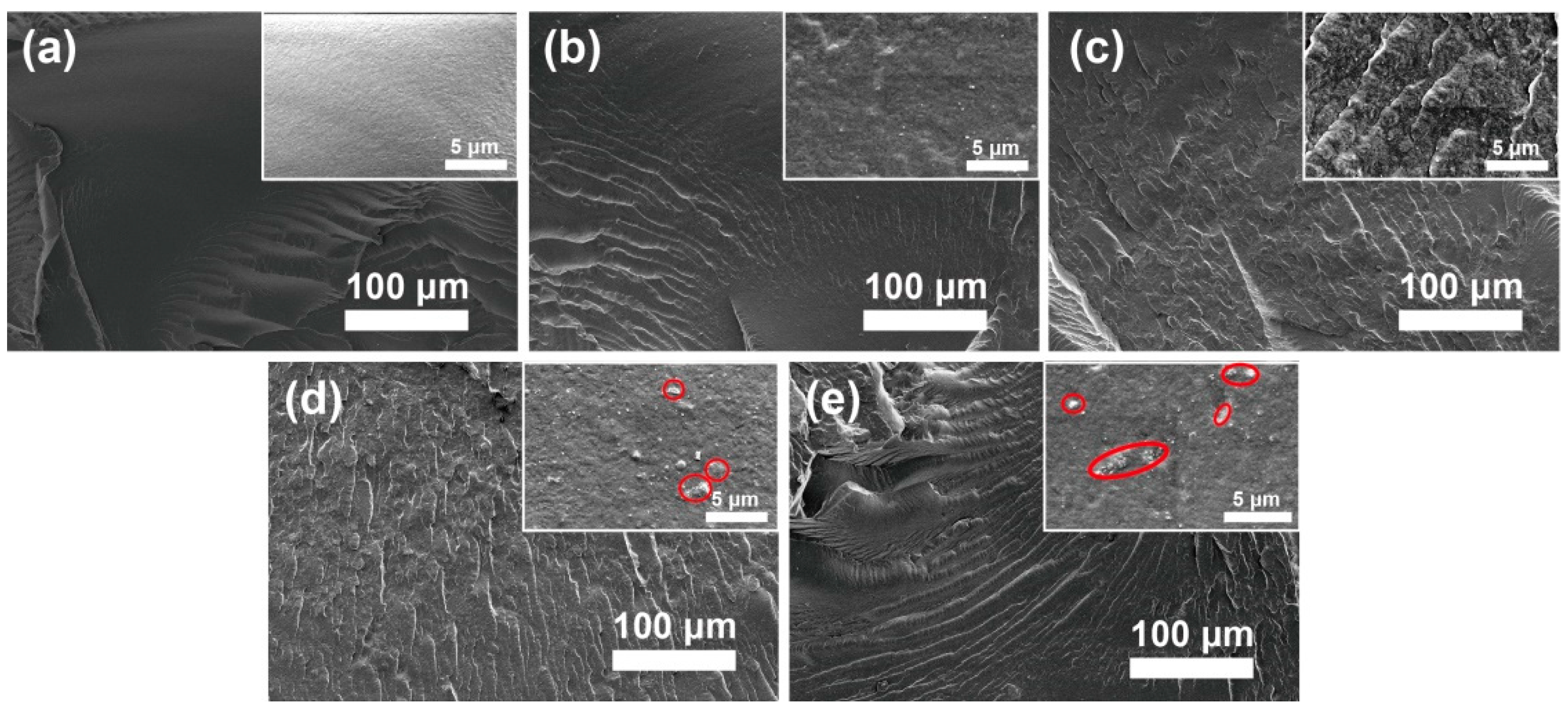
The fracture surface morphology of the PBAT nanocomposite was evaluated by using SEM, as shown in Figure 3. It is observed that MgO@CTAB-0 in Figure 3a exhibits a smooth surface due to its brittle fracture of PBAT. In addition, the fracture surfaces of the PBAT nanocomposites become rougher (Figure 3b–e) with the increasing MgO@CTAB loading, indicating that the presence of rigid MgO@CTAB nanoparticles contributes to the stress transformation [25]. Some aggregations (red circles) are observed on the fracture surface in the high-resolution SEM images when the content of MgO@CTAB is increased to 5 wt%.
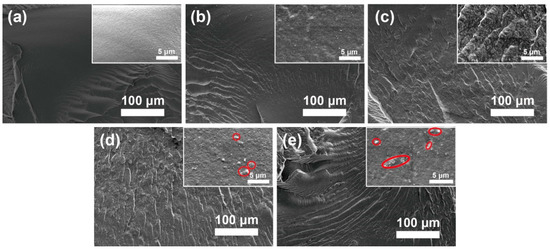
Figure 3.
Fracture surface SEM images of poly(butylene adipate-co-terephthalate) (PBAT) nanocomposites. (a) MgO@CTAB-0, (b) MgO@CTAB-1, (c) MgO@CTAB-3, (d) MgO@CTAB-5, and (e) MgO@CTAB-7.
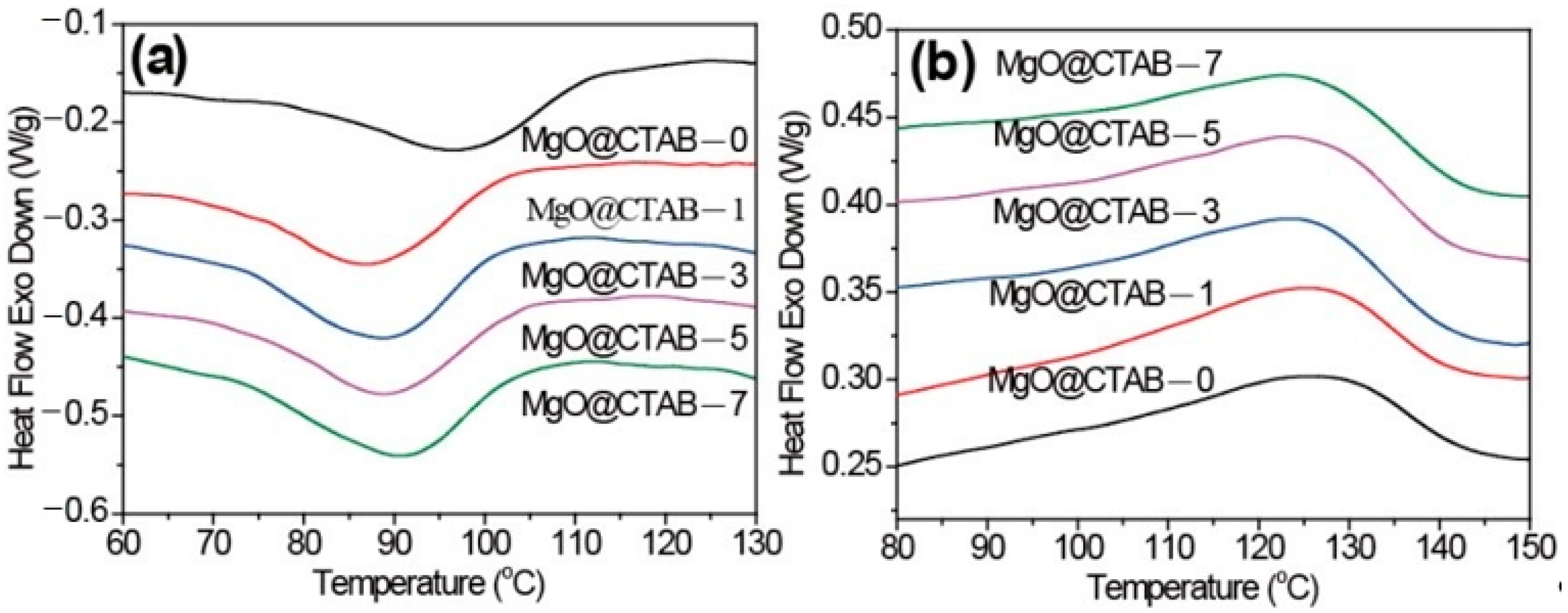
The melting and crystallization behaviors of pure PBAT and its nanocomposites are shown in Figure 4. The peak crystallization temperature (Tc), crystallization enthalpy (ΔHc), the peak melting temperature (Tp), the melting enthalpy (ΔHm), and the crystallinity (χ) are summarized in Table 1. The degree of crystallinity was calculated by the following equation:
where ΔHm represents to the melting enthalpy, Wf is the weight ratio of MgO@CTAB, and ΔH0 stands for the 100% melting enthalpy of PBAT (114 J g−1) [26].
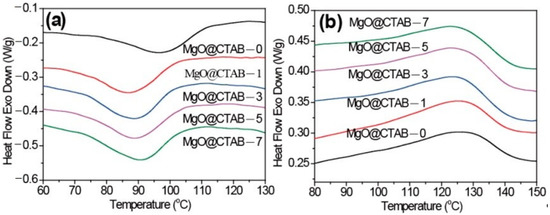
Figure 4.
Differential scanning calorimetry (DSC) thermograms of PBAT/MgO@CTAB nanocomposites. (a) First cooling, (b) second heating.

Table 1.
DSC thermograms of PBAT and its nanocomposites.
In Figure 4a, it is observed that the initial crystallization temperatures and Tc of PBAT nanocomposites shift to lower values. Meanwhile, the ΔHc values of PBAT nanocomposites increase slightly as compared with those of pure PBAT. These results indicate that the presence of MgO@CTAB contributes to the inhibition of crystal nucleation during the crystallization process. The Tp of PBAT nanocomposites in Figure 4b exhibits a decreasing trend with the increasing content of MgO@CTAB. Moreover, the crystallinity values of PBAT nanocomposite are all higher than those of pure PBAT.
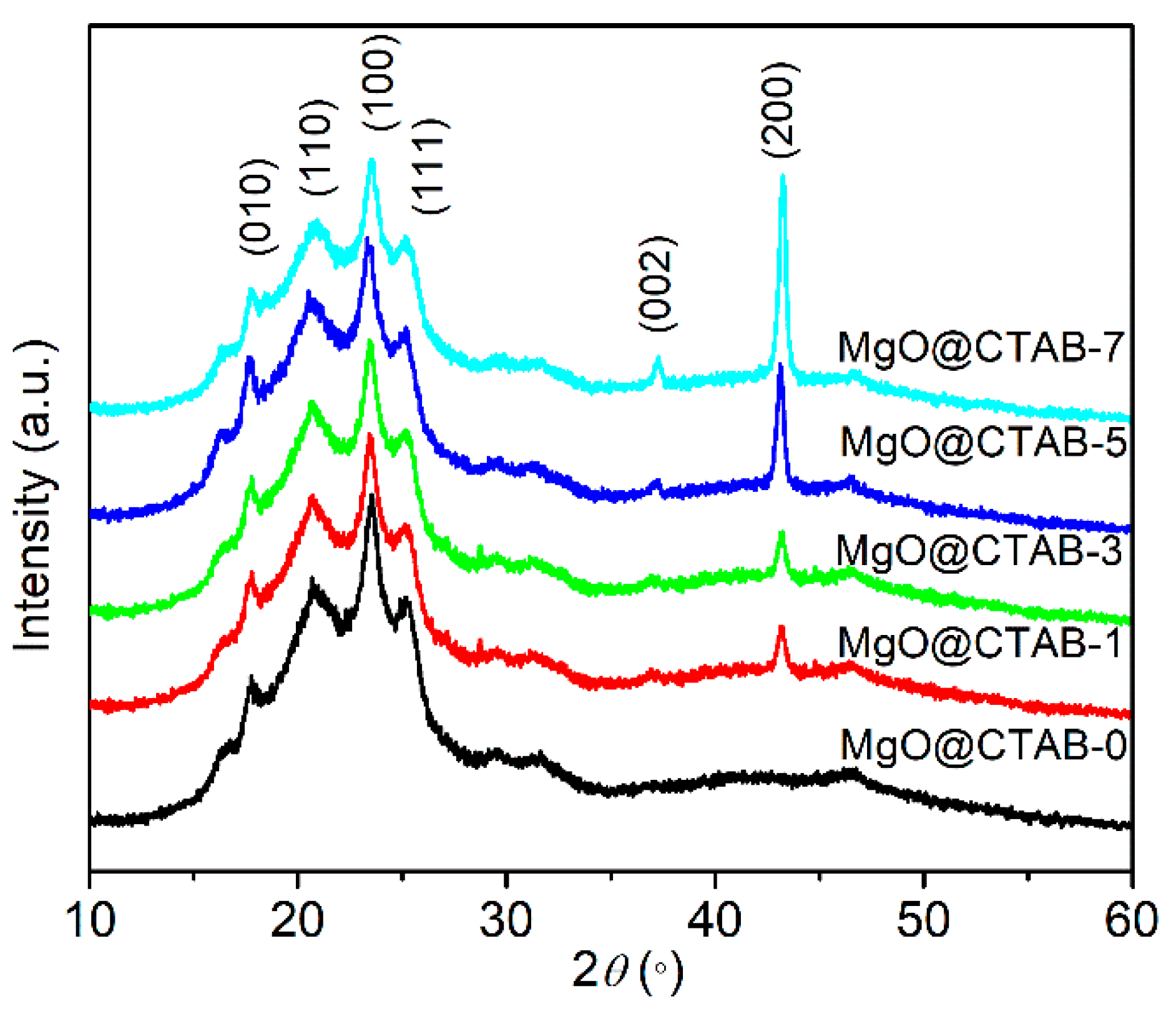
The XRD patterns of PBAT nanocomposites are shown in Figure 5. Four diffraction peaks at 17.4°, 20.4°, 23.3°, and 25.1° are observed in MgO@CTAB-0, which correspond to the (010) (110) (100) and (111) planes of PBAT, respectively [27]. Furthermore, the intensity of the characteristic peaks at 37.3° and 43.2° gradually increases as the content of MgO@CTAB increases from 1 to 7 wt%, which are related to the (002) and (200) planes of MgO. In addition, it is noted that there is no significant shift or change in the diffraction peaks between pure PBAT and its nanocomposites.
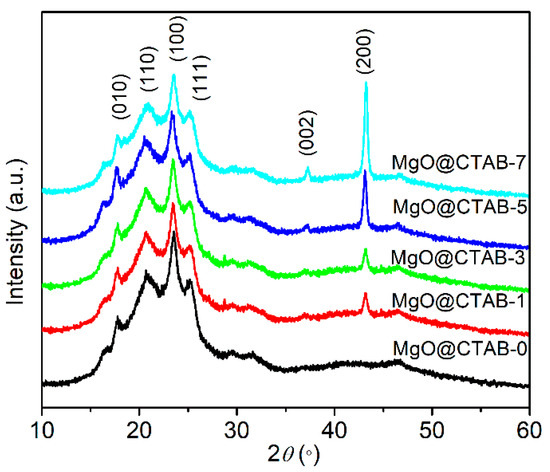
Figure 5.
XRD patterns of PBAT nanocomposites with different loadings of MgO@CTAB.
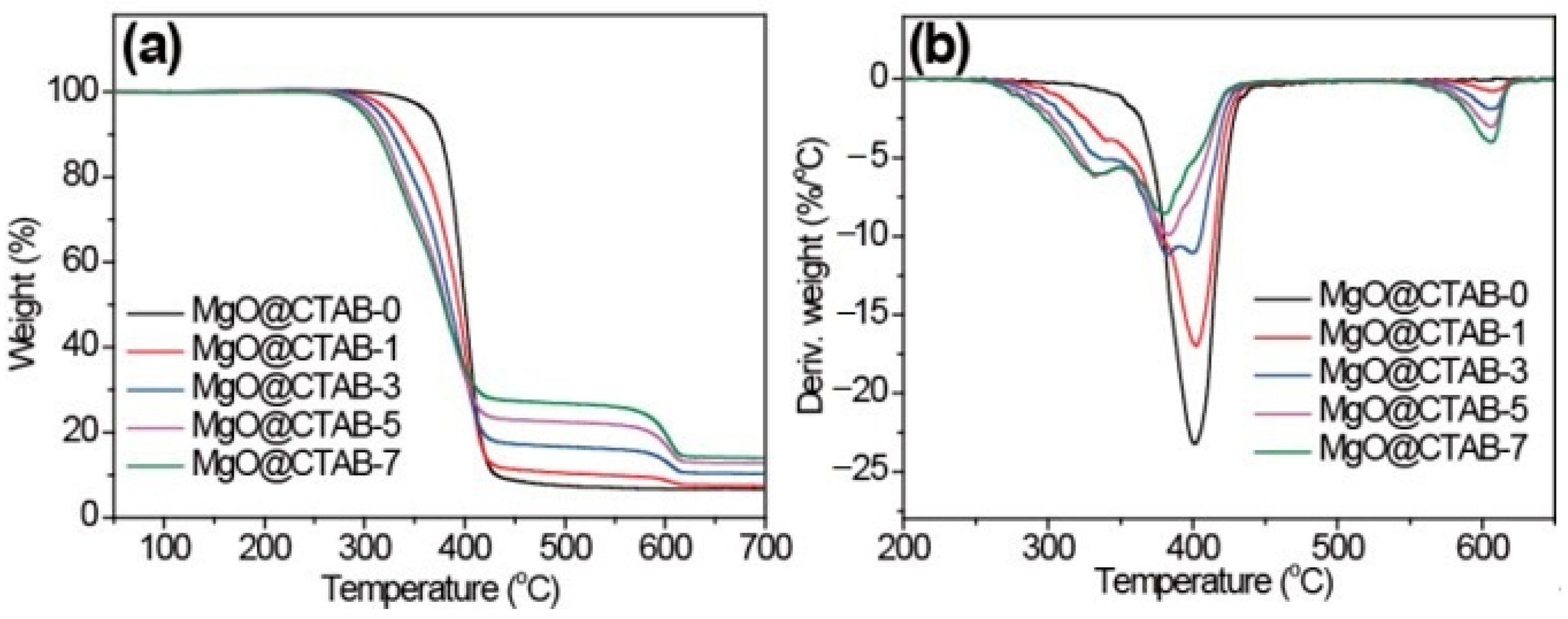
The effects of MgO@CTAB on the thermal stability of PBAT were investigated through TGA, and the results are shown in Figure 6. The initial decomposition temperatures (T10), peak decomposition temperatures (Tp), and char yields at 700 °C are listed in Table 2. In Figure 6, it is observed that the decomposition curves shift to lower temperatures with the increasing loading of MgO@CTAB. The T10 of MgO@CTAB-7 decreases from 375.2 to 313.7 °C as compared with that of MgO@CTAB-0. In addition, the values of Tp exhibit a similar decreasing trend. The decrease in thermal stability is mainly attributed to the catalytic effects of MgO, which is consistent with other reports [28]. Furthermore, the thermal decomposition of coated CTAB also resulted in weight loss. As expected, the char yields of PBAT nanocomposites at 700 °C increase gradually with the increasing content of MgO@CTAB.
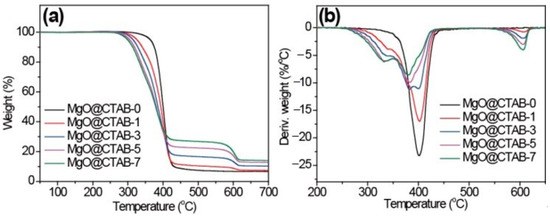
Figure 6.
TGA curves of PBAT/MgO@CTAB nanocomposites. (a) TGA, (b) DTG.

Table 2.
Thermal stability of PBAT and its nanocomposites.
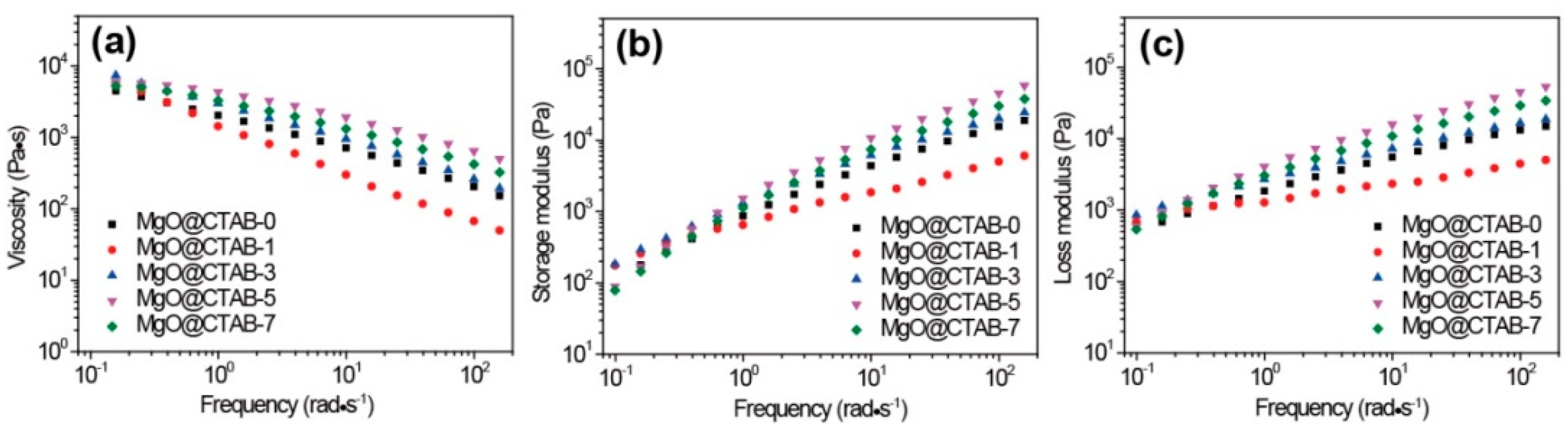
The effects of MgO@CTAB on the rheological properties of PBAT were evaluated by taking dynamic rheological measurements. The complex viscosity (η*), storage modulus (G′), and loss modulus (G″) as a function of frequency at 150 °C are shown in Figure 7. In Figure 7a, the η* of all samples exhibits a typical shear thinning behavior. It is worth noting that the values of η* and G′ of MgO@CTAB-1 decrease significantly as compared with those of pure PBAT, which is consistent with the reported PBAT composite [29]. This may be ascribed to the presence of CTAB because it is a small molecule that improves the flowability of PBAT. The storage modulus of the PBAT nanocomposite in Figure 7b increases with the increasing content of MgO@CTAB. However, the enhancement of the storage modulus is not proportional to the content of MgO@CTAB. Enhancement of the storage modulus reaches its maximum value at 5 wt% of MgO@CTAB and then decreases with further loadings. This is due to the fact that the enhancement of MgO@CTAB is more profound than the inverse effect of the aggregation of MgO@CTAB in the PBAT matrix at the loading of 5 wt% MgO@CTAB. Figure 7c shows that the values of G″ decrease concomitantly with G′, indicating that the structure of the PBAT nanocomposites is most affected by filler content.
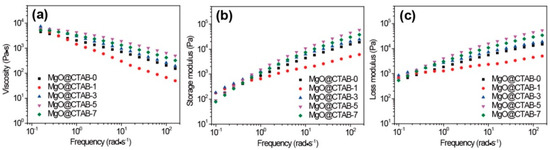
Figure 7.
Dynamic rheological properties of PBAT nanocomposites with various contents of MgO@CTAB. (a) Complex viscosity, (b) storage modulus, and (c) loss modulus as a function of frequency.
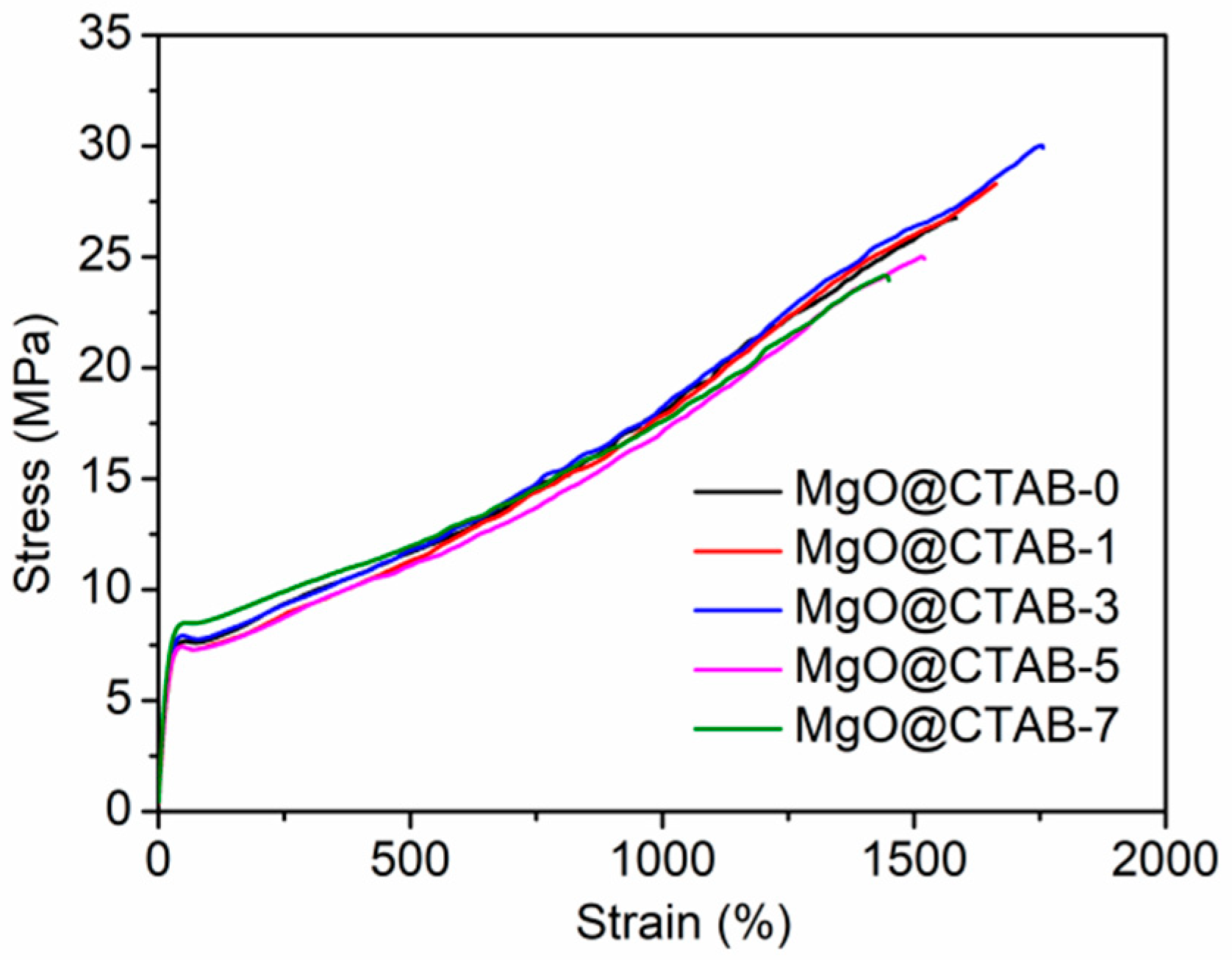
The typical strain versus stress curves of PBAT and its nanocomposites are presented in Figure 8. The tensile stress, Young’s modulus, and elongation at break are summarized in Table 3. It can be observed that the strain–stress curves of all samples in Figure 8 can be divided into three regions, namely, elastic, plastic deformation, and strain hardening regions. The elastic region exhibits a linear change with recoverable deformation, followed by the plastic deformation region, in which neck forming would occur. The strain hardening region undergoes a strain hardening phenomenon with the continuous increase in the stress. It is clear that the Young’s modulus of the PBAT/MgO@CTAB nanocomposite increases with the increasing content of MgO@CTAB because MgO@CTAB particles serve as rigid fillers to transform the stress [30]. The tensile stress of PBAT nanocomposite achieves a maximum value of 29.90 MPa with the addition of 3 wt% MgO@CTAB, followed by a decreasing tendency due to the aggregation of MgO@CTAB, as revealed by SEM images. In addition, the elongation at break of PBAT nanocomposites increases slightly with the addition of a low content of MgO@CTAB. With the further increasing of nanofiller content, the elongation at break of PBAT decreases significantly. This is because the aggregation of MgO@CTAB causes stress concentration, which decreases the ductility.
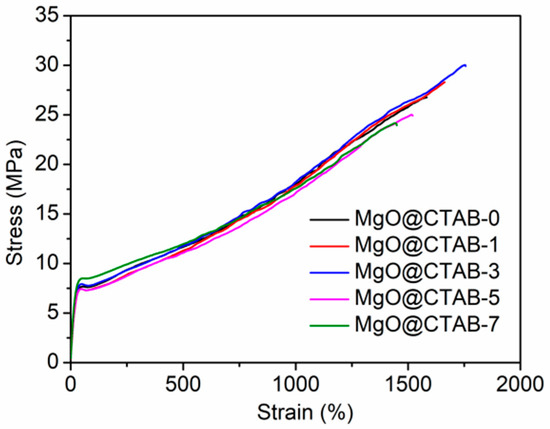
Figure 8.
Tensile stress versus strain of PBAT nanocomposites.

Table 3.
Tensile properties of PBAT nanocomposites.
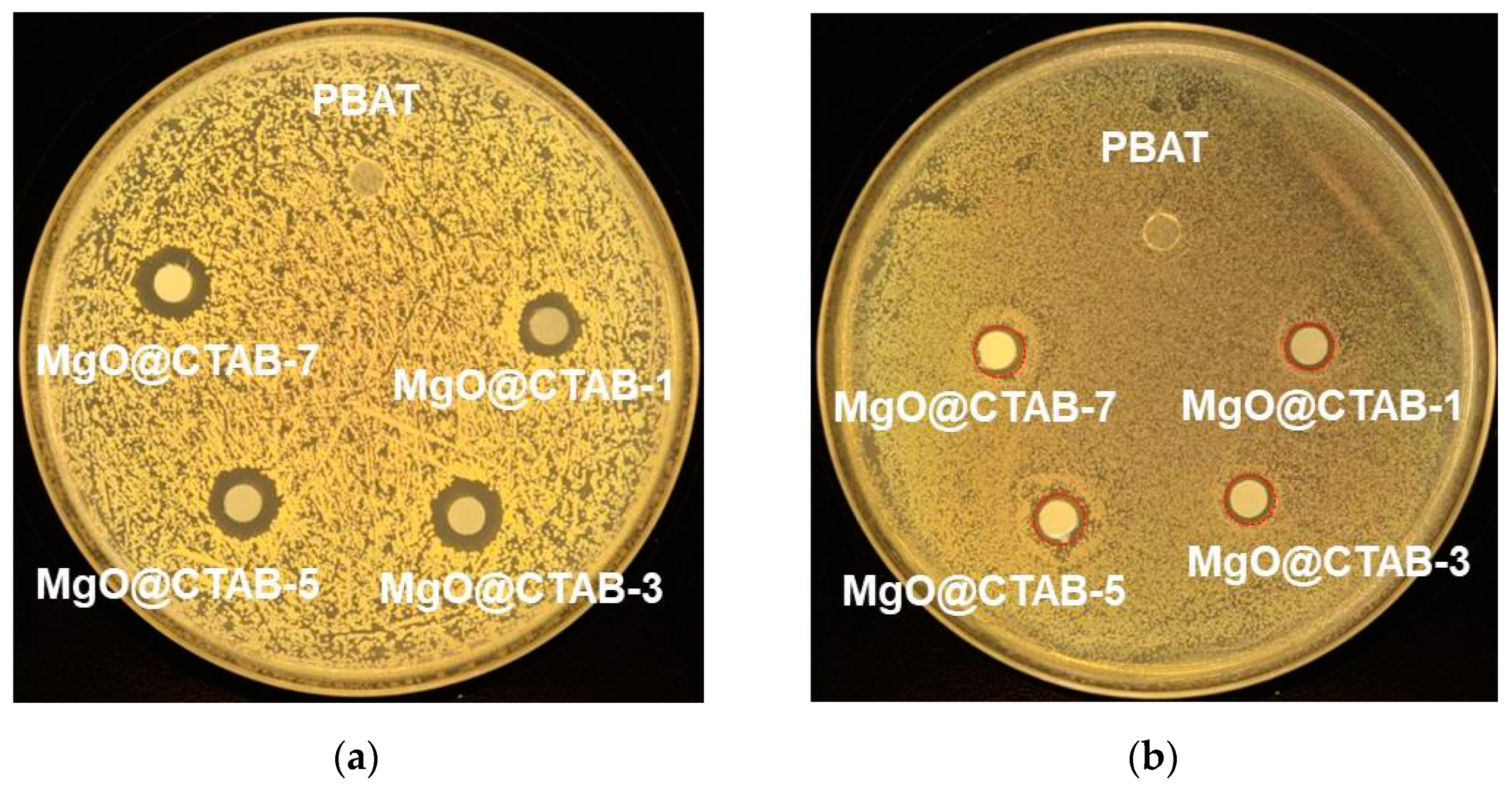
The antimicrobial activity of the PBAT nanocomposite films against S. aureus and E. coli is shown in Figure 9, and the bacterial inhibition zone data are listed in Table 4. It is clear that no inhibition zone is observed for MgO@CTAB-0 film, indicating that pure PBAT film has no antibacterial activity against S. aureus and E. coli. The addition of MgO@CTAB endows the PBAT nanocomposite with antibacterial activity [10,14]. Moreover, the bacterial inhibition zone increases gradually with the increasing content of MgO@CTAB. These results can be ascribed to the superior antibacterial properties of MgO. However, PBAT/MgO@CTAB exhibits better antibacterial efficiency against S. aureus than against E. coli, which is similar to other studies in the literature [31,32,33,34]. This phenomenon is ascribed to the fact that the peptidoglycan membrane of the Gram-positive bacteria S. aureus is much thicker than that of the Gram-negative bacteria E. coli, and as a result, the released Mg ions do not easily penetrate the peptidoglycan membrane and disrupt the essential enzyme systems in the bacteria.
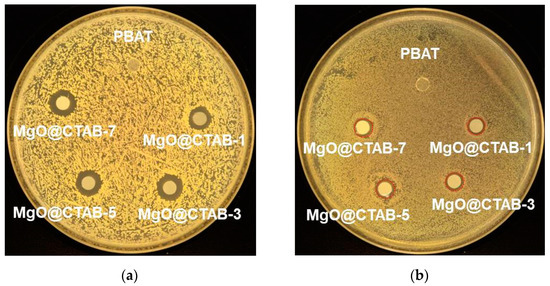
Figure 9.
Photographs of antimicrobial test results of PBAT and its nanocomposite against (a) S. aureus, (b) E. coli.

Table 4.
Antimicrobial activity of PBAT nanocomposite films against S. aureus and E. coli.
4. Conclusions
In this work, MgO@CTAB nanoparticles were synthesized and then mixed with a PBAT matrix using the melt compounding method. SEM observation and dynamical rheological measurement revealed that aggregation of MgO@CTAB occurred when the content of MgO@CTAB exceeded 5 wt%. PBAT nanocomposites containing 3 wt% MgO@CTAB exhibited the highest tensile strength of 27.90 MPa and a maximum elongation at break of 1773.95%. The presence of MgO@CTAB resulted in the poor thermal stability of PBAT due to its catalytic effect. In addition, MgO@CTAB enhanced the crystallinity of PBAT. The incorporation of MgO@CTAB endowed PBAT with antibacterial activity against both the Gram-positive bacteria S. aureus and the Gram-negative bacteria E. coli. The bacterial inhibition zone showed that PBAT/MgO@CTAB nanocomposites had better antibacterial performance against S. aureus than E. coli.
Author Contributions
Investigation, X.W., L.C., S.F., and X.L.; writing—original draft preparation, X.W.; methodology, X.W. and S.F.; writing—review and editing, Y.L.; supervision, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research and APC was funded by the Natural Science Foundation of Hunan Province [No. 2019JJ50132], the Innovation Platform Open Fund of Hunan Province [No. 18K079].
Institutional Review Board Statement
This study didn’t involve humans or animals.
Informed Consent Statement
This study didn’t involve patients.
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Herrera, R.; Franco, L.; Rodriguez-Galan, A.; Puiggali, J. Characterization and degradation behavior of poly(butylene adipate-co-terephthalate)s. J. Polym. Sci. Part. A Polym. Chem. 2002, 40, 4141–4157. [Google Scholar] [CrossRef]
- Jiang, L.; Wolcott, M.P.; Zhang, J.W. Study of biodegradable polyactide/poly(butylene adipate-co-terephthalate) blends. Biomacromolecules 2006, 7, 199–207. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W. Preparation of antibacterial poly(lactide)/poly(butylene adipate-co-terephthalate) composite films incorporated with grapefruit seed extract. Int. J. Biol. Macromol. 2018, 120, 846–852. [Google Scholar] [CrossRef]
- Liu, Y.T.; Yuan, Y.; Duan, S.Q.; Li, C.; Hu, B.; Liu, A.P.; Wu, D.T.; Cui, H.Y.; Lin, L.; He, J.L.; et al. Preparation and characterization of chitosan films with three kinds of molecular weight for food packaging. Int. J. Biol. Macromol. 2020, 155, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, T.; He, H.B.; Wu, X.H.; Cao, X.W.; Jin, J.; Sun, Q.J.; Roy, V.A.L.; Li, R.K.Y. Rhelogical and antibacterial performance of sodium alginate/zinc oxide composite coating for cellulosic paper. Colloids Surf. B 2018, 167, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Spiridon, I.; Anghel, N.C.; Darie-Nita, R.N.; Iwanczuk, A.; Ursu, R.G.; Spiridon, I.A. New composites based on starch/Ecoflex (R)/biomass wastes: Mechanical thermal, morphological and antimicrobial properties. Int. J. Biol. Macromol. 2020, 156, 1435–1444. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W. Effect of types of zinc oxide nanoparticles on structural, mechanical and antibacterial properties of poly(lactide)/poly(butylene adipate-co-terephthalate) composite films. Food Packag. Shelf. 2019, 21, 100327. [Google Scholar] [CrossRef]
- Venkatesan, R.; Rajeswari, N.; Tamilselvi, A. Antimicrobial, mechanical, barrier, and thermal properties of bio-based poly (butylene adipate-co-terephthalate) (PBAT)/Ag2O nanocomposite films for packaging application. Polym. Advan. Technol. 2018, 29, 61–68. [Google Scholar] [CrossRef]
- Luo, S.L.; Zhang, P.; Gao, D. Preparation and properties of antimicrobial poly(butylene adipate-co-terephthalate)/TiO2 nanocomposites films. J. Macrom. Sci. B 2020, 59, 248–261. [Google Scholar] [CrossRef]
- Anicic, N.; Vukomanovic, M.; Koklic, T.; Suvorov, D. Fewer defects in the surface slows the hydrolysis rate, decreases the ROS generation potential, and improves the non-ROS antimicrobial activity of MgO. Small 2018, 14, 1800205. [Google Scholar] [CrossRef] [PubMed]
- Makhluf, S.; Dror, R.; Nitzan, Y.; Abramovich, Y.; Jelinek, R.; Gedanken, A. Microwave-assisted synthesis of nanocrystalline MgO and its use as a bacteriocide. Adv. Funct. Mater. 2005, 15, 1708–1715. [Google Scholar] [CrossRef]
- El-Shaer, A.; Abdelfatah, M.; Mahmoud, K.R.; Momay, S.; Eraky, M.R. Correlation between photoluminescence and positron annihilation lifetime spectroscopy to characterize defects in calcined MgO nanoparticles as a first step to explain antibacterial activity. J. Alloys Compd. 2020, 817, 152799. [Google Scholar] [CrossRef]
- Wang, Y.; Cen, C.; Chen, J.; Fu, L. MgO/carboxymethyl chitosan nanocomposite improves thermal stability, waterproof and antibacterial performance for food packaging. Carbohyd. Polym. 2020, 236, 116078. [Google Scholar] [CrossRef]
- Swaroop, C.; Shukla, M. Nano-magnesium oxide reinforced polylactic acid biofilms for food packaging applications. Int. J. Biol. Macromol. 2018, 113, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Swaroop, C.; Shukla, M. Development of blown polylactic acid-MgO nanocomposite films for food packaging. Compos. Part A Appl. S. 2019, 124, 105482. [Google Scholar] [CrossRef]
- Muhammad, S.; Siddiq, M.; Niazi, J.H.; Qureshi, A. Role of quaternary ammonium compound immobilized metallic graphene oxide in PMMA/PEG membrane for antibacterial, antifouling and selective gas permeability properties. Polym. Bull. 2018, 75, 5695–5712. [Google Scholar] [CrossRef]
- Chang, Z.M.; Wang, Z.; Lu, M.M.; Shao, D.; Yue, J.; Yang, D.; Li, M.Q.; Dong, W.F. Janus silver mesoporous silica nanobullets with synergistic antibacterial functions. Colloids Surf. B 2017, 157, 199–206. [Google Scholar] [CrossRef]
- Sasikumar, M.; Ganeshkumar, A.; Chandraprabha, M.N.; Rajaram, R.; Krishna, R.H.; Ananth, N.; Sivakumar, P. Investigation of antimicrobial activity of CTAB assisted hydrothermally derived nano BaTiO3. Mater. Res. Express. 2019, 6, 025408. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Swain, S.; Giri, L.; Neogi, S. Fabrication of magnesium oxide nanoparticles by solvent alteration and their bactericidal applications. J. Mater. Chem. B 2019, 7, 4141–4152. [Google Scholar] [CrossRef]
- Venkatesan, R.; Rajeswari, N. ZnO/PBAT nanocomposite films: Investigation on the mechanical and biological activity for food packaging. Polym. Advan. Technol. 2017, 28, 20–27. [Google Scholar] [CrossRef]
- Li, M.; Zhou, S.Q.; Xu, M.Y. Graphene oxide supported magnesium oxide as an efficient cathode catalyst for power generation and wastewater treatment in single chamber microbial fuel cells. Chem. Eng. J. 2017, 328, 106–116. [Google Scholar] [CrossRef]
- Li, L.X.; Xu, D.; Li, X.Q.; Liu, W.C.; Jia, Y. Excellent fluoride removal properties of porous hollow MgO microspheres. New J. Chem. 2014, 38, 5445–5452. [Google Scholar] [CrossRef]
- de Barrosa, H.R.; Piovan, L.; Sassaki, G.L.; Sabry, D.D.; Mattoso, N.; Nunes, A.M.; Meneghetti, M.R.; Riegel-Vidotti, I.C. Surface interactions of gold nanorods and polysaccharides: From clusters to individual nanoparticles. Carbohydr. Polym. 2016, 152, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhao, W.J.; Sun, Q.J.; Yu, B.; Yin, X.M.; Cao, X.W.; Feng, Y.H.; Li, R.K.Y.; Qu, J.P. Surface treatment of two dimensional MXene for poly (vinylidene fluoride) nanocomposites with tunable dielectric permittivity. Compos. Commun. 2020, 100562. [Google Scholar] [CrossRef]
- Wu, W.; Wu, C.K.; Peng, H.Y.; Sun, Q.J.; Zhou, L.; Zhuang, J.Q.; Cao, X.W.; Roy, V.A.L.; Li, R.K.Y. Effect of nitrogen-doped graphene on morphology and properties of immiscible poly(butylene succinate)/polylactide blends. Compos. Part B Eng. 2017, 113, 300–307. [Google Scholar] [CrossRef]
- Moustafa, H.; Guizani, C.; Dupont, C.; Martin, V.; Jeguirim, M.; Dufresne, A. Utilization of torrefied coffee grounds as reinforcing agent to produce high-quality biodegradable PBAT composites for food packaging applications. ACS Sustain. Chem. Eng. 2017, 5, 1906–1916. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, C.L.; Zheng, S.M.; Li, W.; Li, B.B.; Xie, X.L. Poly (butylene adipate-co-terephthalate)/magnesium oxide/silver ternary composite biofilms for food packaging application. Food Packag. Shelf. 2020, 24, 100487. [Google Scholar] [CrossRef]
- Xiang, S.; Feng, L.D.; Bian, X.C.; Li, G.; Chen, X.S. Evaluation of PLA content in PLA/PBAT blends using TGA. Polym. Test. 2020, 81, 106211. [Google Scholar] [CrossRef]
- Fourati, Y.; Tarres, Q.; Mutje, P.; Boufi, S. PBAT/thermoplastic starch blends: Effect of compatibilizers on the rheological, mechanical and morphological properties. Carbohydr. Polym. 2018, 199, 51–57. [Google Scholar] [CrossRef]
- Zhao, H.; She, W.; Shi, D.; Wu, W.; Zhang, Q.C.; Li, R.K.Y. Polyurethane/POSS nanocomposites for superior hydrophobicity and high ductility. Compos. Part B Eng. 2019, 177, 107441. [Google Scholar] [CrossRef]
- Nguyen, N.Y.T.; Grelling, N.; Wetteland, C.L.; Rosario, R.; Liu, H.N. Antimicrobial activities and mechanisms of magnesium oxide nanoparticles (nMgO) against pathogenic bacteria, yeasts, and biofilms. Sci. Rep. UK 2018, 8, 16260. [Google Scholar] [CrossRef] [PubMed]
- Baig, U.; Gondal, M.A.; Rehman, S.; Akhtar, S. Facile synthesis, characterization of nano-tungsten trioxide decorated with silver nanoparticles and their antibacterial activity against water-borne gram-negative pathogens. Appl. Nanosci. 2020, 10, 851–860. [Google Scholar] [CrossRef]
- Baig, U.; Gondal, M.A.; Ansari, M.A.; Dastageer, M.A.; Sajid, M.; Falath, W.S. Rapid synthesis and characterization of advanced ceramic-polymeric nanocomposites for efficient photocatalytic decontamination of hazardous organic pollutant under visible light and inhibition of microbial biofilm. Ceram. Int. 2020, 47, 4737–4748. [Google Scholar] [CrossRef]
- Baig, U.; Ansari, M.A.; Gondal, M.A.; Akhtar, S.; Khan, F.A.; Falath, W.S. Single step production of high-purity copper oxide-titanium dioxide nanocomposites and their effective antibacterial and anti-biofilm activity against drug-resistant bacteria. Mater. Sci. Eng. C Mater. 2020, 113, 110992. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).