Synthesis of Magnetic Ferrocene-Containing Polymer with Photothermal Effects for Rapid Degradation of Methylene Blue
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Chemicals
2.2. Instruments
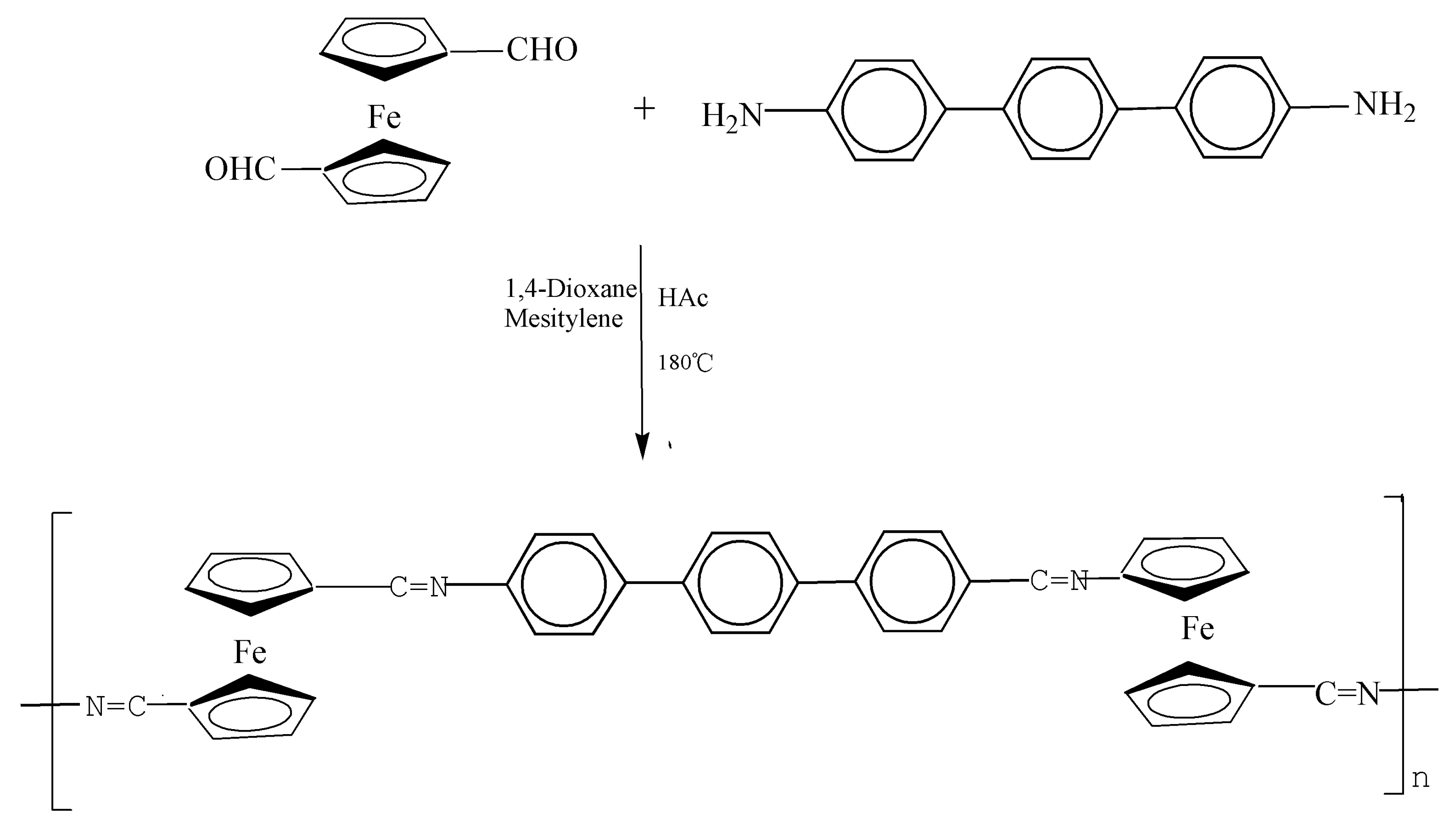
2.3. Fabrication of Polymer
2.4. Adsorption Performance of the Materials to MB
2.5. Photo-Thermocatalytic Degradation Experiment
3. Results and Discussion
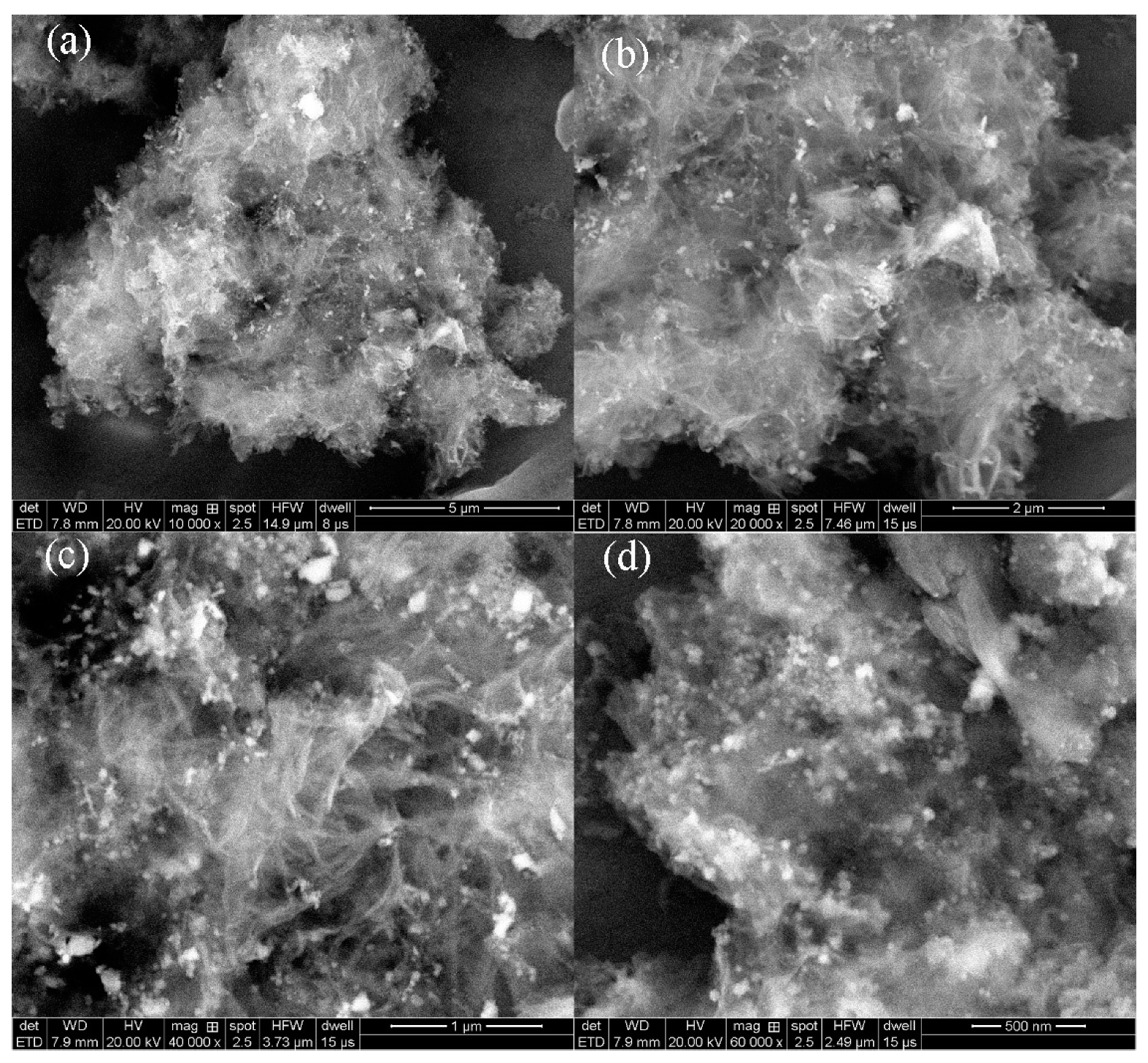
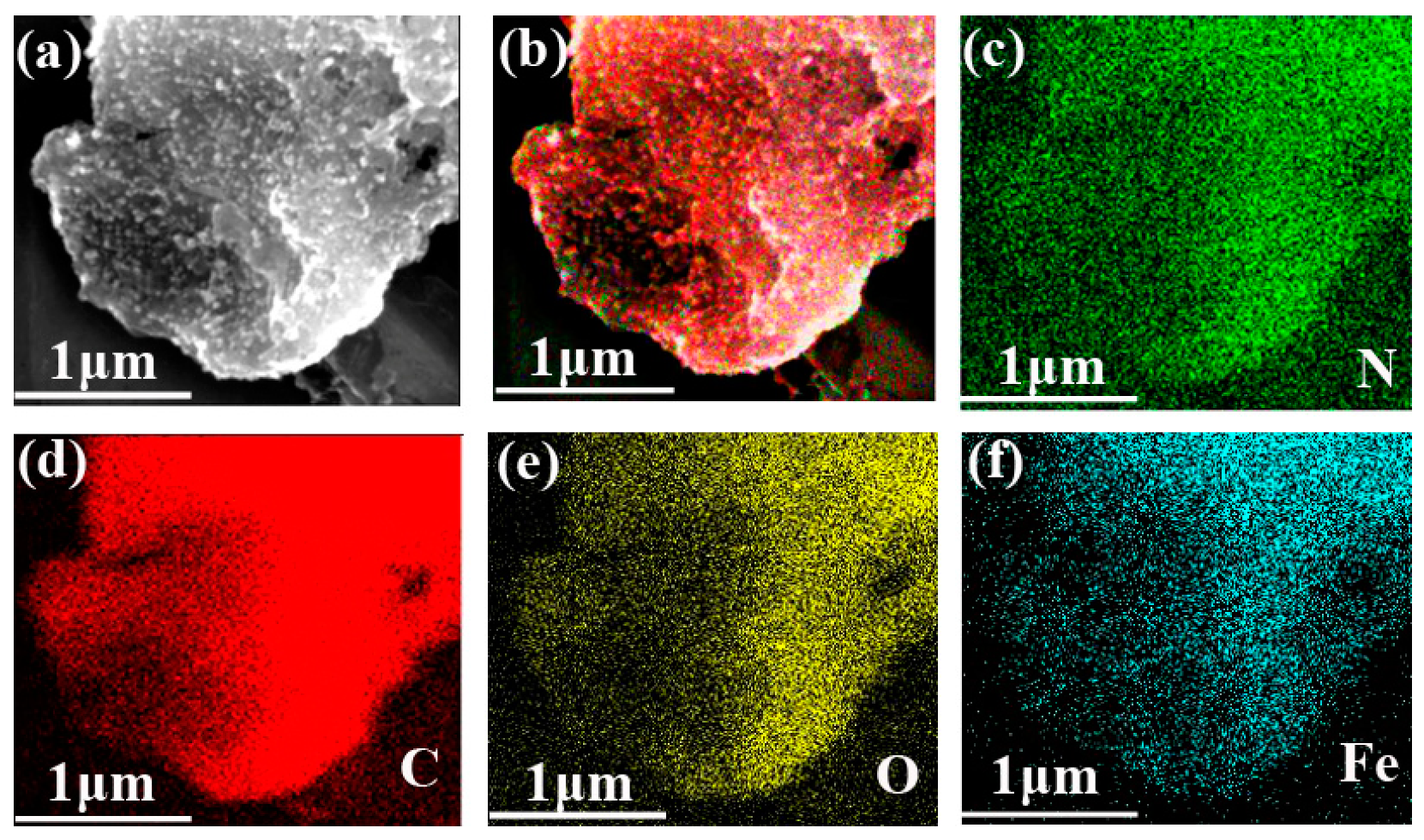
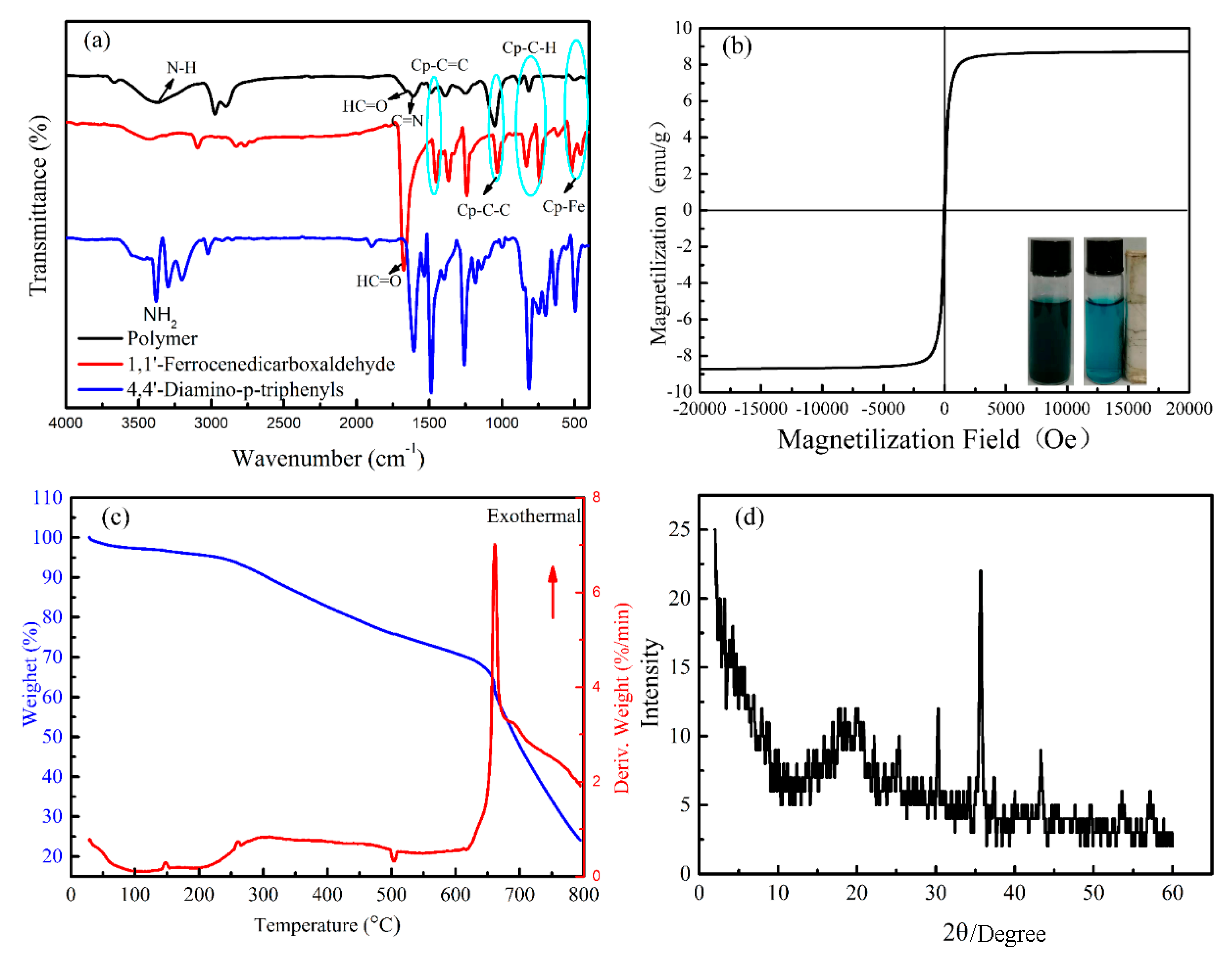
3.1. Preparation and Characterization
3.2. Photothermal Property
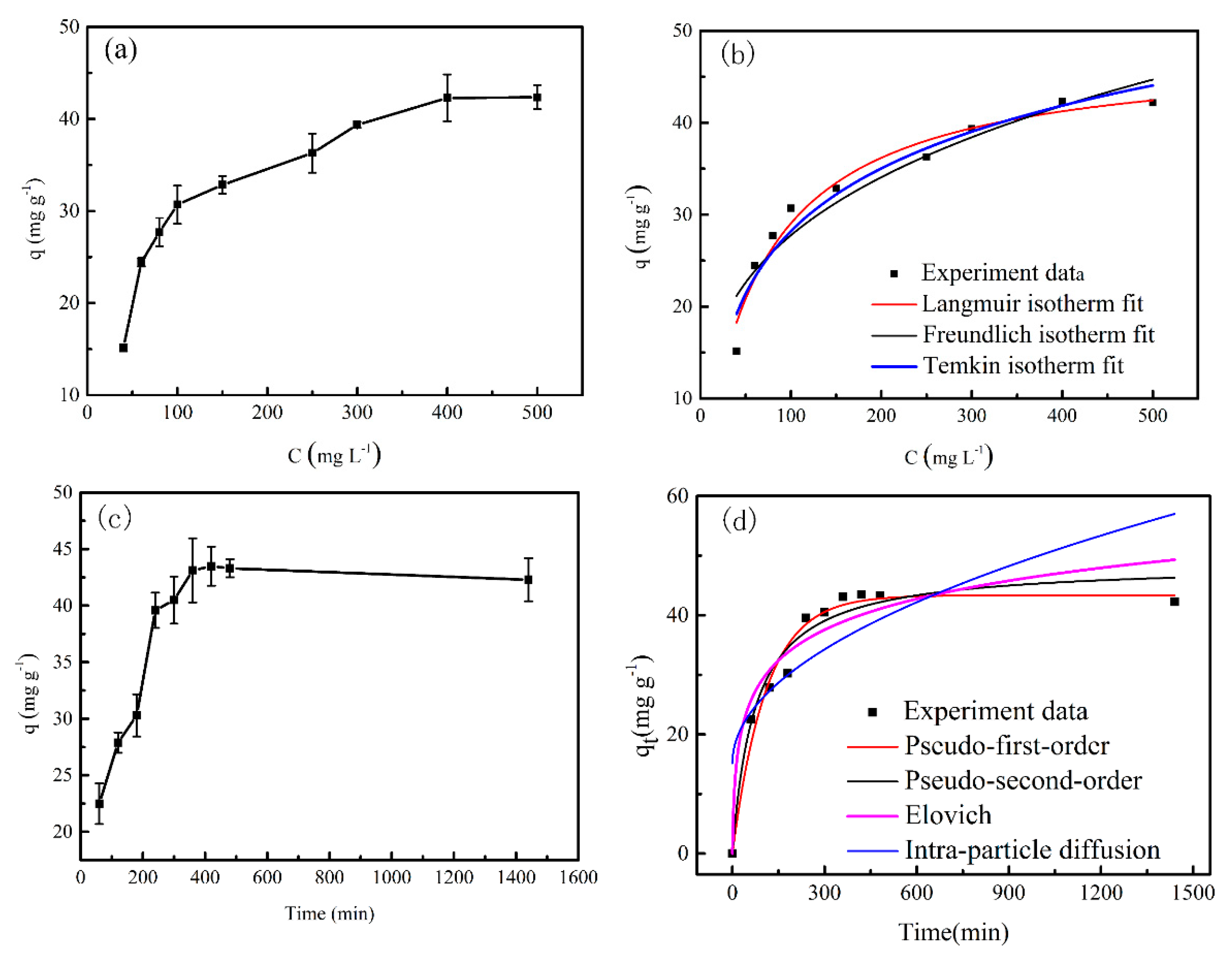
3.3. Adsorption Property
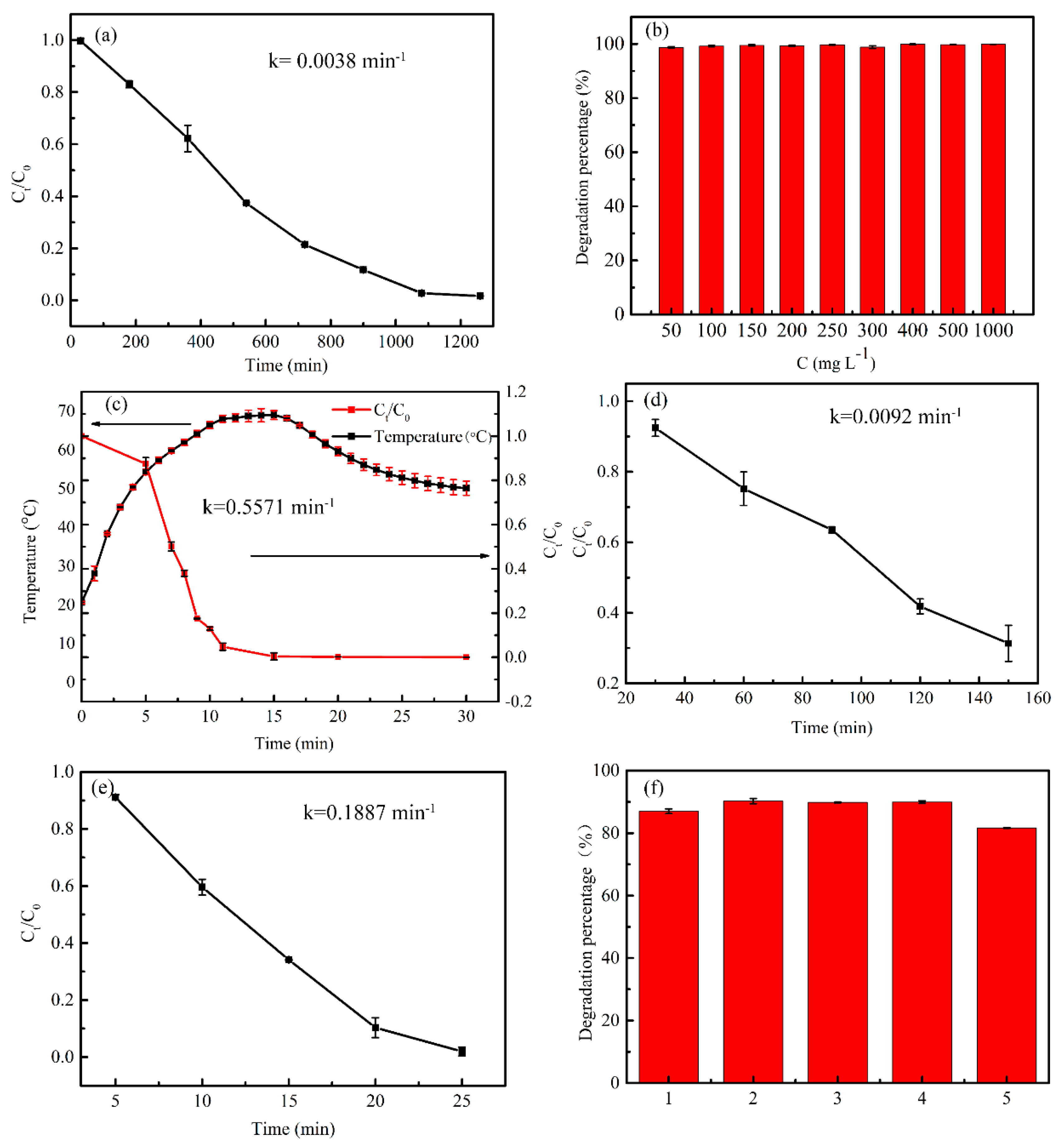
3.4. Catalytic Property
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, W.; Zhu, W.; Du, W.; Liu, X.; Zhang, X.; Dong, H. Cocrystals Strategy towards Novel Materials for Near-infrared Photothermal Conversion and Imaging. Angew. Chem. 2018, 57, 3963–3967. [Google Scholar]
- Tian, X.; Zhu, H.; Meng, X.; Wang, J.; Zheng, C.; Xia, Y.; Xiong, Z. Amphiphilic Calcium Alginate Carbon Aerogels: Broad-Spectrum Adsorbents for Ionic and Solvent Dyes with Multiple Functions for Decolorized Oil–Water Separation. ACS Sustain. Chem. Eng. 2020, 8, 12755–12767. [Google Scholar] [CrossRef]
- Long, Q.; Zhang, Z.; Qi, G.; Wang, Z.; Chen, Y.; Liu, Z. Fabrication of Chitosan Nanofiltration Membranes by the Film Casting Strategy for Effective Removal of Dyes/Salts in Textile Wastewater. ACS Sustain. Chem. Eng. 2020, 8, 2512–2522. [Google Scholar] [CrossRef]
- Alammar, A.; Park, S.H.; Ibrahim, I.; Arun, D.; Holtzl, T.; Dumée, L.F.; Lim, H.N.; Szekely, G. Architecting neonicotinoid-scavenging nanocomposite hydrogels for environmental remediation. Appl. Mater. Today 2020, 21, 100878. [Google Scholar] [CrossRef]
- Li, J.P.; Ma, R.M.; Lu, Y.; Wu, Z.X.; Su, M.L.; Jin, K.X.; Qin, D.C.; Zhang, R.; Bai, R.H.; He, S.; et al. A gravity-driven high-flux catalytic filter prepared using a naturally three-dimensional porous rattan biotemplate decorated with Ag nanoparticles. Green Chem. 2020, 22, 6846–6854. [Google Scholar] [CrossRef]
- Qian, X.; Zhang, J.; Gu, Z.; Chen, Y. Nanocatalysts-Augmented Fenton Chemical Reaction for Nanocatalytic Tumor Therapy. Biomaterials 2019, 211, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Chen, Z.; Zhang, F.; Guo, W.; Lin, H.; Qu, F. Construction of Z-scheme MoSe2/CdSe hollow nanostructure with enhanced full spectrum photocatalytic activity. Appl. Catal. B Environ. 2019, 244, 76–86. [Google Scholar] [CrossRef]
- Zhao, T.; Xinga, Z.; Xiu, Z.; Li, Z.; Chen, P.; Zhu, Q.; Zhou, W. Synergistic effect of surface plasmon resonance, Ti3+ and oxygen vacancy defects on Ag/MoS2/TiO2-x ternary heterojunctions with enhancing photothermal catalysis for low-temperature wastewater degradation. J. Hazard. Mater. 2019, 364, 117–124. [Google Scholar] [CrossRef]
- Sha, Y.; Zhang, Y.; Xu, E.; Wang, Z.; Zhu, T.; Craig, S.L.; Tang, C. Quantitative and Mechanistic Mechanochemistry in Ferrocene Dissociation. ACS Macro Lett. 2018, 7, 1174–1179. [Google Scholar]
- Sha, Y.; Zhang, Y.; Zhu, T.; Tan, S.; Cha, Y.; Craig, S.L.; Tang, C. Ring-Closing Metathesis and Ring-Opening Metathesis Polymerization toward Main-Chain Ferrocene-Containing Polymers. Macromolecules 2018, 51, 9131–9139. [Google Scholar] [CrossRef]
- Horikoshi, R.; Mochida, T. Ferrocene-Containing Coordination Polymers: Ligand Design and Assembled Structures. Eur. J. Inorg. Chem. 2010, 2010, 5355–5371. [Google Scholar] [CrossRef]
- Komorowska-Durka, M.; Dimitrakis, G.; Bogdał, D.; Stankiewicz, A.I.; Stefanidis, G.D. A concise review on microwave-assisted polycondensation reactions and curing of polycondensation polymers with focus on the effect of process conditions. Chem. Eng. J. 2015, 264, 633–644. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Chen, Y.; Yin, M.; Jia, T.; He, S.; Deng, Q.; Wang, S. Carbon Tube Clusters with Nanometer Walls Thickness, Micrometer Diameter from Biomass, and Its Adsorption Property as Bioadsorbent. ACS Sustain. Chem. Eng. 2018, 7, 858–866. [Google Scholar] [CrossRef]
- Jaseela, P.K.; Garvasis, J.; Joseph, A. Selective adsorption of methylene blue (MB) dye from aqueous mixture of MB and methyl orange (MO) using mesoporous titania (TiO2)—Poly vinyl alcohol (PVA) nanocomposite. J. Mol. Liq. 2019, 286, 110908. [Google Scholar] [CrossRef]
- Teimuri-Mofrad, R.; Esmati, S.; Rabiei, M.; Gholamhosseini-Nazari, M. Ferrocene-containing ionic liquid supported on silica nanospheres (SiO2@Imid-Cl@Fc) as a mild and efficient heterogeneous catalyst for the synthesis of pyrano[3,2-b]pyran derivatives under ultrasound irradiation. J. Chem. Res. 2018, 42, 7–12. [Google Scholar]
- Teimuri-Mofrad, R.; Abbasi, H.; Hadi, R. Graphene oxide-grafted ferrocene moiety via ring opening polymerization (ROP) as a supercapacitor electrode material. Polymer 2019, 167, 138–145. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, H.; Song, Y.; Zhang, S.; Wang, M.; Jia, C.; Tian, J.-Y.; He, L.; Zhang, X.; Liu, C.-S. Fe(III)-based metal–organic framework-derived core–shell nanostructure: Sensitive electrochemical platform for high trace determination of heavy metal ions. Biosens. Bioelectron. 2017, 94, 358–364. [Google Scholar]
- Cheng, X.; Deng, X.; Wang, P.; Liu, H. Coupling TiO2 nanotubes photoelectrode with Pd nano-particles and reduced graphene oxide for enhanced photocatalytic decomposition of diclofenac and mechanism insights. Sep. Purif. Technol. 2015, 154, 51–59. [Google Scholar] [CrossRef]
- Shankar, S.; Gowthaman, N.S.K.; John, S.A. Synthesis of albumin capped gold nanoparticles and their direct attachment on glassy carbon electrode for the determination of nitrite ion. J. Electroanal. Chem. 2018, 828, 33–40. [Google Scholar] [CrossRef]
- El-Nagar, G.A.; Hassan, M.A.; Fetyan, A.; Kayarkatte, M.K.; Lauermann, I.; Roth, C. A promising N-doped carbon-metal oxide hybrid electrocatalyst derived from crustacean’s shells: Oxygen reduction and oxygen evolution. Appl. Catal. B Environ. 2017, 214, 137–147. [Google Scholar]
- Wang, F.-M.; Cheng, H.-M.; Wu, H.-C.; Chu, S.-Y.; Cheng, C.-S.; Yang, C.-R. Novel SEI formation of maleimide-based additives and its improvement of capability and cyclicability in lithium ion batteries. Electrochim. Acta 2009, 54, 3344–3351. [Google Scholar] [CrossRef]
- He, S.; Rong, Q.; Niu, H.; Cai, Y. Construction of a superior visible-light-driven photocatalyst based on a C3N4 active centre-photoelectron shift platform-electron withdrawing unit triadic structure covalent organic framework. Chem. Commun. 2017, 53, 9636–9639. [Google Scholar]
- Liu, S.; Xin, Z.J.; Lei, Y.J.; Yang, Y.; Yan, X.Y.; Lu, Y.B.; Li, C.B.; Wang, H.Y. Thin Copper-Based Film for Efficient Electrochemical Hydrogen Production from Neutral Aqueous Solutions. ACS Sustain. Chem. Eng. 2017, 5, 7496–7501. [Google Scholar] [CrossRef]
- Zhao, P.; Chen, H.; Ge, S.; Yoshikawa, K. Effect of the hydrothermal pretreatment for the reduction of NO emission from sewage sludge combustion. Appl. Energy 2013, 111, 199–205. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Yun, J.; Kong, J. Novel ferrocene-containing organosilicon polymers and uniform microspheres prepared by free radical copolymerization: Precursors for magnetic Si-C-Fe-(O) nanomaterials. Mater. Des. 2018, 144, 86–97. [Google Scholar] [CrossRef]
- Woodbridge, C.M.; Pugmire, D.L.; Johnson, R.C.; Boag, N.M.; Langell, M.A. HREELS and XPS Studies of Ferrocene on Ag (100). J. Phys. Chem. B 2000, 104, 3085–3093. [Google Scholar] [CrossRef]
- Eyley, S.; Shariki, S.; Dale, S.E.; Bending, S.; Marken, F.; Thielemans, W. Ferrocene-decorated nanocrystalline cellulose with charge carrier mobility. Langmuir 2012, 28, 6514–6519. [Google Scholar]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Chen, C.; Yang, G.L.; Wu, Q.H.; Tian, J.; Hao, E.H.; Cao, H.L.; Gao, Y.; Zhang, W.A. Inhibiting Radiative Transition-Mediated Multifunctional Polymeric Nanoplatforms for Highly Efficient Tumor Phototherapeutics. ACS Appl. Mater. Interfaces 2020, 12, 44523–44533. [Google Scholar]
- Xiao, L.H.; Chen, X.; Yang, X.Y.; Sun, J.H.; Geng, J.X. Recent Advances in Polymer-Based Photothermal Materials for Biological Applications. ACS Appl. Polym. Mater. 2020, 2, 4273–4288. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, W.; Yu, S.; Zhu, Y.; Zhang, R.; Tay, J.H. Adsorption of anion polyacrylamide from aqueous solution by polytetrafluoroethylene (PTFE) membrane as an adsorbent: Kinetic and isotherm studies. J. Colloid Interface Sci. 2019, 544, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Piri, F.; Mollahosseini, A.; khadir, A.; Hosseini, M.M. Enhanced adsorption of dyes on microwave-assisted synthesized magnetic zeolite-hydroxyapatite nanocomposite. J. Environ. Chem. Eng. 2019, 7, 103338. [Google Scholar] [CrossRef]
- Chu, C.; Ryberg, E.C.; Loeb, S.K.; Suh, M.-J.; Kim, J.-H. Water Disinfection in Rural Areas Demands Unconventional Solar Technologies. Acc. Chem. Res. 2019, 52, 1187–1195. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Zhang, C.; Chen, Y.; Deng, Q. Synthesis of Magnetic Ferrocene-Containing Polymer with Photothermal Effects for Rapid Degradation of Methylene Blue. Polymers 2021, 13, 558. https://doi.org/10.3390/polym13040558
Zhu W, Zhang C, Chen Y, Deng Q. Synthesis of Magnetic Ferrocene-Containing Polymer with Photothermal Effects for Rapid Degradation of Methylene Blue. Polymers. 2021; 13(4):558. https://doi.org/10.3390/polym13040558
Chicago/Turabian StyleZhu, Wenhui, Caiyun Zhang, Yali Chen, and Qiliang Deng. 2021. "Synthesis of Magnetic Ferrocene-Containing Polymer with Photothermal Effects for Rapid Degradation of Methylene Blue" Polymers 13, no. 4: 558. https://doi.org/10.3390/polym13040558






