Facile Post Treatment of Ag Nanowire/Polymer Composites for Flexible Transparent Electrodes and Thin Film Heaters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
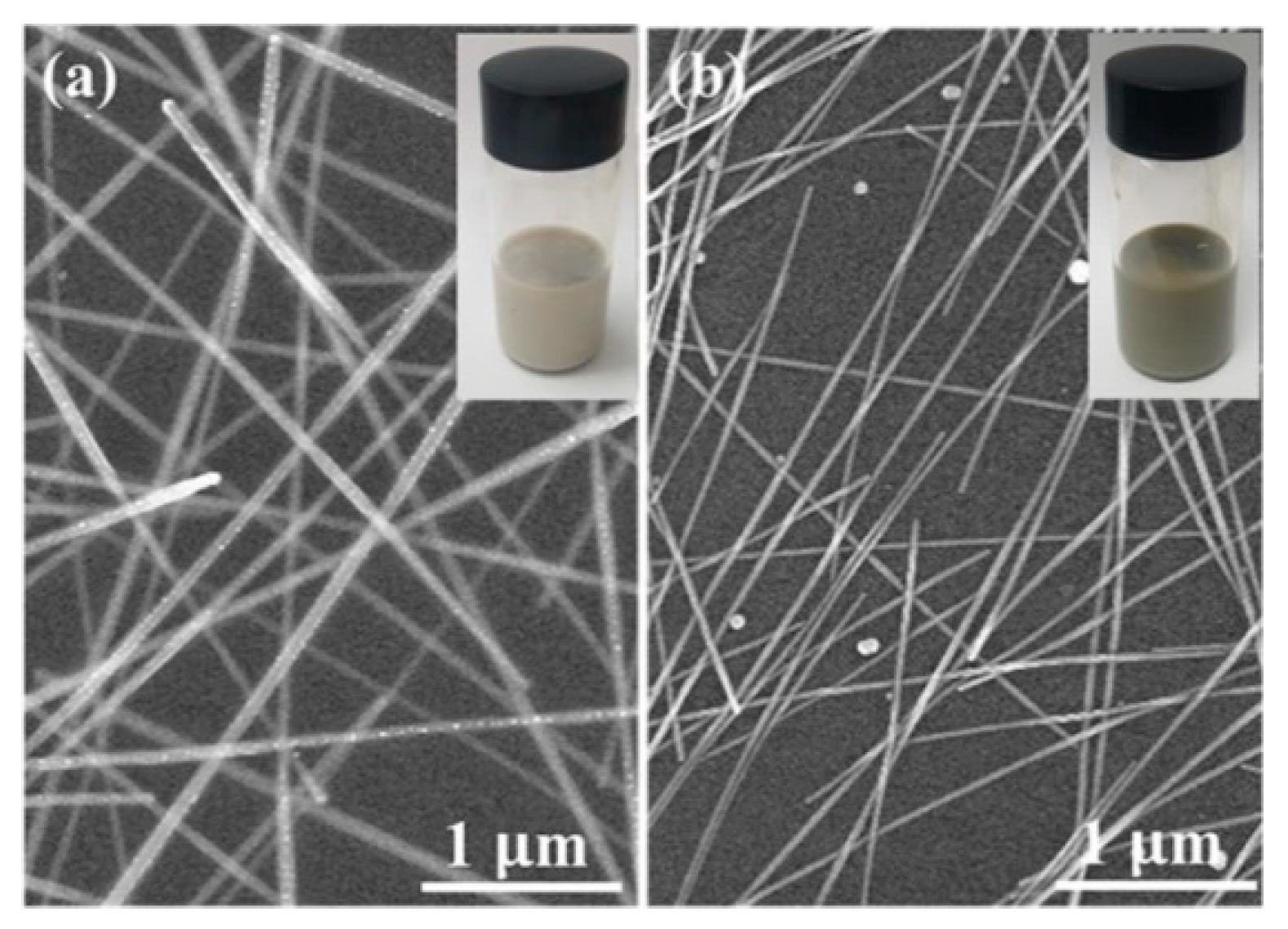
2.2. Controlled Synthesis of Ag NWs
2.3. Fabrication and Post Treatment of Ag NWs Network
2.4. Characterizations
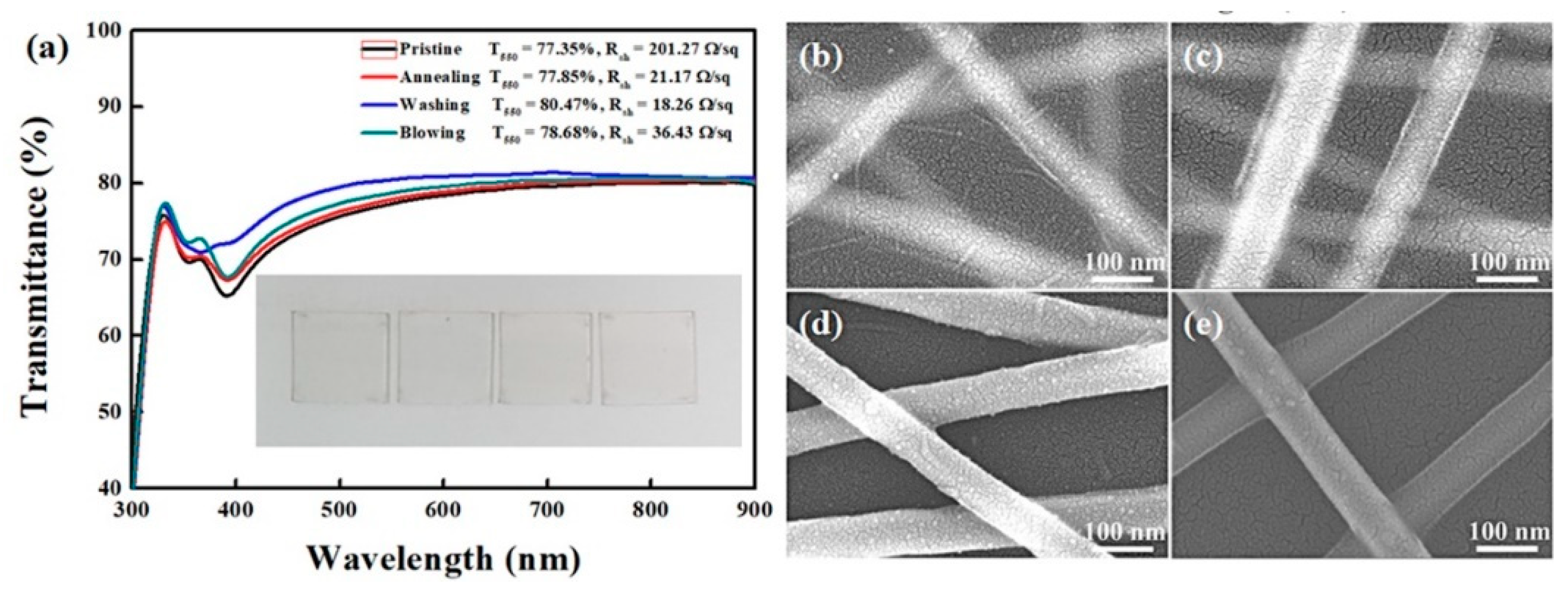
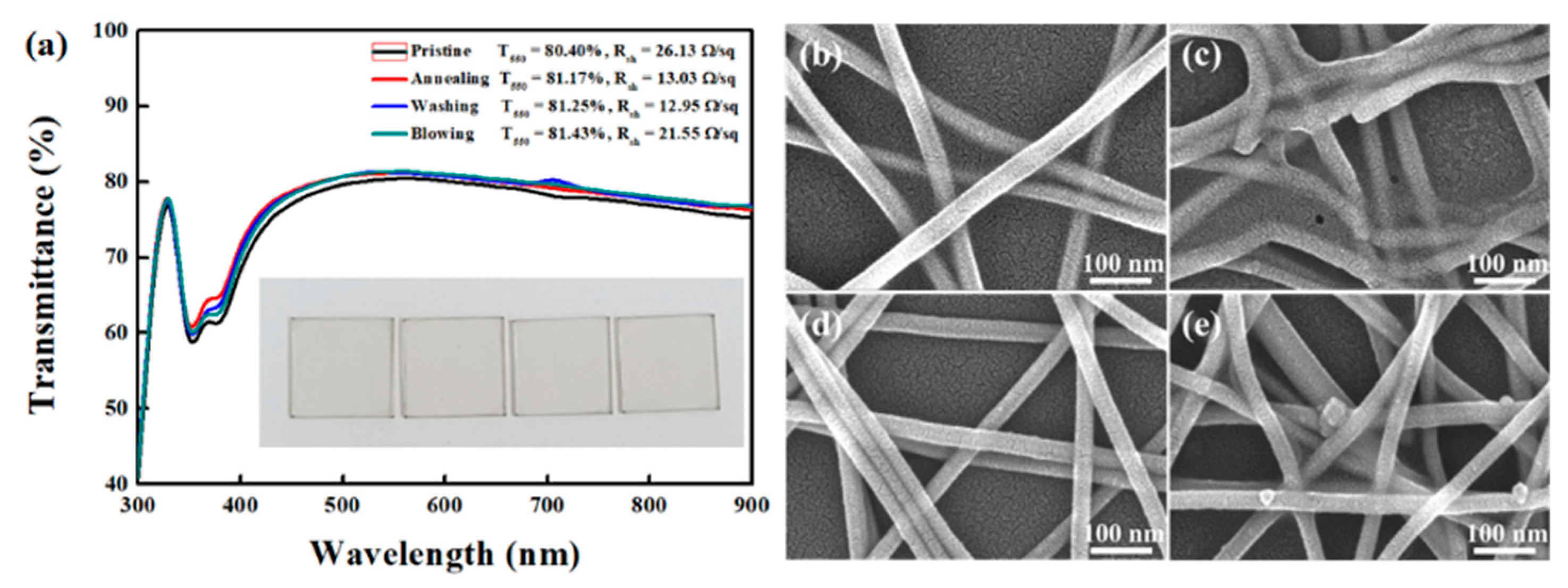
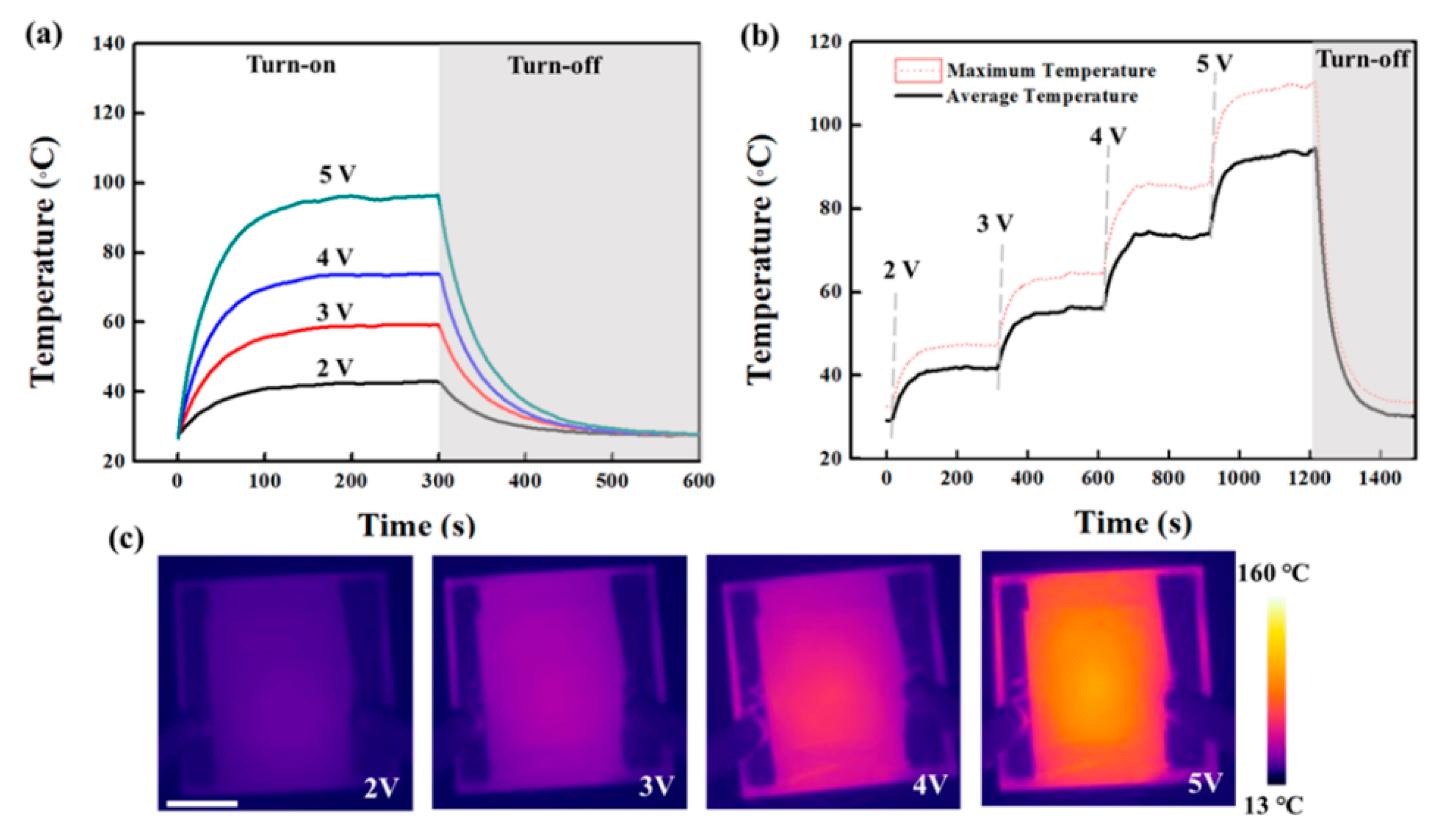
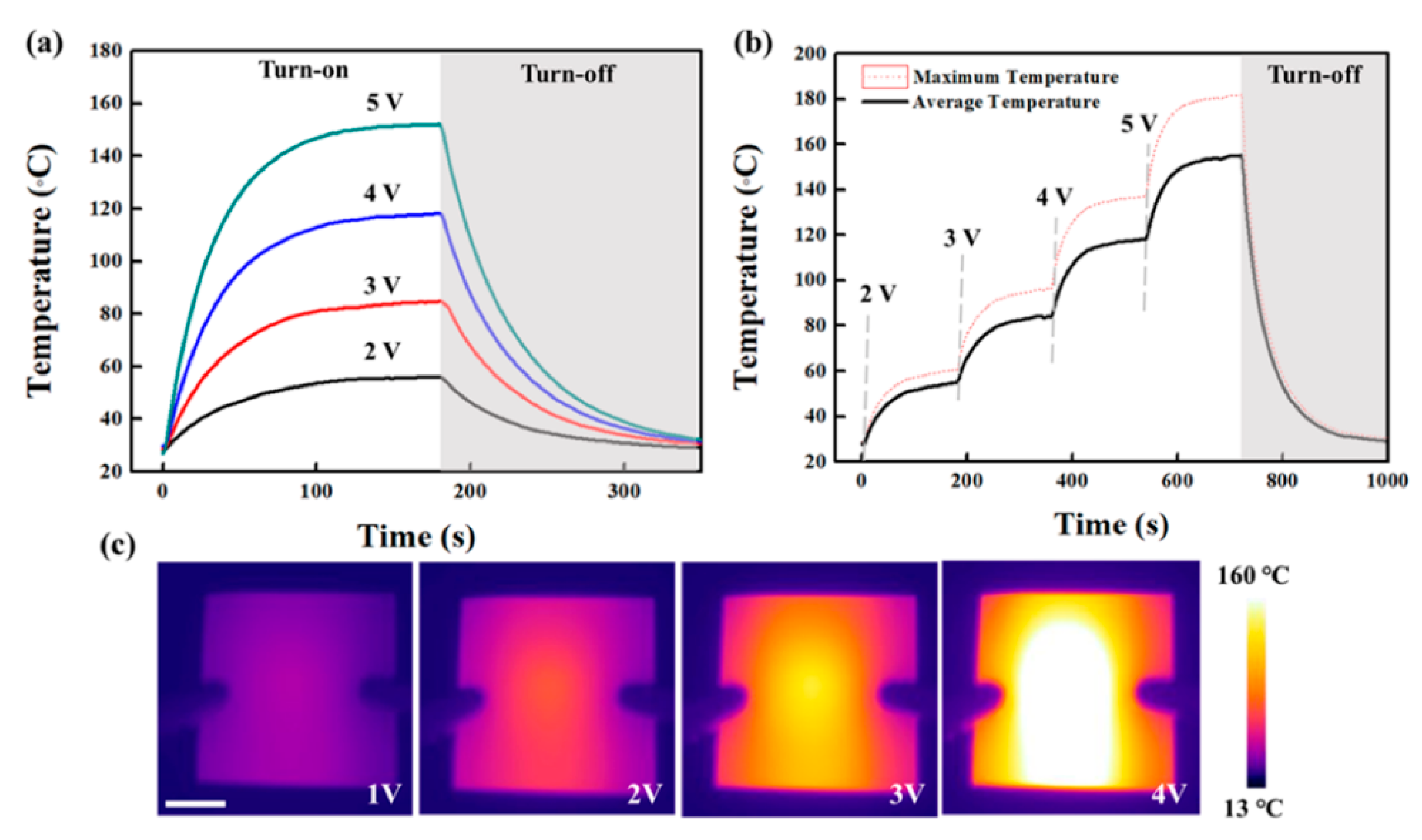
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, Z.; Li, L.; Zhang, Q.; Hu, W.; Pei, Q. Silver nanowire—Polymer composite electrodes for efficient polymer solar cells. Adv. Mater. 2011, 23, 4453–4457. [Google Scholar] [CrossRef]
- Song, J.; Son, D.; Kim, J.; Yoo, Y.J.; Lee, G.J.; Wang, L.; Choi, M.K.; Yang, J.; Lee, M.; Do, K. Wearable Force Touch Sensor Array Using a Flexible and Transparent Electrode. Adv. Funct. Mater. 2017, 27, 1605286. [Google Scholar] [CrossRef]
- Lipomi, D.J.; Vosgueritchian, M.; Tee, B.C.; Hellstrom, S.L.; Lee, J.A.; Fox, Z.; Bao, C.H. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat. Nanotech. 2011, 6, 788. [Google Scholar] [CrossRef]
- Li, L.; Yu, Z.; Hu, W.; Chang, C.; Chen, Q.; Pei, Q. Efficient Flexible Phosphorescent Polymer Light—Emitting Diodes Based on Silver Nanowire--Polymer Composite Electrode. Adv. Mater. 2011, 23, 5563–5567. [Google Scholar] [CrossRef]
- Kim, S.; Yim, J.; Wang, X.; Bradley, D.D.; Lee, S.; de Mello, J.C. Spin and Spray Deposited Single Walled Carbon Nanotube Electrodes for Organic Solar Cells. Adv. Funct. Mater. 2010, 20, 2310–2316. [Google Scholar] [CrossRef]
- Ellmer, K. Past achievements and future challenges in the development of optically transparent electrodes. Nat. Photon. 2012, 6, 809. [Google Scholar] [CrossRef]
- Lee, J.; Lee, P.; Lee, H.B.; Hong, S.; Lee, I.; Yeo, J.; Lee, S.S.; Kim, T.; Lee, D.; Ko, S.H. Room Temperature Nanosoldering of a Very Long Metal Nanowire Network by Conducting--Polymer--Assisted Joining for a Flexible Touch--Panel Application. Adv. Funct. Mater. 2013, 23, 4171–4176. [Google Scholar] [CrossRef]
- Hecht, D.S.; Hu, L.; Irvin, G. Emerging Transparent Electrodes Based on Thin Films of Carbon Nanotubes, Graphene, and Metallic Nanostructures. Adv. Mater. 2011, 23, 1482–1513. [Google Scholar] [CrossRef]
- Wassei, J.K.; Kaner, R.B. Graphene, a promising transparent conductor. Mater. Today 2010, 13, 52–59. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, Q.; Li, L.; Chen, Q.; Niu, X.; Liu, J.; Pei, Q. Highly Flexible Silver Nanowire Electrodes for Shape Memory Polymer Light Emitting Diodes. Adv. Mater. 2011, 23, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, L.; Tong, K.; Ren, Z.; Hu, W.; Niu, X.; Chen, Y.; Pei, Q. Silver Nanowire Percolation Network Soldered with Graphene Oxide at Room Temperature and Its Application for Fully Stretchable Polymer Light-Emitting Diodes. ACS Nano 2014, 8, 1590–1600. [Google Scholar] [CrossRef]
- Hu, L.; Kim, H.S.; Lee, J.; Peumans, P.; Cui, Y. Scalable Coating and Properties of Transparent, Flexible, Silver Nanowire Electrodes. ACS Nano 2010, 4, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; An, K.; Won, P.; Ka, Y.; Hwang, H.; Moon, H.; Kwon, Y.; Hong, S.; Kim, C.; Lee, C.; et al. A dual-scale metal nanowire network transparent conductor for highly efficient and flexible organic light emitting diodes. Nanoscale 2017, 9, 1978–1985. [Google Scholar] [CrossRef]
- Elshaarawy, R.F.M.; Seif, G.A.; El-Naggar, M.E.; Mostafa, T.B.; El-Sawi, E.A. In-situ and ex-situ synthesis of poly-(imidazolium vanillyl)-grafted chitosan/silver nanobiocomposites for safe antibacterial finishing of cotton fabrics. Eur. Polym. J. 2019, 116, 210–221. [Google Scholar] [CrossRef]
- Hussein, J.; El-Naggar, M.E.; Latif, A.L.; Medhat, D.; Bana, M.E.; Refaat, E.; Morsy, S. Solvent-free and one-pot synthesis of silver and zinc oxide nanoparticles:Activity toward cell membrane component and insulin signaling pathway inexperimental diabetes. Colloids Surf. B Biointerfaces 2018, 170, 76–84. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.E.; Hebeish, S.S.A.A. Bactericidal finishing of loomstate, scoured and bleached cotton fibres via sustainable in-situ synthesis of silver nanoparticles. Int. J. Biolog. Macromol. 2018, 106, 1192–1202. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Wassel, A.R.; Shoueir, K. Visible-light driven photocatalytic effectiveness for solid-state synthesis of ZnO/natural clay/TiO2 nanoarchitectures towards complete decolorization of methylene blue from aqueous solution. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100425. [Google Scholar]
- Khattab, T.A.; El-Naggar, M.E.; Abdelrahman, M.S.; Aldalbahi, A.; Hatshan, M.R. Simple Development of Novel Reversible Colorimetric Thermometer Using Urea Organogel Embedded with Thermochromic Hydrazone Chromophore. Chemosensors 2020, 8, 132. [Google Scholar] [CrossRef]
- Shoueir, K.; Wassel, A.R.; Ahmed, M.K.; El-Naggard, M.E. Encapsulation of extremely stable polyaniline onto Bio-MOF: Photo-activated antimicrobial and depletion of ciprofloxacin from aqueous solutions. J. Photochem. Photobiol. A 2020, 400, 112703. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, Y.; Mayers, B.T.; Herricks, T.; Xia, Y. Uniform Silver Nanowires Synthesis by Reducing AgNO3 with Ethylene Glycol in the Presence of Seeds and Poly(Vinyl Pyrrolidone). Chem. Mater. 2002, 14, 4736–4745. [Google Scholar] [CrossRef]
- Wu, H.; Hu, L.; Rowell, M.W.; Kong, D.; Cha, J.J.; McDonough, J.R.; Zhu, J.; Yang, Y.; McGehee, M.D.; Cui, Y. Electrospun Metal Nanofiber Webs as High-Performance Transparent Electrode. Nano Lett. 2010, 10, 4242–4248. [Google Scholar] [CrossRef]
- Garnett, E.C.; Cai, W.; Cha, J.J.; Mahmood, F.; Connor, S.T.; Christoforo, M.G.; Cui, Y.; McGehee, M.D.; Brongersma, M.L. Self-limited plasmonic welding of silver nanowire junctions. Nat. Mater. 2012, 11, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Rafizadeh, E.; Mani, A.; Kazeminezhad, M. The effects of intermediate and postannealing phenomena on the mechanical properties and microstructure of constrained groove pressed copper sheet. Mater. Sci. Eng. A 2009, 515, 162–168. [Google Scholar] [CrossRef]
- Gaynor, W.; Burkhard, G.F.; McGehee, M.D.; Peumans, P. Smooth Nanowire/Polymer Composite Transparent Electrodes. Adv. Mater. 2011, 23, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Langley, D.; Lagrange, M.; Giusti, G.; Jimenez, C.; Bréchet, Y.; Nguyen, N.D.; Bellet, D. Metallic nanowire networks: Effects of thermal annealing on electrical resistance. Nanoscale 2014, 6, 13535–13543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zhao, B.; Hu, L. PVP Protective Mechanism of Ultrafine Silver Powder Synthesized by Chemical Reduction Processes. J. Sol. State Chem. 1996, 121, 105–110. [Google Scholar] [CrossRef]
- Hong, S.; Lee, H.; Lee, J.; Kwon, J.; Han, S.; Suh, Y.D.; Cho, H.; Shin, J.; Yeo, J.; Ko, S.H. Highly stretchable and transparent metal nanowire heater for wearable electronics applications. Adv. Mater. 2015, 27, 4744–4751. [Google Scholar] [CrossRef]
- Park, J.; Hong, S.; Jang, J.; Kim, H. Blue Laser Annealing of Ag Nanowire Electrodes for Flexible Thin Film Heaters. ECS J. Sol. State Sci. Tech. 2017, 6, 746–751. [Google Scholar] [CrossRef]
- Wang, P.; Chen, S.; Su, C.; Liao, Y. Direct printed silver nanowire thin film patterns for flexible transparent heaters with temperature gradients. RSC Adv. 2015, 5, 98412–98418. [Google Scholar] [CrossRef]
- Huang, Q.; Shen, W.; Fang, X.; Chen, G.; Guo, J.; Xu, W.; Tan, R.; Song, W. Highly flexible and transparent film heaters based on polyimide films embedded with silver nanowires. RSC Adv. 2015, 5, 45836–45842. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, I.S.; Lee, H.D.; Hong, S.I.; Lee, W.; Jung, J.W. Facile Post Treatment of Ag Nanowire/Polymer Composites for Flexible Transparent Electrodes and Thin Film Heaters. Polymers 2021, 13, 586. https://doi.org/10.3390/polym13040586
Jin IS, Lee HD, Hong SI, Lee W, Jung JW. Facile Post Treatment of Ag Nanowire/Polymer Composites for Flexible Transparent Electrodes and Thin Film Heaters. Polymers. 2021; 13(4):586. https://doi.org/10.3390/polym13040586
Chicago/Turabian StyleJin, In Su, Hee Dong Lee, Seok Il Hong, Woosung Lee, and Jae Woong Jung. 2021. "Facile Post Treatment of Ag Nanowire/Polymer Composites for Flexible Transparent Electrodes and Thin Film Heaters" Polymers 13, no. 4: 586. https://doi.org/10.3390/polym13040586






