Polycaprolactone-Based Scaffolds Facilitates Osteogenic Differentiation of Human Adipose-Derived Stem Cells in a Co-Culture System
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Adipose-Derived Stem Cells (ASCs) Isolation and Culture
2.2. Human Osteoblasts (HOB) Isolation and Culture
2.3. PCL-HA Scaffolds Preparation and Fabrication
2.4. Co-Culture Set-Up
2.5. Morphology and Cell Proliferation Analysis
2.6. Alkaline Phosphatase (ALP) Analysis
2.7. Calcium Deposition Analysis
2.8. Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
2.9. Statistical Analysis
3. Results
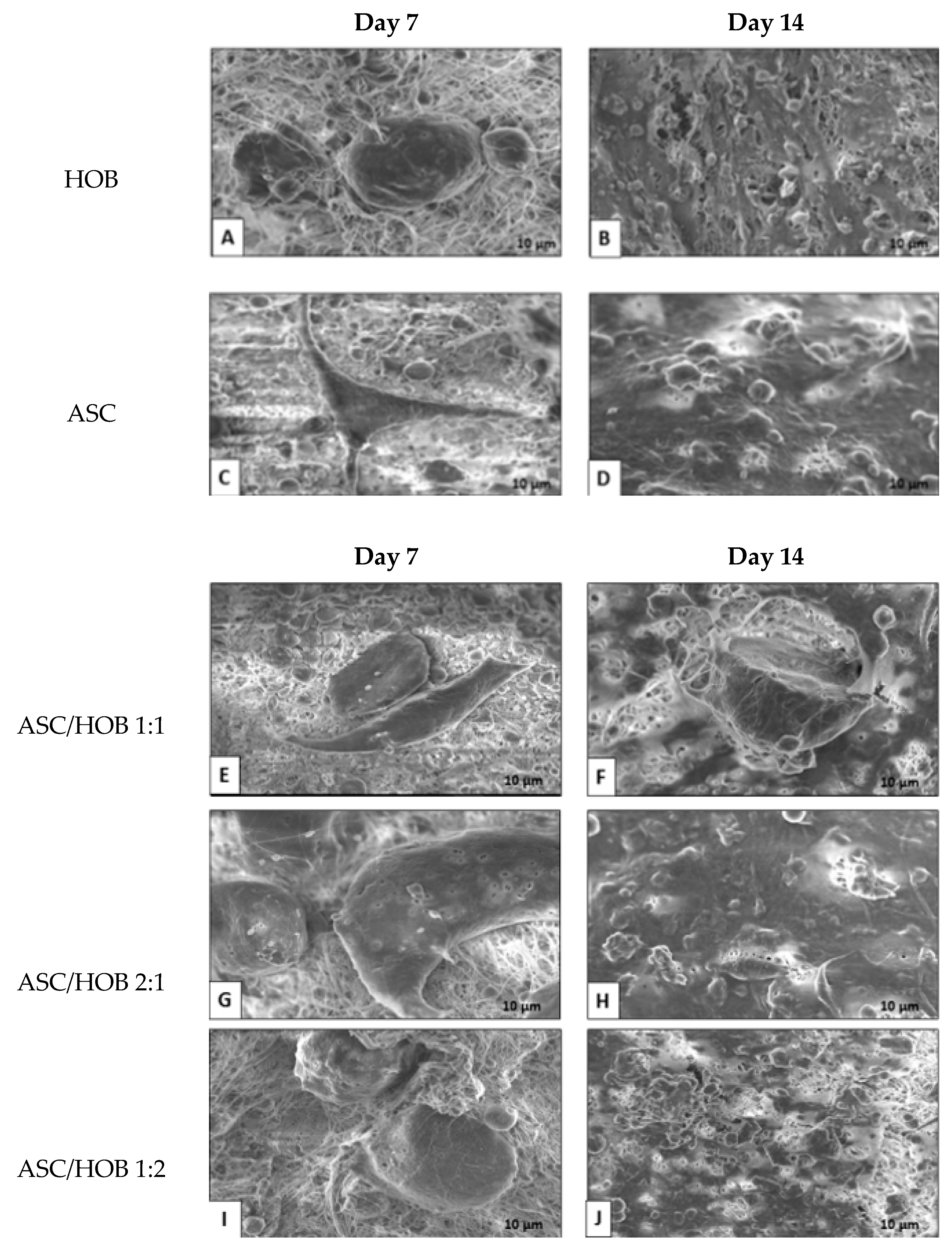
3.1. Cell Morphology in PCL-HA
3.2. Cell Proliferation Ability in PCL-HA Increased with Co-Cultured ASC/HOB at 2:1 Ratio
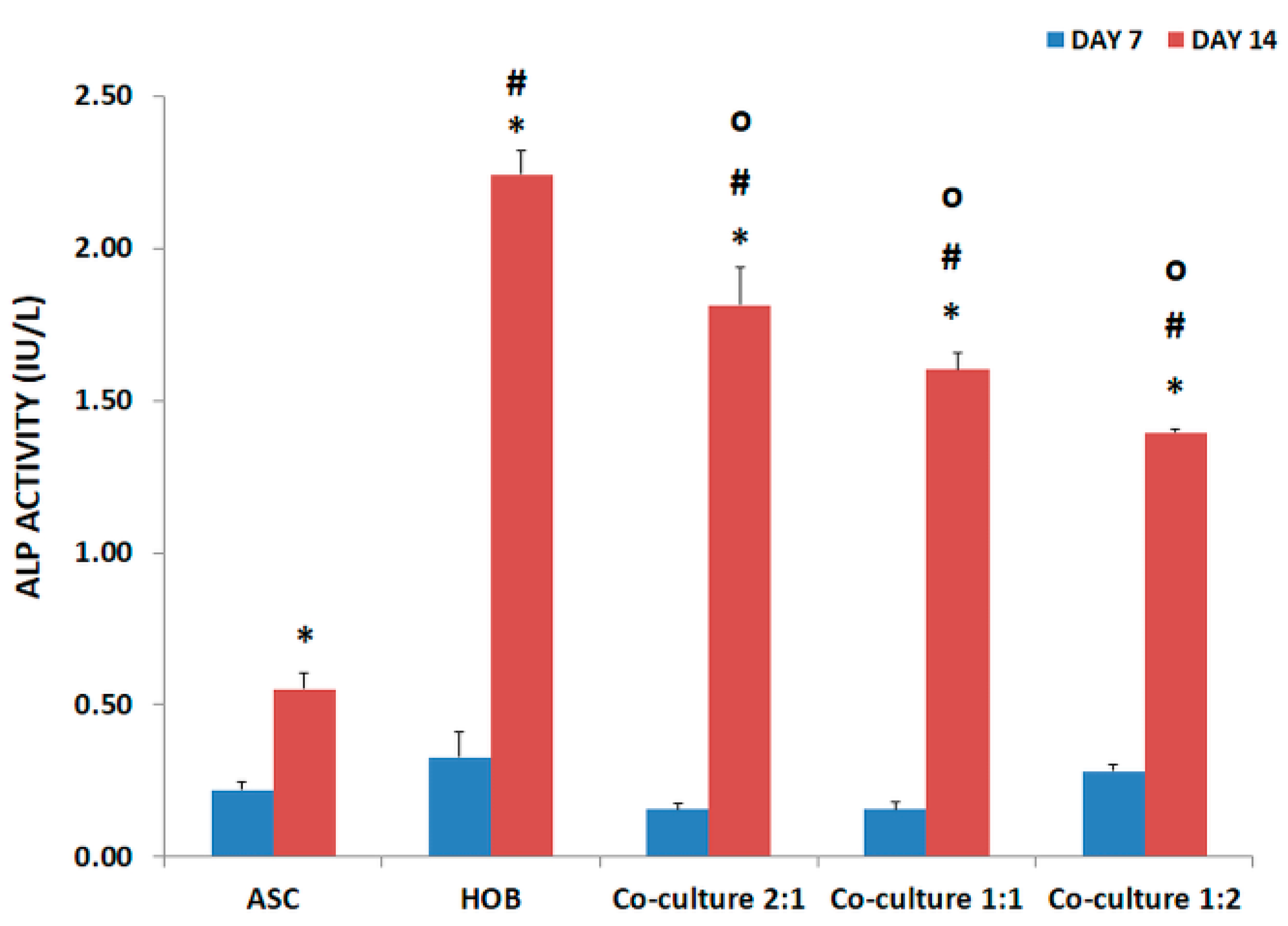
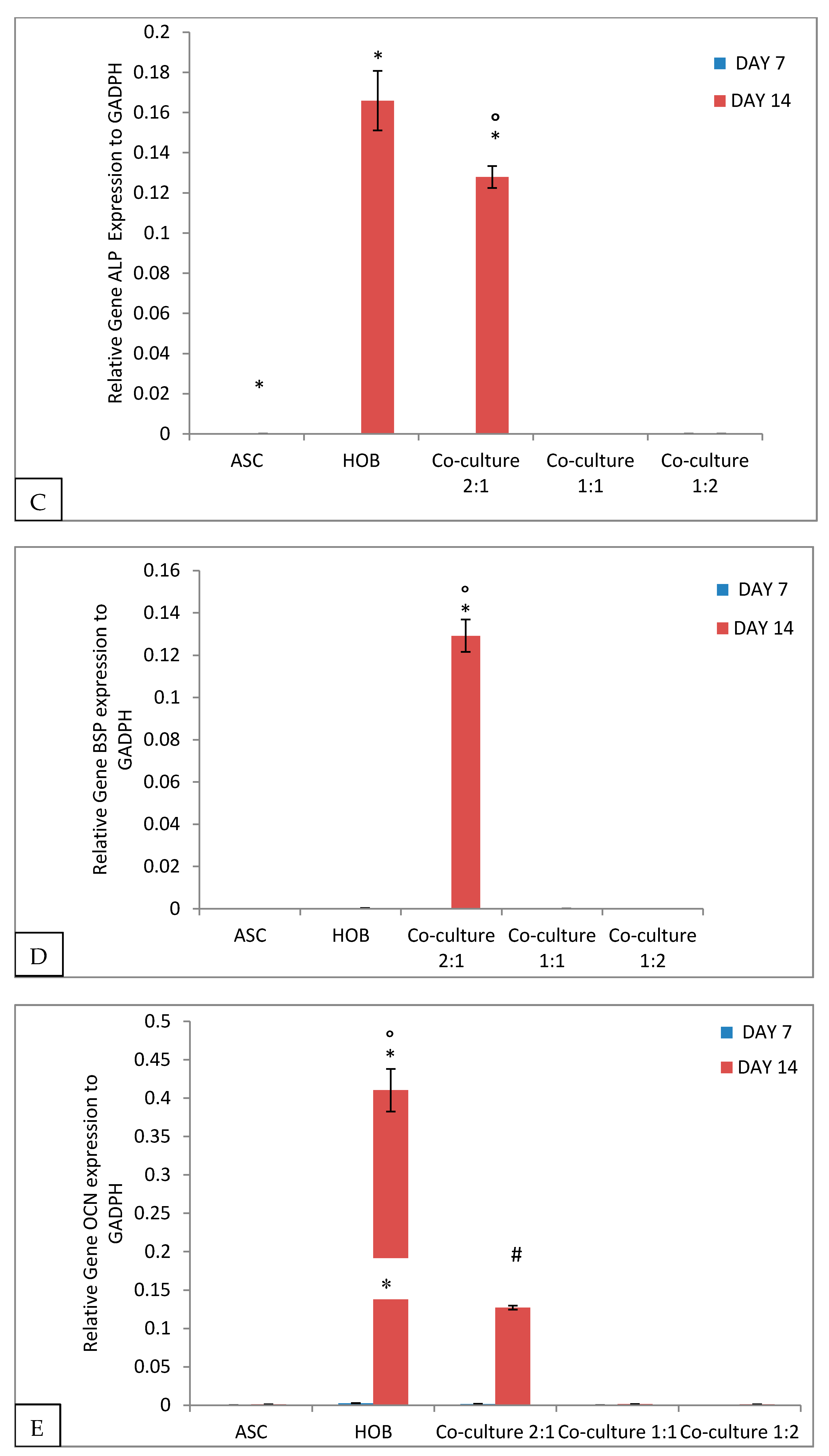
3.3. ALP Activity Increased in Co-Cultured ASC/HOB Seeded in PCL-HA Scaffold
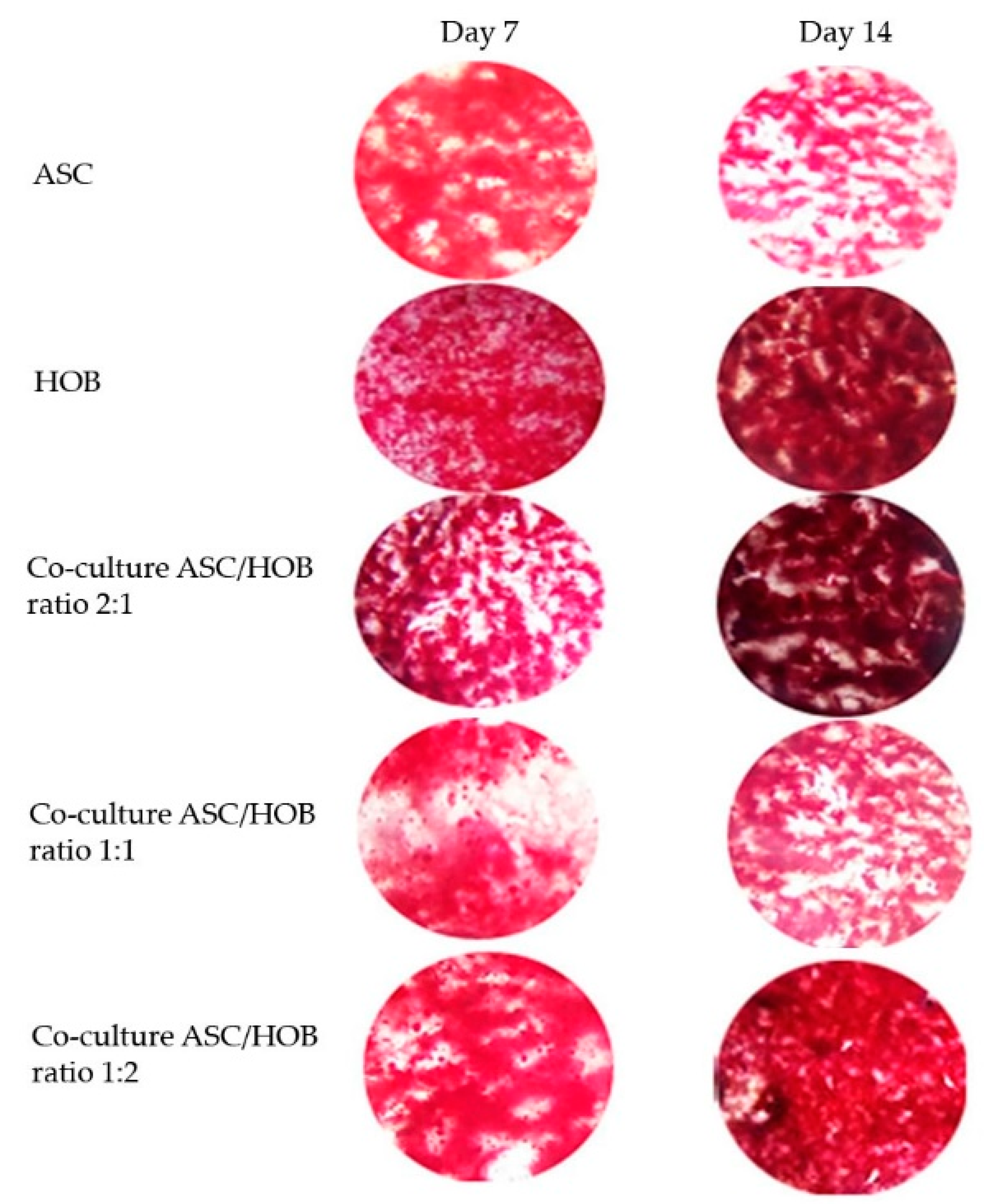
3.4. Calcium Deposition Was Enhanced in Co-Cultured ASC/HOB Seeded at 2:1 in PCL-HA Scaffold
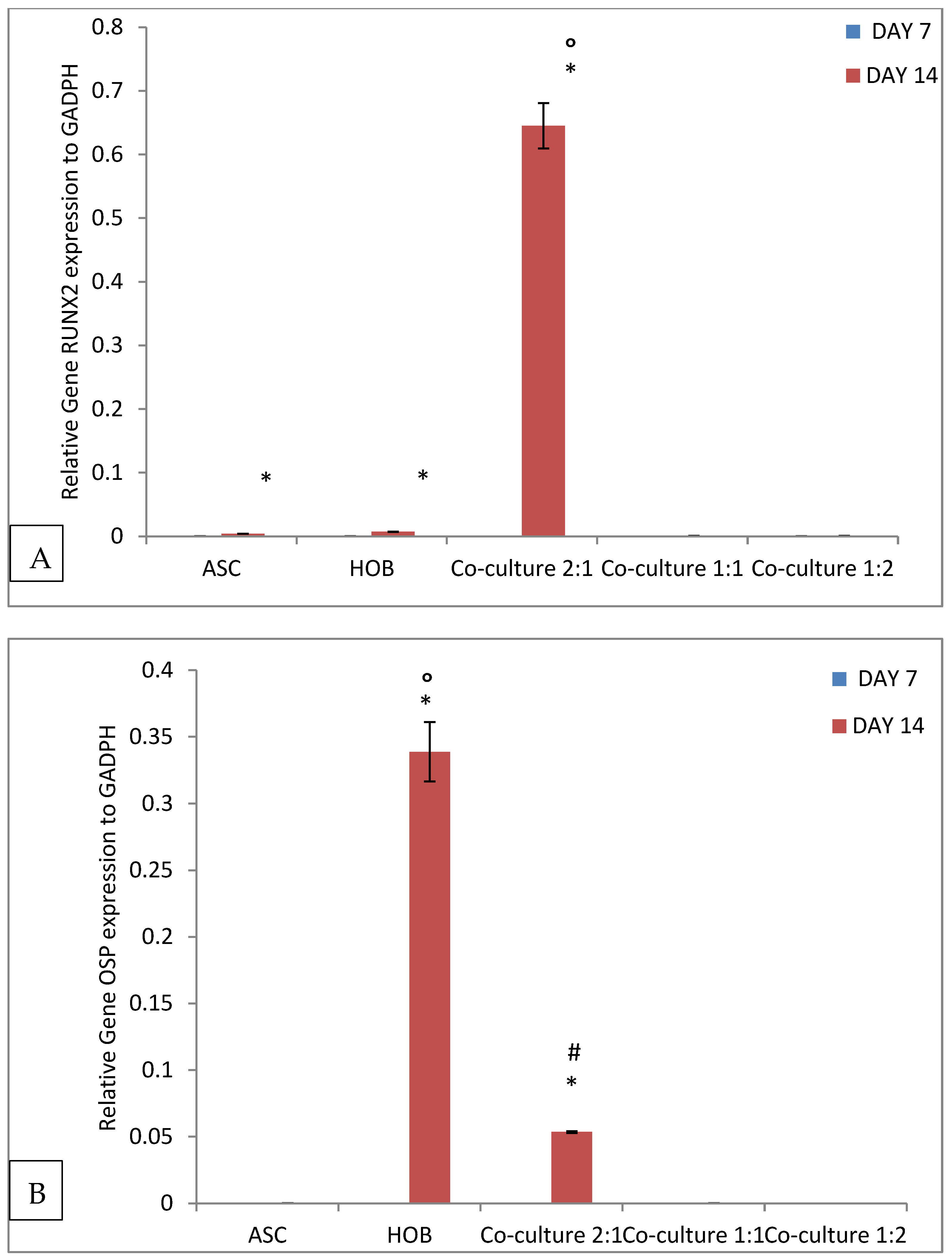
3.5. Alteration of Gene Expression in Cells Seeded in PCL-HA Scaffold
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, A.E.; Allen, K.D.; Golightly, Y.M.; Goode, A.P.; Jordan, J.M. A systematic review of recommendations and guidelines for the management of osteoarthritis: The Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin. Arthritis. Rheum. 2014, 43, 701–712. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.; Zhu, Y.; Han, J. Therapeutic application of mesenchymal stem cells in bone and joint diseases. Clin. Exp. Med. 2014, 14, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Rozila, I.; Azari, P.; Munirah, S.; Wan Safwani, W.K.Z.; Gan, S.N.; Nur Azurah, A.G.; Jahendran, J.; Pingguan-Murphy, B.; Chua, K.H. Differential osteogenic potential of human adipose-derived stem cells co-cultured with human osteoblasts on polymeric microfiber scaffolds. J. Biomed. Mater. Res. Part A 2016, 104, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ma, T. Perfusion regulation of hMSC microenvironment and osteogenic differentiation in 3D scaffold. Biotechnol. Bioeng. 2012, 109, 252–261. [Google Scholar] [CrossRef]
- Habibovic, P.; Yuan, H.; van der Valk, C.M.; Meijer, G.; van Blitterswijk, C.A.; de Groot, K. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials 2005, 26, 3562–3575. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.Y.; Chou, Y.C.; Lai, Y.H.; Lin, Y.T.; Lu, C.J.; Liu, S.J. Fabrication of drug-eluting nano-hydroxylapatite filled polycaprolactone nanocomposites using solution-extrusion 3D printing technique. Polymers 2021, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.E.; Han, J.; Jeong, S.; Lim, J.W.; Lee, M.C.; Son, H.; Kim, H.B.; Choung, Y.H.; Seonwoo, H.; et al. 3D-printed poly(ε-caprolactone)/hydroxyapatite scaffolds modified with alkaline hydrolysis enhance osteogenesis in vitro. Polymers 2021, 13, 257. [Google Scholar] [CrossRef]
- Barradas, A.M.; Monticone, V.; Hulsman, M.; Danoux, C.; Fernandes, H.; Tahmasebi Birgani, Z.; Barrère-de Groot, F.; Yuan, H.; Reinders, M.; Habibovic, P.; et al. Molecular mechanisms of biomaterial-driven osteogenic differentiation in human mesenchymal stromal cells. Integr. Biol. 2013, 5, 920–931. [Google Scholar] [CrossRef][Green Version]
- Hesaraki, S.; Nazarian, H.; Pourbaghi-Masouleh, M.; Borhan, S. Comparative study of mesenchymal stem cells osteogenic differentiation on low-temperature biomineralized nanocrystalline carbonated hydroxyapatite and sintered hydroxyapatite. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 108–118. [Google Scholar] [CrossRef]
- Venugopal, J.; Rajeswari, R.; Shayanti, M.; Low, S.; Bongso, A.; Giri Dev, V.R.; Choon, A.T.; Ramakrishna, S. Electrospray hydroxyapatite on polymer nanofibers to differentiate mesenchymal stem cells to osteogenesis. J. Biomater. Sci. 2013, 24, 170–184. [Google Scholar] [CrossRef]
- Zhang, X.; Chang, W.; Lee, P.; Wang, Y.; Yang, M.; Li, J.; Kumbar, S.G.; Yu, X. Polymer-ceramic spiral structured scaffolds for bone tissue engineering: Effect of hydroxyapatite composition on human fetal osteoblasts. PLoS ONE 2014, 9, e85871. [Google Scholar] [CrossRef]
- Akram, H.; Azari, P.; Wan Abas, W.A.B.; Zain, N.M.; Gan, S.N.; Yahya, R.; Wong, C.S.; Pingguan-Murphy, B. An in-vitro study on the proliferative potential of rat bone marrow stem cells on electrospun fibrous polycaprolactone scaffolds containing micro-hydroxyapatite particles. Mater. Res. Innov. 2014, 18, 520–524. [Google Scholar] [CrossRef]
- Eosoly, S.; Vrana, N.E.; Lohfeld, S.; Hindie, M.; Looney, L. Interaction of cell culture with composition effects on the mechanical properties of polycaprolactone-hydroxyapatite scaffolds fabricated via selective laser sintering (SLS). Mater. Sci. Eng. C 2012, 32, 2250–2257. [Google Scholar] [CrossRef]
- Goers, L.; Freemont, P.; Polizzi, K.M. Co-culture systems and technologies: Taking synthetic biology to the next level. J. R. Soc. Interface 2014, 11, 20140065. [Google Scholar] [CrossRef]
- Akahane, M.; Ueha, T.; Shimizu, T.; Inagaki, Y.; Kido, A.; Imamura, T.; Kawate, K.; Tanaka, Y. Increased osteogenesis with hydroxyapatite constructs combined with serially-passaged bone marrow-derived mesenchymal stem cells. Stem. Cell Discov. 2012, 2, 133–140. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, T.J.; Cho, D.W.; Kim, B.S. Solid free-form fabrication-based PCL/HA scaffolds fabricated with a multi-head deposition system for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2010, 21, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Herliansyah, M.K.; Hamdi, M.; Ide-Ektessabi, A.; Wildan, M.W. Fabrication of bovine bone hydroxyapatite effect of the material shapes and calcination temperature. ISTECS J. Sci. Technol. Pol. VIII 2006, 25, 1345–8981. [Google Scholar]
- Ooi, C.Y.; Hamdi, M.; Ramesh, S. Properties of hydroxyapatite produced by annealing of bovine bone. Ceram. Int. 2007, 33, 1171–1177. [Google Scholar] [CrossRef]
- Rh Owen, G.; Dard, M.; Larjava, H. Hydoxyapatite/beta-tricalcium phosphate biphasic ceramics as regenerative material for the repair of complex bone defects. J. Biomed. Mater. Res. Part B 2018, 106, 2493–2512. [Google Scholar] [CrossRef]
- Faia-Torres, A.B.; Guimond-Lischer, S.; Rottmar, M.; Charnley, M.; Goren, T.; Maniura-Weber, K.; Spencer, N.D.; Reis, R.L.; Textor, M.; Neves, N.M. Differential regulation of osteogenic differentiation of stem cells on surface roughness gradients. Biomaterials 2014, 35, 9023–9032. [Google Scholar] [CrossRef]
- Hoemann, C.D.; El-Gabalawy, H.; McKee, M.D. In Vitro osteogenesis assays: Influence of the primary cell source on alkaline phosphatase activity and mineralization. Pathol. Biol. 2009, 57, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Kyllönen, L.; Haimi, S.; Mannerström, B.; Huhtala, H.; Rajala, K.M.; Skottman, H.; Sándor, G.K.; Miettinen, S. Effects of different serum conditions on osteogenic differentiation of human adipose stem cells in vitro. Stem. Cell Res. Ther. 2013, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Venugopal, J.R.; Sundarrajan, S.; Mukherjee, S.; Ramakrishna, S. Precipitation of nanohydroxyapatite on PLLA/PBLG/Collagen nanofibrous structures for the differentiation of adipose derived stem cells to osteogenic lineage. Biomaterials 2012, 33, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Lopes, J.; Canhao, H.; Fonseca, J.E. Osteoblast and bone formation. Acta Reum. Port. 2007, 32, 103–110. [Google Scholar]
- Monfoulet, L.; Malaval, L.; Aubin, J.E.; Rittling, S.R.; Gadeau, A.P.; Fricain, J.C.; Chassande, O. Bone sialoprotein, but not osteopontin, deficiency impairs the mineralization of regenerating bone during corticol defect healing. Bone 2010, 46, 447–452. [Google Scholar] [CrossRef]
- Ding, J.; Ghali, O.; Lencel, P.; Broux, O.; Chauveau, C.; Devedjian, J.C.; Hardouin, P.; Magne, D. TNF-α and IL-1β inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci. 2009, 84, 499–504. [Google Scholar] [CrossRef]
- Wan Safwani, W.K.Z.; Makpol, S.; Sathapan, S.; Chua, K.H. Alteration of gene expression levels during osteogenic induction of human adipose-derived stem cells in long-term culture. Cell Tissue Bank 2012, 14, 289–301. [Google Scholar] [CrossRef]
- Wang, L.; Dormer, N.H.; Bonewald, L.F.; Detamore, M.S. Osteogenic differentiation of human umbilical cord mesenchymal stromal cells in polyglycolic acid scaffolds. Tissue Eng. Part A 2010, 16, 1937–1948. [Google Scholar] [CrossRef]


| Gene | Accession No. | Primer (5′ → 3′) Sense and Antisense | PCR Product Size (bp) |
|---|---|---|---|
| Glyceraldehyde-3-phosphate dehydrogenase (GADPH) | NM_002046 | R 5′-TCC CTG AGC TGA ACG GGA AG-3′ | 217 |
| F 5′-GGA GGA GTG GGT GTC GCT GT-3′ | |||
| Osteopontin (OSP) | NM_001040060 | R 5′-ATCCATGTGGTCATGGCTTT-3′ | 219 |
| F 5′-CACCTGTGCCATACCAGTTAAAC-3′ | |||
| Osteocalcin (OCN) | NM_199173 | R 5′-CTGAAAGCCGATGTGGTCAG-3′ | 191 |
| F 5′-GTGCAGAGTCCAGCAAAGGT-3′ | |||
| Bone-sialoprotein (BSP) | NM_004967 | R 5′-CTCGGTAATTGTCCCCACGA-3′ | 208 |
| F 5′-GGGCACCTCGAAGACAACAA-3′ | |||
| Runt-2 (RUNX) | NM_004348 | R 5′-CACTCTGGCTTTGGGAAGAG-3′ | 182 |
| F 5′-GCAGTTCCCAAGCATTTCATC-3′ | |||
| alkaline phosphatase (ALP) | NM_000478 | R 5′-AGGGGAACTTGTCCATCTCC-3′ | 200 |
| F 5′-GTACTGGCGAGACCAAGCGCA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozila, I.; Azari, P.; Munirah, S.; Safwani, W.K.Z.W.; Pingguan-Murphy, B.; Chua, K.H. Polycaprolactone-Based Scaffolds Facilitates Osteogenic Differentiation of Human Adipose-Derived Stem Cells in a Co-Culture System. Polymers 2021, 13, 597. https://doi.org/10.3390/polym13040597
Rozila I, Azari P, Munirah S, Safwani WKZW, Pingguan-Murphy B, Chua KH. Polycaprolactone-Based Scaffolds Facilitates Osteogenic Differentiation of Human Adipose-Derived Stem Cells in a Co-Culture System. Polymers. 2021; 13(4):597. https://doi.org/10.3390/polym13040597
Chicago/Turabian StyleRozila, Ismail, Pedram Azari, Sha’ban Munirah, Wan Kamarul Zaman Wan Safwani, Belinda Pingguan-Murphy, and Kien Hui Chua. 2021. "Polycaprolactone-Based Scaffolds Facilitates Osteogenic Differentiation of Human Adipose-Derived Stem Cells in a Co-Culture System" Polymers 13, no. 4: 597. https://doi.org/10.3390/polym13040597
APA StyleRozila, I., Azari, P., Munirah, S., Safwani, W. K. Z. W., Pingguan-Murphy, B., & Chua, K. H. (2021). Polycaprolactone-Based Scaffolds Facilitates Osteogenic Differentiation of Human Adipose-Derived Stem Cells in a Co-Culture System. Polymers, 13(4), 597. https://doi.org/10.3390/polym13040597







