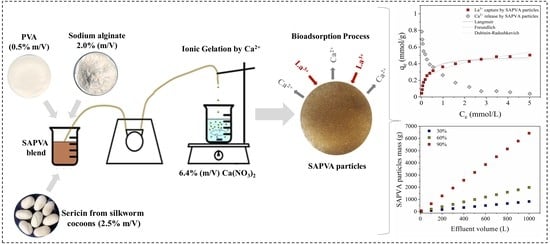
Equilibrium, Thermodynamic, Reuse, and Selectivity Studies for the Bioadsorption of Lanthanum onto Sericin/Alginate/Poly(vinyl alcohol) Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Alginate and Sericin Particles Chemically Crosslinked with Poly(vinyl alcohol)
2.2. Metallic Ion Concentration Quantification
2.3. Bioadsorption Assays in Batch System
2.3.1. Equilibrium Study
2.3.2. Regeneration Study
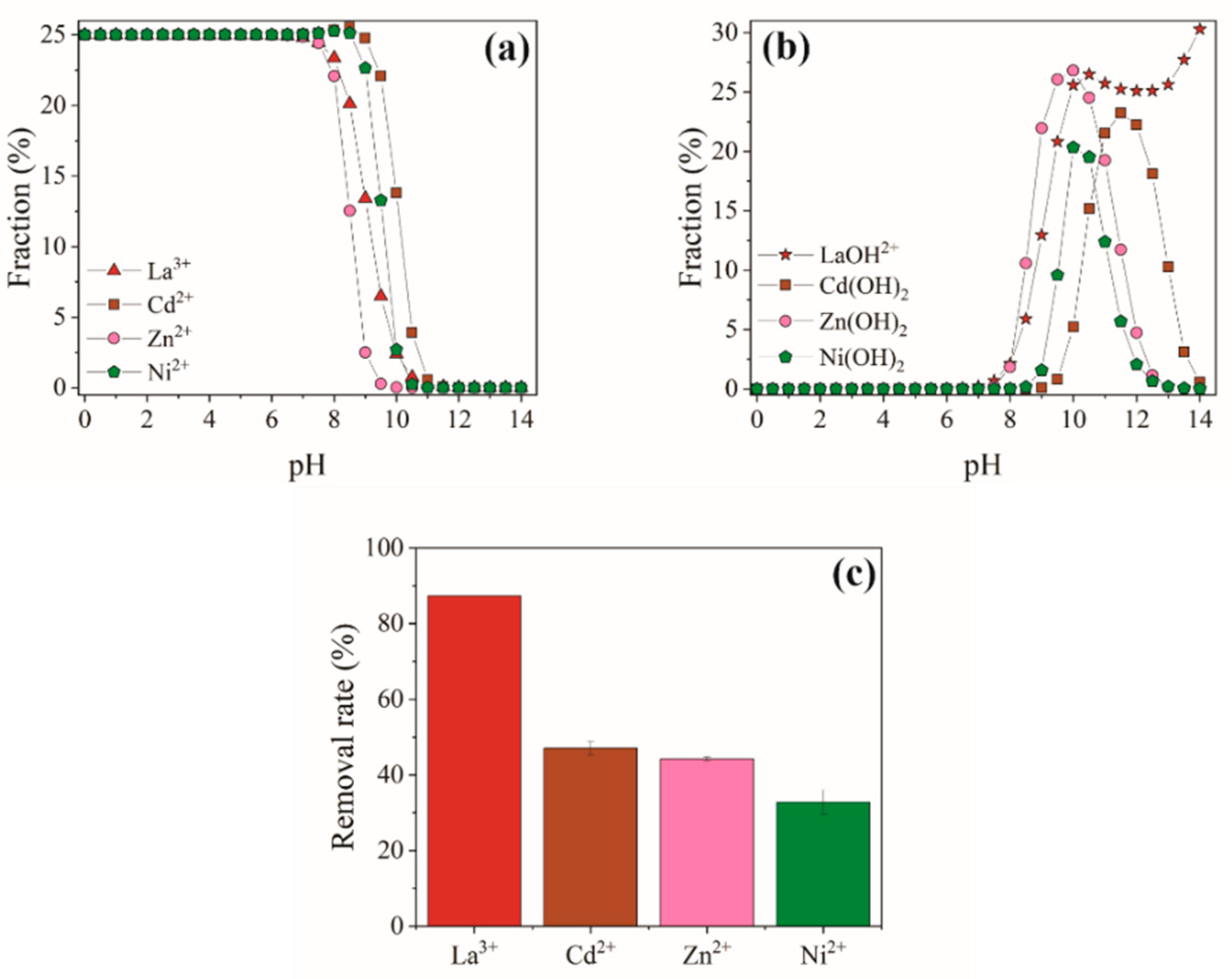
2.3.3. Selectivity Study
2.4. Theory and Mathematical Modeling
2.4.1. Isotherm Models
2.4.2. Thermodynamic Study
2.4.3. Calculation of R2 and AICc
2.5. Characterization Methods
3. Results and Discussion
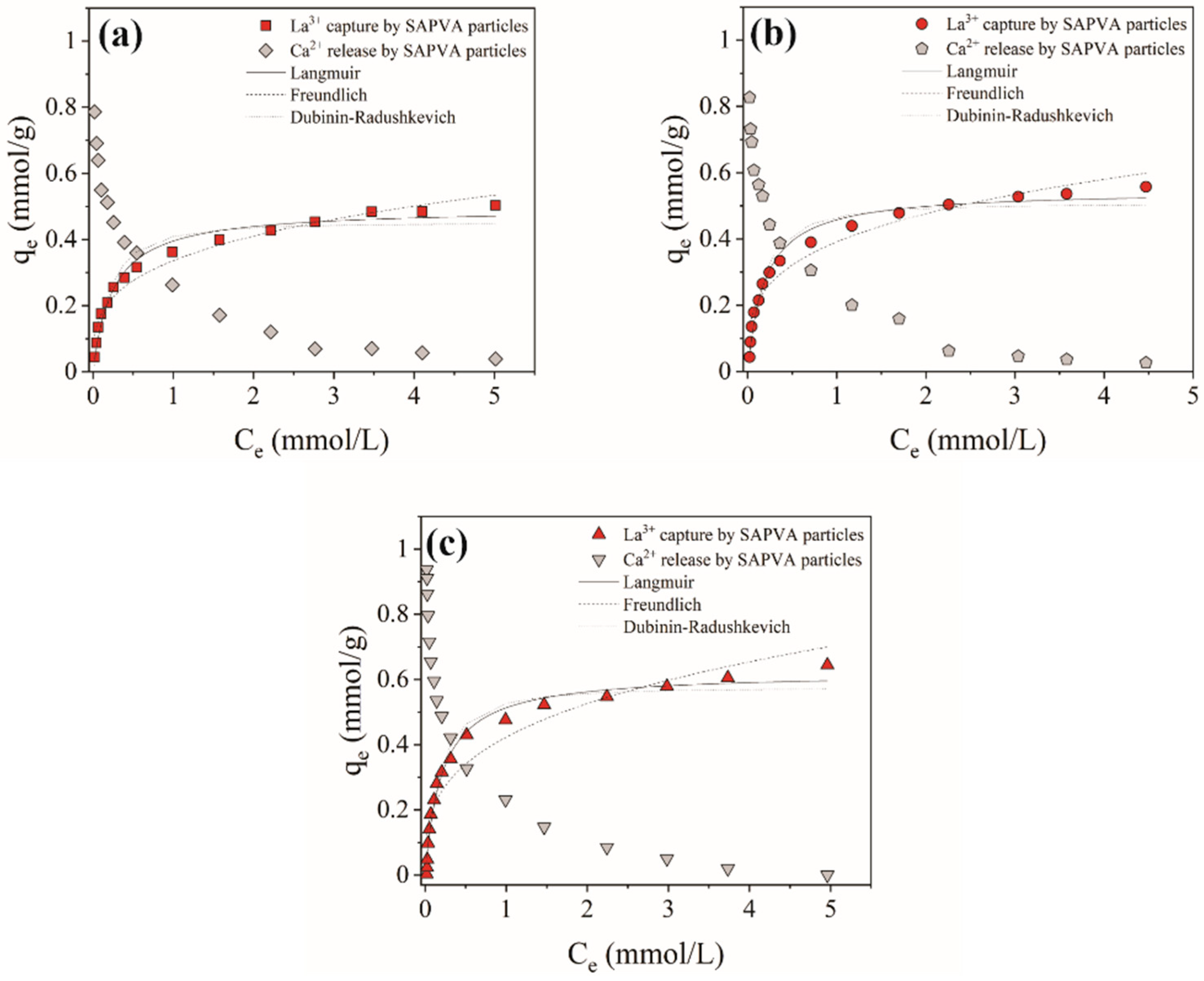
3.1. Bioadsorption Equilibrium
3.2. Thermodynamic Quantities
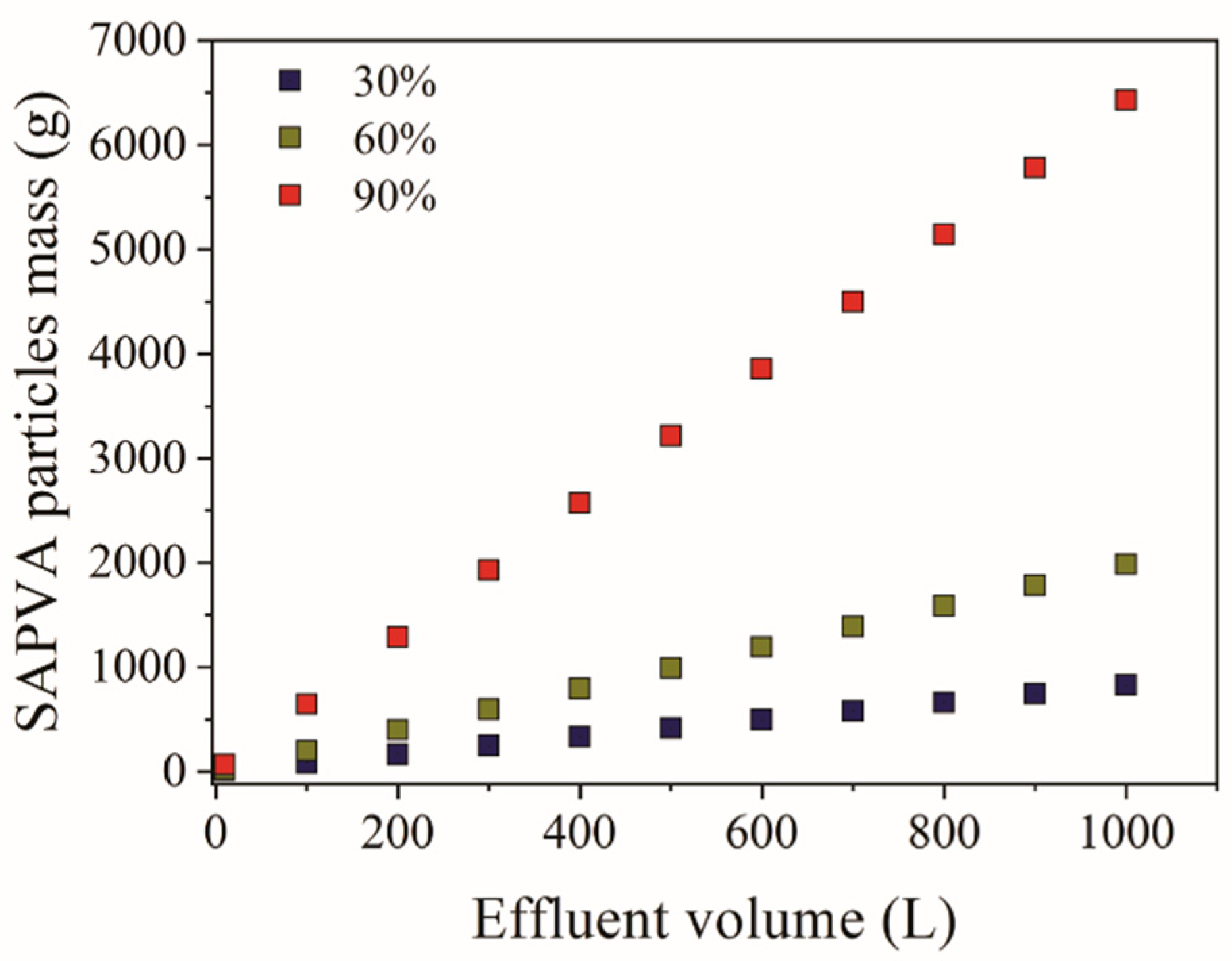
3.3. Simplified Batch Design
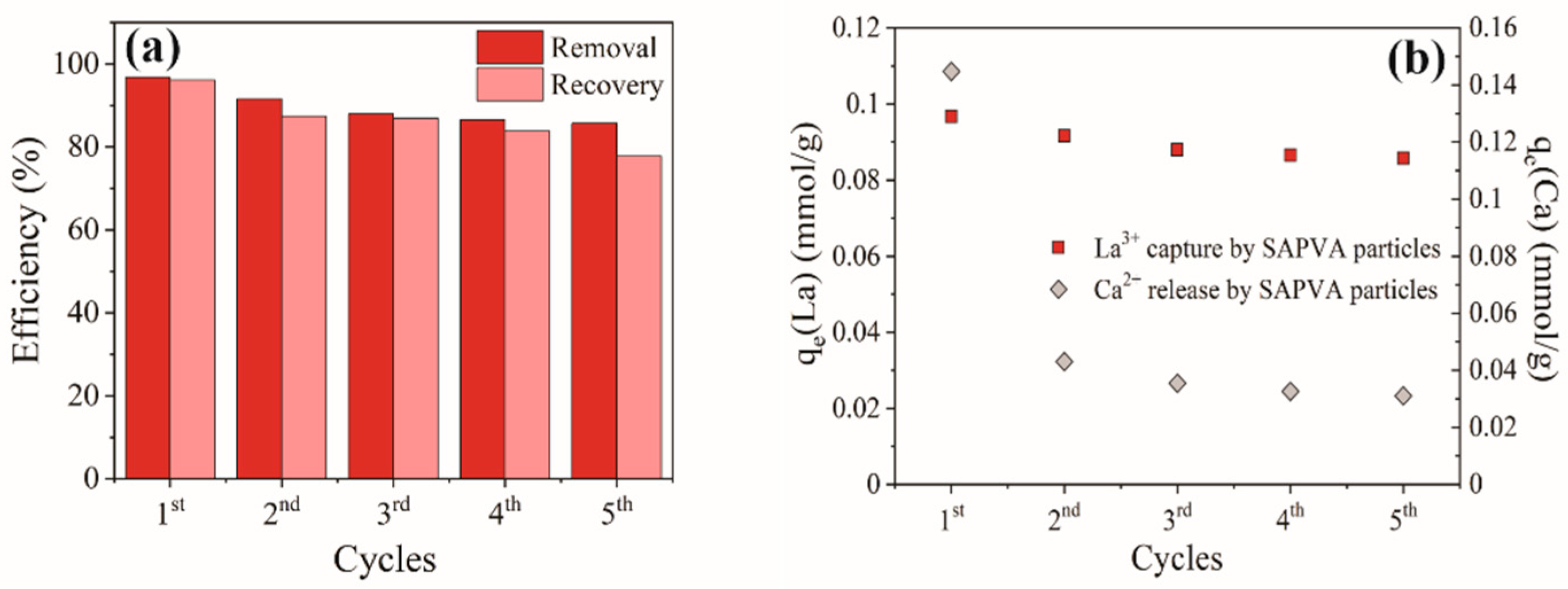
3.4. Regeneration Cycles
3.5. Selectivity
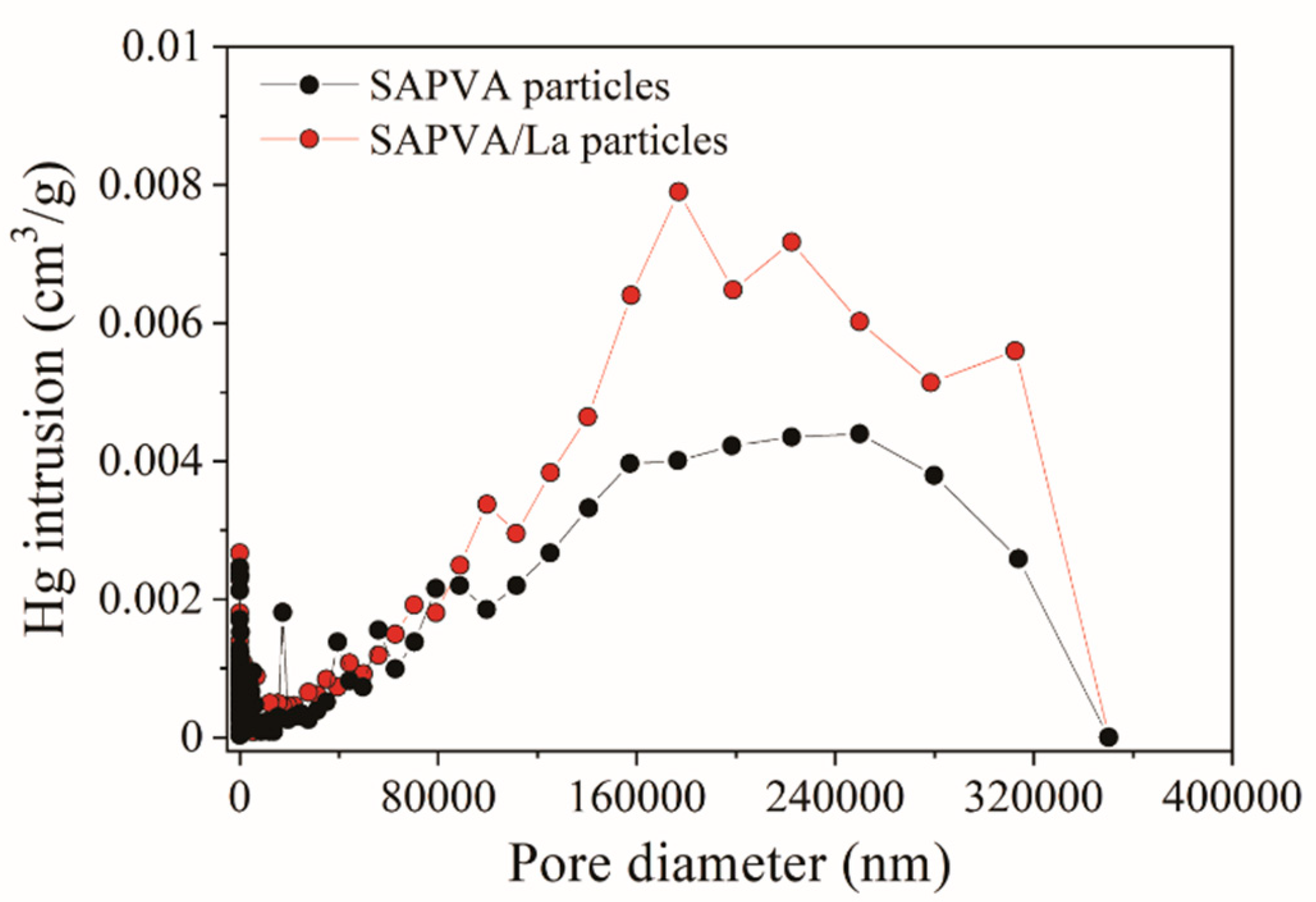
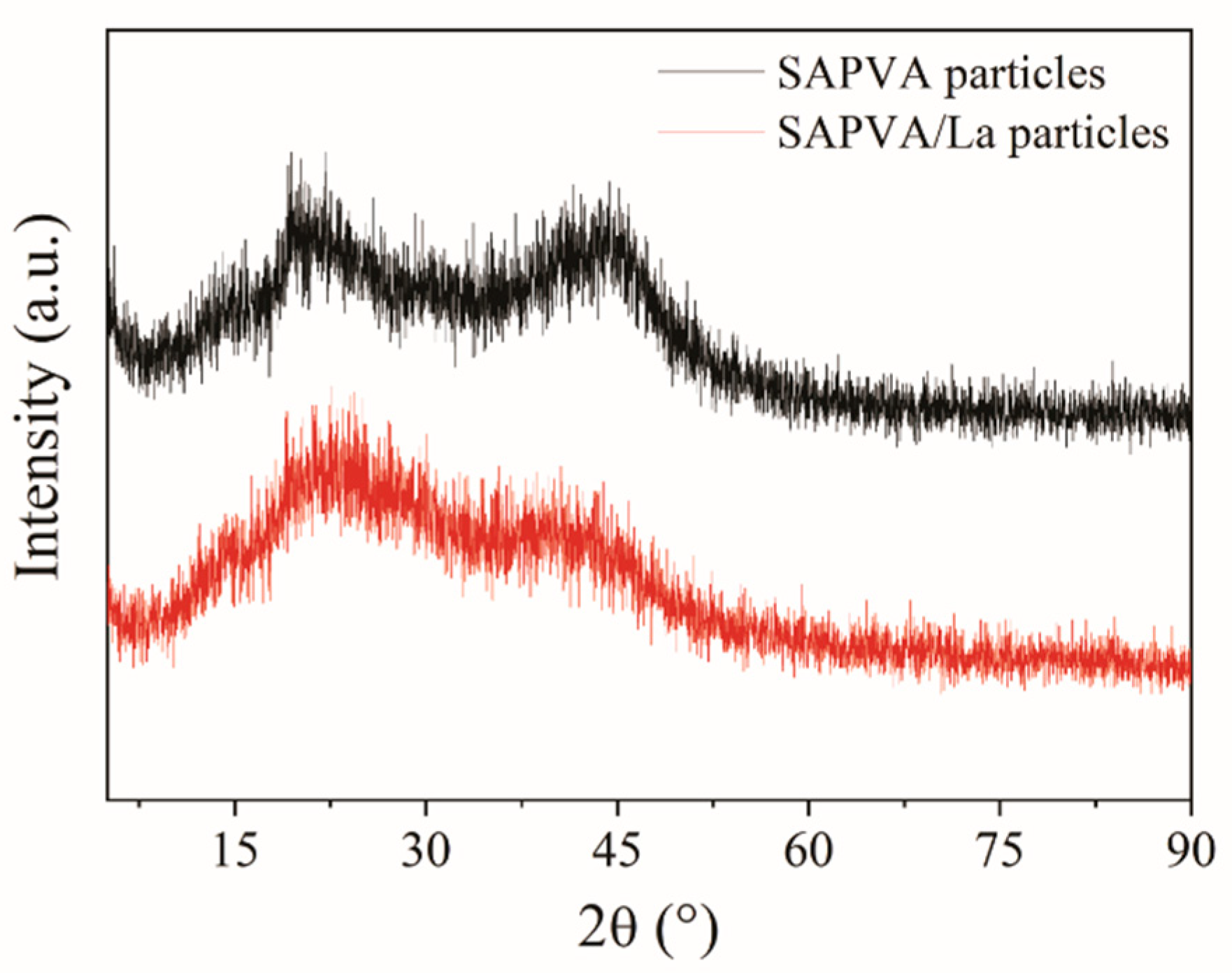
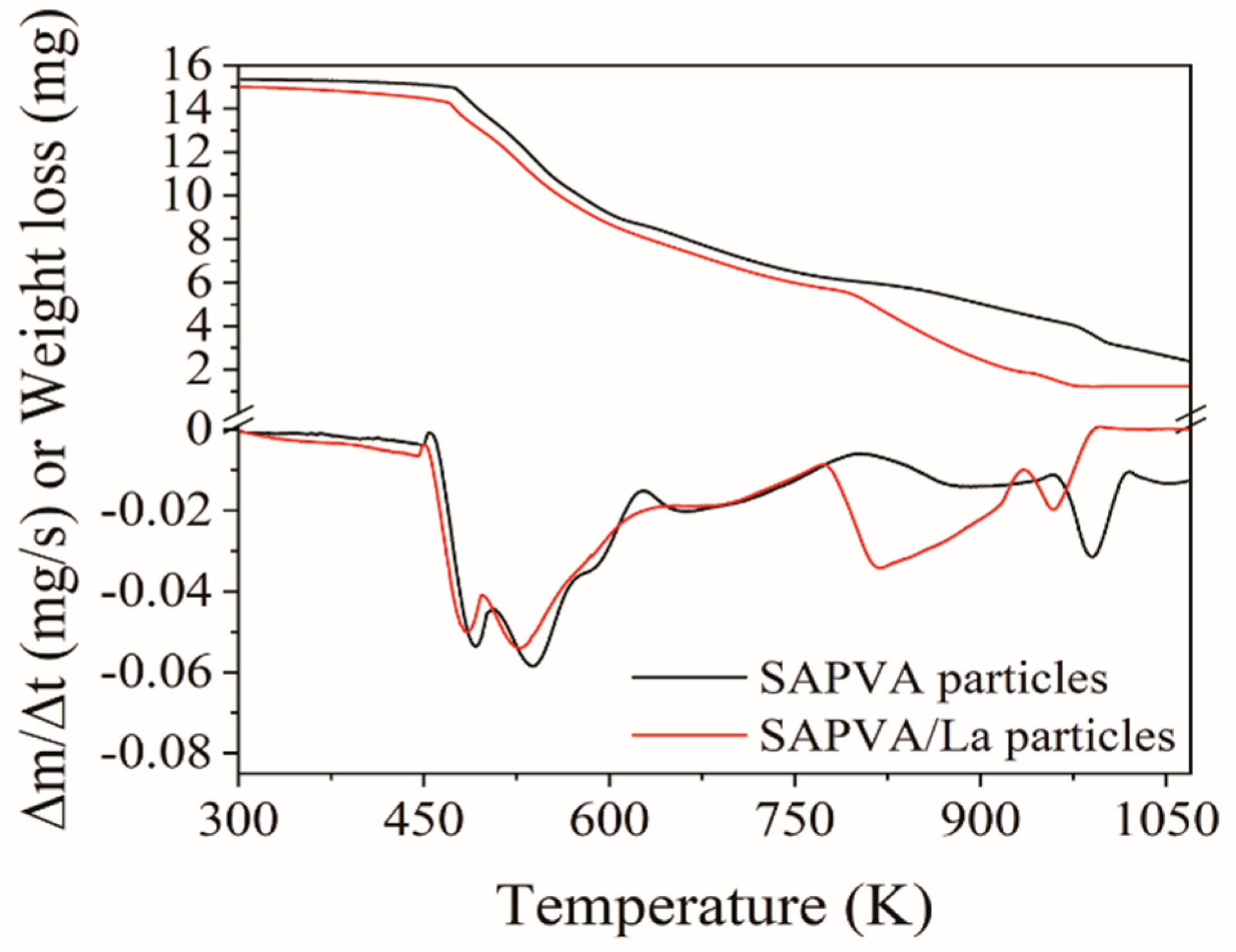
3.6. Biocomposite Characterization
3.7. Practical Applications and Future Research Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- da Costa, T.B.; da Silva, M.G.C.; Vieira, M.G.A. Recovery of rare-earth metals from aqueous solutions by bio/adsorption using non-conventional materials: A review with recent studies and promising approaches in column applications. J. Rare Earths 2020, 38, 339–355. [Google Scholar] [CrossRef]
- Cao, X.; Wang, Q.; Wang, S.; Man, R. Preparation of a novel polystyrene-poly(hydroxamic acid) copolymer and its adsorption properties for rare earth metal ions. Polymers 2020, 12, 1905. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Kim, H.Y.; Park, Y.; Shin, H.S.; Ghodake, G.; Bharagava, R.N.; Mulla, S.I.; Kim, D.S.; Saratale, G.D. Hydrometallurgical process for the recovery of yttrium from spent fluorescent lamp: Leaching and crystallization experiments. J. Clean. Prod. 2020, 261, 121009. [Google Scholar] [CrossRef]
- Saratale, G.D.; Kim, H.Y.; Saratale, R.G.; Kim, D.S. Liquid–liquid extraction of yttrium from the sulfate leach liquor of waste fluorescent lamp powder: Process parameters and analysis. Miner. Eng. 2020, 152, 106341. [Google Scholar] [CrossRef]
- Li, C.; Ma, H.; Venkateswaran, S.; Hsiao, B.S. Sustainable carboxylated cellulose filters for efficient removal and recovery of lanthanum. Environ. Res. 2020, 188. [Google Scholar] [CrossRef] [PubMed]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Iftekhar, S.; Ramasamy, D.L.; Srivastava, V.; Asif, M.B.; Sillanpää, M. Understanding the factors affecting the adsorption of Lanthanum using different adsorbents: A critical review. Chemosphere 2018, 204, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yang, H.; Yan, C.; Luo, W.; Li, X.; Zhao, J. Synthesis of carboxylic acid functionalized diatomite with a micro-villous surface via UV-induced graft polymerization and its adsorption properties for Lanthanum(III) ions. Colloids Surf. A Physicochem. Eng. Asp. 2016, 501, 9–16. [Google Scholar] [CrossRef]
- Barros, Ó.; Costa, L.; Costa, F.; Lago, A.; Rocha, V.; Vipotnik, Z.; Silva, B.; Tavares, T. Recovery of rare earth elements from wastewater towards a circular economy. Molecules 2019, 24, 1005. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Honaker, R.Q. Rare earth elements recovery using staged precipitation from a leachate generated from coarse coal refuse. Int. J. Coal Geol. 2018, 195, 189–199. [Google Scholar] [CrossRef]
- Fontana, D.; Pietrelli, L. Separation of middle rare earths by solvent extraction using 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester as an extractant. J. Rare Earths 2009, 27, 830–833. [Google Scholar] [CrossRef]
- Yaftian, M.R.; Burgard, M.; Dieleman, C.B.; Matt, D. Rare-earth metal-ion separation using a supported liquid membrane mediated by a narrow rim phosphorylated calix[4]arene. J. Membr. Sci. 1998, 144, 57–64. [Google Scholar] [CrossRef]
- Urbanski, T.S.; Fornari, P.; Abbruzzese, C. The extraction of cerium(III) and lanthanum(III) from chloride solutions with LIX 54. Hydrometallurgy 1996, 40, 169–179. [Google Scholar] [CrossRef]
- Das, N.; Das, D. Recovery of rare earth metals through biosorption: An overview. J. Rare Earths 2013, 31, 933–943. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Lima, E.C. Adsorption of rare earth metals: A review of recent literature. J. Mol. Liq. 2016, 221, 954–962. [Google Scholar] [CrossRef]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.S.; Park, J.M. The past, present, and future trends of biosorption. Biotechnol. Bioprocess Eng. 2010, 15, 86–102. [Google Scholar] [CrossRef]
- Dhankhar, R.; Hooda, A. Fungal biosorption-an alternative to meet the challenges of heavy metal pollution in aqueous solutions. Environ. Technol. 2011, 32, 467–491. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef]
- Song, D.; Park, S.J.; Kang, H.W.; Park, S.B.; Han, J.I. Recovery of lithium(I), strontium(II), and lanthanum(III) using Ca-alginate beads. J. Chem. Eng. Data 2013, 58, 2455–2464. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, L.; Wang, L.; Zhu, B.; Fan, L. Adsorption of lanthanum by magnetic alginate-chitosan gel beads. J. Chem. Technol. Biotechnol. 2011, 86, 345–352. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, S.; Zhao, K.; Wang, Z.; Xu, S.; Liang, Z.; Wu, K. Adsorption of La3+ and Ce3+ by poly-γ-glutamic acid crosslinked with polyvinyl alcohol. J. Rare Earths 2015, 33, 884–891. [Google Scholar] [CrossRef]
- Wu, D.; Gao, Y.; Li, W.; Zheng, X.; Chen, Y.G.; Wang, Q. Selective Adsorption of La3+ Using a Tough Alginate-Clay-Poly(n-isopropylacrylamide) Hydrogel with Hierarchical Pores and Reversible Re-Deswelling/Swelling Cycles. ACS Sustain. Chem. Eng. 2016, 4, 6732–6743. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, J.; Zhang, L.; Wu, Q.; Yang, Y. Lanthanum adsorption using iron oxide loaded calcium alginate beads. Hydrometallurgy 2010, 101, 76–83. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Daher, A.M.; Abd El-Fatah, A.I.L.; Abd El Monem, H.; Khalil, M.M.H. Biosorption of lanthanum from aqueous solutions using magnetic alginate beads. J. Dispers. Sci. Technol. 2017, 38, 145–151. [Google Scholar] [CrossRef]
- das Graças Santos, N.T.; da Silva, M.G.C.; Vieira, M.G.A. Development of novel sericin and alginate-based biosorbents for precious metal removal from wastewater. Environ. Sci. Pollut. Res. 2019, 26, 28455–28469. [Google Scholar] [CrossRef]
- Costa, T.B.; Silva, M.G.C.; Vieira, M.G.A. Development of a natural polymeric bioadsorbent based on sericin, alginate and poly(vinyl alcohol) for the recovery of ytterbium from aqueous solutions. J. Clean. Prod. 2021, 279, 123555. [Google Scholar] [CrossRef]
- Zhang, Y.Q. Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 2002, 20, 91–100. [Google Scholar] [CrossRef]
- Cao, T.T.; Zhang, Y.Q. Processing and characterization of silk sericin from Bombyx mori and its application in biomaterials and biomedicines. Mater. Sci. Eng. C 2016, 61, 940–952. [Google Scholar] [CrossRef]
- Dash, B.C.; Mandal, B.B.; Kundu, S.C. Silk gland sericin protein membranes: Fabrication and characterization for potential biotechnological applications. J. Biotechnol. 2009, 144, 321–329. [Google Scholar] [CrossRef]
- Aramwit, P.; Siritientong, T.; Srichana, T. Potential applications of silk sericin, a natural protein from textile industry by-products. Waste Manag. Res. 2012, 30, 217–224. [Google Scholar] [CrossRef]
- Alnaief, M.; Alzaitoun, M.A.; García-González, C.A.; Smirnova, I. Preparation of biodegradable nanoporous microspherical aerogel based on alginate. Carbohydr. Polym. 2011, 84, 1011–1018. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef] [Green Version]
- Cuadros, T.R.; Erices, A.A.; Aguilera, J.M. Porous matrix of calcium alginate/gelatin with enhanced properties as scaffold for cell culture. J. Mech. Behav. Biomed. Mater. 2015, 46, 331–342. [Google Scholar] [CrossRef]
- Kim, J.O.; Park, J.K.; Kim, J.H.; Jin, S.G.; Yong, C.S.; Li, D.X.; Choi, J.Y.; Woo, J.S.; Yoo, B.K.; Lyoo, W.S.; et al. Development of polyvinyl alcohol-sodium alginate gel-matrix-based wound dressing system containing nitrofurazone. Int. J. Pharm. 2008, 359, 79–86. [Google Scholar] [CrossRef] [PubMed]
- da Costa, T.B.; da Silva, M.G.C.; Vieira, M.G.A. Crosslinked alginate/sericin particles for bioadsorption of ytterbium: Equilibrium, thermodynamic and regeneration studies. Int. J. Biol. Macromol. 2020, 165, 1911–1923. [Google Scholar] [CrossRef] [PubMed]
- Gimenes, M.L.; Liu, L.; Feng, X. Sericin/poly(vinyl alcohol) blend membranes for pervaporation separation of ethanol/water mixtures. J. Membr. Sci. 2007, 295, 71–79. [Google Scholar] [CrossRef]
- das Graças Santos, N.T.; Moraes, L.F.; da Silva, M.G.C.; Vieira, M.G.A. Recovery of gold through adsorption onto sericin and alginate particles chemically crosslinked by proanthocyanidins. J. Clean. Prod. 2020, 253, 119925. [Google Scholar] [CrossRef]
- da Silva, T.L.; da Silva, A.C.; Vieira, M.G.A.; Gimenes, M.L.; da Silva, M.G.C. Biosorption study of copper and zinc by particles produced from silk sericin—Alginate blend: Evaluation of blend proportion and thermal cross-linking process in particles production. J. Clean. Prod. 2016, 137, 1470–1478. [Google Scholar] [CrossRef]
- Mukherji, A.K. Simultaneous spectrophotometric determination of thorium and the rare earths with Xylenol Orange. Microchem. J. 1966, 11, 243–254. [Google Scholar] [CrossRef]
- de Vargas Brião, G.; da Silva, M.G.C.; Vieira, M.G.A. Neodymium recovery from aqueous solution through adsorption/desorption onto expanded vermiculite. Appl. Clay Sci. 2020, 198, 105825. [Google Scholar] [CrossRef]
- de Andrade, J.R.; da Silva, M.G.C.; Gimenes, M.L.; Vieira, M.G.A. Bioadsorption of trivalent and hexavalent chromium from aqueous solutions by sericin-alginate particles produced from Bombyx mori cocoons. Environ. Sci. Pollut. Res. 2018, 25, 25967–25982. [Google Scholar] [CrossRef]
- Costa, T.B.; da Silva, M.G.C.; Vieira, M.G.A. Evaluation of metal affinity of lanthanum using different alternative bio/adsorbent materials. Chem. Eng. Trans. 2019, 74, 1129–1134. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; John Wiley & Sons: New York, NY, USA, 1984; ISBN 0-471-86606-7. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Dubinin, M.M.; Radushkevich, L.V. Equation of the Characteristic Curve of Activated Charcoal. Proc. Acad. Sci. USSR Phys. Chem. Sect. 1947, 55, 331–333. [Google Scholar]
- Kilislioglu, A.; Bilgin, B. Thermodynamic and kinetic investigations of uranium adsorption on amberlite IR-118H resin. Appl. Radiat. Isot. 2003, 58, 155–160. [Google Scholar] [CrossRef]
- Milonjić, S.K. A consideration of the correct calculation of thermodynamic parameters of adsorption. J. Serbian Chem. Soc. 2007, 72, 1363–1367. [Google Scholar] [CrossRef]
- Young, D.M.; Crowell, A.D.; Rice, S.A. Physical Adsorption of Gases. Phys. Today 1963, 16, 80–82. [Google Scholar] [CrossRef]
- Bonate, P.; Steimer, J.-L. Pharmacokinetics Pharmacodynamics Modeling Simulation; Springer: San Antonio, TX, USA, 2011; ISBN 9780387271972. [Google Scholar]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Dang, V.B.H.; Doan, H.D.; Dang-Vu, T.; Lohi, A. Equilibrium and kinetics of biosorption of cadmium(II) and copper(II) ions by wheat straw. Bioresour. Technol. 2009, 100, 211–219. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Sert, Ş.; Kütahyali, C.; Inan, S.; Talip, Z.; Çetinkaya, B.; Eral, M. Biosorption of lanthanum and cerium from aqueous solutions by Platanus orientalis leaf powder. Hydrometallurgy 2008, 90, 13–18. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, C.; Chen, Y.; Zhu, B.; Yang, Y.; Wang, Q.; Ye, W. Preparation, characterization and adsorptive study of rare earth ions using magnetic GMZ bentonite. Appl. Clay Sci. 2012, 62–63, 87–93. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, B.; Wu, D.; Wang, Q.; Yang, Y.; Ye, W.; Guo, J. Eu(III) adsorption using di(2-thylhexly) phosphoric acid-immobilized magnetic GMZ bentonite. Chem. Eng. J. 2012, 181–182, 387–396. [Google Scholar] [CrossRef]
- Wu, D.; Sun, Y.; Wang, Q. Adsorption of lanthanum (III) from aqueous solution using 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester-grafted magnetic silica nanocomposites. J. Hazard. Mater. 2013, 260, 409–419. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, S.B.; Marwani, H.M.; Asiri, A.M. SnO2-TiO2 nanocomposites as new adsorbent for efficient removal of La(III) ions from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2014, 45, 1964–1974. [Google Scholar] [CrossRef]
- Birungi, Z.S.; Chirwa, E.M.N. The kinetics of uptake and recovery of lanthanum using freshwater algae as biosorbents: Comparative analysis. Bioresour. Technol. 2014, 160, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Galhoum, A.A.; Mafhouz, M.G.; Abdel-Rehem, S.T.; Gomaa, N.A.; Atia, A.A.; Vincent, T.; Guibal, E. Cysteine-Functionalized chitosan magnetic nano-based particles for the recovery of light and heavy rare earth metals: Uptake kinetics and sorption isotherms. Nanomaterials 2015, 5, 154–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Huang, L.; Long, Z.; Feng, Z.; Wang, L. Adsorption ability of rare earth elements on clay minerals and its practical performance. J. Rare Earths 2016, 34, 543–548. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Meng, Y.; Wang, X.; Yin, D.; Sillanpää, M. An EDTA-β-cyclodextrin material for the adsorption of rare earth elements and its application in preconcentration of rare earth elements in seawater. J. Colloid Interface Sci. 2016, 465, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Ashour, R.M.; Abdelhamid, H.N.; Abdel-Magied, A.F.; Abdel-Khalek, A.A.; Ali, M.M.; Uheida, A.; Muhammed, M.; Zou, X.; Dutta, J. Rare Earth Ions Adsorption onto Graphene Oxide Nanosheets. Solvent Extr. Ion Exch. 2017, 35, 91–103. [Google Scholar] [CrossRef]
- Kano, N.; Pang, M.; Deng, Y.; Imaizumi, H. Adsorption of Rare Earth Elements (REEs) onto Activated Carbon Modified with Potassium Permanganate (KMnO4). J. Appl. Solut. Chem. Model. 2017, 6, 51–61. [Google Scholar] [CrossRef]
- Iftekhar, S.; Srivastava, V.; Sillanpää, M. Enrichment of lanthanides in aqueous system by cellulose based silica nanocomposite. Chem. Eng. J. 2017, 320, 151–159. [Google Scholar] [CrossRef]
- Iftekhar, S.; Srivastava, V.; Casas, A.; Sillanpää, M. Synthesis of novel GA-g-PAM/SiO2 nanocomposite for the recovery of rare earth elements (REE) ions from aqueous solution. J. Clean. Prod. 2018, 170, 251–259. [Google Scholar] [CrossRef]
- Lee, Y.R.; Yu, K.; Ravi, S.; Ahn, W.S. Selective Adsorption of Rare Earth Elements over Functionalized Cr-MIL-101. ACS Appl. Mater. Interfaces 2018, 10, 23918–23927. [Google Scholar] [CrossRef]
- Saha, P.; Chowdhury, S. Insight into Adsorption Thermodynamics. Thermodynamics 2011, 16, 349–364. [Google Scholar] [CrossRef] [Green Version]
- Tolba, A.A.; Mohamady, S.I.; Hussin, S.S.; Akashi, T.; Sakai, Y.; Galhoum, A.A.; Guibal, E. Synthesis and characterization of poly(carboxymethyl)-cellulose for enhanced La(III) sorption. Carbohydr. Polym. 2017, 157, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhou, C.; Wang, S.; Man, R. Adsorption Properties for La(III), Ce(III), and Y(III) with Poly(6-acryloylamino-hexyl hydroxamic acid) Resin. Polymers 2021, 13, 3. [Google Scholar] [CrossRef]
- Coelho, C.M.; de Andrade, J.R.; da Silva, M.G.C.; Vieira, M.G.A. Removal of propranolol hydrochloride by batch biosorption using remaining biomass of alginate extraction from Sargassum filipendula algae. Environ. Sci. Pollut. Res. 2020, 27, 16599–16611. [Google Scholar] [CrossRef]
- Antonelli, R.; Malpass, G.R.P.; Da Silva, M.G.C.; Vieira, M.G.A. Adsorption of ciprofloxacin onto thermally modified bentonite clay: Experimental design, characterization, and adsorbent regeneration. J. Environ. Chem. Eng. 2020, 8, 104553. [Google Scholar] [CrossRef]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Varshini, C.J.S.; Das, N. Recovery of lanthanum(III) from aqueous solution using biosorbents of plant and animal origin: Batch and column studies. Miner. Eng. 2014, 69, 40–56. [Google Scholar] [CrossRef]
- Jeon, C.; Nah, I.W.; Hwang, K.Y. Adsorption of heavy metals using magnetically modified alginic acid. Hydrometallurgy 2007, 86, 140–146. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, J.; Pan, F.; Zhou, H.; Yang, X.; Li, W.; Liu, H. Adsorption properties toward trivalent rare earths by alginate beads doping with silica. Ind. Eng. Chem. Res. 2013, 52, 3453–3461. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, J.; Wei, X.; Huo, F.; Li, W.; Hu, Q.; Liu, H. Adsorption of rare earths (III) by calcium alginate-poly glutamic acid hybrid gels. J. Chem. Technol. Biotechnol. 2014, 89, 969–977. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Nagura, M.; Ohnishi, R.; Gitoh, Y.; Ohkoshi, Y. Structures and physical properties of cross-linked sericin membranes. J. Insect Biotechnol. Sericology 2001, 70, 149–153. [Google Scholar] [CrossRef]
- Zhang, X.; Khan, M.M.R.; Yamamoto, T.; Tsukada, M.; Morikawa, H. Fabrication of silk sericin nanofibers from a silk sericin-hope cocoon with electrospinning method. Int. J. Biol. Macromol. 2012, 50, 337–347. [Google Scholar] [CrossRef]
- Patel, N.; Lalwani, D.; Gollmer, S.; Injeti, E.; Sari, Y.; Nesamony, J. Development and evaluation of a calcium alginate based oral ceftriaxone sodium formulation. Prog. Biomater. 2016, 5, 117–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, L.; Chen, N.; Feng, C.; Gao, Y.; Li, M. Xanthate-modified magnetic chitosan/poly (vinyl alcohol) adsorbent: Preparation, characterization, and performance of Pb(II) removal from aqueous solution. J. Taiwan Inst. Chem. Eng. 2017, 78, 485–492. [Google Scholar] [CrossRef]
- De Freitas, G.R.; Vieira, M.G.A.; Da Silva, M.G.C. Batch and Fixed Bed Biosorption of Copper by Acidified Algae Waste Biomass. Ind. Eng. Chem. Res. 2018, 57, 11767–11777. [Google Scholar] [CrossRef]
- das Gracas Santos, N.T.; Landers, R.; da Silva, M.G.C.; Vieira, M.G.A. Adsorption of Gold Ions onto Sericin and Alginate Particles Chemically Crosslinked by Proanthocyanidins: A Complete Fixed-Bed Column Study. Ind. Eng. Chem. Res. 2020, 59, 318–328. [Google Scholar] [CrossRef]

| Models | Parameters | Temperature | ||
|---|---|---|---|---|
| 298 K | 313 K | 328 K | ||
| Experimental | qmax (mmol/g) | 0.503 | 0.557 | 0.644 |
| Langmuir | qmax (mmol/g) | 0.495 | 0.548 | 0.621 |
| kL (L/mmol) | 3.950 | 5.011 | 4.762 | |
| RL | 0.025 | 0.019 | 0.020 | |
| R2 | 0.984 | 0.984 | 0.984 | |
| AICc | −121.628 | −117.638 | −113.298 | |
| Freundlich | n | 0.328 | 0.382 | 0.423 |
| R2 | 3.184 | 3.203 | 3.183 | |
| AICc | 0.949 | 0.931 | 0.915 | |
| kF [(mmol/g)·(L/mmol)1/n] | −101.268 | −92.426 | −85.024 | |
| Dubinin– Radushkevich | qm (mmol/g) | 0.450 | 0.507 | 0.574 |
| kDR (mol²/J2) | 3.23 × 10−8 | 2.50 × 10−8 | 2.41 × 10−8 | |
| E (kJ/mol) | 3.937 | 4.472 | 4.557 | |
| R2 | 0.964 | 0.973 | 0.977 | |
| AICc | −107.119 | −108.465 | −107.041 | |
| Bio/Adsorbents | qmax (mmol/g) | References |
|---|---|---|
| Platanus orientalis leaf powder | 0.206 * | Sert et al. [55] |
| Magnetic GMZ bentonite | 0.132 * | Wu et al. [56] |
| P204 immobilized magnetic GMZ bentonite | 0.292 * | Chen et al. [57] |
| P507 functionalized magnetic | 0.402 * | Wu et al. [58] |
| SnO2–TiO2 nanocomposites | 0.488 * | Rahman et al. [59] |
| Stichococcus bacillaris | 0.367 * | Birungi et al. [60] |
| Chlorella vulgaris | 0.537 * | |
| Cys@CHI-magnetic | 0.129 * | Galhoum et al. [61] |
| Kaolin | 0.012 * | Xiao et al. [62] |
| EDTA-β-cyclodextrin | 0.343 | Zhao et al. [63] |
| GO nanosheets | 0.617 * | Ashour et al. [64] |
| KMnO4–AC | 0.001 * | Kano et al. [65] |
| CLN/SiO2 | 0.212 * | Iftekhar et al. [66] |
| GA-g-PAM/SiO2 | 0.057 * | Iftekhar et al. [67] |
| MIL-101-PMIDA | 0.269 * | Lee et al. [68] |
| SAPVA particles | 0.503 | This study |
| 0.557 | ||
| 0.644 |
| T (K) | ΔH (kJ/mol) | ΔS (J/mol·K) | ΔG (kJ/mol) | Ea (kJ/mol) |
|---|---|---|---|---|
| 298 | +15.372 | +110.543 | −17.586 | 17.851 |
| 313 | −19.244 | 17.976 | ||
| 328 | −20.902 | 18.101 |
| qe (mmol/g) | ΔHST (kJ/mol) | R2 |
|---|---|---|
| 0.394 | 17.84 | 0.999 |
| 0.438 | 12.63 | 0.981 |
| 0.476 | 11.17 | 0.981 |
| Coexisting Ions | ||
|---|---|---|
| La3+ | 0.686 | - |
| Cd2+ | 0.089 | 7.702 |
| Zn2+ | 0.079 | 8.655 |
| Ni2+ | 0.049 | 13.987 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa, T.B.; da Silva, M.G.C.; Vieira, M.G.A. Equilibrium, Thermodynamic, Reuse, and Selectivity Studies for the Bioadsorption of Lanthanum onto Sericin/Alginate/Poly(vinyl alcohol) Particles. Polymers 2021, 13, 623. https://doi.org/10.3390/polym13040623
da Costa TB, da Silva MGC, Vieira MGA. Equilibrium, Thermodynamic, Reuse, and Selectivity Studies for the Bioadsorption of Lanthanum onto Sericin/Alginate/Poly(vinyl alcohol) Particles. Polymers. 2021; 13(4):623. https://doi.org/10.3390/polym13040623
Chicago/Turabian Styleda Costa, Talles Barcelos, Meuris Gurgel Carlos da Silva, and Melissa Gurgel Adeodato Vieira. 2021. "Equilibrium, Thermodynamic, Reuse, and Selectivity Studies for the Bioadsorption of Lanthanum onto Sericin/Alginate/Poly(vinyl alcohol) Particles" Polymers 13, no. 4: 623. https://doi.org/10.3390/polym13040623
APA Styleda Costa, T. B., da Silva, M. G. C., & Vieira, M. G. A. (2021). Equilibrium, Thermodynamic, Reuse, and Selectivity Studies for the Bioadsorption of Lanthanum onto Sericin/Alginate/Poly(vinyl alcohol) Particles. Polymers, 13(4), 623. https://doi.org/10.3390/polym13040623