A Bibliometric Analysis of the Global Trend of Using Alginate, Gelatine, and Hydroxyapatite for Bone Tissue Regeneration Applications
Abstract
:1. Introduction
2. Alginate, Gelatine, and Hydroxyapatite for Bone Tissue Regeneration
2.1. Alginate
2.2. Gelatine
2.3. Hydroxyapatite
2.4. Scaffold Preparation
2.5. Current Studies Related to Alginate, Gelatine, and Hydroxyapatite
3. Bibliometric Analysis on Scopus Database
- Bibliometric data cannot be interpreted as a holistic response to quality measurement. For example, the number of citations of an article does not necessarily mean that it is of high quality, but symbolizes its impact or usefulness.
- In publications, not only English language articles are published but also many different languages.
- A bibliometric analysis does not include whole research areas and does not index all publications.
- The number of citations is highly dissimilar between disciplines. So, a direct comparison cannot be made using it.
4. Global Trend
5. Conclusions and Final Considerations
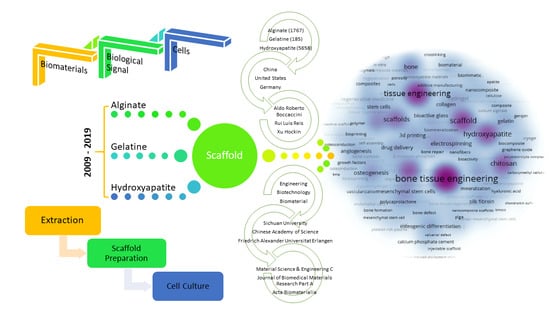
- A total of 7446 publications with the keywords ‘‘bone tissue” and scaffold were found, while 1767 (alginate), 185 (gelatine), 5658 (hydroxyapatite) papers with the specific sub keywords were determined from 2009 to 2019.
- Article type comes into prominence as the dominant category in terms of the type of publication.
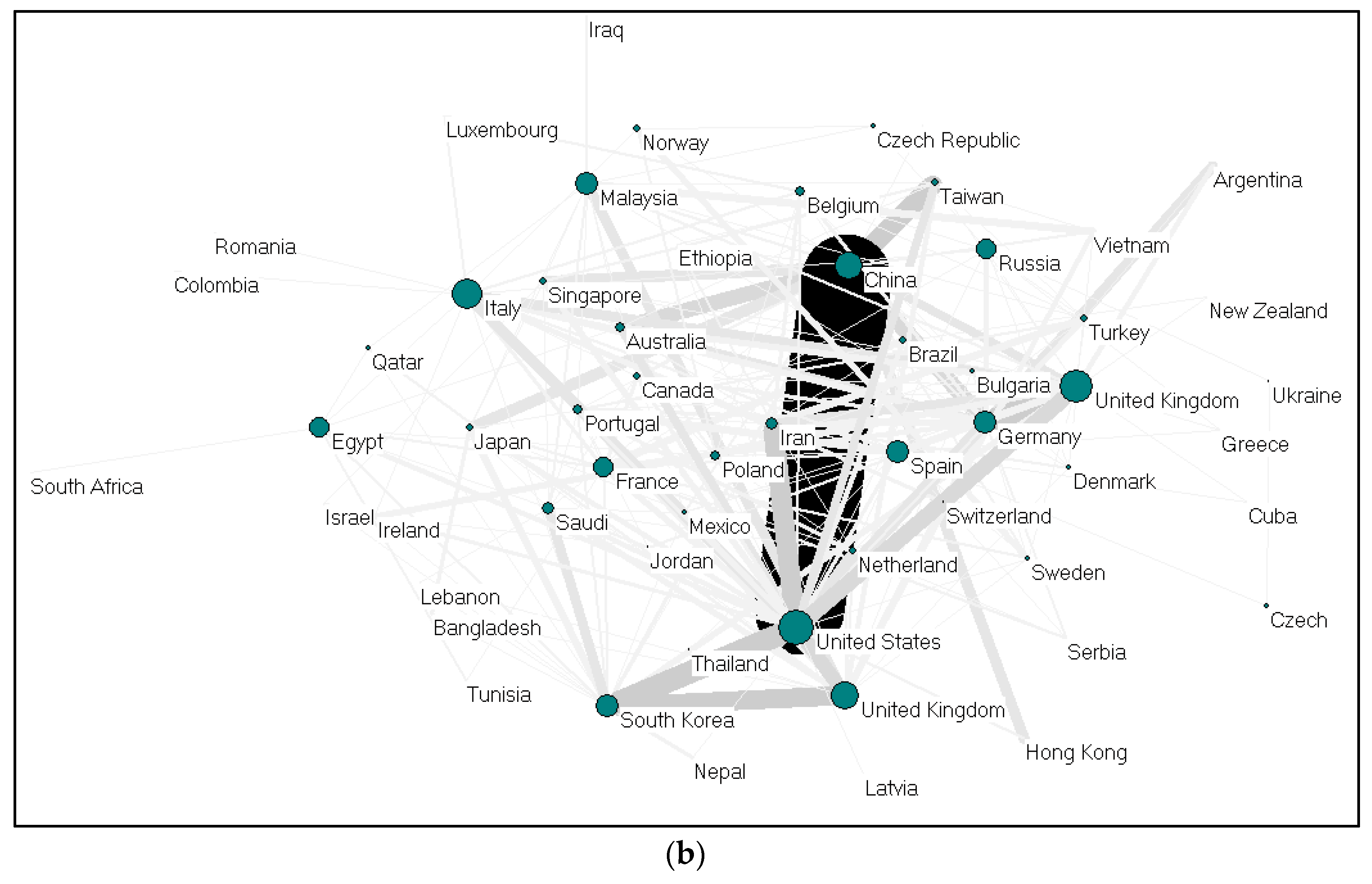
- China and the United States are the most productive countries, according to the total publication criteria.
- While Boccaccini Aldo Roberto, from Germany, is the most productive author in terms of publication number, Ramakrishna Seeram is the most productive author considering the average citations per article, with 63.74 points.
- The most preferred keywords are bone tissue engineering, scaffold and bone regeneration for alginate.
- Over 94.14% of the publications were published in English.
- Material Science and Engineering C takes the leading place, with 89 (alginate), 12 (gelatine), and 288 (hydroxyapatite) publications and a 10.2 impact factor.
- In moving the related research forward, a better interpretation of bibliometric analysis needs to evolve. More information on this type of research would facilitate the production of a greater degree of precision on this subject.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neuss, S.; Apel, C.; Buttler, P.; Denecke, B.; Dhanasingh, A.; Ding, X.; Grafahrend, D.; Groger, A.; Hemmrich, K.; Herr, A.; et al. Assessment of stem cell/biomaterial combinations for stem cell-based tissue engineering. Biomaterials 2008, 29, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, H.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials 2006, 27, 6064–6082. [Google Scholar] [CrossRef]
- Tabata, Y. Biomaterials technology for tissue engineering applications. J. R. Soc. Interface 2009, 6, 311–324. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, F.J. Biomaterials and scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Hickey, R.J.; Pelling, A.E. Cellulose biomaterials for tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Li, H.; Xia, P.; Kong, W.; Chang, Y.; Fu, C.; Wang, K.; Yang, X.; Qi, Z. Applications of fibrin-based hydrogels for nerve protection and regeneration after spinal cord injury. J. Biol. Eng. 2020, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.T.; Venkatarama, R.S.; Grasman, J.M. Recent study in injury models to study skeletal muscle regeneration and repair. Bioengineering 2020, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jia, Y.; Cai, H.; Wang, W.; Sun, C. Functional patch combined with surface electromyographic biofeedback for post-stroke dysphagia. Chin. J. Tissue Eng. Res. 2020, 24, 4697–4701. [Google Scholar]
- Grawish, M.E.; Grawish, L.M.; Grawish, H.M.; Grawish, M.M.; El-Negoly, S.A. Challenges of engineering biomimetic dental and paradental tissues. Tissue Eng. Reg. Med. 2020, 17, 403–421. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials, and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef]
- Nosrati, H.; Pourmotabed, S.; Sharifi, E. A review on some natural biopolymers and their applications in angiogenesis and tissue engineering. J. Appl. Biotechnol. Rep. 2018, 5, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Pryjmakova, J.; Kaimlova, M.; Hubacek, T.; Svorcik, V.; Siegel, J. Nanostructured materials for artificial tissue replacements. Int. J. Mol. Sci. 2020, 21, 2521. [Google Scholar] [CrossRef] [Green Version]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in regenerative medicine and tissue engineering innovation and transformation of medicine. Stem Cells Int. 2018, 2018, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saberianpour, S.; Heidarzadeh, M.; Geranmayeh, M.H.; Hosseinkhani, H.; Rahbarghazi, R.; Nouri, M. Tissue engineering strategies for the induction of angiogenesis using biomaterials. J. Biol. Eng. 2018, 12, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [Green Version]
- Han, F.; Wang, J.; Ding, L.; Hu, Y.; Li, W.; Yuan, Z.; Guo, Q.; Zhu, C.; Yu, L.; Wang, H.; et al. Tissue engineering and regenerative medicine: Achievements, future, and sustainability in Asia. Front. Bioeng. Biotechnol. 2020, 8, 83. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Lode, A.; Akkineni, A.R.; Gelinsky, M. Concentrated gelatin/alginate composites for fabrication of predesigned scaffolds with a favorable cell response by 3D plotting. RSC Adv. 2015, 5, 43480–43488. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, C.; Dinda, A.K.; Potdar, P.D.; Chou, C.; Mishra, N.C. Fabrication and characterization of novel nano-biocomposite scaffold of chitosan-gelatin-alginate-hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. C 2016, 64, 416–427. [Google Scholar] [CrossRef]
- Yan, J.; Miao, Y.; Tan, H.; Zhou, T.; Ling, Z.; Chen, Y.; Xing, X.; Hu, X. Injectable alginate/hydroxyapatite gel scaffold combined with gelatin microspheres for drug delivery and bone tissue engineering. Mater. Sci. Eng. C 2016, 63, 274–284. [Google Scholar] [CrossRef]
- Ventakesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.; Kim, S. Alginate composite for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar]
- Abdulghani, S.; Mitchell, G.R. Biomaterials for in situ tissue regeneration: A review. Biomolecules 2019, 9, 750. [Google Scholar] [CrossRef] [Green Version]
- Yunos, D.M.; Bretcanu, O.; Boccaccini, A.R. Polymer-bioceramic composites for tissue engineering scaffolds. J. Mater. Sci. 2008, 43, 4433–4442. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 1–19. [Google Scholar] [CrossRef]
- Shuai, C.; Yu, L.; Feng, P.; Gao, C.; Peng, S. Interfacial reinforcement in bioceramic/biopolymer composite bone scaffold: The role of coupling agent. Colloids Surf. B Biointerfaces 2020, 193, 111083. [Google Scholar] [CrossRef]
- Sowmya, S.; Bumgardener, J.D.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Role of nanostructured biopolymers and bioceramics in enamel, dentin, and periodontal tissue regeneration. Prog. Polym. Sci. 2013, 38, 1748–1772. [Google Scholar] [CrossRef]
- Gombotz, W.R.; Wee, S.F. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998, 31, 267–285. [Google Scholar] [CrossRef]
- Xing, M.; Cao, Q.; Wang, Y.; Xiao, H.; Zhao, J.; Zhang, Q.; Ji, A.; Song, S. Advances in research on the bioactivity of alginate oligosaccharides. Mar. Drugs 2020, 18, 144. [Google Scholar] [CrossRef] [Green Version]
- Queen, D.; Orsted, H.; Sanada, H.; Sussman, G. A dressing history. Int. Wound J. 2004, 1, 59–77. [Google Scholar] [CrossRef]
- Chee, S.Y.; Wong, C.L. Extraction and characterization of alginate from brown seaweed (Fucales, Phaeophyceae) collected from Port Dickson, Peninsular Malaysia. J. Appl. Phycol. 2011, 23, 191–196. [Google Scholar] [CrossRef]
- Leal, D.; Matsuhiro, B.; Rossi, M.; Caruso, F. FT-IR spectra of alginic acid block fractions in three species of brown seaweeds. Carbohydr. Res. 2008, 343, 308–316. [Google Scholar] [CrossRef]
- Youssouf, L.; Lallemand, L.; Giraud, P.; Soule, F.; Bhaw-Luximon, A.; Meilhac, O.; D’Heelencourt, C.L.; Jhurry, D.; Couprie, J. Ultrasound-assisted extraction and structural characterization by NMR of alginates and carrageenans from seaweeds. Carbohydr. Polym. 2017, 166, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.E.; Nuge, T.; Yeow, T.K.; Nordin, N.; Prasad, R.G.S.V. Gelatin based scaffolds for tissue engineering: A review. Polym. Res. J. 2015, 9, 15–32. [Google Scholar]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalians gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Herpandi, H.; Huda, N.; Adzitey, F. Fish bone and scale as a potential source of halal gelatin. J. Fish. Aquat. Sci. 2011, 6, 379–389. [Google Scholar]
- Johns, P.; Courts, A. (Eds.) Relationship between collagen and gelatin. In The Science and Technology of Gelatin; Academic Press: New York, NY, USA, 1977; pp. 137–178. [Google Scholar]
- Abedinia, A.; Ariffin, F.; Huda, N.; Nafchi, A.M. Extraction and characterization of gelatin from the feet of Pekin duck (Anas platyrhynchos domestica) as affected by acid, alkaline, and enzyme pretreatment. Int. J. Biol. Macromol. 2017, 98, 586–594. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2001, 22, 87–96. [Google Scholar] [CrossRef]
- Granito, R.N.; Renno, A.C.M.; Yamamura, H.; Almeida, M.C.; Ruiz, P.L.M.; Ribeiro, D.A. Hydroxyapatite from fish for bone tissue engineering: A promising approach. Int. J. Mol. Cell. Med. 2018, 7, 80–90. [Google Scholar]
- Pon-On, W.; Suntornsaratoon, P.; Charoenphandu, N.; Thongbunchoo, J.; Krishnamra, N.; Tang, I.M. Hydroxyapatite from fish scale for potential use as bone scaffold or regenerative material. Mater. Sci. Eng. C 2016, 62, 183–189. [Google Scholar] [CrossRef]
- Lu, J.; Yu, H.; Chen, C. Biological properties of calcium phosphate biomaterials for bone repair: A review. RSC Adv. 2018, 8, 2015–2033. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.T.; Lu, A.; Watson, E.; Santoro, M.; Melchiorri, A.J.; Grosfeld, E.C.; Beucken, J.J.J.P.; Jansen, J.A.; Scott, D.W.; Fisher, J.P.; et al. Incorporation of fast dissolving glucose porogens and poly(lactic-co-glycolic acid) microparticles within calcium phosphate cements for bone tissue regeneration. Acta Biomater. 2018, 15, 341–350. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Kang, Y.; Yin, G.; Yuan, Q.; Yao, Y.; Huang, Z.; Liao, X.; Yang, B.; Liao, L.; Wang, H. Preparation of poly(L-lactic acid)/β-tricalcium phosphate scaffold for bone tissue engineering without organic solvent. Mater. Lett. 2008, 62, 2029–2032. [Google Scholar] [CrossRef]
- Mozafari, M.; Mostarzadeh, F.; Rabiee, M.; Azami, M.; Maleknia, S.; Tahriri, M.; Mostarzadeh, Z.; Nezafati, N. Development of microporous nanocomposite scaffolds of gelatin/bioactive glass prepared through layer solvent casting combined with lamination technique for bone tissue engineering. Ceram. Int. 2010, 36, 2431–2439. [Google Scholar] [CrossRef]
- Sola, A.; Bertacchini, J.; D’Avella, D.; Anselmi, L.; Maraldi, T.; Marmiroli, S.; Messori, M. Development of solvent-casting particulate leaching (SCPL) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Mater. Sci. Eng. C 2019, 96, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Lee, O.J.; Lee, M.C.; Moon, B.M.; Ju, H.W.; Lee, J.M.; Kim, J.; Kim, D.W.; Park, C.H. Fabrication of 3D porous silk scaffolds by particulate (salt/sucrose) leaching for bone tissue reconstruction. Int. J. Biol. Macromol. 2015, 78, 215–223. [Google Scholar] [CrossRef]
- Sadiasa, A.; Nguyen, T.H.; Lee, B. In vitro and in vivo evaluation of porous PCL-PLLA 3D polymer scaffolds fabricated via salt leaching method for bone tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2014, 25, 150–167. [Google Scholar] [CrossRef]
- Sohier, J. A novel method to obtain protein release from porous polymer scaffolds: Emulsion coating. J. Control. Release 2003, 87, 57–68. [Google Scholar] [CrossRef]
- Fu, Q.; Rahaman, M.N.; Bal, B.S.; Brown, R.F.; Day, D.E. Mechanical and in vitro performance of 13-93 bioactive glass scaffolds prepared by a polymer foam replication technique. Acta Biomater. 2008, 6, 1854–1864. [Google Scholar] [CrossRef]
- Fu, H.; Fu, Q.; Zhou, N.; Huang, W.; Rahaman, M.N.; Wang, D.; Liu, X. In vitro evaluation of borate-based bioactive glass scaffolds prepared by a polymer foam replication method. Mater. Sci. Eng. C 2009, 29, 2275–2281. [Google Scholar] [CrossRef]
- Rodrigues, S.C.; Salgado, C.L.; Sahu, A.; Garcia, M.P.; Fernandes, M.H.; Monteiro, F.J. Preparation and characterization of collagen-nanohydroxyapatite biocomposite scaffolds by cryogelation method for bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2013, 101A, 1080–1094. [Google Scholar] [CrossRef]
- Bolgen, N.; Yang, Y.; Korkusuz, P.; Guzel, E.; El Haj, A.J.; Piskin, E. Three-dimensional ingrowth of bone cells within biodegradable cryogel scaffolds in bioreactors at different regimes. Tissue Eng. Part A 2008, 14, 1743–1750. [Google Scholar] [CrossRef]
- Wu, S.; Ma, S.; Zhang, C.; Cao, G.; Wu, D.; Gao, C.; Lakshmanan, S. Cryogel biocomposite containing chitosan-gelatin/cerium-zinc doped hydroxyapatite for bone tissue engineering. Saudi J. Biol. Sci. 2020, 27, 2638–2644. [Google Scholar] [CrossRef]
- Ramay, H.R.; Zhang, M. Preparation of porous hydroxyapatite scaffolds by combination of gel-casting and polymer sponge method. Biomaterials 2003, 24, 3293–3302. [Google Scholar] [CrossRef]
- Nie, L.; Chen, D.; Suo, J.; Zou, P.; Feng, S.; Yang, Q.; Yang, S.; Ye, S. Physicochemical characterization and biocompatibility in vitro of biphasic calcium phosphate/polyvinyl alcohol scaffolds prepared by freeze-drying method for bone tissue engineering. Colloids Surf. B Biointerfaces 2012, 100, 169–176. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Ma, G.; Yang, D.; Nie, J. The effect of the prefrozen process on properties of a chitosan/hydroxyapatite/poly(methyl methacrylate) composite prepared by freeze drying method used for bone tissue engineering. RSC Adv. 2015, 5, 79679–79686. [Google Scholar] [CrossRef]
- Shahbarazab, Z.; Teimouri, A.; Chermahini, A.N.; Azadi, M. Fabrication and characterization of nanobiocomposite scaffold of zein/chitosan/nanohydroxyapatite prepared by freeze-drying method for bone tissue engineering. Int. J. Biol. Macromol. 2018, 108, 1017–1027. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Venugopal, J.; Ramakrishna, S. Electrospun nanostructured scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 2884–2893. [Google Scholar] [CrossRef]
- Hang, J.; Castano, O.; Kim, H. Electrospun materials as potential platforms for bone tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1065–1083. [Google Scholar]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Bendtsen, S.T.; Quinnell, S.P.; Wei, M. Development of a novel alginate-polyvinyl alcohol-hydroxyapatite hydrogel for 3D bioprinting bone tissue engineered scaffolds. J. Biomed. Mater. Res. Part A 2017, 105, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Chimene, D.; Miller, L.; Cross, L.M.; Jaiswal, M.K.; Singh, I.; Gaharwar, A.K. Nanoengineered osteconductive bioink for 3D bioprinting bone tissue. ACS Appl. Mater. Interfaces 2020, 12, 15976–15988. [Google Scholar] [CrossRef]
- Murphy, C.; Kolan, K.; Li, W.; Semon, J.; Day, D.; Leu, M. 3D bioprinting of stem cells and polymer/bioactive glass composite scaffolds for bone tissue engineering. Int. J. Bioprinting 2017, 3, 54–64. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Garske, D.S.; Schmidt-Bleek, K.; Ellinghaus, A.; Dienelt, A.; Gu, L.; Mooney, D.J.; Duda, G.N.; Cipitria, A. Alginate Hydrogels for In Vivo Bone Regeneration: The Immune Competence of the Animal Model Matters. Tissue Eng. Part A 2020, 26, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, Y.; Roohani, I.; No, Y.J.; Madafiglio, G.; Chang, F.; Zhang, F.; Lu, Z.; Zreiqat, H. Nature-inspired topographies on hydroxyapatite surfaces regulate stem cells behaviour. Bioact. Mater. 2021, 6, 1107–1117. [Google Scholar] [CrossRef]
- Kruppke, B.; Farack, J.; Wagner, A.; Beckmann, S.; Heinemann, C.; Glenske, K.; Rößler, S.; Wiesmann, H.; Wenisch, S.; Hanke, T. Gelatine modified monetite as a bone substitute material: An in vitro assessment of bone biocompatibility. Acta Biomater. 2016, 32, 275–285. [Google Scholar] [CrossRef]
- Mahmoud, E.M.; Sayed, M.; El-Kady, A.M.; Elsayed, H.; Naga, S.M. In vitro and in vivo study of naturally derived alginate/hydroxyapatite bio composite scaffolds. Int. J. Biol. Macromol. 2020, 165, 1346–1360. [Google Scholar] [CrossRef]
- Ye, Q.; Zhang, Y.; Dai, K.; Chen, X.; Read, H.M.; Zeng, L.; Hang, F. Three dimensional printed bioglass/gelatin/alginate composite scaffolds with promoted mechanical strength, biomineralization, cell responses and osteogenesis. J. Mater. Sci. Mater. Med. 2020, 31, 77. [Google Scholar] [CrossRef]
- Ho, H.V.; Tripathi, G.; Gwon, J.; Lee, S.; Lee, B. Novel TOCNF reinforced injectable alginate/β-tricalcium phosphate microspheres for bone regeneration. Mater. Des. 2020, 194, 108892. [Google Scholar] [CrossRef]
- Przekora, A.; Kazimierczak, P.; Wojcik, M. Ex vivo determination of chitosan/curdlan/hydroxyapatite biomaterial osseointegration with the use of human trabecular bone explant: New method for biocompatibility testing of bone implants reducing animal tests. Mater. Sci. Eng. C 2021, 119, 111612. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Tao, H.; Jin, L.; Wan, Z.; Dai, F.; Xiang, W.; Deng, H. Carboxymethyl chitosan/sodium alginate-based micron-fibers fabricated by emulsion electrospinning for periosteal tissue engineering. Mater. Des. 2020, 194, 108849. [Google Scholar] [CrossRef]
- Reakasame, S.; Jin, A.; Zheng, K.; Qu, M.; Boccaccini, A.R. Biofabrication and Characterization of Alginate Dialdehyde-Gelatin Microcapsules Incorporating Bioactive Glass for Cell Delivery Application. Macromol. Biosci. 2020, 20, 2000138. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Zhang, M.; He, J.; Zheng, B.; Liu, F.; Zhao, Z. Use of Silver Nanoparticle–Gelatin/Alginate Scaffold to Repair Skull Defects. Coatings 2020, 10, 948. [Google Scholar] [CrossRef]
- Benedini, L.; Laiuppa, J.; Santillán, G.; Baldini, M.; Messina, P. Antibacterial alginate/nano-hydroxyapatite composites for bone tissue engineering: Assessment of their bioactivity, biocompatibility, and antibacterial activity. Mater. Sci. Eng. C 2020, 115, 111101. [Google Scholar] [CrossRef] [PubMed]
- Abouzeid, R.E.; Khiari, R.; Salama, A.; Diab, M.; Beneventi, D.; Dufresne, A. In situ mineralization of nano-hydroxyapatite on bifunctional cellulose nanofiber/polyvinyl alcohol/sodium alginate hydrogel using 3D printing. Int. J. Biol. Macromol. 2020, 160, 538–547. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact. Mater. 2021, 6, 490–502. [Google Scholar] [CrossRef]
- Gautam, S.; Sharma, C.; Purohit, S.D.; Singh, H.; Dinda, A.K.; Potdar, P.D.; Chou, C.; Mishra, N.C. Gelatin-polycaprolactone-nanohydroxyapatite electrospun nanocomposite scaffold for bone tissue engineering. Mater. Sci. Eng. C 2021, 119, 111588. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, B.; Vijayalakshmi, U. In Vitro bioactivity, biocompatibility and corrosion resistance of multi-ionic (Ce/Si) co-doped hydroxyapatite porous coating on Ti-6Al-4 V for bone regeneration applications. Mater. Sci. Eng. C 2021, 119, 111620. [Google Scholar]
- Volkov, A.V.; Muraev, A.A.; Zharkova, I.I.; Voivona, V.V.; Akoulina, A.A.; Zhuikov, V.A.; Khaydapova, D.D.; Chesnokova, D.V.; Menshikh, K.A.; Dudun, A.A.; et al. Poly(3-hydroxybutyrate)/hydroxyapatite/alginate scaffolds seeded with mesenchymal stem cells enhance the regeneration of critical-sized bone defect. Mater. Sci. Eng. C 2020, 114, 110991. [Google Scholar] [CrossRef]
- Lima, D.B.; Souza, M.A.A.; Lima, G.G.; Souto, E.P.F.; Oliveira, H.M.L.; Fook, M.V.L.; Sa, M.J.C. Injectable bone substitute based on chitosan with polyethylene glycol polymeric solution and biphasic calcium phosphate microspheres. Carbohydr. Polym. 2020, 245, 116575. [Google Scholar] [CrossRef]
- Sharmila, G.; Muthukumaran, C.; Kirthika, S.; Keerthana, S.; Kumar, N.M.; Jeyanthi, J. Fabrication and characterization of Spinacia oleracea extract incorporated alginate/carboxymethyl cellulose microporous scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 156, 430–437. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, J.; Lin, B.; Li, J.; Wang, H.; Wang, D.; Pang, L.; Zeng, X.; Wang, H.; Zhang, Y. A novel 3D printed bioactive scaffolds with enhanced osteogenic inspired by ancient Chinese medicine HYSA for bone repair. Exp. Cell Res. 2020, 394, 112139. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, A. Statistical Bibliography or Bibliometrics? J. Doc. 1969, 25, 348–349. [Google Scholar]
- Belter, C.W. Bibliometric indicators: Opportunities and limits. J. Med. Lib. Assoc. 2015, 103, 219–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, J.; Chi, R. Intercultural relations: A bibliometric survey. Int. J. Intercult. Relat. 2013, 37, 133–145. [Google Scholar] [CrossRef]
- Pajek: Analysis and Visualization of Very Large Networks. Available online: mrvar.fdv.uni-lj.si/pajek/ (accessed on 15 December 2020).
- VOSviewer. Available online: www.vosviewer.com (accessed on 30 December 2020).






| Keywords | Publications (1969–2019) | Publications (2009–2019) |
|---|---|---|
| Alginate | 35,572 | 23,038 |
| Gelatine | 3853 | 1544 |
| Hydroxyapatite | 58,489 | 31,349 |
| Scaffold | 133,383 | 107,064 |
| Bone Tissue | 34,198 | 22,270 |
| Alginate + Scaffold + Bone Tissue | 315 | 284 |
| Gelatine + Scaffold + Bone Tissue | 24 | 23 |
| Hydroxyapatite + Scaffold + Bone Tissue | 2920 | 2404 |
| Alginate + Hydroxyapatite + Scaffold + Bone Tissue | 105 | 94 |
| Gelatine + Hydroxyapatite + Scaffold + Bone Tissue | 13 | 13 |
| Alginate + Gelatine + Scaffold + Bone Tissue | 4 | 4 |
| Alginate + Gelatine + Hydroxyapatite + Scaffold + Bone Tissue | 2 | 2 |
| Alginate | Gelatine | Hydroxyapatite | Alg | Gel | Hyd | |
|---|---|---|---|---|---|---|
| Article | 1329 | 135 | 4526 | 75.20% | 73.00% | 80.00% |
| Conference Paper | 40 | 6 | 342 | 2.30% | 3.20% | 6.04% |
| Review | 282 | 32 | 516 | 16.00% | 17.30% | 9.12% |
| Book Chapter | 113 | 12 | 242 | 6.40% | 6.49% | 4.28% |
| Other | 3 | 0 | 32 | 0.00% | 0.00% | 0.01% |
| Total | 1767 | 185 | 5658 |
| Number of Publication | Percentage % | Number of Citation | H-Index (Rank) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | Alg | Gel | Hyd | Alg | Gel | Hyd | Alg | Gel | Hyd | Alg | Gel | Hyd |
| China | 511 | 40 | 1617 | 28.92 | 21.62 | 28.58 | 9166 | 858 | 21,910 | 54 (2) | 16 (2) | 82 (2) |
| United States | 338 | 12 | 973 | 19.13 | 6.49 | 17.20 | 13,097 | 412 | 26,727 | 67 (1) | 9 (5) | 101 (1) |
| India | 181 | 18 | 389 | 10.24 | 9.73 | 6.88 | 5208 | 782 | 8714 | 41 (4) | 11 (4) | 58 (4) |
| South Korea | 144 | 11 | 423 | 8.15 | 5.95 | 7.48 | 3747 | 427 | 9621 | 34 (5) | 8 (6) | 56 (5) |
| Iran | 131 | 13 | 404 | 7.41 | 7.03 | 7.14 | 2953 | 225 | 6688 | 32 (6) | 9 (5) | 45 (7) |
| Germany | 114 | 26 | 349 | 6.45 | 14.05 | 6.17 | 5067 | 863 | 10,273 | 43 (3) | 17 (1) | 63 (3) |
| Italy | 92 | 21 | 352 | 5.21 | 11.35 | 6.22 | 3541 | 512 | 9190 | 31 (7) | 12 (3) | 55 (6) |
| United Kingdom | 81 | 6 | 272 | 4.58 | 3.24 | 4.81 | 4312 | 148 | 8804 | 34 (5) | 5 (8) | 55 (6) |
| Portugal | 52 | 4 | 128 | 2.94 | 2.16 | 2.26 | 2353 | 97 | 4433 | 27 (8) | 4 (9) | 42 (8) |
| Turkey | 51 | 9 | 128 | 2.89 | 4.86 | 2.26 | 830 | 106 | 2006 | 18 (9) | 6 (7) | 27 (9) |
| Total | 1767 | 185 | 5658 | 100 | 100 | 100 | ||||||
| Number of Publication | Total Citation | Citation/Article | H-Index | |||
|---|---|---|---|---|---|---|
| Authors | Alginate | Gelatine | Hydroxyapatite | |||
| Boccaccini Aldo Roberto | 50 | 11 | 111 | 44,249 | 35.74 | 92 |
| Reis Rui Luis | 21 | 2 | 55 | 45,911 | 37.30 | 99 |
| Xu Hockin H.K. | 19 | 0 | 23 | 10,777 | 61.23 | 67 |
| Chang Jiang | 18 | 2 | 39 | 21,026 | 44.93 | 80 |
| Selvamurugan Nagarajan | 18 | 2 | 29 | 7704 | 56.23 | 47 |
| Wu Chengtie | 17 | 3 | 42 | 12,183 | 48.35 | 64 |
| Venkatesan Jayachandran | 16 | 2 | 25 | 4274 | 34.19 | 34 |
| Kim Se Kwon | 15 | 2 | 24 | 30,725 | 43.15 | 92 |
| Zhao Liang | 15 | 0 | 16 | 1304 | 27.17 | 20 |
| Ramakrishna Seeram | 15 | 1 | 43 | 83,183 | 63.74 | 137 |
| Roether Judith A. | 14 | 3 | 21 | 5132 | 42.77 | 36 |
| Weir Michael D. | 14 | 0 | 18 | 2001 | 12.91 | 23 |
| Gelinsky Michael | 13 | 5 | 23 | 6007 | 24.03 | 43 |
| Azami Mahmoud | 12 | 1 | 27 | 1827 | 25.38 | 28 |
| Weng Jie | 12 | 0 | 21 | 6546 | 24.80 | 42 |
| Alginate | Gelatine | Hydroxyapatite | |||
|---|---|---|---|---|---|
| Word | Frequency | Word | Frequency | Word | Frequency |
| Bone | 1189 | Saffold | 104 | Bone | 3655 |
| Tissue | 847 | Bone | 103 | Scaffold | 3340 |
| Engineering | 697 | Tissue | 81 | Tissue | 2394 |
| Scaffold | 981 | Engineering | 61 | Engineering | 1996 |
| Cell | 359 | Composite | 28 | Composite | 1048 |
| Regeneration | 329 | Hydroxyapatite | 27 | Regeneration | 799 |
| Composite | 252 | Regeneration | 26 | Hydroxyapatite | 714 |
| Stem | 220 | Cell | 24 | Stem | 628 |
| Porous | 143 | Porous | 21 | Porous | 600 |
| 3D | 138 | Bioactive | 20 | Phosphate | 454 |
| Calcium | 133 | 3D | 18 | Vitro | 432 |
| Phosphate | 130 | Characterization | 16 | Bioactive | 413 |
| Mesenshymal | 126 | Glass | 15 | Calcium | 397 |
| Differentiation | 108 | Vitro | 14 | Properties | 388 |
| Vitro | 102 | Gelatine | 7 | Mesenchymal | 379 |
| Material Science & Engineering C | Journal of Biomedical Materials Research Part A | Acta Biomaterialia | International Journal of Biological Macromolecules | Biomaterials | RSC Advances | Colloids and Surfaces B Biointerfaces | Carbohydrate Polymers | Chinese Journal of Tissue Engineering Research | Journal of Material Science Materials in Medicine | |
|---|---|---|---|---|---|---|---|---|---|---|
| Alginate | 89 | 79 | 62 | 58 | 46 | 42 | 34 | 33 | 31 | 31 |
| Gelatine | 12 | 11 | 7 | 8 | 2 | 3 | 1 | 2 | 2 | 3 |
| Hydroxyapatite | 288 | 267 | 198 | 95 | 127 | 93 | 71 | 48 | 109 | 153 |
| CiteScore (2019) | 10.2 | 6.6 | 11.8 | 6.9 | 18.7 | 6.5 | 7.1 | 11.7 | 0.1 | 4.9 |
| Institutions | Number of Publications | Total Citation | H-Index | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Alg | Gel | Hyd | Alg | Gel | Hyd | Alg | Gel | Hyd | |
| Sichuan University | 61 | 4 | 167 | 1551 | 127 | 3755 | 26 | 3 | 36 |
| Chinese Academy of Science | 53 | 3 | 148 | 1524 | 165 | 4319 | 22 | 3 | 40 |
| Friedrich Alexander-Universitat Erlangen | 51 | 13 | 115 | 3027 | 304 | 4526 | 25 | 10 | 40 |
| Amirkabir University of Technology | 36 | 8 | 92 | 877 | 132 | 1972 | 19 | 6 | 30 |
| National University of Singapore | 23 | 2 | 82 | 946 | 46 | 3134 | 15 | 2 | 32 |
| Shanghai JiaoTong University | 26 | 4 | 77 | 551 | 60 | 1964 | 13 | 3 | 26 |
| Central South University | 12 | 0 | 67 | 313 | 0 | 1347 | 7 | 0 | 23 |
| Tehran University of Medical Science | 22 | 0 | 65 | 624 | 0 | 1486 | 12 | 0 | 26 |
| Universidade do Minho | 24 | 3 | 63 | 1549 | 83 | 2803 | 16 | 3 | 31 |
| South China University of Technology | 24 | 0 | 63 | 555 | 0 | 1421 | 14 | 0 | 26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussin, M.S.F.; Mohd Serah, A.; Azlan, K.A.; Abdullah, H.Z.; Idris, M.I.; Ghazali, I.; Mohd Shariff, A.H.; Huda, N.; Zakaria, A.A. A Bibliometric Analysis of the Global Trend of Using Alginate, Gelatine, and Hydroxyapatite for Bone Tissue Regeneration Applications. Polymers 2021, 13, 647. https://doi.org/10.3390/polym13040647
Hussin MSF, Mohd Serah A, Azlan KA, Abdullah HZ, Idris MI, Ghazali I, Mohd Shariff AH, Huda N, Zakaria AA. A Bibliometric Analysis of the Global Trend of Using Alginate, Gelatine, and Hydroxyapatite for Bone Tissue Regeneration Applications. Polymers. 2021; 13(4):647. https://doi.org/10.3390/polym13040647
Chicago/Turabian StyleHussin, Mohamed Saiful Firdaus, Aludin Mohd Serah, Khairul Azri Azlan, Hasan Zuhudi Abdullah, Maizlinda Izwana Idris, Ihwan Ghazali, Amir Husni Mohd Shariff, Nurul Huda, and Azrul Abidin Zakaria. 2021. "A Bibliometric Analysis of the Global Trend of Using Alginate, Gelatine, and Hydroxyapatite for Bone Tissue Regeneration Applications" Polymers 13, no. 4: 647. https://doi.org/10.3390/polym13040647
APA StyleHussin, M. S. F., Mohd Serah, A., Azlan, K. A., Abdullah, H. Z., Idris, M. I., Ghazali, I., Mohd Shariff, A. H., Huda, N., & Zakaria, A. A. (2021). A Bibliometric Analysis of the Global Trend of Using Alginate, Gelatine, and Hydroxyapatite for Bone Tissue Regeneration Applications. Polymers, 13(4), 647. https://doi.org/10.3390/polym13040647