Morphology and Crystallinity of Urea-Formaldehyde Resin Adhesives with Different Molar Ratios
Abstract
1. Introduction
2. Experiment
2.1. Material
2.2. Experiment Methods
2.2.1. Preparation of UF Resin
2.2.2. Performance of UF Resin
2.2.3. Polarizing Optical Microscope (POM)
2.2.4. X-Ray Diffraction (XRD)
2.2.5. Transmission Electron Microscope (TEM)
2.2.6. Scanning Electron Microscope (SEM)
2.2.7. Fourier Infrared Spectrometer (FT-IR)
3. Results and Discussion
3.1. Properties of UF Resin with Different Mole Ratios F/U
3.2. Crystalline Changes of UF Resin with Different Molar Ratios F/U
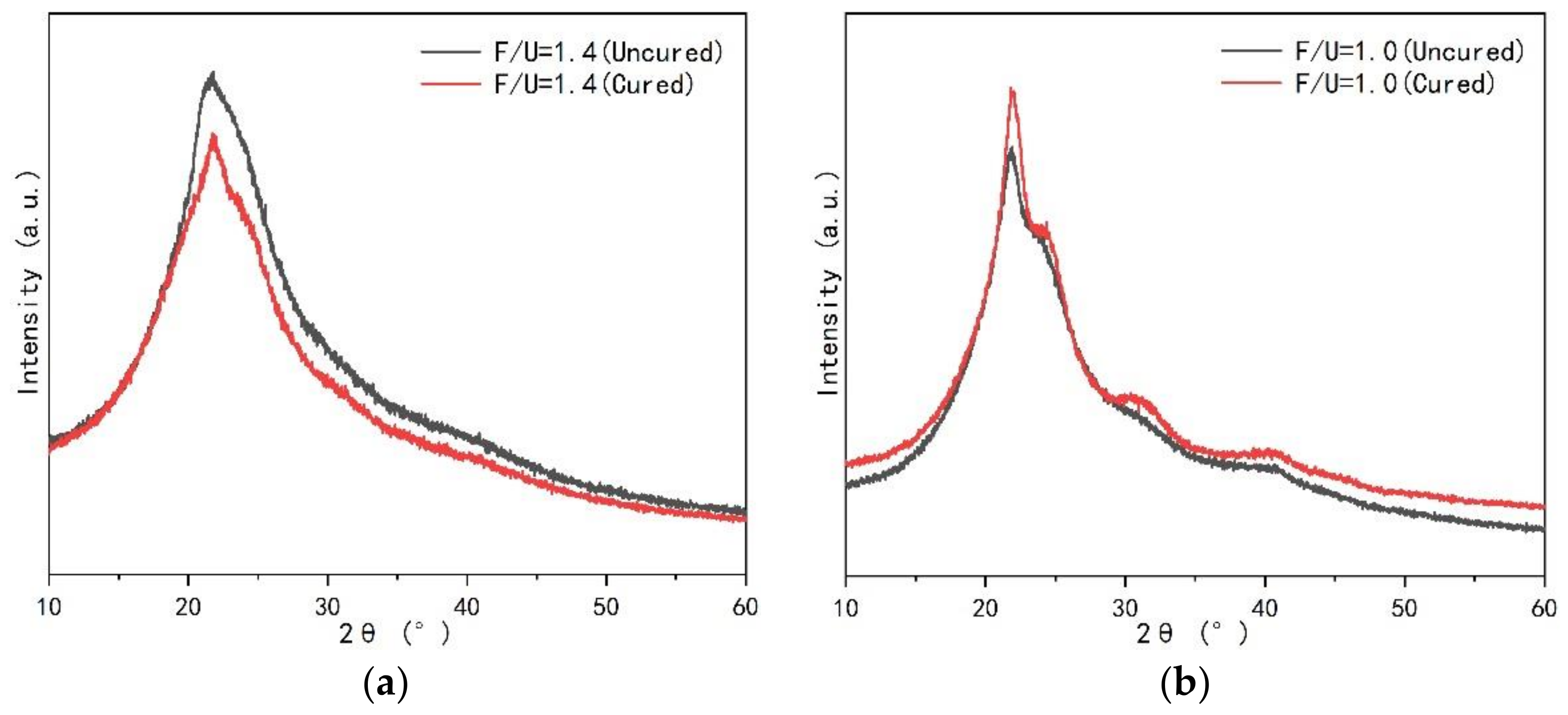
3.2.1. Crystalline Morphology of UF Resin
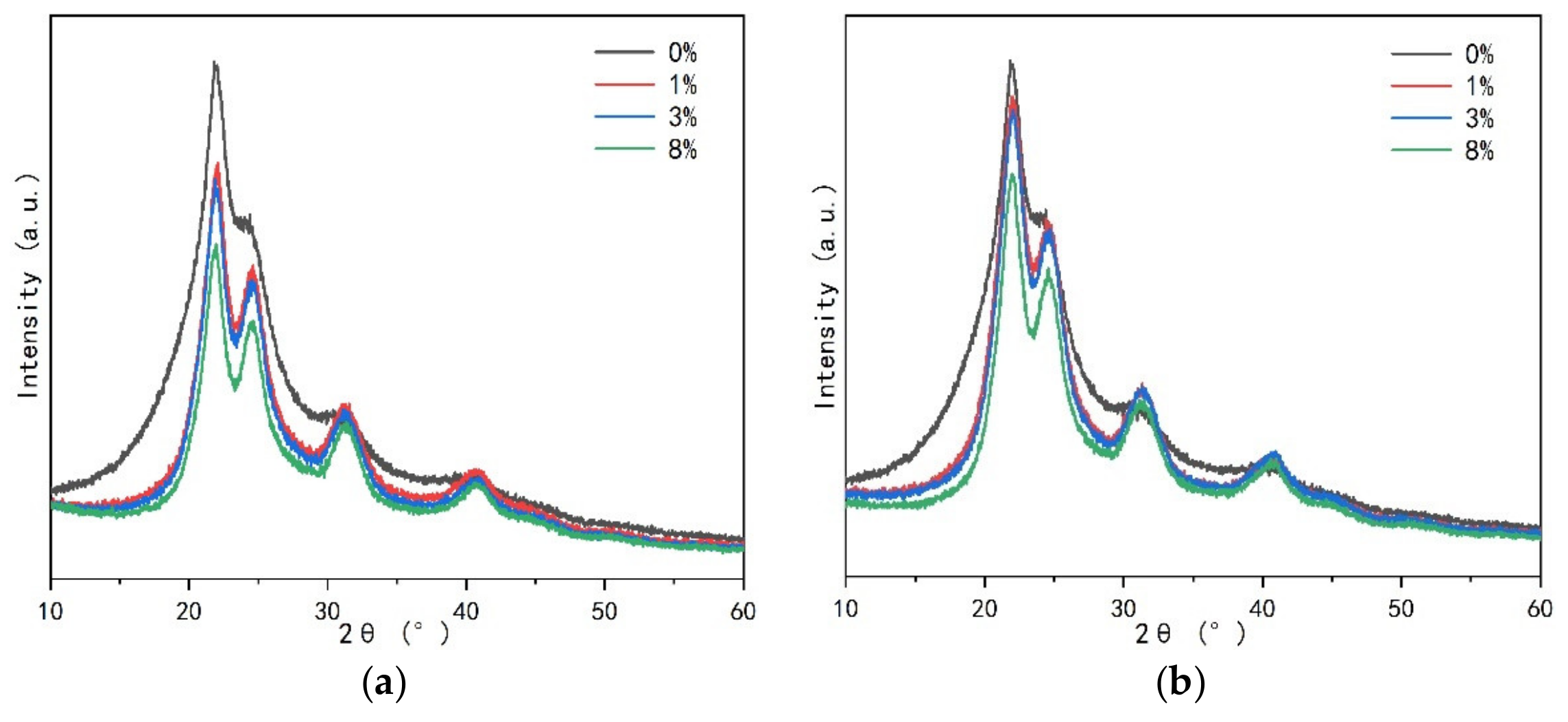
3.2.2. Crystalline Regions of UF Resin
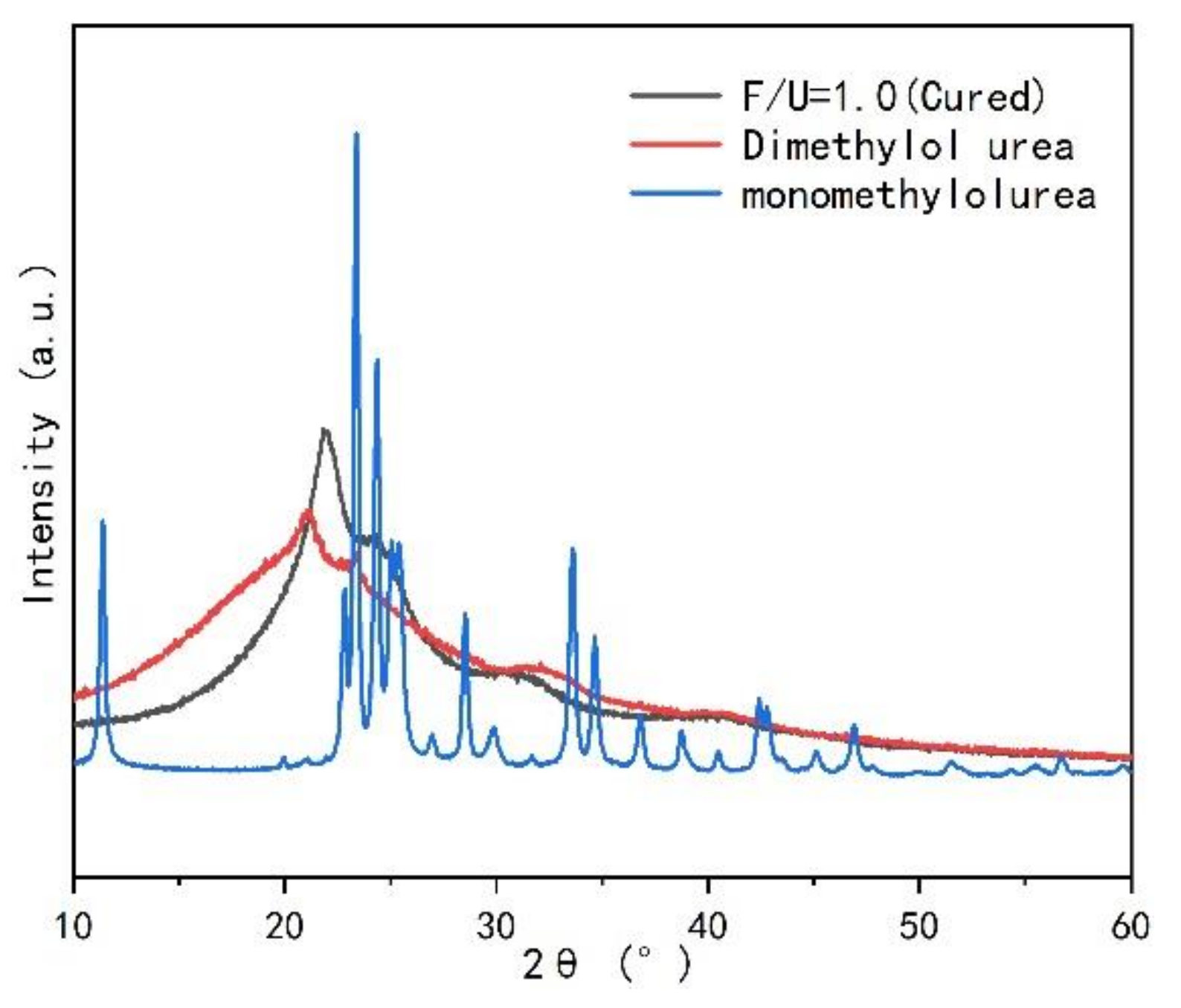
3.2.3. Crystal Characteristics of UF Resin
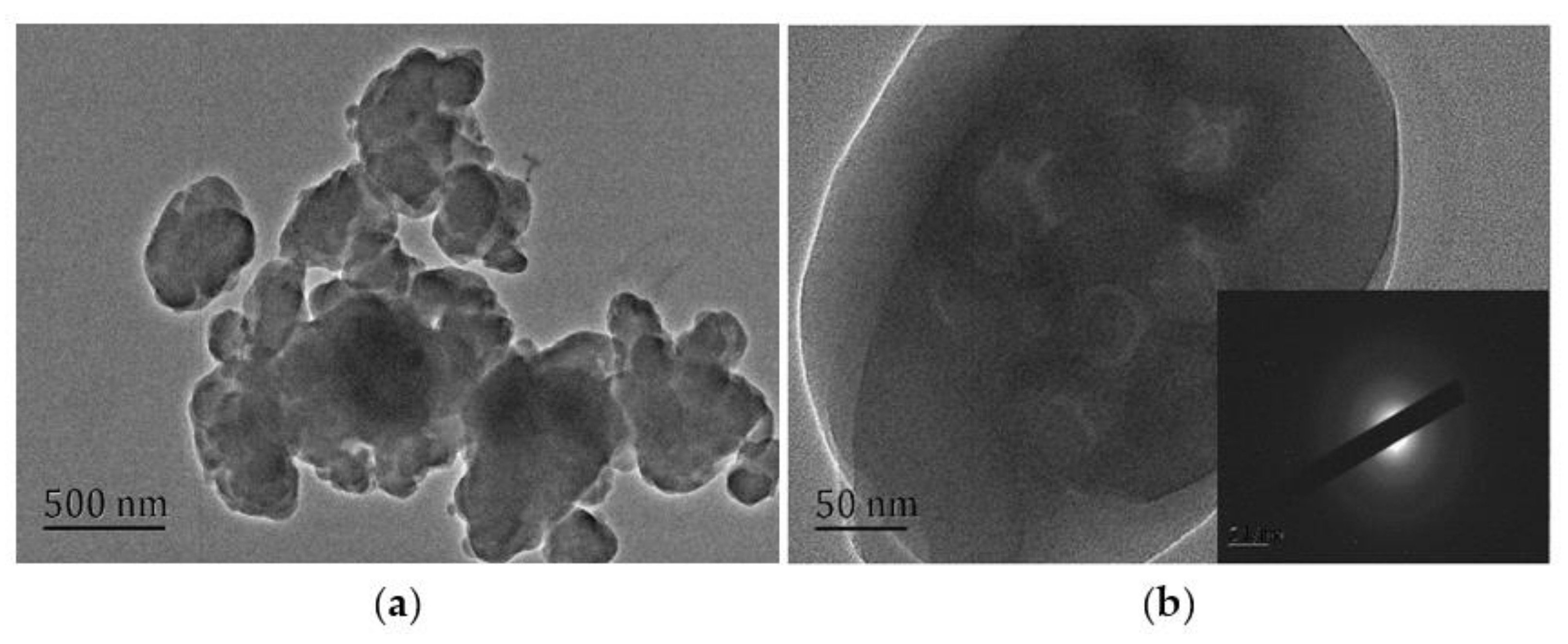
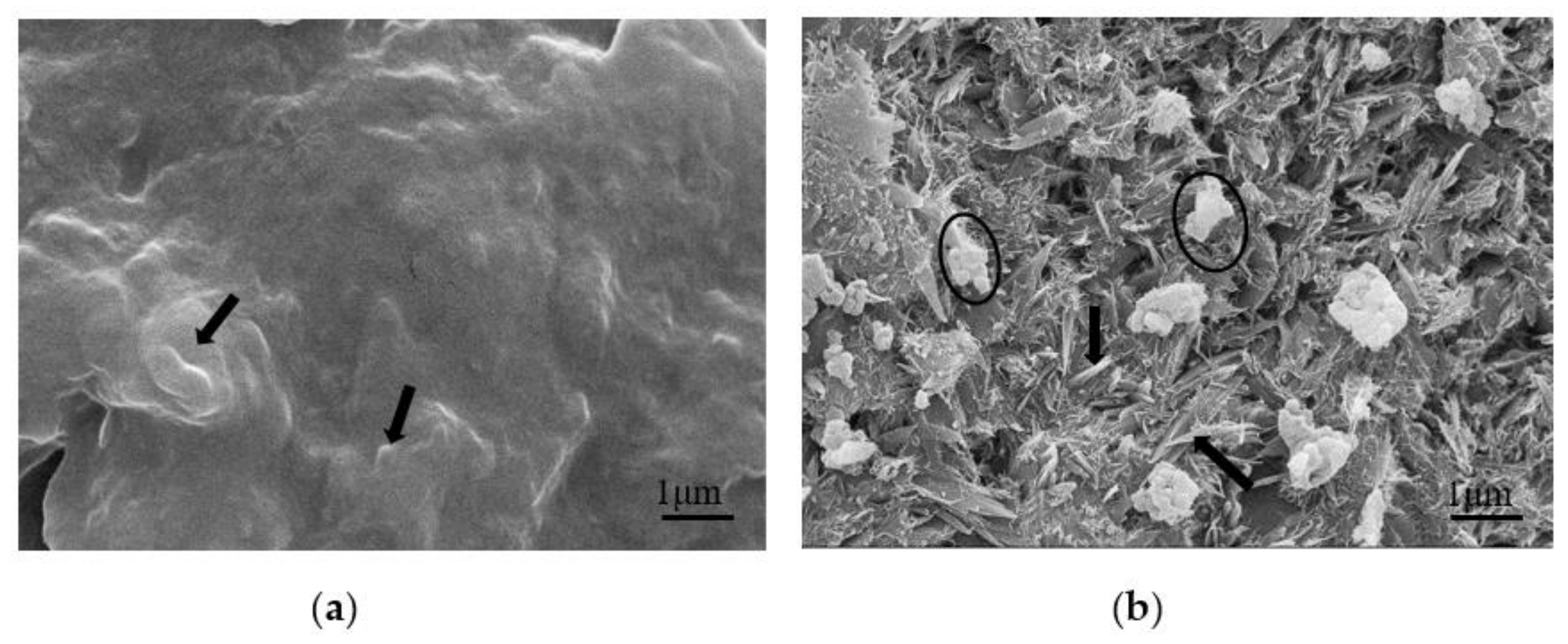
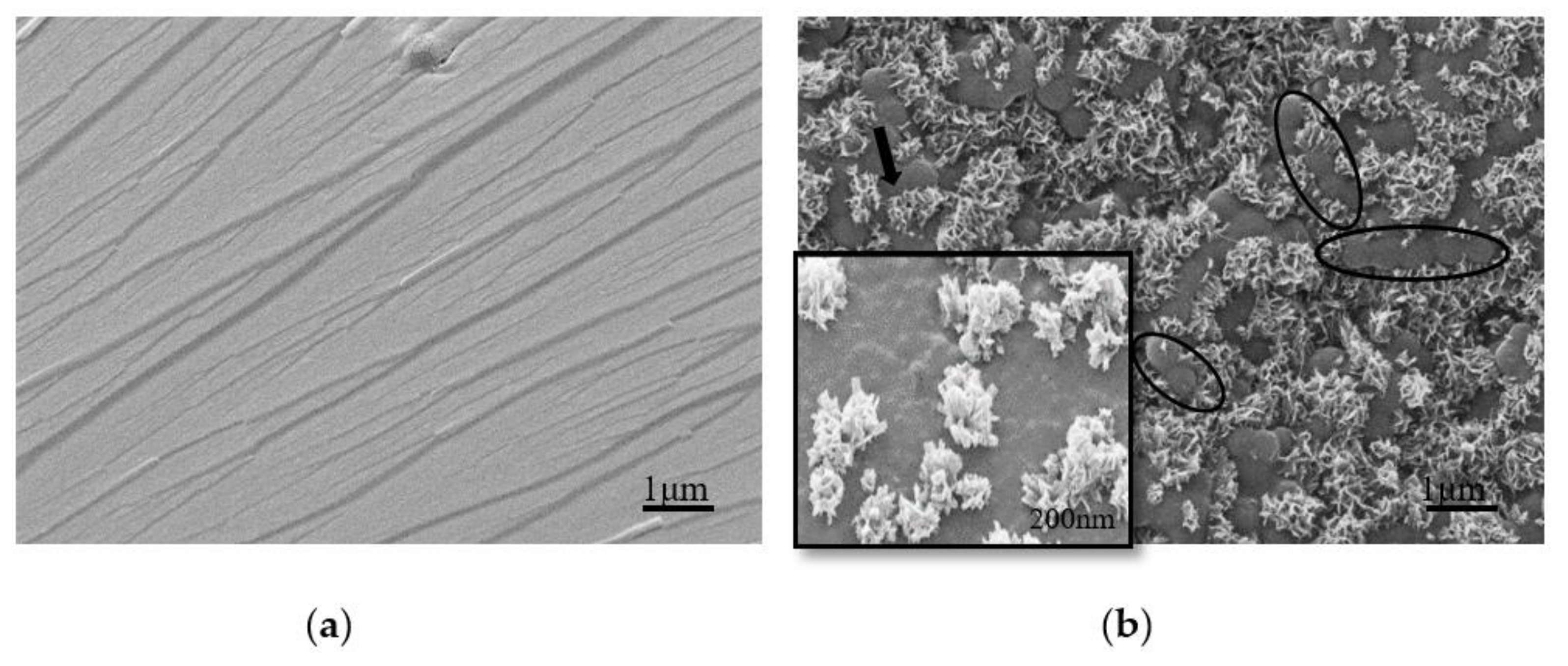
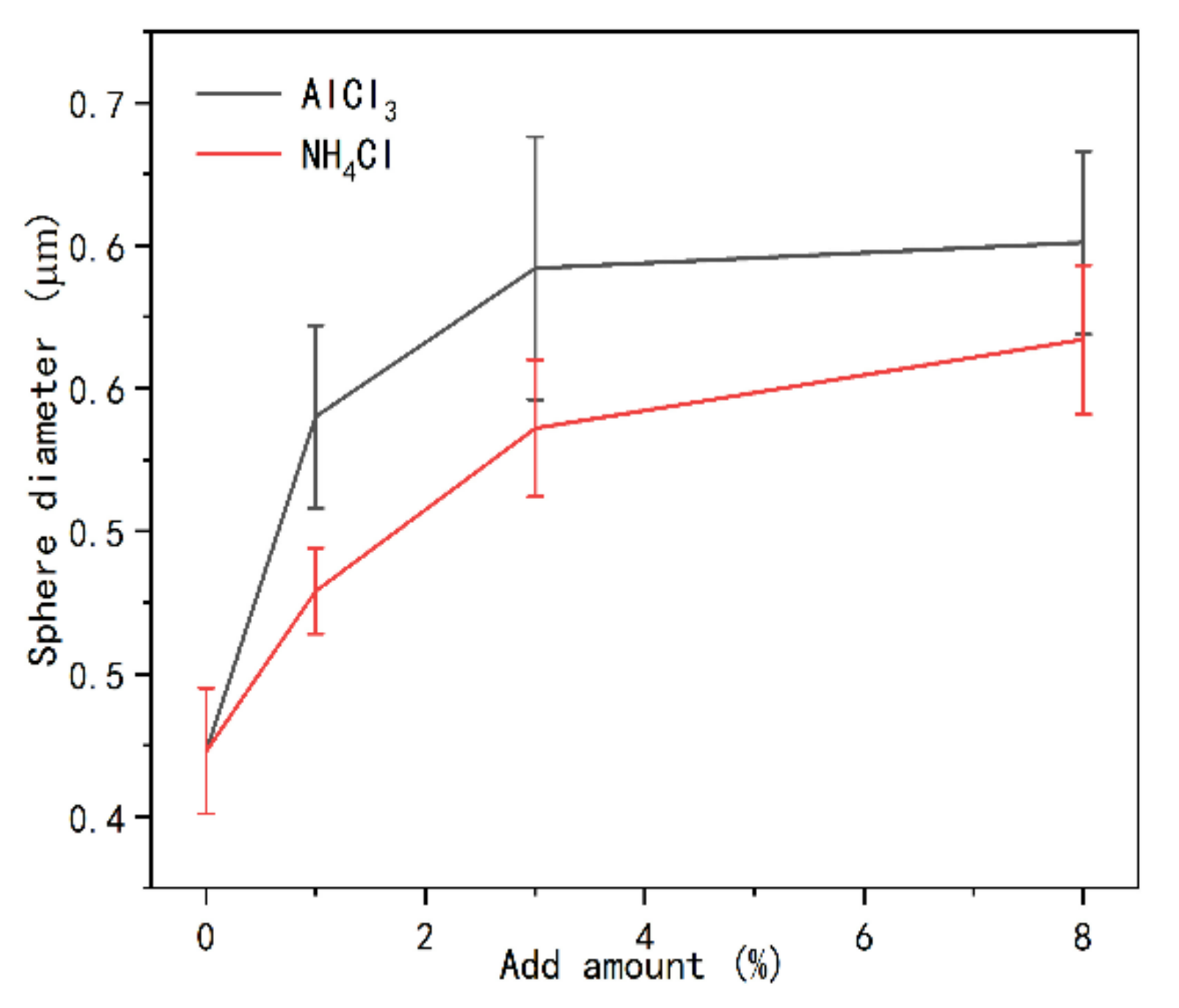
3.3. Morphological Changes of UF Resins with Different Molar Ratios F/U
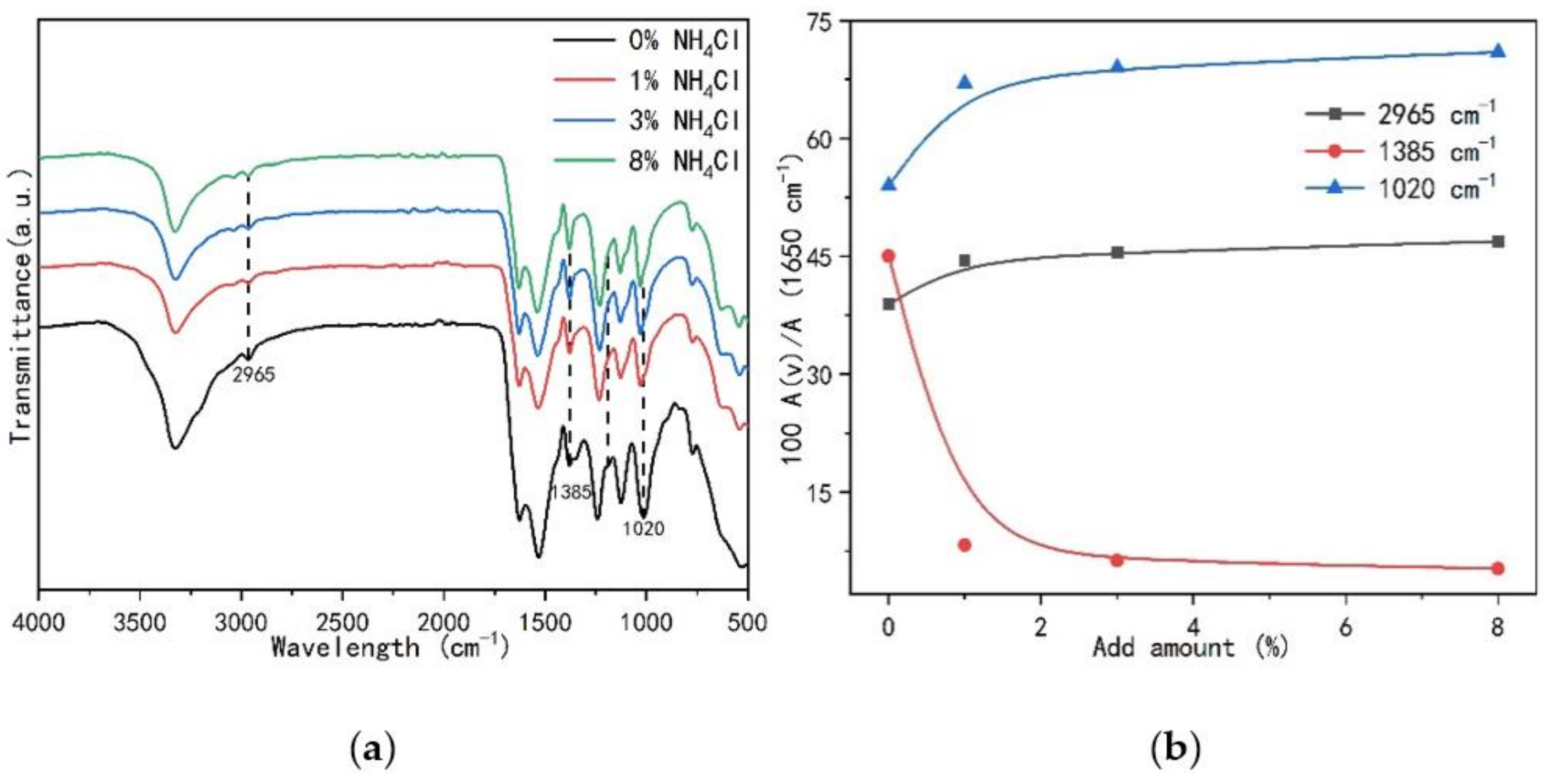
3.4. Structural Changes of UF Resins with Different Molar Ratios F/U
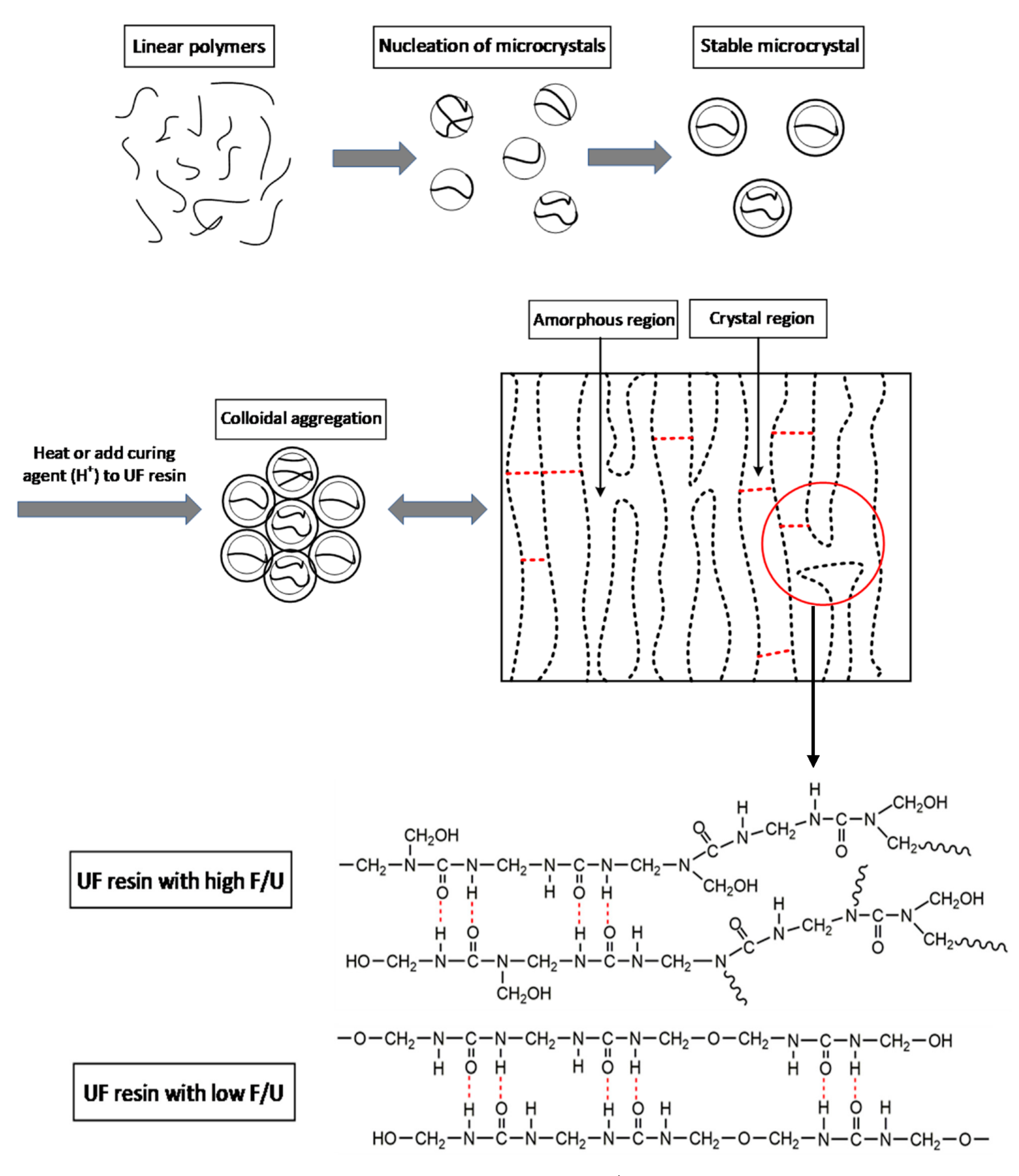
3.5. Crystallization Model of Urea–Formaldehyde Resin during Curing Process
4. Conclusions
- (1)
- The UF resin with high F/U (1.4) showed amorphous, while the resin with low F/U (1.0) showed crystallization region. The common crystallization peak and the position of additional peak did not change before and after curing, and at the same time, it did not change with the change of curing agent type and addition.
- (2)
- The cured UF resin with a low F/U (1.0) has the characteristics of polycrystal and body-centered cubic cell.
- (3)
- The change of crystallinity may be related to the appearance of spherical particles, which may be developed from flat crystals to a more advanced level, and the existence of curing agent significantly promotes the development of crystal region. Spherical particles are part of the crystal, or the nucleation site of the primary crystal.
- (4)
- The branched chain structure of UF resin with high F/U may affect the curing presentation mode of the resin, such as crystallinity, morphology and so on. The linear segment may affect the orderly development of the crystal region or the packing of spherical particles.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dorieh, A.; Mahmoodi, N.O.; Mamaghani, M.; Pizzi, A. Effect of different acids during the synthesis of urea-formaldehyde adhesives and the mechanical properties of medium-density fiberboards bonded with them. J. Appl. Polym. Sci. 2019, 136, 14. [Google Scholar] [CrossRef]
- Li, T.; Guo, X.; Liang, J. Competitive formation of the methylene and methylene ether bridges in the urea–formaldehyde reaction in alkaline solution: A combined experimental and theoretical study. Wood Sci. Technol. 2015, 49, 475–493. [Google Scholar] [CrossRef]
- Li, D.G. Urea Formaldehyde Resin, 1st ed.; Chemical Industry Press: Beijing, China, 2002; pp. 15–60. [Google Scholar]
- Lubis, M.A.R.; Park, B.D. Enhancing the performance of low molar ratio urea- formaldehyde resin adhesives via in-situ modification with intercalated nanoclay. J. Adhes. 2020, 32, 158. [Google Scholar] [CrossRef]
- Kim, W.J.; Terada, N.; Nomura, T. Effect of formaldehyde on the expression of adhesion molecules in nasal microvascular endothelial cells: The role of formaldehyde in the pathogenesis of sick building syndrome. Clin. Exp. Allergy 2010, 32, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Boran, S.; Usta, M.; Gümüşkaya, E. Decreasing formaldehyde emission from medium density fiberboard panels produced by adding different amine compounds to urea formaldehyde resin. Int. J. Adhes. Adhes. 2011, 31, 674–678. [Google Scholar] [CrossRef]
- Byung, D.P.; Causin, V. Crystallinity and domain size of cured urea–formaldehyde resin adhesives with different formaldehyde/urea mole ratios. Eur. Polym. J. 2013, 49, 532–537. [Google Scholar]
- Jeong, B.; Park, B.D. Effect of molecular weight of urea–formaldehyde resins on their cure kinetics, interphase, penetration into wood, and adhesion in bonding wood. Wood Sci. Technol. 2019, 53, 665–685. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Xiao, H.; Xu, S.F.; Liu, F.; Xie, J.L.; Qi, J.Q. Significant evaluation of three factors affecting the pre-curing behavior of urea formaldehyde resin: Temperature, solid content, and pH. J. Therm. Anal. Calorim. 2018, 133, 1463–1470. [Google Scholar] [CrossRef]
- Lubis, M.A.R.; Park, B.D. Analysis of the hydrolysates from cured and uncured urea-formaldehyde (UF) resins with two F/U mole ratios. Holzforschung 2018, 72, 759–768. [Google Scholar] [CrossRef]
- Pavlo, B.; Ján, S.; Gregory, N.; František, K.; Nataliya, B. Characteristics of beech bark and its effect on properties of UF adhesive and on bonding strength and formaldehyde emission of plywood panels. Eur. J. Wood Wood Prod. 2021, 2, 83. [Google Scholar]
- Dong, Y.H.; Gao, Q.; Zhang, Y. Study on Curing Behavior of Low Molar Ratio Urea-Formaldehyde Resins with Different Curing Agents. Adv Mat Res. 2011, 150, 965–968. [Google Scholar] [CrossRef]
- Nuryawan, A.; Singh, A.P.; Zanetti, M. Insights into the development of crystallinity in liquid urea-formaldehyde resins. Int. J. Adhes. Adhes. 2017, 72, 62–69. [Google Scholar] [CrossRef]
- Stuligross, J.; Koutsky, J.A. A Morphological Study of Urea-Formaldehyde Resins. J. Adhes. 1985, 18, 281–299. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Park, B.D. Determination of Crystallinity of Thermosetting Urea-Formaldehyde Resins Using Deconvolution Method. Macromol. Res. 2020, 28, 1598–1607. [Google Scholar] [CrossRef]
- Myers, G.E. Formaldehyde Emission from Particleboard and plywood Paneling-Measurement, Mechanism, and Product Standards. For. Prod. J. 1983, 33, 27–37. [Google Scholar]
- Park, B.D.; Jeong, H.W. Hydrolytic stability and crystallinity of cured urea-formaldehyde resin adhesives with different formaldehyde/urea mole ratios. Int. J. Adhes. Adhes 2011, 31, 524–529. [Google Scholar] [CrossRef]
- Pratt, T.J.; Johns, W.E.; Rammon, R.M. A Novel Concept on the Structure of Cured Urea Formaldehyde Resin. J. Adhes. 1985, 17, 275–295. [Google Scholar] [CrossRef]
- Motter, W.K. The Formation of the Colloidal Phase in Low Mole Ratio Urea-Formaldehyde Resins; Washington State University: Washington, DC, USA, 1990. [Google Scholar]
- Dunker, A.K.; John, W.E.; Rammon, R. Slightly Bizarre Protein Chemistry: Urea-Formaldehyde Resin from a Biochemical Perspective. J. Adhes. 1986, 19, 153–176. [Google Scholar] [CrossRef]
- Johns, W.E.; Dunker, A.K. Urea-Formaldehyde Resins, 2nd ed.; Addison-Wesley: Bergen, NJ, USA, 1986; pp. 126–148. [Google Scholar]
- Wibowo, E.S.; Park, B.D.; Causin, V. Hydrogen-Bond-Induced Crystallization in Low-Molar-Ratio Urea–Formaldehyde Resins during Synthesis. Ind. Eng. Chem. Res. 2020, 59, 29. [Google Scholar] [CrossRef]
- Pizzi, A. A molecular mechanics approach to the adhesion of urea-formaldehyde resins to cellulose. Part 2. Amorphous vs. crystalline Cellulose I. J. Adhes. Sci. Technol. 1990, 4, 589–595. [Google Scholar] [CrossRef]
- Dazmiri, M.K.; Kiamahalleh, M.V.; Dorieh, A. Effect of the initial F/U molar ratio in urea-formaldehyde resins synthesis and its influence on the performance of medium density fiberboard bonded with them. Int. J. Adhes. Adhes. 2019, 95, 1024. [Google Scholar]
- Levendis, D.; Pizzi, A.; Ferg, E. The Correlation of Strength and Formaldehyde Emission with the Crystalline/Amorphous Structure of UF Resins. Holzforschung 1992, 46, 263–269. [Google Scholar] [CrossRef]
- Wang, H.; Cao, M.; Li, T.H.; Yang, L.; Duan, Z.G.; Zhou, X.J.; Du, G.B. Characterization of the Low Molar Ratio Urea-Formaldehyde Resin with 13C-NMR and ESI-MS: Negative Effects of the Post-Added Urea on the Urea-Formaldehyde Polymers. Polymers 2018, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Ferg, E.E.; Pizzi, A.; Levendis, D.C. 13C NMR analysis method for urea–formaldehyde resin strength and formaldehyde emission. J. Appl. Polym. Sci. 2010, 50, 907–915. [Google Scholar] [CrossRef]
- Singh, A.P.; Causin, V.; Nuryawan, A. Morphological, chemical and crystalline features of urea–formaldehyde resin cured in contact with wood. Eur. Polym. J. 2014, 56, 185–193. [Google Scholar] [CrossRef]
- Fan, D.B.; Li, J.Z.; Mao, A. Curing Characteristics of Low Molar Ratio Urea-Formaldehyde Resins. J. Adhes. Interface. 2006, 7, 45–52. [Google Scholar]
- Liu, C.; Luo, J.; Li, X. Effects of Compounded Curing Agents on Properties and Performance of Urea Formaldehyde Resin. J. Polym. Environ. 2018, 26, 158–165. [Google Scholar] [CrossRef]
- Sun, Q.N.; Hse, C.Y.; Todd, F.S. Effect of different catalysts on urea–formaldehyde resin synthesis. J. Appl. Polym. Sci. 2014, 131, 160. [Google Scholar] [CrossRef]
- Park, B.D.; Singh, A.P.; Nuryawan, A. MRT Letter: High resolution SEM imaging of nano-architecture of cured urea-formaldehyde resin using plasma coating of osmium. Microsc. Res. Tech. 2013, 76, 1108–1111. [Google Scholar] [CrossRef]
- Despres, A.; Pizzi, A. Colloidal aggregation of aminoplastic polycondensation resins: Urea–formaldehyde versus melamine–formaldehyde and melamine–urea–formaldehyde resins. J. Appl. Polym. Sci. 2010, 100, 1406–1412. [Google Scholar] [CrossRef]
- Li, A.P.; Kan, C.Y.; Du, Y. Research on the unstable structure of urea-formaldehyde resin under acidic conditions. Chem. J. Chin. Univ. 2006, 27, 204–206. [Google Scholar]
- Park, B.-D.; Jeong, H.-W.; Lee, S.M. Morphology and chemical elements detection of cured urea–formaldehyde resins. J. Appl. Polym. Sci. 2011, 120, 1475–1482. [Google Scholar]
- Ferra, J.M.M.; Adélio, M.M.; Costa, M.R.N. A study on the colloidal nature of urea-formaldehyde resins and its relation with adhesive performance. J. Appl. Polym. Sci. 2010, 118, 1956–1968. [Google Scholar]
- George, E.M. Investigation of urea–formaldehyde polymer cure by infrared. J. Appl. Polym. Sci. 2010, 26, 747–764. [Google Scholar]






| F/U Mole Ratio | Solid Content (%) | Viscosity (mPa.s) | Curing Time (s) | Free Formaldehyde (%) |
|---|---|---|---|---|
| 1.4 | 52.0 | 42 | 81 | 0.45 |
| 1.0 | 55.6 | 48 | 122 | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhang, Y. Morphology and Crystallinity of Urea-Formaldehyde Resin Adhesives with Different Molar Ratios. Polymers 2021, 13, 673. https://doi.org/10.3390/polym13050673
Li J, Zhang Y. Morphology and Crystallinity of Urea-Formaldehyde Resin Adhesives with Different Molar Ratios. Polymers. 2021; 13(5):673. https://doi.org/10.3390/polym13050673
Chicago/Turabian StyleLi, Ji, and Yifu Zhang. 2021. "Morphology and Crystallinity of Urea-Formaldehyde Resin Adhesives with Different Molar Ratios" Polymers 13, no. 5: 673. https://doi.org/10.3390/polym13050673
APA StyleLi, J., & Zhang, Y. (2021). Morphology and Crystallinity of Urea-Formaldehyde Resin Adhesives with Different Molar Ratios. Polymers, 13(5), 673. https://doi.org/10.3390/polym13050673







