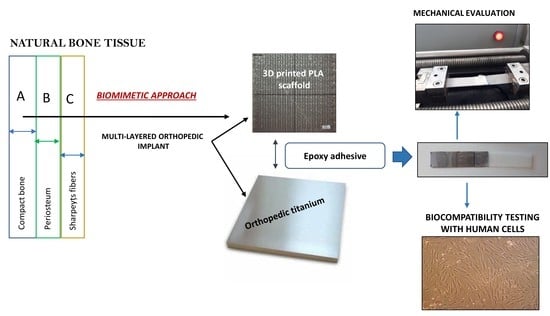
Gradient 3D Printed PLA Scaffolds on Biomedical Titanium: Mechanical Evaluation and Biocompatibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Manufacturing Methods
2.2.1. Adjustment of Key Printing Parameters
2.2.2. Titanium Functionalization
2.2.3. Single Lap Joints
2.3. Characterization
2.3.1. Optical Images and Pore Volume
- (1)
- Filament diameter: 1.75 mm
- (2)
- Filament used to 3D print the scaffold (h): 23.75 mm
- (3)
- The volume of the printed scaffold and the theoretical volume are:Vprinted scaffold: π × r2 × h = 57.1 mm3, where π is 3.14; r is the radius and h the heightVtheoretical: 85 mm3 (is a solid scaffold 10 × 10 × 0.85)
- (4)
- The calculated porosity is thereof calculated:Vtheoretical − Vprinted scaffold = Vporosity = 27.9 mm3Vporosity (%) = 32.82%
2.3.2. Mechanical Evaluation
2.3.3. Evaluation of Substrates Biocompatibility
3. Results
3.1. Microscopy and Pore Volume
3.2. Mechanical Evaluation: Adjustment of Printing Parameters
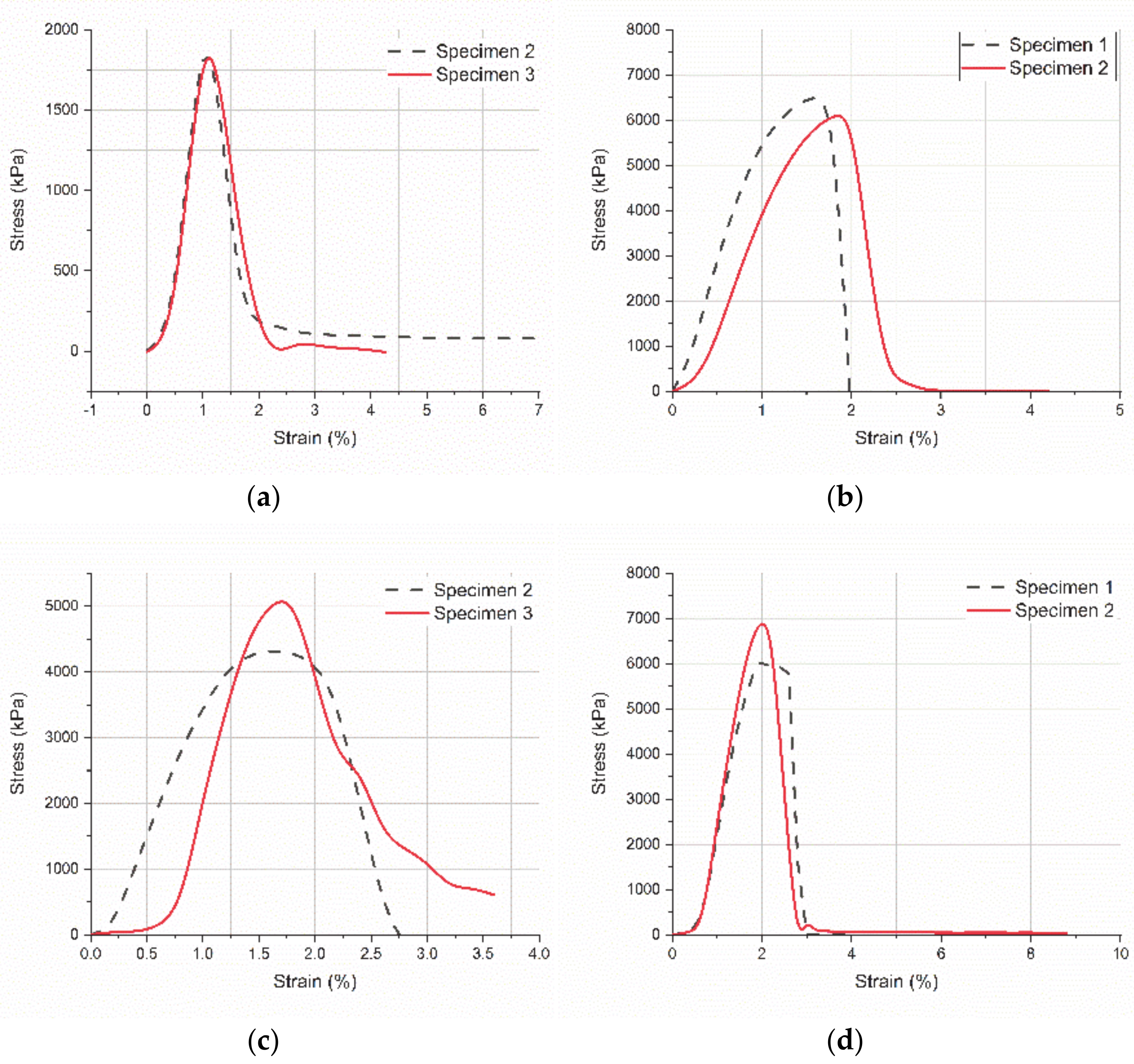
3.3. Micro-Tensile Testing of Scaffolds
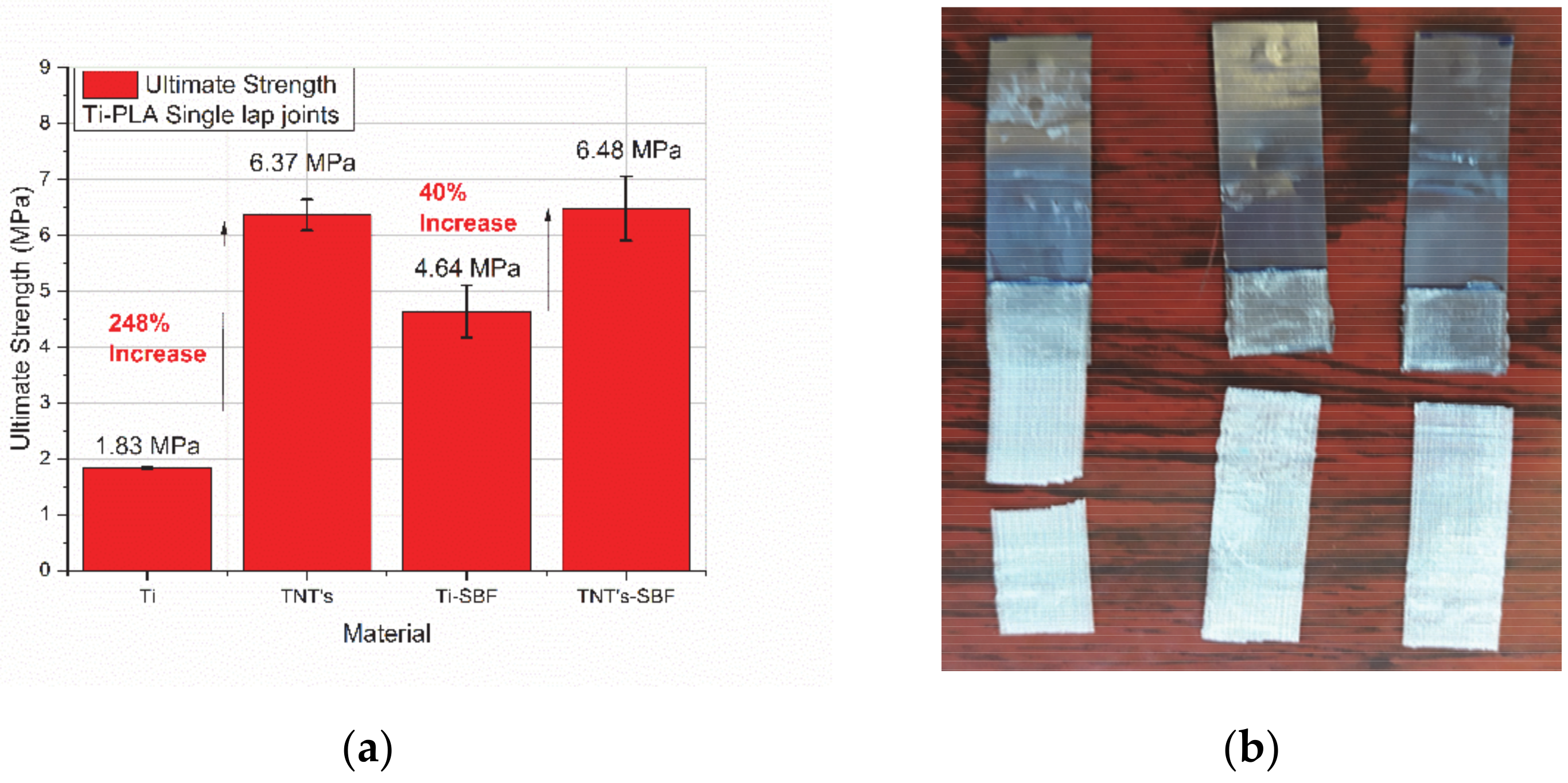
3.4. Micro-Tensile Testing of Titanium-PLA Single Lap Joints
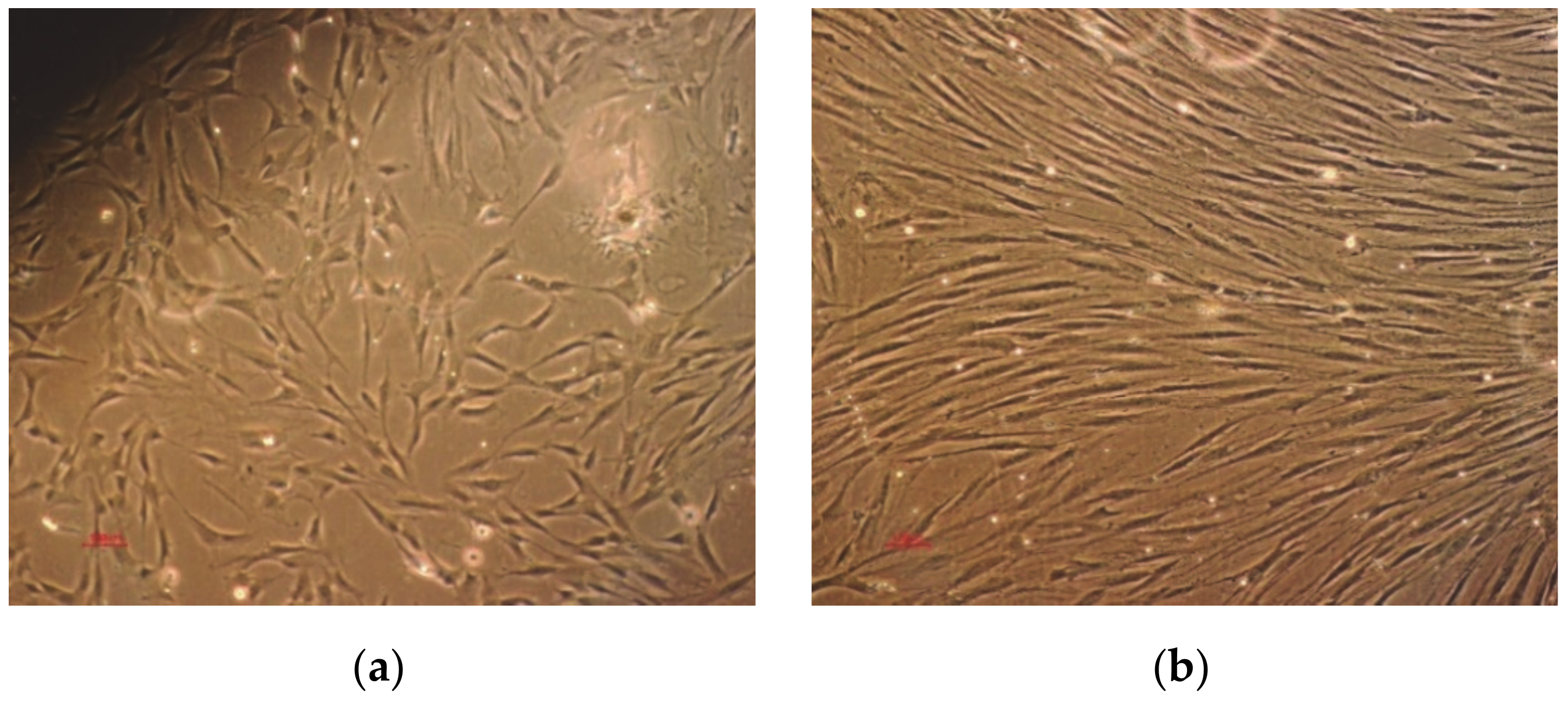
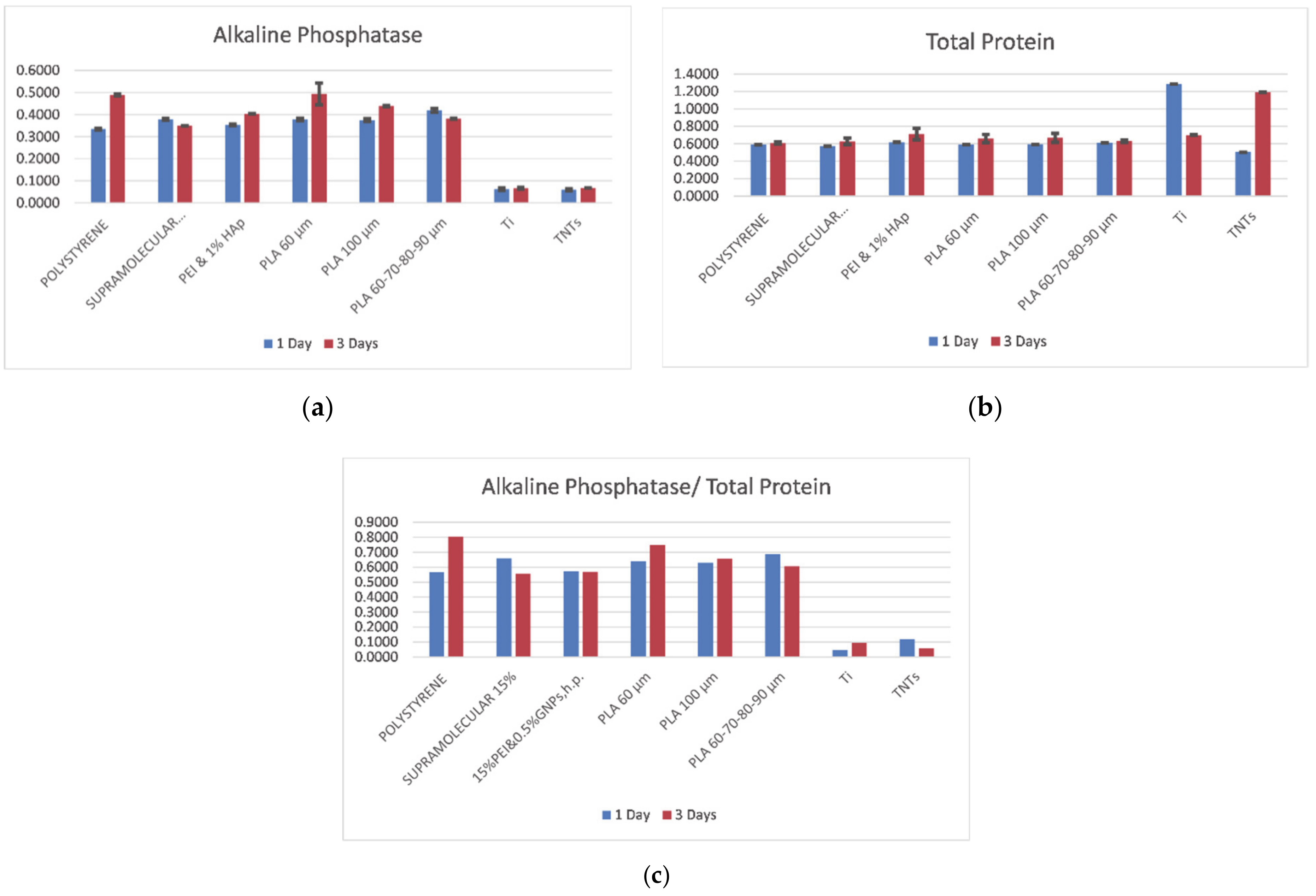
3.5. Biocompatibility
4. Conclusions
- The most influent parameters in the 3D printing manufacturing process were the temperature of the printing components (nozzle, chamber, bed), and the printing speed, which should be low.
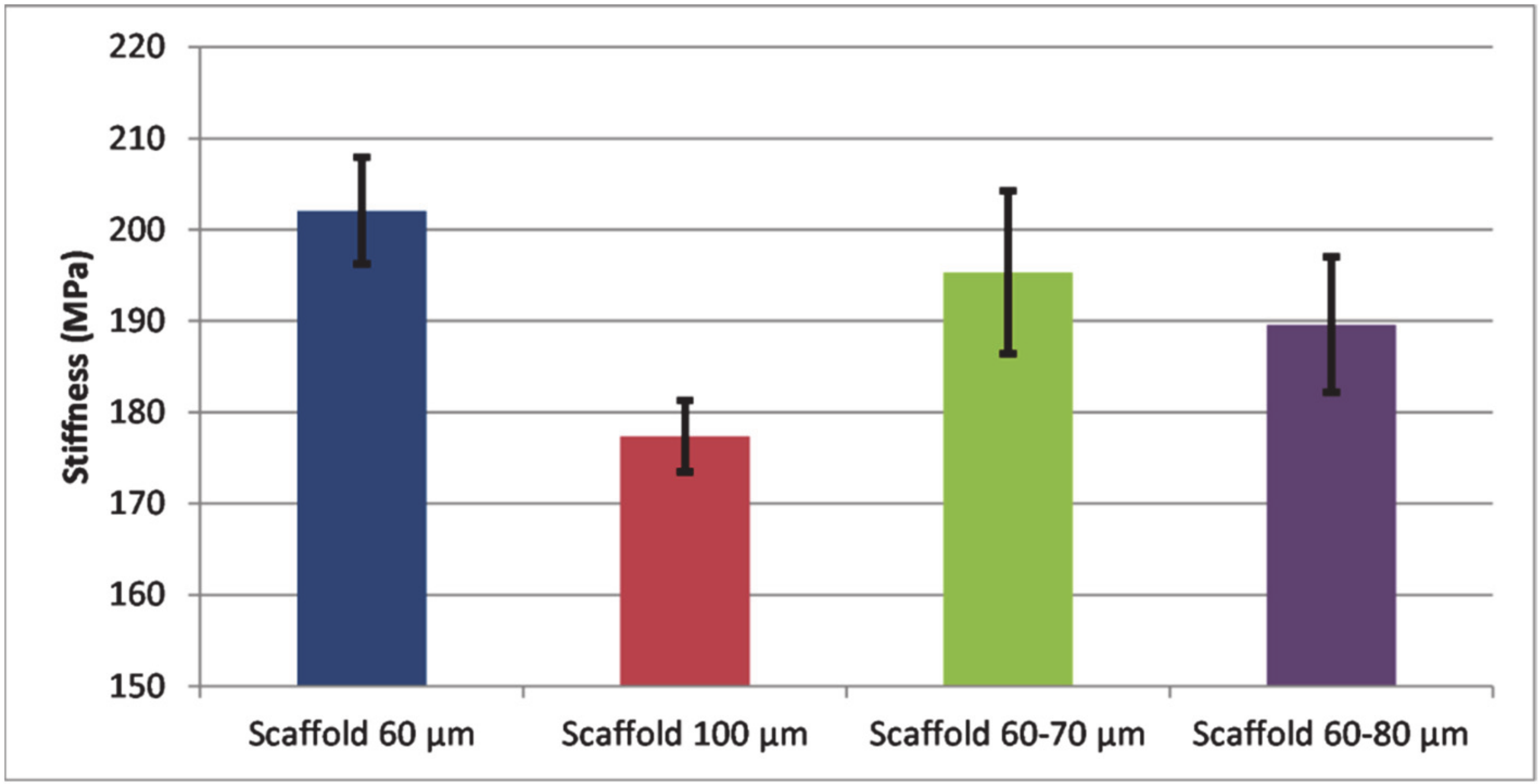
- Single and double-layered scaffolds (60, 100, 60–70, and 60–80 μm side lengths) were tested in micro-tensile mode. The highest stiffness values were detected in single-layered samples with smaller pores (60 μm side length) due to an increased mass of their architecture. The study revealed that in terms of mechanical performance, single-layered scaffolds with small pore dimeter/side length present a more stable architecture for orthopedic implantology applications, where materials are subjected to complex loadings.
- Scaffolds with 60–70 μm side lengths showed overall balance, with a moderate porosity and an improved stiffness (34% porosity and 190 MPa stiffness).
- Small pore dimensions are preferred to manufacture synthetic orthopedic components. On the other hand, higher pore volume allows liquid diffusibility which is demanded in applications such as drug delivery systems. Scaffolds with larger pores or with gradient porosity along their thickness remain of interest when multi-functionality is targeted.
- 3D printed PLA scaffolds with 100 μm side length of the pores were added on pure titanium and on TNTs (titania nanotubes) by using a silicone-based adhesive. Titanium-PLA single lap joints were tested in micro-tensile mode to observe the general performance and to measure the adhesion strength of the overlap area, before and after immersion in a simulated body fluid. A 248% increase in the ultimate strength of the dry joints was found with the addition of TNTs on titanium surface and a 40% increase of the ultimate strength was found for the same specimens after 8 h immersion in SBF.
- The study showed that the adhesion of the coating/ scaffold to the medical titanium depends very much on the surface treatment of the metal and that highly organized, nano-level structures result in improved adhesion strength between the metal and the plastic coating.
- A preliminary biocompatibility study indicated that the 3D printed PLA scaffolds induced positive behavior in primary osteoblasts cells comparing to all the other tested substrates (tissue culture polystyrene, metals, electrospun membranes). It was found that primary osteoblasts prefer smaller pore diameter/ side lengths in a single-layered structure, for short incubation periods. A 30% to 50% increase of expressed ALP was found in cells seeded on plastic substrates comparing to the metallic ones. This proves that the addition of plastic coatings on metallic implants is highly recommended.
- In between all substrates, PLA with 60 μm pore side length promotes ALP release by cells, especially after 3 days of incubation. The superiority of this structure may be explained through the existence of an extended surface area for osteoblasts to adhere; at the same time, the small pore architecture allows cells to migrate and create tissue-networks along scaffold’ thickness. On the other hand, small dimeters may limit cell proliferation for long incubation periods.
- Conventional titanium generally entraps cells and limits their development, which has been confirmed through the present investigation. The optimum solution is, therefore, the addition of a plastic scaffold on the metallic prosthesis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, H.; Suonan, A.; Zhou, J.; Yuan, Q.; Liu, L.; Zhao, X.; Lou, X.; Yang, C.; Li, D.; Zhang, Y. PEEK (Polyether-ether-ketone) and its composite materials in orthopedic implantation. Arab. J. Chem. 2021, 14, 102977. [Google Scholar] [CrossRef]
- Kumta, P.N.; Yu, G.; Roy, A. Biomimetic Plywood Motifs for Bone Tissue Engineering. U.S. Patent 2021/0000602 A1, 7 January 2021. [Google Scholar]
- Papenburg, B.J.; Liu, J.; Higuera, G.A.; Barradas, A.M.C.; de Boer, J.; van Blitterswijk, C.A.; Wessling, M.; Stamatialis, D. Development and analysis of multi-layer scaffolds for tissue engineering. Biomaterials 2009, 30, 6228–6239. [Google Scholar] [CrossRef]
- Sabino, R.M.; Mondini, G.; Kipper, M.G.; Martins, A.F.; Popat, K.C. Tanfloc/heparin polyelectrolyte multilayers improve osteogenic differentiation of adipose-derived stem cells on titania nanotube surfaces. Carbohydrate Polymers 2021, 251, 117079. [Google Scholar] [CrossRef]
- Taherian, M.H.; Rezazadeh, M.; Taji, A. Optimum Surface Roughness for Titanium Coated PEEK Produced by Electron Beam PVD for Orthopedic Applications. Mater. Technol. 2021, 1–14. [Google Scholar] [CrossRef]
- Seidi, A.; Ramalingam, M.; Elloumi-Hannachi, I.; Ostrovidov, S.; Khademhosseini, A. Gradient biomaterials for soft-to-hard interface tissue engineering. Acta Biomater. 2011, 7, 1441–1451. [Google Scholar] [CrossRef]
- Deliormanlı, A.M.; Türk, M.; Atmaca, H. Preparation and characterization of PCL-coated porous hydroxyapatite scaffolds in the presence of MWCNTs and graphene for orthopedic applications. J. Porous Mater. 2019, 26, 247–259. [Google Scholar] [CrossRef]
- Kiran, A.S.K.; Kizhakeyild, A.; Ramalingam, R.; Vermad, N.K.; Lakshminarayanane, R.; Kumara, T.S.S.; Dobleb, M.; Ramakrishna, S. Drug loaded electrospun polymer/ceramic composite nanofibrous coatings on titanium for implant related infections. Ceram. Int. 2019, 45, 18710–18720. [Google Scholar] [CrossRef]
- Al Aboody, S.M. Electrospun fabrication and direct coating of bio-degradable fibrous composite on orthopedic Titanium implant: Synthesis and Characterizations. Mater. Res. Express 2021, 8, 015307. [Google Scholar] [CrossRef]
- Anjos, S.D.; Mavropoulos, E.; Alves, G.G.; Costa, A.M.; Hausen, M.A.; Spiegel, C.N.; Longuinho, M.M.; Mir, M.; Granjeiro, J.M.; Rossi, A.M. Impact of crystallinity and crystal size of nanostructured carbonated hydroxyapatite on pre-osteoblast in vitro biocompatibility. J. Biomed. Mater. Res. 2019, 107, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Song, K. In Vitro Fabrication and Biocompatibility Assay of a Biomimetic Osteoblastic Niche. Appl. Biochem. Biotechnol. 2019, 189, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Liu, X.; Ma, P.X. Polymeric Scaffolds for Bone Tissue Engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Song, W.; Yee, K.; Lee, K.-Y.; Tagarielli, V.L. Measurements of the mechanical response of unidirectional 3D-printed PLA. Mater. Des. 2017, 123, 154–164. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A. Integrated ternary bionanocomposites with superior mechanical performance via the synergistic role of graphene and plasma treated carbon nanotubes. Compos. B. Eng. 2019, 168, 550–559. [Google Scholar] [CrossRef]
- Aleš, G.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A.; et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11. [Google Scholar] [CrossRef]
- Papanicolaou, G.C.; Portan, D.V.; Petropoulos, G.N.; Kontaxis, L.C. Effect of TiO2 Nanotubes Developed on Pure Titanium Substrates on the Mechanical Performance of Titanium-Titanium Single-Lap Adhesive Joints. Cienc. Tecnol. Mater. 2016, 28, 130–137. [Google Scholar]
- Portan, D.V.; Papaefthymiou, K.; Arvanita, E.; Jiga, G.; Papanicolaou, G.C. A combined statistical and microscopic analysis of TiO2 nanotubes synthesized under different electrochemical anodizing conditions. J. Mater. Sci. 2012, 47, 4696–4705. [Google Scholar] [CrossRef]
- Kostopoulos, V.; Kotrotsos, A.; Fouriki, K.; Kalarakis, A.; Portan, D.V. Fabrication and Characterization of Polyetherimide Electrospun Scaffolds Modified with Graphene Nano-Platelets and Hydroxyapatite Nano-Particles. Int. J. Mol. Sci. 2020, 21, 583. [Google Scholar] [CrossRef]
- Portan, D.V.; Kroustalli, A.A.; Deligianni, D.D.; Papanicolaou, G.C. On the biocompatibility between TiO2 nanotubes layer and human osteoblasts. J. Biomed. Mater. Res. A 2012, 100A, 2546–2553. [Google Scholar] [CrossRef]
- Feier, A.M.; Manu, D.R.; Strnad, G.; Dobreanu, M.; Rusu, O.M.; Portan, D.; Bataga, T. A Step Forward Standardization of Biocompatibility Testing on Tissue Culture Polystyrene. Mater. Plast. 2018, 55, 303–307. [Google Scholar] [CrossRef]
- Entezari, A.; Roohani, I.; Li, G.; Dunstan, C.R.; Rognon, P.; Li, Q.; Jiang, X.; Zreiqat, H.; Li, G. Architectural Design of 3D Printed Scaffolds Controls the Volume and Functionality of Newly Formed Bone. Adv. Healthc. Mater. 2019, 8, 1801353. [Google Scholar] [CrossRef]
- Decuir, F.; Phelan, K.; Hollins, B.C. 32nd Southern Biomedical Engineering Conference (SBEC). 32nd Southern Biomedical Engineering Conference (SBEC), Shreveport, LA, USA, 11–13 March 2016. [Google Scholar] [CrossRef]
- Velioglu, Z.B.; Pulat, D.; Demirbakan, B.; Ozcan, B.; Bayrak, E.; Erisken, C. 3D-printed poly(lactic acid) scaffolds for trabecular bone repair and regeneration: Scaffold and native bone characterization. Connect. Tissue Res. 2019, 60, 274–282. [Google Scholar] [CrossRef]
- Zhao, H.; Li, L.; Ding, S.; Liu, C.; Ai, J. Effect of porous structure and pore size on mechanical strength of 3D-printed comby scaffolds. Mater. Lett. 2018, 223, 21–24. [Google Scholar] [CrossRef]
- Papanicolaou, G.C.; Kosmidou, T.V.; Vatalis, A.S.; Delides, C.C. Water absorption mechanism and some anomalous effects on the mechanical and viscoelastic behavior of an epoxy system. J. Appl. Polym. Sci. 2006, 99, 1328–1339. [Google Scholar] [CrossRef]
- Hua, W.; Kar, P.; Roy, P.; Bu, L.; Shoute, L.C.T.; Kumar, P.; Shankar, K. Resistance of Superhydrophobic Surface-Functionalized TiO2 Nanotubes to Corrosion and Intense Cavitation. Nanomaterials 2018, 8, 783. [Google Scholar] [CrossRef]
- Liu, G.; Du, K.; Wang, K. Surface wettability of TiO2 nanotube arrays prepared by electrochemical anodization. Appl. Surf. Sci. 2016, 388, 313–320. [Google Scholar] [CrossRef]
- Sappia, L.; Felice, B.; Sanchez, M.A.; Martí, M.; Madrida, R.; Pividori, M.I. Electrochemical sensor for alkaline phosphatase as biomarker for clinical and in vitro applications. Sens. Actuators B Chem. 2019, 281, 221–228. [Google Scholar] [CrossRef]
- He, P.; Wang, X.L.; Ning, C.Y. Regulation of osteoblast functions on titanium surfaces with different micro/nanotopographies and compositions. Sci. China Technol. Sci. 2019, 62, 559–568. [Google Scholar] [CrossRef]
- Portan, D.V.; Deligianni, D.D.; Papanicolaou, G.C.; Kostopoulos, V.; Psarras, G.C.; Tyllianakis, M. Combined Optimized Effect of a Highly Self-Organized Nanosubstrate and an Electric Field on Osteoblast Bone Cells Activity. Biomed. Res. Int. 2019, 7574635. [Google Scholar] [CrossRef]
- Portan, D.V.; Deligianni, D.D.; Deligianni, K.; Kroustalli, A.A.; Tyllianakis, M.; Papanicolaou, G.C. Modeling of the interaction between osteoblasts and biocompatible substrates as a function of adhesion strength. J. Biomed. Mater. Res. A 2018, 106, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Pamula, E.; Filová, E.; Bacáková, L.; Lisa, V.; Adamczyk, D. Resorbable polymeric scaffolds for bone tissue engineering: The influence of their microstructure on the growth of human osteoblast-like MG 63 cells. J. Biomed. Mater. Res. A 2009, 89, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Katagiri, H.; Kono, M.; Schaller, B.; Iizuka, T.; Safi, A.F. The impact of the size of bone substitute granules on macrophage and osteoblast behaviors in vitro. Clin. Oral Investig. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zamani, Y.; Amoabediny, G.; Mohammadi, J.; Zandieh-Doulabi, B.; Klein-Nulend, J.; Helder, M.N. Increased Osteogenic Potential of Pre-Osteoblasts on Three-Dimensional Printed Scaffolds Compared to Porous Scaffolds for Bone Regeneration. Iran. Biomed. J. 2021, 25, 78–87. [Google Scholar]




| Pore Side Length (μm) | Dimensions | Filament Length (mm) | Vscaffold | VPLA (%) | Vporosity (%) |
|---|---|---|---|---|---|
| 60 | 10 × 10 × 0.85 | 23.75 | 85 | 67.22 | 32.78 |
| 60–70 | 10 × 10 × 0.45 | 12.16 | 45 | 65.02 | 34.98 |
| 60–80 | 10 × 10 × 0.45 | 11.87 | 45 | 63.48 | 36.52 |
| 60–70–80–90 | 10 × 10 × 0.85 | 23.46 | 85 | 66.40 | 33.6 |
| 100 | 10 × 10 × 0.85 | 21.5 | 85 | 60.84 | 39.16 |
| Sample No. | Tnozzle(°C) | Tchamber(°C) | V(mm/s) | R(mm) | Tbed(°C) | Rinfill | D(%) | E (GPa) | Tensile strength (MPa) |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 220 | 40 | 60 | 0.12 | 80 | ±45 | 100 | 2.45 | 33.88 |
| 1 | 220 | 40 | 20 | 0.1 | 80 | 0/90 | 90 | 2.49 | 34.30 |
| 2 | 190 | 40 | 20 | 0.12 | 30 | 0/90 | 100 | 2.78 | 38.82 |
| 3 | 190 | 40 | 60 | 0.1 | 30 | ±45 | 90 | 2.06 | 23.10 |
| 4 | 220 | 30 | 20 | 0.1 | 30 | ±45 | 100 | 2.72 | 36.99 |
| 5 | 220 | 30 | 60 | 0.12 | 30 | 0/90 | 90 | 2.42 | 33.33 |
| 6 | 190 | 30 | 60 | 0.12 | 80 | 0/90 | 100 | 2.63 | 36.53 |
| 7 | 190 | 30 | 20 | 0.1 | 80 | ±45 | 90 | 2.31 | 31.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portan, D.V.; Ntoulias, C.; Mantzouranis, G.; Fortis, A.P.; Deligianni, D.D.; Polyzos, D.; Kostopoulos, V. Gradient 3D Printed PLA Scaffolds on Biomedical Titanium: Mechanical Evaluation and Biocompatibility. Polymers 2021, 13, 682. https://doi.org/10.3390/polym13050682
Portan DV, Ntoulias C, Mantzouranis G, Fortis AP, Deligianni DD, Polyzos D, Kostopoulos V. Gradient 3D Printed PLA Scaffolds on Biomedical Titanium: Mechanical Evaluation and Biocompatibility. Polymers. 2021; 13(5):682. https://doi.org/10.3390/polym13050682
Chicago/Turabian StylePortan, Diana V., Christos Ntoulias, Georgios Mantzouranis, Athanassios P. Fortis, Despina D. Deligianni, Demosthenes Polyzos, and Vassilis Kostopoulos. 2021. "Gradient 3D Printed PLA Scaffolds on Biomedical Titanium: Mechanical Evaluation and Biocompatibility" Polymers 13, no. 5: 682. https://doi.org/10.3390/polym13050682
APA StylePortan, D. V., Ntoulias, C., Mantzouranis, G., Fortis, A. P., Deligianni, D. D., Polyzos, D., & Kostopoulos, V. (2021). Gradient 3D Printed PLA Scaffolds on Biomedical Titanium: Mechanical Evaluation and Biocompatibility. Polymers, 13(5), 682. https://doi.org/10.3390/polym13050682