Characterization and Biotechnological Potential of Intracellular Polyhydroxybutyrate by Stigeoclonium sp. B23 Using Cassava Peel as Carbon Source
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cassava Peel Pretreatment
2.2. Cassava Peel Hydrolysis
2.3. Media and Growth Conditions
2.4. Extraction and Quantification of PHB Content
2.5. Statistical Analysis
2.6. PHB Characterization
2.6.1. Scanning Electron Microscopy (SEM)
2.6.2. Differential Scanning Calorimetry (DSC)
2.6.3. Thermogravimetric Analysis (TGA)
2.6.4. X-ray Diffraction Analysis (DRX)
2.7. PHB Nanoparticles Preparation
Fish Embryo Acute Toxicity (FET) Test
3. Results and Discussion
3.1. Glucose Composition of CPH during Cultivation
3.2. Biomass and PHB Yield
3.3. PHB Nanoparticles Toxicity
3.4. Characterization Analysis
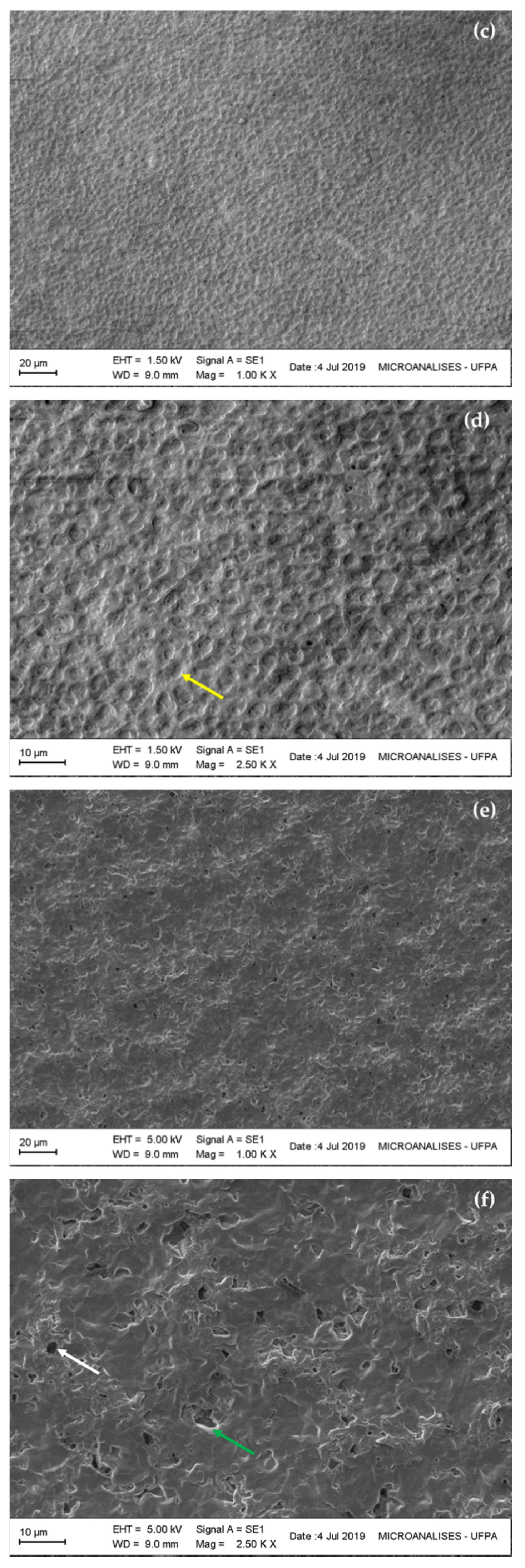
3.4.1. Morphology of Microalgal PHB
3.4.2. Thermal and Degradation Behaviour of Microalgal PHB
3.4.3. Cristallinity of Microalgal PHB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, G.-Q.; Patel, M.K. Plastics derived from biological sources: Present and future: A technical and environmental review. Chem. Rev. 2011, 112, 2082–2099. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.; An, Y.-J. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: A review. Environ. Pollut. 2018, 240, 387–395. [Google Scholar] [CrossRef]
- Yates, M.R.; Barlow, C.Y. Life cycle assessments of biodegradable, commercial biopolymers—A critical review. Resour. Conserv. Recycl. 2013, 78, 54–66. [Google Scholar] [CrossRef]
- Gradíssimo, D.G.; Xavier, L.P.; Santos, A.V. Cyanobacterial polyhydroxyalkanoates: A sustainable alternative in circular economy. Molecules 2020, 25, 4331. [Google Scholar] [CrossRef]
- Jendrossek, D.; Pfeiffer, D. New insights in the formation of polyhydroxyalkanoate granules (carbonosomes) and novel functions of poly(3-hydroxybutyrate). Environ. Microbiol. 2014, 16, 2357–2373. [Google Scholar] [CrossRef]
- Kushwah, B.S.; Singh, V. Towards understanding polyhydroxyalkanoates and their use. J. Polym. Res. 2016, 23, 1–14. [Google Scholar] [CrossRef]
- Reddy, C.S.K.; Ghai, R.; Kalia, V. Polyhydroxyalkanoates: An overview. Bioresour. Technol. 2003, 87, 137–146. [Google Scholar] [CrossRef]
- Lee, I.; Kim, M.K.; Chang, H.N.; Park, Y.H. Regulation of poly-β-hydroxybutyrate biosynthesis by nicotinamide nucleotide in Alcaligenes eutrophus. FEMS Microbiol. Lett. 1995, 131, 35–39. [Google Scholar] [CrossRef]
- Mitra, R.; Xu, T.; Xiang, H.; Han, J. Current developments on polyhydroxyalkanoates synthesis by using halophiles as a promising cell factory. Microb. Cell Factories 2020, 19, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, Y.S.; Alrumman, S.A.; Otaif, K.A.; Alamri, S.A.; Mostafa, M.S.; Sahlabji, T. Production and characterization of bioplastic by polyhydroxybutyrate accumulating erythrobacter aquimaris isolated from mangrove rhizosphere. Molecules 2020, 25, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Możejko-Ciesielska, J.; Kiewisz, R. Bacterial polyhydroxyalkanoates: Still fabulous? Microbiol. Res. 2016, 192, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Raza, Z.A.; Khalil, S.; Abid, S. Recent progress in development and chemical modification of poly(hydroxybutyrate)-based blends for potential medical applications. Int. J. Biol. Macromol. 2020, 160, 77–100. [Google Scholar] [CrossRef] [PubMed]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef] [Green Version]
- Gadgil, B.S.T.; Killi, N.; Rathna, G.V.N. Polyhydroxyalkanoates as biomaterials. MedChemComm 2017, 8, 1774–1787. [Google Scholar] [CrossRef]
- Sharma, V.; Sehgal, R.; Gupta, R. Polyhydroxyalkanoate (PHA): Properties and modifications. Polymer 2021, 212, 123161. [Google Scholar] [CrossRef]
- Dobrogojski, J.; Spychalski, M.; Luciński, R.; Borek, S. Transgenic plants as a source of polyhydroxyalkanoates. Acta Physiol. Plant. 2018, 40, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Abdo, S.M.; Ali, G.H. Analysis of polyhydroxybutrate and bioplastic production from microalgae. Bull. Natl. Res. Cent. 2019, 43, 1–4. [Google Scholar] [CrossRef]
- Costa, S.S.; Miranda, A.L.; de Morais, M.G.; Costa, J.A.V.; Druzian, J.I. Microalgae as source of polyhydroxyalkanoates (PHAs)—A review. Int. J. Biol. Macromol. 2019, 131, 536–547. [Google Scholar] [CrossRef]
- Cassuriaga, A.; Freitas, B.; Morais, M.; Costa, J. Innovative polyhydroxybutyrate production by Chlorella fusca grown with pentoses. Bioresour. Technol. 2018, 265, 456–463. [Google Scholar] [CrossRef]
- Das, S.K.; Sathish, A.; Stanley, J. Production of biofuel and bioplastic from chlorella pyrenoidosa. Mater. Today Proc. 2018, 5, 16774–16781. [Google Scholar] [CrossRef]
- Roja, K.; Sudhakar, D.R.; Anto, S.; Mathimani, T. Extraction and characterization of polyhydroxyalkanoates from marine green alga and cyanobacteria. Biocatal. Agric. Biotechnol. 2019, 22, 101358. [Google Scholar] [CrossRef]
- Chaogang, W.; Zhangli, H.; Anping, L.; Baohui, J. Biosynthesis of Poly-3-Hydroxybutyrate (PHB) in the transgenic green alga Chlamydomonas reinhardtii. J. Phycol. 2010, 46, 396–402. [Google Scholar] [CrossRef]
- Kavitha, G.; Kurinjimalar, C.; Sivakumar, K.; Kaarthik, M.; Aravind, R.; Palani, P.; Rengasamy, R. Optimization of polyhydroxybutyrate production utilizing waste water as nutrient source by Botryococcus braunii Kütz using response surface methodology. Int. J. Biol. Macromol. 2016, 93, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Mourão, M.M.; Gradíssimo, D.G.; Santos, A.V.; Schneider, M.P.C.; Faustino, S.M.M.; Vasconcelos, V.; Xavier, L.P. Optimization of polyhydroxybutyrate production by amazonian microalga Stigeoclonium sp. B23. Biomolecules 2020, 10, 1628. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Saratale, G.D.; Cho, S.K.; Kim, D.S.; Ghodake, G.S.; Kadam, A.; Kumar, G.; Bharagava, R.N.; Banu, R.; Shin, H.S. Pretreatment of kenaf (Hibiscus cannabinus L.) biomass feedstock for polyhydroxybutyrate (PHB) production and characterization. Bioresour. Technol. 2019, 282, 75–80. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Varjani, S.; Cho, S.-K.; Ghodake, G.S.; Kadam, A.; Mulla, S.I.; Bharagava, R.N.; Kim, D.-S.; Shin, H.S. Development of ultrasound aided chemical pretreatment methods to enrich saccharification of wheat waste biomass for polyhydroxybutyrate production and its characterization. Ind. Crop. Prod. 2020, 150, 112425. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.-K.; Ghodake, G.S.; Shin, H.-S.; Saratale, G.D.; Park, Y.; Lee, H.-S.; Bharagava, R.N.; Kim, D.-S. Utilization of noxious weed water hyacinth biomass as a potential feedstock for biopolymers production: A novel approach. Polymers 2020, 12, 1704. [Google Scholar] [CrossRef]
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xie, L.; Yin, Z.; Khanal, S.K.; Zhou, Q. Biorefinery approach for cassava-based industrial wastes: Current status and opportunities. Bioresour. Technol. 2016, 215, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Tang, L.; Zhang, J.; Mao, Z.; Jiang, L. Optimization of thermal-dilute sulfuric acid pretreatment for enhancement of methane production from cassava residues. Bioresour. Technol. 2011, 102, 3958–3965. [Google Scholar] [CrossRef] [PubMed]
- Zeoula, L.M.; Neto, S.F.C.; Branco, A.F.; Prado, I.N.D.; Dalponte, A.O.; Kassies, M.; Fregadolli, F.L. Mandioca e resíduos das farinheiras na alimentação de ruminantes: pH, Concentração de N-NH3 e eficiência microbiana. Rev. Bras. Zootec. 2002, 31, 1582–1593. [Google Scholar] [CrossRef] [Green Version]
- Poomipuk, N.; Reungsang, A.; Plangklang, P. Poly-β-hydroxyalkanoates production from cassava starch hydrolysate by Cupriavidus sp. KKU38. Int. J. Biol. Macromol. 2014, 65, 51–64. [Google Scholar] [CrossRef]
- Bumbak, F.; Cook, S.; Zachleder, V.; Hauser, S.; Kovar, K. Best practices in heterotrophic high-cell-density microalgal processes: Achievements, potential and possible limitations. Appl. Microbiol. Biotechnol. 2011, 91, 31–46. [Google Scholar] [CrossRef] [Green Version]
- Lackner, M.; Kamravamanesh, D.; Krampl, M.; Itzinger, R.; Paulik, C.; Chodak, I.; Herwig, C. Characterization of photosynthetically synthesized poly(3-hydroxybutyrate) using a randomly mutated strain of Synechocystis sp. PCC 6714. Int. J. Biobased Plast. 2019, 1, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Ansari, S.; Fatma, T. Cyanobacterial Polyhydroxybutyrate (PHB): Screening, optimization and characterization. PLoS ONE 2016, 11, e0158168. [Google Scholar] [CrossRef] [Green Version]
- Hong, K.; Beld, J.; Davis, T.D.; Burkart, M.D.; Palenik, B. Screening and characterization of polyhydroxyalkanoate granules, and phylogenetic analysis of polyhydroxyalkanoate synthase gene PhaC in cyanobacteria. J. Phycol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Allen, M.M. Simple conditions for growth of unicellular blue-green algae on plates. J. Phycol. 1968, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kotai, J. Instructions for preparation of modified nutrient solution Z8 for algae. Nor. Inst. Water Res. 1972, 69, 1–5. [Google Scholar]
- Getachew, A.; Woldesenbet, F. Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res. Notes 2016, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kuntzler, S.G.; De Almeida, A.C.A.; Costa, J.A.V.; De Morais, M.G. Polyhydroxybutyrate and phenolic compounds microalgae electrospun nanofibers: A novel nanomaterial with antibacterial activity. Int. J. Biol. Macromol. 2018, 113, 1008–1014. [Google Scholar] [CrossRef]
- Salgaonkar, B.B.; Bragança, J.M. Utilization of sugarcane bagasse by halogeometricum borinquense strain E3 for biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Bioengineering 2017, 4, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakeri, F.; Shakeri, S.; Hojjatoleslami, M. Preparation and characterization of carvacrol loaded polyhydroxybutyrate nanoparticles by nanoprecipitation and dialysis methods. J. Food Sci. 2014, 79, N697–N705. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation and Development. Test No. 236: Fish Embryo Acute Toxicity (FET) Test. OECD Guidel. Test. Chem. OECD Publ. 2013, 2, 1–22. [Google Scholar]
- Kim, J.; Park, C.; Kim, T.-H.; Lee, M.; Kim, S.; Kim, S.-W.; Lee, J. Effects of various pretreatments for enhanced anaerobic digestion with waste activated sludge. J. Biosci. Bioeng. 2003, 95, 271–275. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Luttenton, M.R.; Lowe, R.L. Response of a lentic periphyton community to nutrient enrichment at low n:p ratios. J. Phycol. 2006, 42, 1007–1015. [Google Scholar] [CrossRef]
- Marks, J.C.; Lowe, R.L. The independent and interactive effects of snail grazing and nutrient enrichment on structuring periphyton communities. Hydrobiologia 1989, 185, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Chanprateep, S. Current trends in biodegradable polyhydroxyalkanoates. J. Biosci. Bioeng. 2010, 110, 621–632. [Google Scholar] [CrossRef]
- Curien, G.; Flori, S.; Villanova, V.; Magneschi, L.; Giustini, C.; Forti, G.; Matringe, M.; Petroutsos, D.; Kuntz, M.; Finazzi, G. The water to water cycles in microalgae. Plant Cell Physiol. 2016, 57, 1354–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Yu, X.; Li, T.; Xiong, X.; Chen, S. Induction of D-xylose uptake and expression of NAD(P)H-linked xylose reductase and NADP + -linked xylitol dehydrogenase in the oleaginous microalga Chlorella sorokiniana. Biotechnol. Biofuels 2014, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Hondo, S.; Takahashi, M.; Osanai, T.; Matsuda, M.; Hasunuma, T.; Tazuke, A.; Nakahira, Y.; Chohnan, S.; Hasegawa, M.; Asayama, M. Genetic engineering and metabolite profiling for overproduction of polyhydroxybutyrate in cyanobacteria. J. Biosci. Bioeng. 2015, 120, 510–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saratale, R.G.; Cho, S.-K.; Saratale, G.D.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Bharagava, R.N.; Kumar, G.; Kim, D.S.; Mulla, S.I.; et al. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685. [Google Scholar] [CrossRef]
- Bell, I.R.; Ives, J.A.; Wayne, B.J. Nonlinear effects of nanoparticles: Biological variability from hormetic doses, small particle sizes, and dynamic adaptive interactions. Dose Response 2013, 12, 202–232. [Google Scholar] [CrossRef] [Green Version]
- Vranic, S.; Shimada, Y.; Ichihara, S.; Kimata, M.; Wu, W.; Tanaka, T.; Boland, S.; Tran, L.; Ichihara, G. Toxicological evaluation of SiO2 nanoparticles by zebrafish embryo toxicity test. Int. J. Mol. Sci. 2019, 20, 882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAdam, B.; Fournet, M.B.; McDonald, P.; Mojicevic, M. Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef]
- Frone, A.N.; Nicolae, C.A.; Eremia, M.C.; Tofan, V.; Ghiurea, M.; Chiulan, I.; Radu, E.; Damian, C.M.; Panaitescu, D.M. Low molecular weight and polymeric modifiers as toughening agents in poly(3-Hydroxybutyrate) films. Polymers 2020, 12, 2446. [Google Scholar] [CrossRef]
- Magara, G.; Khan, F.R.; Pinti, M.; Syberg, K.; Inzirillo, A.; Elia, A.C. Effects of combined exposures of fluoranthene and polyethylene or polyhydroxybutyrate microplastics on oxidative stress biomarkers in the blue mussel (Mytilus edulis). J. Toxicol. Environ. Health Part A 2019, 82, 616–625. [Google Scholar] [CrossRef]
- Zhao, H.; Cui, Z.; Sun, X.; Turng, L.-S.; Peng, X. Morphology and properties of injection molded solid and microcellular polylactic acid/polyhydroxybutyrate-Valerate (PLA/PHBV) blends. Ind. Eng. Chem. Res. 2013, 52, 2569–2581. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.-S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, mechanical and morphological characterization of plasticized PLA–PHB blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Ansari, N.F.; Annuar, M.S.M.; Murphy, B.P. A porous medium-chain-length poly(3-hydroxyalkanoates)/hydroxyapatite composite as scaffold for bone tissue engineering. Eng. Life Sci. 2016, 17, 420–429. [Google Scholar] [CrossRef]
- Anbukarasu, P.; Sauvageau, D.; Elias, A. Tuning the properties of polyhydroxybutyrate films using acetic acid via solvent casting. Sci. Rep. 2015, 5, 17884. [Google Scholar] [CrossRef] [Green Version]
- Sudesh, K. Polyhydroxyalkanoates from Palm Oil: Biodegradable Plastics (SpringerBriefs in Microbiology), 2013th ed.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Janigová, I.; Lacík, I.; Chodák, I. Thermal degradation of plasticized poly(3-hydroxybutyrate) investigated by DSC. Polym. Degrad. Stab. 2002, 77, 35–41. [Google Scholar] [CrossRef]
- Thiré, R.M.D.S.M.; Arruda, L.C.; Barreto, L.S. Morphology and thermal properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/attapulgite nanocomposites. Mater. Res. 2011, 14, 340–344. [Google Scholar] [CrossRef] [Green Version]
- Corre, Y.-M.; Bruzaud, S.; Audic, J.-L.; Grohens, Y. Morphology and functional properties of commercial polyhydroxyalkanoates: A comprehensive and comparative study. Polym. Test. 2012, 31, 226–235. [Google Scholar] [CrossRef]
- Biradar, G.G.; Shivasharana, C.T.; Kaliwal, B.B. Characterization of polyhydroxybutyrate (PHB) Produced by novel bacterium Lysinibacillus sphaericus BBKGBS6 isolated from soil. J. Polym. Environ. 2018, 26, 1685–1701. [Google Scholar] [CrossRef]
- Costa, S.S.; Miranda, A.L.; Andrade, B.B.; Assis, D.D.J.; Souza, C.O.; de Morais, M.G.; Costa, J.A.V.; Druzian, J.I. Influence of nitrogen on growth, biomass composition, production, and properties of polyhydroxyalkanoates (PHAs) by microalgae. Int. J. Biol. Macromol. 2018, 116, 552–562. [Google Scholar] [CrossRef]
- Babruwad, P.R.; Prabhu, S.U.; Upadhyaya, K.P.; Hungund, B.S. Production and characterization of thermostable polyhydroxybutyrate from Bacillus cereus PW3A. J. Biochem. Technol. 2016, 6, 990–995. [Google Scholar]
- Kovalcik, A.; Obruca, S.; Kalina, M.; Machovsky, M.; Enev, V.; Jakesova, M.; Sobkova, M.; Marova, I. Enzymatic hydrolysis of poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) scaffolds. Materials 2020, 13, 2992. [Google Scholar] [CrossRef] [PubMed]
- Assis, D.D.J.; Gomes, G.V.P.; Pascoal, D.R.D.C.; Pinho, L.S.; Chaves, L.B.O.; Druzian, J.I. Simultaneous biosynthesis of polyhydroxyalkanoates and extracellular polymeric substance (EPS) from crude glycerol from biodiesel production by different bacterial strains. Appl. Biochem. Biotechnol. 2016, 180, 1110–1127. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.N.; Lee, M.Y.; Park, W.H. Thermal stabilization of poly(3-hydroxybutyrate) by poly(glycidyl methacrylate). J. Appl. Polym. Sci. 2002, 83, 2945–2952. [Google Scholar] [CrossRef]
- Koller, M.; Salerno, A.; Braunegg, G. Polyhydroxyalkanoates: Basics, production and applications of microbial biopolyesters. In Bio-Based Plastics: Materials and Applications; Wiley: Hoboken, NJ, USA, 2013; pp. 137–170. [Google Scholar]
- Anbukarasu, P.; Sauvageau, D.; Elias, A.L. Enzymatic degradation of dimensionally constrained polyhydroxybutyrate films. Phys. Chem. Chem. Phys. 2017, 19, 30021–30030. [Google Scholar] [CrossRef] [PubMed]
- Bhati, R.; Mallick, N. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production by the diazotrophic cyanobacterium Nostoc muscorum Agardh: Process optimization and polymer characterization. Algal Res. 2015, 7, 78–85. [Google Scholar] [CrossRef]
- Napolitano, S.; Wübbenhorst, M. Slowing down of the crystallization kinetics in ultrathin polymer films: A Size or an interface effect? Macromolecules 2006, 39, 5967–5970. [Google Scholar] [CrossRef]









| Cultivation | CPH (g/L) | NaNO3 (g/L) |
|---|---|---|
| 1 | 10 | 0.5 |
| 2 | 5.0 | 1.0 |
| 3 | 1.0 | 1.5 |
| Medium | NaNO3 (g) | BMP (g/L) | PHB (%) | PPHB (g/L) |
|---|---|---|---|---|
| Z8/100%NaNO3 | 46.7 | 1.53 ± 0.09 | 0.92 ± 0.01 | 0.014 ± 0.001 |
| Z8/25%NaNO3 | 11.675 | 0.80 ± 0.06 | 12.16 ± 1.28 | 0.098 ± 0.005 |
| Z8/2.5%NaNO3 | 1.1675 | 0.52 ± 0.06 | 8.90 ± 1.96 | 0.046 ± 0.006 |
| DSC Characterization | ||||||||
|---|---|---|---|---|---|---|---|---|
| PHB | Tg (°C) | Tm (°C) | (J/g) | Xc (%) | Td (°C) | Tonset (°C) | Tendset (°C) | (J/g) |
| BG-110 | 44.32 | 168.31 | 19.74 | 13.46 | 271.72 | 252.90 | 302.08 | 186.70 |
| Cultivation 1 | 46.04 | 164.09 | 11.03 | 7.52 | 254.20 | 225.58 | 289.19 | 142.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourão, M.M.; Xavier, L.P.; Urbatzka, R.; Figueiroa, L.B.; Costa, C.E.F.d.; Dias, C.G.B.T.; Schneider, M.P.C.; Vasconcelos, V.; Santos, A.V. Characterization and Biotechnological Potential of Intracellular Polyhydroxybutyrate by Stigeoclonium sp. B23 Using Cassava Peel as Carbon Source. Polymers 2021, 13, 687. https://doi.org/10.3390/polym13050687
Mourão MM, Xavier LP, Urbatzka R, Figueiroa LB, Costa CEFd, Dias CGBT, Schneider MPC, Vasconcelos V, Santos AV. Characterization and Biotechnological Potential of Intracellular Polyhydroxybutyrate by Stigeoclonium sp. B23 Using Cassava Peel as Carbon Source. Polymers. 2021; 13(5):687. https://doi.org/10.3390/polym13050687
Chicago/Turabian StyleMourão, Murilo Moraes, Luciana Pereira Xavier, Ralph Urbatzka, Lucas Barbosa Figueiroa, Carlos Emmerson Ferreira da Costa, Carmen Gilda Barroso Tavares Dias, Maria Paula Cruz Schneider, Vitor Vasconcelos, and Agenor Valadares Santos. 2021. "Characterization and Biotechnological Potential of Intracellular Polyhydroxybutyrate by Stigeoclonium sp. B23 Using Cassava Peel as Carbon Source" Polymers 13, no. 5: 687. https://doi.org/10.3390/polym13050687
APA StyleMourão, M. M., Xavier, L. P., Urbatzka, R., Figueiroa, L. B., Costa, C. E. F. d., Dias, C. G. B. T., Schneider, M. P. C., Vasconcelos, V., & Santos, A. V. (2021). Characterization and Biotechnological Potential of Intracellular Polyhydroxybutyrate by Stigeoclonium sp. B23 Using Cassava Peel as Carbon Source. Polymers, 13(5), 687. https://doi.org/10.3390/polym13050687











