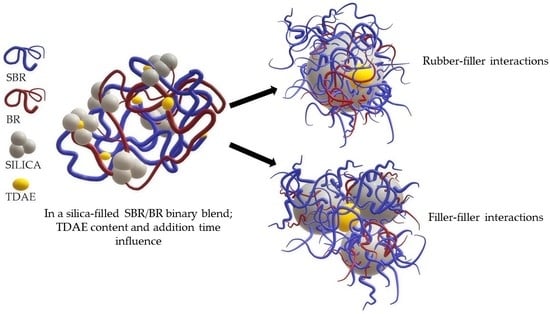
Influence of Treated Distillate Aromatic Extract (TDAE) Content and Addition Time on Rubber-Filler Interactions in Silica Filled SBR/BR Blends
Abstract
1. Introduction
2. Experimental
2.1. Materials and Compounding
2.2. Sample Preparation and Characterization
3. Result and Discussion
3.1. Influence of TDAE Addition Time on Mixing Behavior and Interfacial Interactions
3.2. Influence of TDAE Content on Rubber–Filler and Filler–Filler Interactions
3.3. Investigation of Dynamic Mechanical and Mechanical Properties of SBR/BR Vulcanizates and Their Relation to Rubber Layer L
4. Practical Applications and Future Research Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kobayashi, S.; Müllen, K. Encyclopedia of Polymeric Nanomaterials, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 2222–2227. [Google Scholar]
- Saeed, F.; Ansarifar, A.; Ellis, R.J.; Haile-Meskel, Y.; Irfan, M.S. Two advanced styrene-butadiene/polybutadiene rubber blends filled with a silanized silica nanofiller for potential use in passenger car tire tread compound. J. Appl. Polym. Sci. 2011, 123, 1518–1529. [Google Scholar] [CrossRef]
- Sisanth, K.; Thomas, M.; Abraham, J.; Thomas, S. General introduction to rubber compounding. In Progress in Rubber Nanocomposites; Thomas, S., Hanna, J.M., Eds.; Woodhead Publishing: Cambridge, UK, 2017; Volume 75, pp. 1–39. [Google Scholar]
- Litvinov, V.M.; Orza, R.R.; Kluppel, M.; Van Duin, M.M.; Magusin, P.C.M.M. Rubber–filler interactions and network structure in relation to stress–strain behavior of vulcanized, carbon black filled EPDM. Macromolecules 2011, 44, 4887–4900. [Google Scholar] [CrossRef]
- Choi, S.-S.; Kwon, H.-M.; Kim, Y.; Ko, E.; Kim, E. Determination of bound rubber composition of filled SBR/BR blend com-pounds by analysis of the unbound rubber composition and bound rubber content. Polym. Test 2017, 59, 414–422. [Google Scholar] [CrossRef]
- Tao, Y.-C.; Dong, B.; Zhang, L.-Q.; Wu, Y.-P. Reactions of silica–silane rubber and properties of silane–silica/solution-polymerized styrene–butadiene rubber composite. Rubber Chem. Technol. 2016, 89, 526–539. [Google Scholar] [CrossRef]
- Reuvekamp, L.; Brinke, J.W.; Swaaij, P.J.; Noordermeer, J. Effects of mixing conditions-Reaction of TESPT silane coupling agent during mixing with silica filler and tire rubber. KGK Kautsch. Gummi Kunstst. 2002, 55, 41–47. [Google Scholar]
- Sarkawi, S.S.; Dierkes, W.K.; Noordermeer, J.W.M. Morphology of silica-reinforced natural rubber: The effect of silane coupling agent. Rubber Chem. Technol. 2015, 88, 359–372. [Google Scholar] [CrossRef]
- Choi, S.-S. Influence of storage time and temperature and silane coupling agent on bound rubber formation in filled styrene–butadiene rubber compounds. Polym. Test. 2002, 21, 201–208. [Google Scholar] [CrossRef]
- Maghami, S.; Dierkes, W.K.; Noordermeer, J.W.M. Functionalized SBRs in silica-reinforced tire tread compounds: Evidence for interactions between silica filler and zinc oxide. Rubber Chem. Technol 2016, 89, 559–572. [Google Scholar] [CrossRef]
- Rocha, T.; Schuster, R.H.; Jacobi, M.; Samios, D. Influence of epoxidation on physical properties of SBR and its interaction with precipitated silica. KGK Kautsch. Gummi Kunstst. 2004, 57, 656–661. [Google Scholar]
- Thiele, S.; Kiesekamp, J.; Rulhoff, S.; Bellgardt, D. Modified synthetic rubber for silica & carbon black containing tire treads. KGK Rubberpoint 2011, 64, 36–41. [Google Scholar]
- Meissner, B. Bound rubber and elastomer-filler interaction. Rubber Chem. Technol. 1995, 68, 297–310. [Google Scholar] [CrossRef]
- Meissner, B. Theory of bound rubber. J. Appl. Polym. Sci. 1974, 18, 2483–2491. [Google Scholar] [CrossRef]
- Levresse, P.; Feke, D.; Manas-Zloczower, I. Analysis of the formation of bound poly(dimethylsiloxane) on silica. Polymer 1998, 39, 3919–3924. [Google Scholar] [CrossRef]
- Leblanc, J. Rubber–filler interactions and rheological properties in filled compounds. Prog. Polym. Sci. 2002, 27, 627–687. [Google Scholar] [CrossRef]
- Law, Y.Y.; Feke, D.L.; Manas-Zloczower, I. Thermogravimetric analysis of the kinetics of bound-rubber formation on surface-modified silica. Rubber Chem. Technol. 2014, 87, 311–319. [Google Scholar] [CrossRef]
- Choi, S.-S.; Ko, E. Novel test method to estimate bound rubber formation of silica-filled solution styrene-butadiene rubber compounds. Polym. Test. 2014, 40, 170–177. [Google Scholar] [CrossRef]
- Choi, S.-S. Effect of bound rubber on characteristics of highly filled styrene-butadiene rubber compounds with different types of carbon black. J. Appl. Polym. Sci. 2004, 93, 1001–1006. [Google Scholar] [CrossRef]
- Choi, S.-S. Difference in bound rubber formation of silica and carbon black with styrene-butadiene rubber. Polym. Adv. Technol. 2002, 13, 466–474. [Google Scholar] [CrossRef]
- Pliskin, I.; Tokita, N. Bound rubber in elastomers: Analysis of elastomer-filler interaction and its effect on viscosity and modulus of composite systems. J. Appl. Polym. Sci. 1972, 16, 473–492. [Google Scholar] [CrossRef]
- Kaewsakul, W.; Sahakaro, K.; Dierkes, W.K.; Noordermeer, J.W.M. Factors influencing the flocculation process in sili-ca-reinforced natural rubber compounds. J. Elastomers Plast. 2016, 48, 426–441. [Google Scholar] [CrossRef]
- Le, H.H.; Ilisch, S.; Kasaliwal, G.R.; Radusch, H.-J. Filler phase distribution in rubber blends characterized by thermo-gravimetric analysis of rubber filler gel. Rubber Chem. Technol. 2008, 81, 767–781. [Google Scholar] [CrossRef]
- Le, H.H.; Ilisch, S.; Heidenreich, D.; Osswald, K.; Radusch, H.-J. Phase selective localization of filler in ternary rubber blends. Rubber Chem. Technol. 2011, 84, 41–54. [Google Scholar] [CrossRef]
- Le, H.H.; Parsaker, M.; Sriharish, M.N.; Henning, S.; Menzel, M.; Wiessner, S.; Das, A.; Do, Q.K.; Heinrich, G.; Radusch, H.J. Effect of rubber polarity on selective wetting of carbon nanotubes in ternary blends. Express Polym. Lett. 2015, 9, 960–971. [Google Scholar] [CrossRef]
- Le, H.; Hamann, E.; Ilisch, S.; Heinrich, G.; Radusch, H.-J. Selective wetting and dispersion of filler in rubber composites under influence of processing and curing additives. Polymer 2014, 55, 1560–1569. [Google Scholar] [CrossRef]
- Wunde, M.; Klüppel, M. Influence of phase morphology and filler distribution in NR/BR and NR/SBR blends on fracture mechanical properties. Rubber Chem. Technol. 2016, 89, 588–607. [Google Scholar] [CrossRef]
- Wunde, M.; Klüppel, M. Impact of mixing procedure on phase morphology and fracture mechanical properties of carbon black-filled NR/SBR blends. Contin. Mech. Thermodyn. 2017, 29, 1135–1148. [Google Scholar] [CrossRef]
- Mujtaba, A.; Keller, M.; Ilisch, S.; Radusch, H.J.; Thurn-Albrecht, T.; Saalwächter, K.; Beiner, M. Mechanical properties and cross-link density of styrene–butadiene model composites containing fillers with bimodal particle size distribution. Macromolecules 2012, 45, 6504–6515. [Google Scholar] [CrossRef]
- Mujtaba, A.; Keller, M.; Ilisch, S.; Radusch, H.J.; Beiner, M.; Thurn-Albrecht, T.; Saalwächter, K. Detection of surface-immobilized components and their role in viscoelastic reinforcement of rubber–silica nanocomposites. ACS Macro Lett. 2014, 3, 481–485. [Google Scholar] [CrossRef]
- Chokanandsombat, Y.; Sea-Oui, P.; Sirisinha, C. Influence of aromatic content in rubber processing oils on viscoelastic behaviour and mechanical properties of styrene-butadiene-rubber (SBR) For tyre tread applications. Adv. Mater. Res. 2013, 747, 471–474. [Google Scholar] [CrossRef]
- Petchkaew, A.; Sahakaro, K.; Dierkes, W.; Noordermeer, J. Petroleum-based safe process oils in NR and NR/Sbr blends: Part ill. Effects of oil types and contents on the properties of carbon black filled compounds. KGK Kautsch. Gummi Kunstst. 2015, 68, 20–29. [Google Scholar]
- Ezzoddin, S.; Abbasian, A.; Aman-Alikhani, M.; Ganjali, S.T. The influence of non-carcinogenic petroleum-based process oils on tire compounds’ performance. Iran. Polym. J. 2013, 22, 697–707. [Google Scholar] [CrossRef]
- Null, V. Safe process oils for tires with low environmental impact. KGK Kautsch. Gummi Kunstst. 1999, 52, 6. [Google Scholar]
- Rathi, A.; Hernández, M.; García, S.J.; Dierkes, W.K.; Noordermeer, J.W.M.; Bergmann, C.; Trimbach, J.; Blume, A. Identifying the effect of aromatic oil on the individual component dynamics of S-SBR/BR blends by broadband dielectric spectroscopy. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 842–854. [Google Scholar] [CrossRef]
- Petchkaew, A.; Sahakaro, K.; Dierkes, W.; Noordermeer, J. Petroleum-based safe process oils in NR, SBR and their blends: Study on unfilled compounds. Part II. Properties. KGK Kautsch. Gummi Kunstst. 2013, 66, 21–27. [Google Scholar]
- Global Asia. Available online: http://www.globalasia.co.th/Datasheet/industry_eng/SD-PD-02-2-85%20VIVATEC%20500.pdf (accessed on 8 February 2021).
- Deutsches Institut fur Normung DIN 53529-2. Testing of Rubber and Elastomers; Measurement of Vulcanization Characteristics (Curometry); Evaluation of Cross-Linking Isotherms in Terms of Reaction Kinetics; Deutsches Institut fur Normung: Berlin, Germany, 1983. [Google Scholar]
- Lange, H. Determination of the degree of swelling and crosslinking of extremely small polymer gel quantities by analytical ultracentrifugation. Colloid Polym. Sci. 1986, 264, 488–493. [Google Scholar] [CrossRef]
- Rybiński, P.; Janowska, G. Effect of the spatial network structure and cross-link density of diene rubbers on their thermal stability and fire hazard. J. Therm. Anal. Calorim. 2014, 117, 377–386. [Google Scholar] [CrossRef]
- Flory, P.J. Statistical mechanics of swelling of network structures. J. Chem. Phys. 1950, 18, 108–111. [Google Scholar] [CrossRef]
- Blume, A.; Kiesewetter, J. Determination of the crosslink density of tire tread compounds by different analytical methods. KGK Kautsch. Gummi Kunstst. 2019, 9, 33–42. [Google Scholar]
- Deutsches Institut fur Normung DIN 53513. Determination of the Viscoelastic Properties of Elastomers on Exposure to Forced Vibration at Non-Resonant Frequencies; Deutsches Institut fur Normung E.V. (DIN): Berlin, Germany, 1990. [Google Scholar]
- Deutsches Institut fur Normung E.V. (DIN)53504. Testing of Rubber-Determination of Tensile Strength at Break, Tensile Stress at Yield, Elongation at Break and Stress Values in a Tensile Test; Deutsches Institut fur Normung E.V. (DIN): Berlin, Germany, 2017. [Google Scholar]
- Deutsches Institut fur Normung E.V. (DIN) ISO 7619-1. Rubber, Vulcanized or Thermoplastic-Determination of Indentation Hardness-Part 1: Durometer Method (Shore Hardness); Deutsches Institut fur Normung E.V. (DIN): Berlin, Germany, 2012. [Google Scholar]
- Le, H.H.; Ilisch, S.; Jakob, B.; Radusch, H.-J. Online characterization of the effect of mixing parameters on carbon black dispersion in rubber compounds using electrical conductivity. Rubber Chem. Technol. 2004, 77, 147–160. [Google Scholar] [CrossRef]
- Le, H.H.; Prodanova, I.; Ilisch, S.; Radusch, H.-J. Online electrical conductivity as a measure to characterize the carbon black dispersion in oil containing rubber compounds with a different polarity of rubber. Rubber Chem. Technol. 2004, 77, 815–829. [Google Scholar] [CrossRef]
- Mihara, S.; Datta, R.N.; Noordermeer, J.W.M. Flocculation in silica reinforced rubber compounds. Rubber Chem. Technol. 2009, 82, 524–540. [Google Scholar] [CrossRef]
- Yang, J.; Park, W.; Ryu, C.; Kim, S.J.; Kim, D.I.; Seo, G.; Kim, J.; Chung, C. Estimation of silica flocculation in SBR/BR compounds reinforced with different silica contents from their rheocurves. J. Appl. Polym. Sci. 2020, 137, 48559. [Google Scholar] [CrossRef]
- Leblanc, J.L. Some considerations on optimum mixing with respect to the full development of rubber-carbon black morphology. KGK Kautsch. Gummi Kunstst. 2001, 54, 327–333. [Google Scholar]
- Rodgers, B.; Tallury, S.; Klingensmith, W. Rubber Compounding, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 251–378. [Google Scholar]
- Tscheschel, A.; Lacayo, J.; Stoyan, D. Statistical characterization of TEM images of silica-filled rubber. J. Microsc. 2005, 217, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-J. The role of filler networking in dynamic properties of filled rubber. Rubber Chem. Technol. 1999, 72, 430–448. [Google Scholar] [CrossRef]
- Payne, A.R. Effect of dispersion on the dynamic properties of filler-loaded rubbers. J. Appl. Polym. Sci. 1965, 9, 2273–2284. [Google Scholar] [CrossRef]
- Ouyang, G.B. Modulus, Hysteresis and the Payne Effect; KGK-Kautschuk Gummi Kunstoff: Heidelberg, Germany, 2006. [Google Scholar]
- Fröhlich, J.; Niedermeier, W.; Luginsland, H.D. The effect of filler–filler and filler–elastomer interaction on rubber rein-forcement. Compos. Part A Appl. Sci. Manuf. 2005, 36, 449–460. [Google Scholar] [CrossRef]
- Yang, R.; Song, Y.; Zheng, Q. Payne effect of silica-filled styrene-butadiene rubber. Polymer 2017, 116, 304–313. [Google Scholar] [CrossRef]
- Lin, C.J.; Hergenrother, W.L.; Alexanian, E.; Bohm, G.A. On the filler flocculation in silica-filled rubbers part I. Quantifying and tracking the filler flocculation and polymer-filler interactions in the unvulcanized rubber compounds. Rubber Chem. Technol. 2002, 75, 865–890. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, J.W.; Lee, D.Y.; Seo, K.H. Correlation between the crosslink characteristics and mechanical properties of natural rubber compound via accelerators and reinforcement. Polymers 2020, 12, 2020. [Google Scholar] [CrossRef]
- Hayichelaeh, C. Silica-Reinforced Natural Rubber Tire Compounds with Safe Compounding Ingredients; University Library/University of Twente: Enschede, The Netherlands, 2018. [Google Scholar]
- Jin, J.; Noordermeer, J.W.M.; Dierkes, W.K.; Blume, A. The origin of marching modulus of silica-filled tire tread compounds. Rubber Chem. Technol. 2019, 93, 378–394. [Google Scholar] [CrossRef]
- Torbati-Fard, N.; Hosseini, S.M.; Razzaghi-Kashani, M. Effect of the silica-rubber interface on the mechanical, viscoelastic, and tribological behaviors of filled styrene-butadiene rubber vulcanizates. Polym. J. 2020, 52, 1223–1234. [Google Scholar] [CrossRef]
- Heinrich, G.; Klüppel, M. Recent advances in the theory of filler networking in elastomers. In Advances in Polymer Science; Springer International Publishing: New York, NY, USA, 2007; pp. 1–44. [Google Scholar]









| Components | Content, phr | Addition time, min |
|---|---|---|
| Stage I Mixing | ||
| S-SBR | 80 | 0 |
| BR | 20 | 0 |
| Silica Ultrasil 7000 | 50 | 1 and 1.5 |
| Silane Si75 | 4.2 | 1 and 1.5 |
| TDAE | 0/5/20 | 0/1.5/3 |
| Zinc Oxide | 0.5 | 0.5 |
| Stearic Acid | 3 | 0.5 |
| End Time: 10 min | ||
| Stage II Mixing | ||
| Sulfur | 2.5 | 0 |
| DPG | 1.5 | 0 |
| TBBS | 1.5 | 0 |
| CBS | 1.5 | 0 |
| End time: 5 min | ||
| TDAE Content & Addition Time | |||||
|---|---|---|---|---|---|
| Properties | without TDAE | 5 phr, 0 min | 5 phr, 1.5 min | 5 phr, 3 min | 20 phr, 1.5 min |
| 0.45 | 0.47 | 0.46 | 0.43 | 0.42 | |
| (MPa) | 5.83 ± 0.11 | 5.22 ± 0.18 | 5.06 ± 0.28 | 5.21 ± 0.22 | 3.27 ± 0.05 |
| (MPa) | 13.6 ± 0.16 | 12.4 ± 0.12 | 11.4 ± 0.96 | 12.2 ± 0.13 | 7.55 ± 0.09 |
| (MPa) | 12.9 ± 2.57 | 12.4 ± 1.25 | 12.7 ± 1.14 | 13.2 ± 1.37 | 12.3 ± 0.96 |
| (%) | 191 ± 31.6 | 200 ± 14.0 | 218 ± 16.7 | 210 ± 17.5 | 281 ± 14.8 |
| Hardness (Shore A) | 73.7 ± 1.1 | 71.6 ± 0.6 | 70.6 ± 0.5 | 68.8 ± 0.4 | 65.2 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sökmen, S.; Oßwald, K.; Reincke, K.; Ilisch, S. Influence of Treated Distillate Aromatic Extract (TDAE) Content and Addition Time on Rubber-Filler Interactions in Silica Filled SBR/BR Blends. Polymers 2021, 13, 698. https://doi.org/10.3390/polym13050698
Sökmen S, Oßwald K, Reincke K, Ilisch S. Influence of Treated Distillate Aromatic Extract (TDAE) Content and Addition Time on Rubber-Filler Interactions in Silica Filled SBR/BR Blends. Polymers. 2021; 13(5):698. https://doi.org/10.3390/polym13050698
Chicago/Turabian StyleSökmen, Selin, Katja Oßwald, Katrin Reincke, and Sybill Ilisch. 2021. "Influence of Treated Distillate Aromatic Extract (TDAE) Content and Addition Time on Rubber-Filler Interactions in Silica Filled SBR/BR Blends" Polymers 13, no. 5: 698. https://doi.org/10.3390/polym13050698
APA StyleSökmen, S., Oßwald, K., Reincke, K., & Ilisch, S. (2021). Influence of Treated Distillate Aromatic Extract (TDAE) Content and Addition Time on Rubber-Filler Interactions in Silica Filled SBR/BR Blends. Polymers, 13(5), 698. https://doi.org/10.3390/polym13050698