Investigation of Carbon Nanotube Grafted Graphene Oxide Hybrid Aerogel for Polystyrene Composites with Reinforced Mechanical Performance
Abstract
:1. Introduction
2. Experimental
2.1. Materials
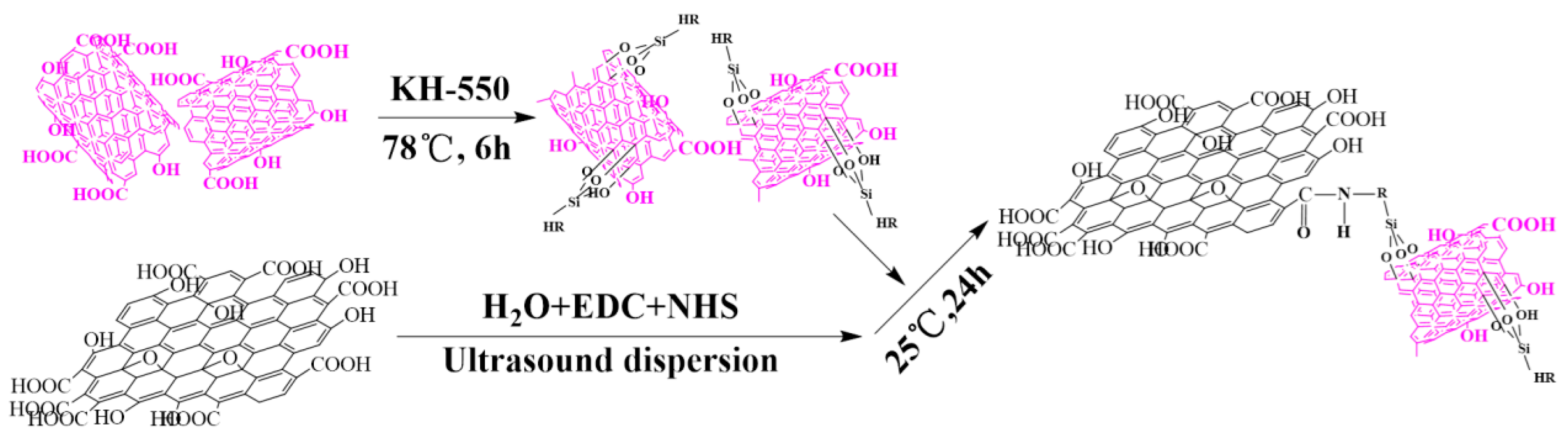
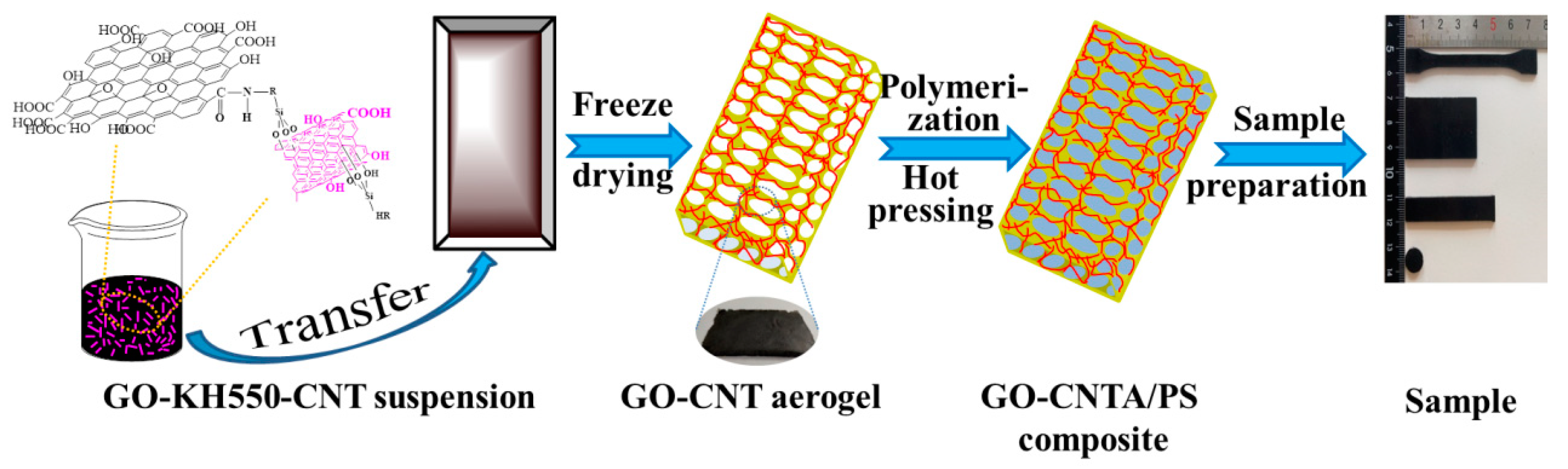
2.2. Preparation of the GO-CNT Aerogel
2.3. Preparation of GO-CNTA/PS Nanocomposites
2.4. Characterization
3. Results and Discussion
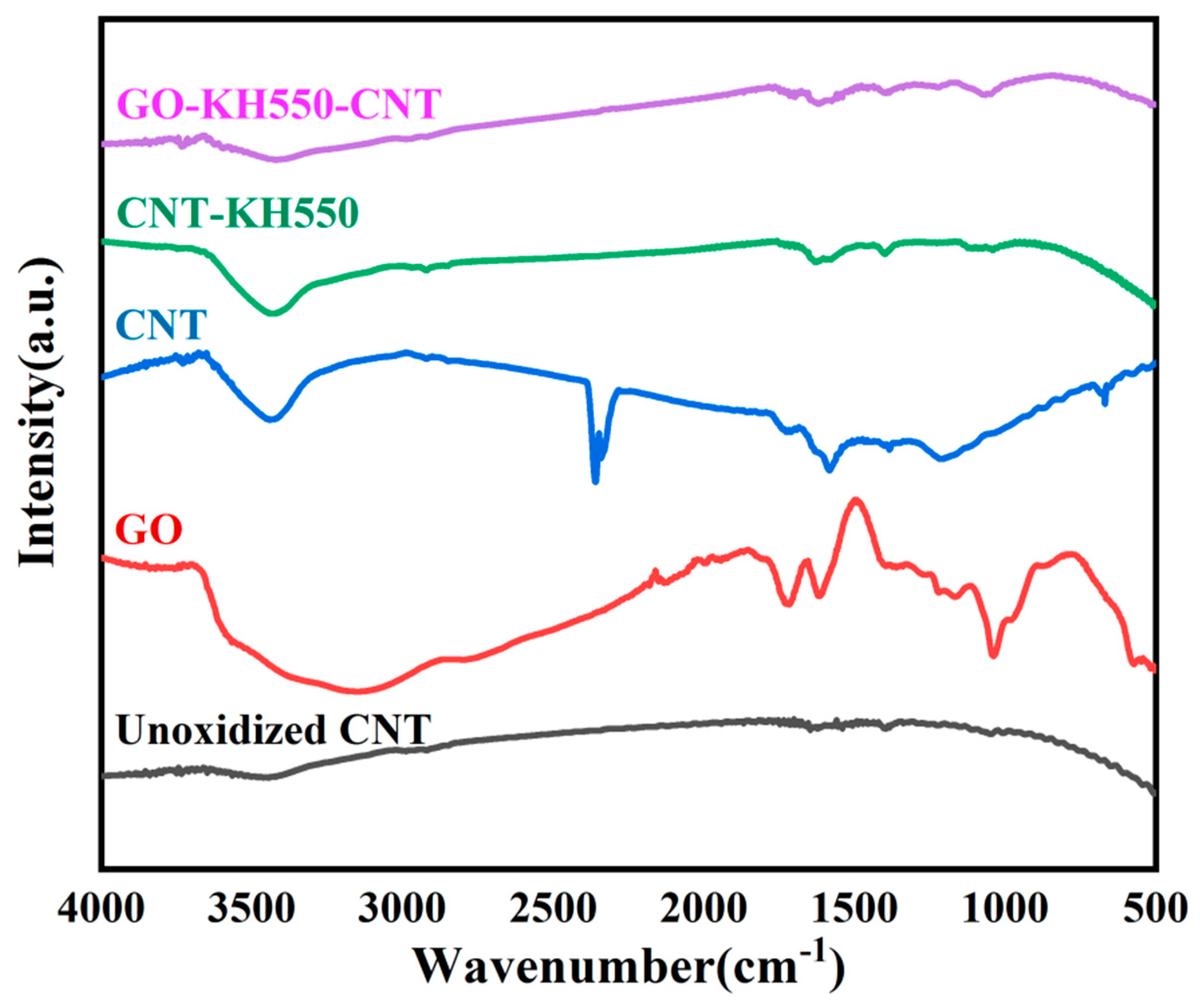
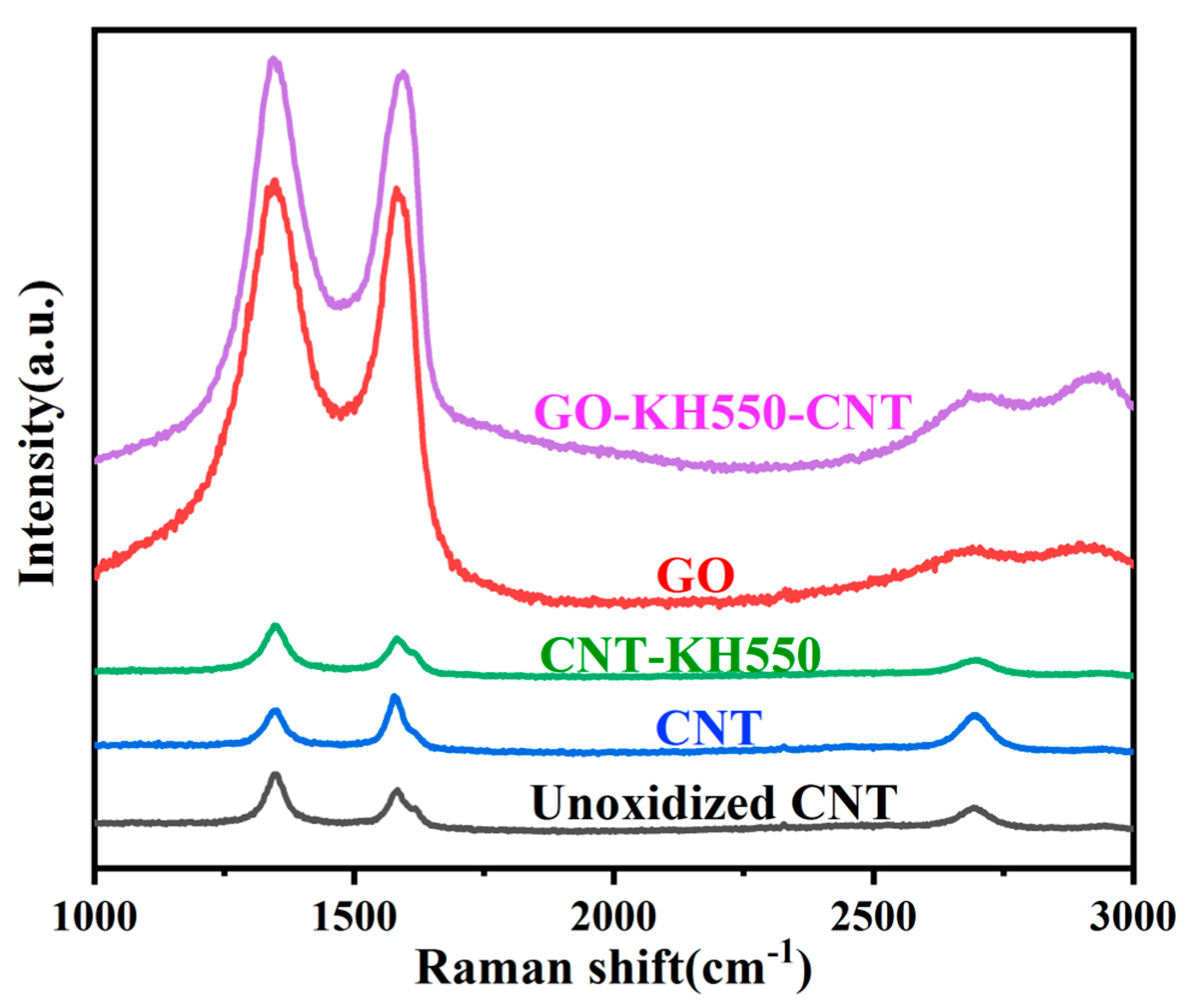
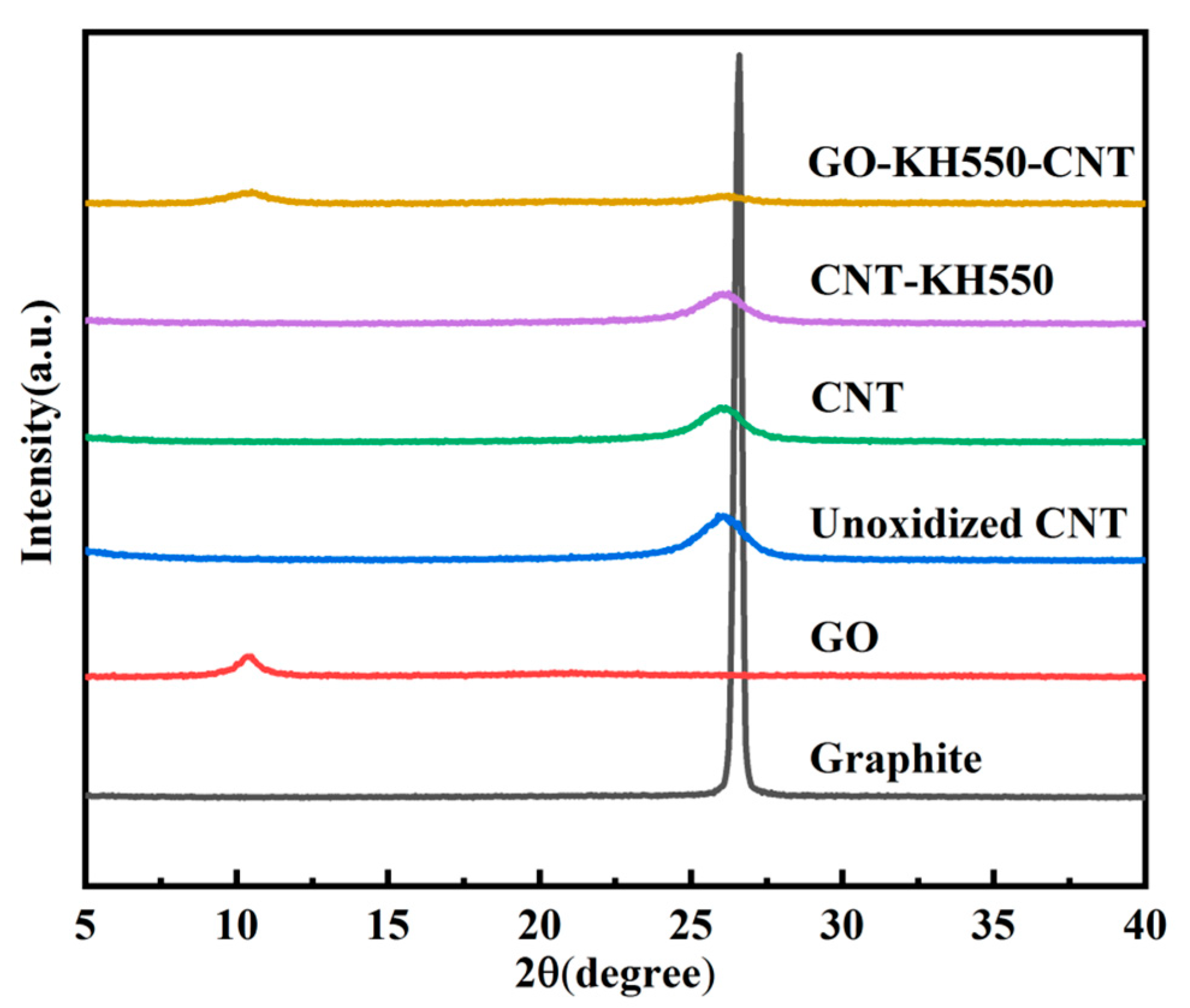
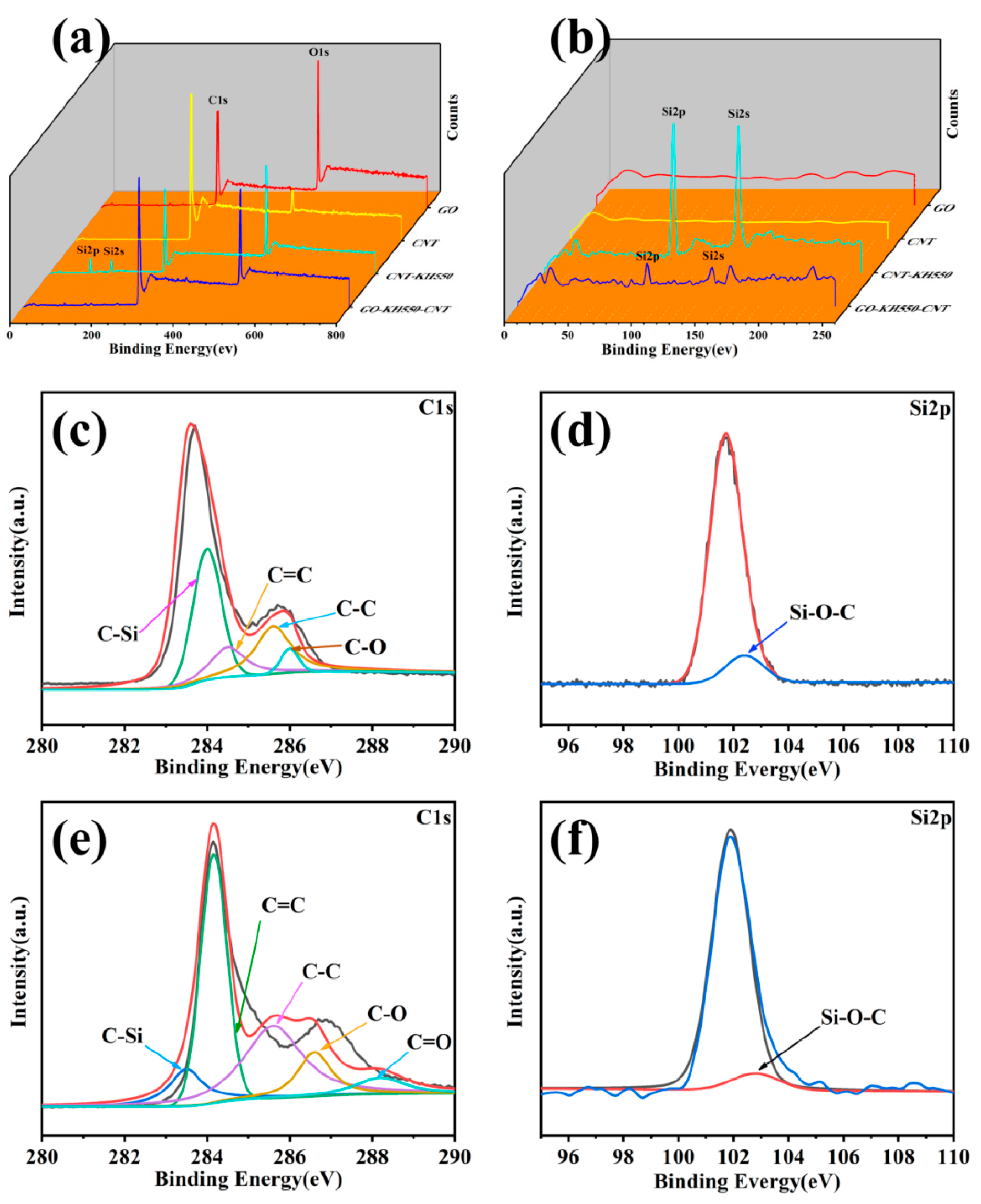
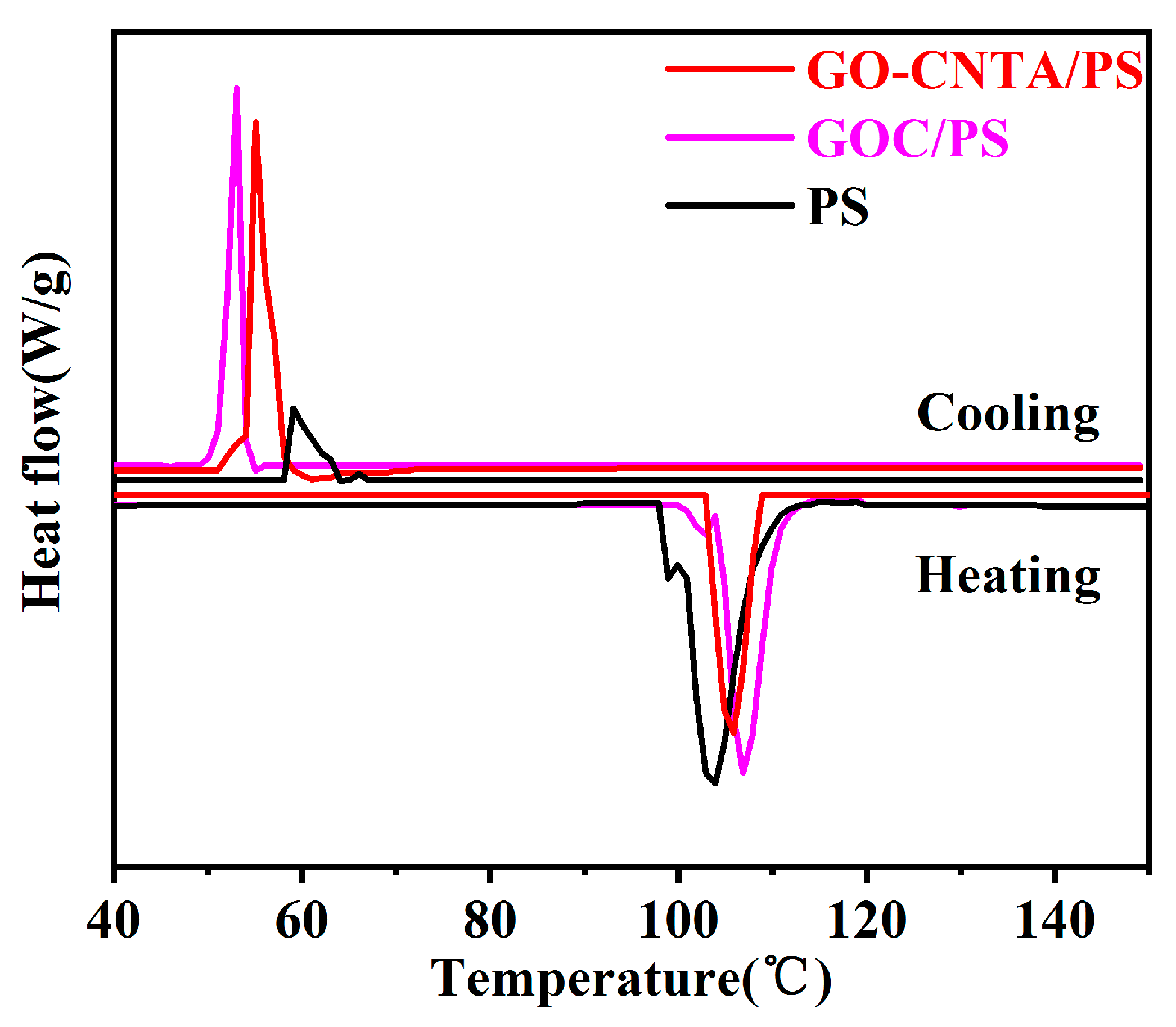
3.1. Chemical Composition and Morphology of GO, CNT, CNT-KH550, and GO-CNT
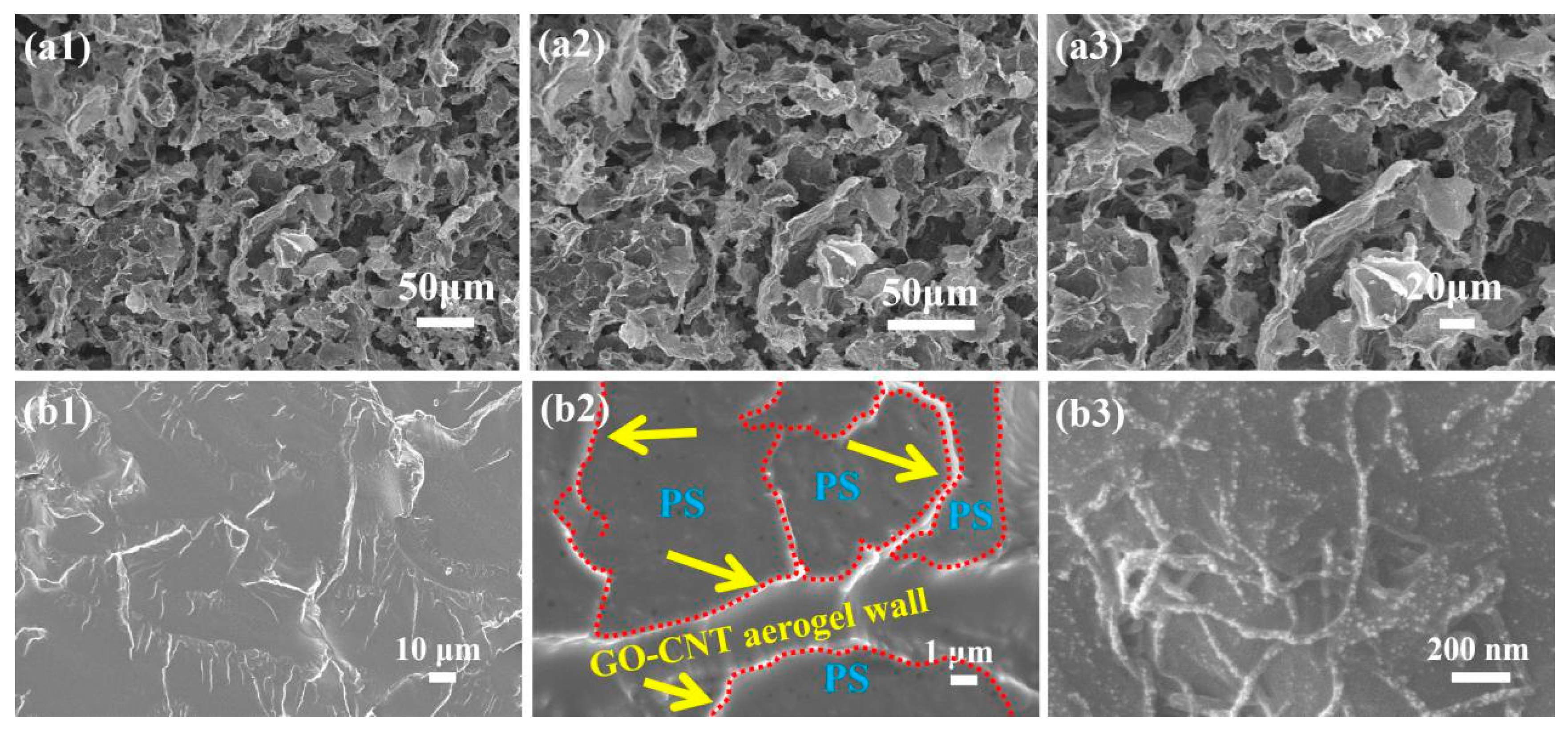
3.2. Morphology and Dispersion of GO-CNT Aerogel and GO-CNTA/PS Nanocomposites
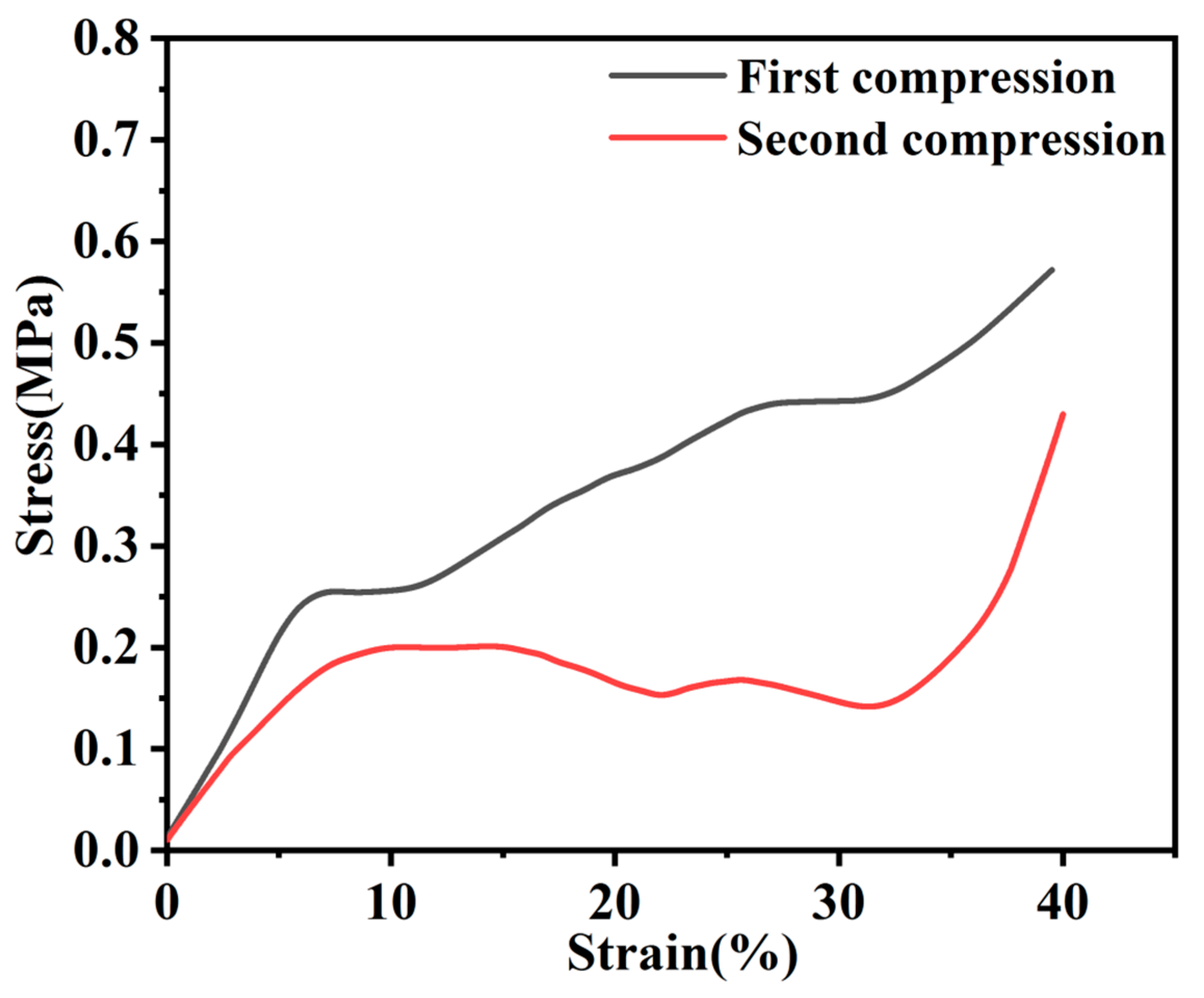
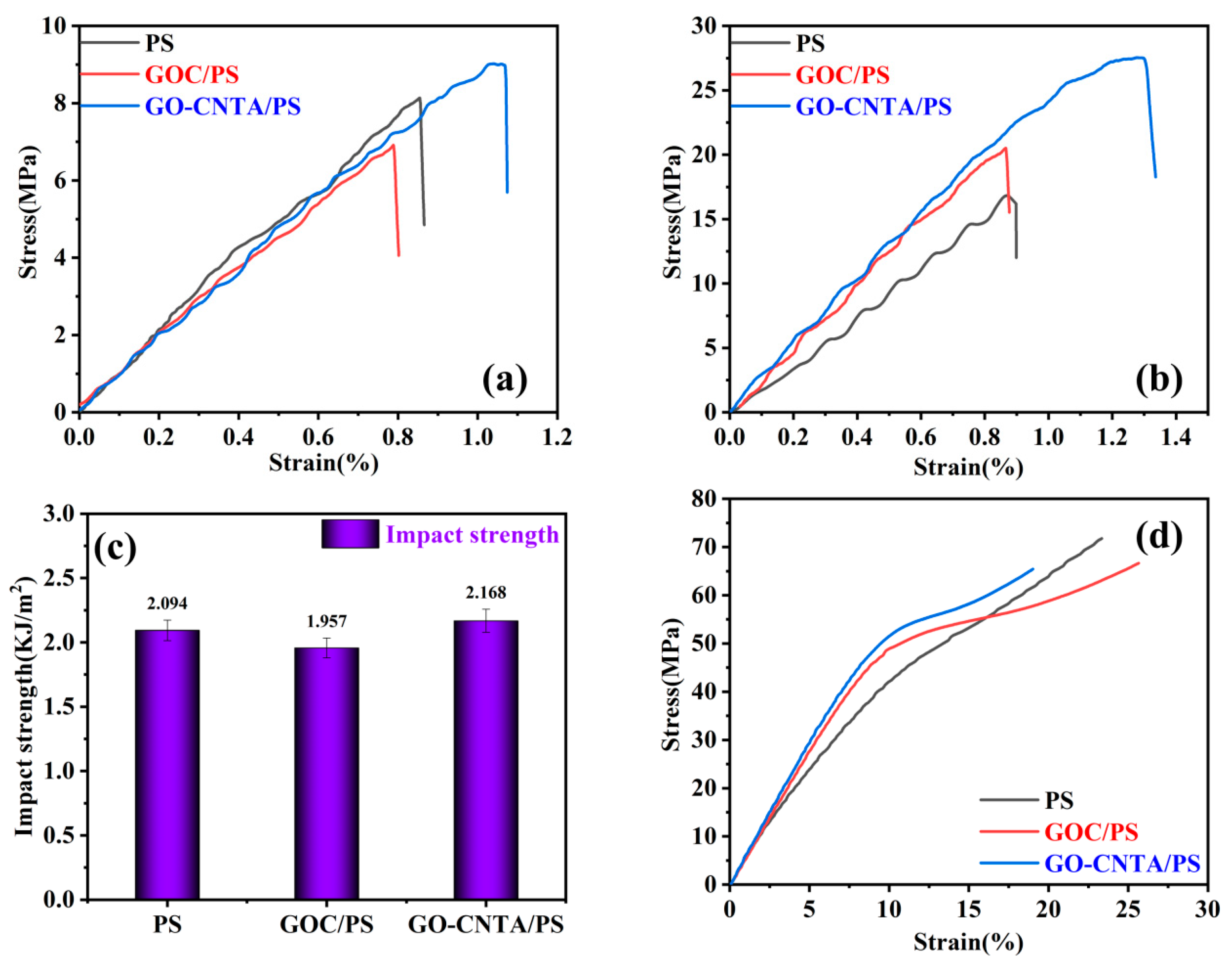
3.3. Mechanical Properties of the Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wanga, E.; Donga, Y.; Islam, Z.; Yu, L.; Liu, F.; Chen, S.; Qi, X.; Zhu, Y.; Fu, Y.; Xu, Z.; et al. Effect of graphene oxide-carbon nanotube hybrid filler on the mechanical property and thermal response speed of shape memory epoxy composites. Compos. Sci. Technol. 2019, 169, 209–216. [Google Scholar] [CrossRef]
- Banisaeid, M. Effect of functionalized carbon nanotubes on the mechanical properties of epoxy-based composites. Full. Nanotub. Carbon Nanostructures 2020, 28, 1–7. [Google Scholar] [CrossRef]
- Surnova, A.; Balkaev, D.; Musin, D.; Amirov, R.; Dimiev, A.M. Fully exfoliated graphene oxide accelerates epoxy resin curing, and results in dramatic improvement of the polymer mechanical properties. Compos. Part B Eng. 2019, 162, 685–691. [Google Scholar] [CrossRef]
- Shen, Y.; Xue, H.; Chen, K.; Zhou, Q.; Zhang, Y. Antimony selenide/graphene oxide composite for sensitive photoelectrochemical detection of DNA methyltransferase activity. J. Mater. Chem. B 2019, 7, 6789–6795. [Google Scholar]
- Ye, S.; Feng, J.; Wu, P. Highly elastic graphene oxide–epoxy composite aerogels via simple freeze-drying and subsequent routine curing. J. Mater. Chem. A 2013, 1, 3495–3502. [Google Scholar] [CrossRef]
- Xiao, W.; Liu, Y.; Guo, S. Composites of Graphene Oxide and Epoxy Resin Assuming Uniform 3D Graphene Oxide Network Structure. RSC Adv. 2016, 6, 86904–86908. [Google Scholar] [CrossRef]
- Zhang, W.; Kong, Q.; Tao, Z.; Wei, J.; Xie, L.; Cui, X. 3D Thermally Cross-Linked Graphene Aerogel–Enhanced Silicone Rubber Elastomer as Thermal Interface Material. Adv. Mater. Interfaces 2019, 6, 1900147–1900155. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, Z.; Wan, W.; Gogotsi, Y.; Qiu, J. Polymer/Graphene Hybrid Aerogel with High Compressibility, Conductivity, and “Sticky” Superhydrophobicity. ACS Appl. Mater. Inter. 2014, 6, 3242–3249. [Google Scholar] [CrossRef]
- Zhao, S.; Yan, Y.; Gao, A.; Shuai, C.; Jian, Z. Flexible Polydimethylsilane Nanocomposites Enhanced with a Three-Dimensional Graphene/Carbon Nanotube Bicontinuous Framework for High-Performance Electromagnetic Interference Shielding. ACS Appl. Mater. Inter. 2018, 10, 26723–26732. [Google Scholar] [CrossRef]
- Hidalgo-Manrique, P.; Yan, S.; Lin, F.; Hong, Q.; Kinloch, I.A.; Chen, X.; Young, R.J.; Zhang, X.; Dai, S. Microstructure and mechanical behaviour of aluminium matrix composites reinforced with graphene oxide and carbon nanotubes. J. Mater. Sci. 2017, 52, 13466–13477. [Google Scholar] [CrossRef]
- Xiumin, L.; Qunfeng, C. Synergistic reinforcing effect from graphene and carbon nanotubes. Compos. Commun. 2018, 10, 122–128. [Google Scholar]
- Song, B.; He, W.; Wang, X.; Zeng, X.; Wong, C.P. Fabrication of Stretchable and Conductive Polymer Nanocomposites based on Interconnected Graphene Aerogel. Compos. Sci. Technol. 2020, 200, 108430. [Google Scholar] [CrossRef]
- Shang, Y.; Li, T.; Zhu, X.; Zhang, Y.; Chen, X.; Zhao, T. Effect of the graphene sheets derived from multistep oxidized carbon nanotubes on the performance of graphene sheets/poly(methyl methacrylate) composites. J. Anal. Appl. Pyrol. 2015, 114, 243–249. [Google Scholar] [CrossRef]
- Cao, Q.; He, F.; Li, Y.; He, Z.; Fan, J.; Wang, R.; Hu, W.; Zhang, K.; Yang, W. Graphene-carbon nanotube hybrid aerogel/polyethylene glycol phase change composite for thermal management, Fullerenes. Full. Nanotub. Carbon Nanostructures 2020, 28, 656–662. [Google Scholar] [CrossRef]
- Zeng, F.; Feng, G.; Nguyen, S.T.; Hai, M.D. Advanced multifunctional graphene aerogel-Poly (methyl methacrylate) composites: Experiments and modeling. Carbon 2015, 81, 396–404. [Google Scholar]
- Chen, Z.; Wu, R.; Liu, Y.; Ha, Y.; Guo, Y.; Sun, D.; Liu, M.; Fang, F. Ultrafine Co Nanoparticles Encapsulated in Carbon-Nanotubes-Grafted Graphene Sheets as Advanced Electrocatalysts for the Hydrogen Evolution Reaction. Adv. Mater. 2018, 30, 1802011. [Google Scholar] [CrossRef]
- Li, Y.; Guo, L.J.; Wang, Y.W.; Li, H.J.; Song, Q. A Novel Multiscale Reinforcement by In-Situ Growing Carbon Nanotubes on Graphene Oxide Grafted Carbon Fibers and Its Reinforced Carbon/Carbon Composites with Improved Tensile Properties. J. Mater. Sci. Technol. 2016, 32, 419–424. [Google Scholar] [CrossRef]
- Li, W.; Dichiara, A.; Bai, J. Carbon nanotube-graphene nanoplatelet hybrids as high-performance multifunctional reinforcements in epoxy composites. Compos. Sci. Technol. 2013, 74, 221–227. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Lei, L.; Lan, T.; Li, Y.; Li, P.; Lin, X.; Liu, R.; Huang, Z.; Fen, X. Chemical vapor deposition-grown carbon nanotubes/graphene hybrids for electrochemical energy storage and conversion. Flatchem 2019, 15, 100091. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Li, P.; Ma, L.; Song, G. Enhancing mechanical performances of polystyrene composites via constructing carbon nanotube/graphene oxide aerogel and hot pressing. Compos. Sci. Technol. 2020, 195, 108191. [Google Scholar] [CrossRef]
- Hsan, N.; Dutta, P.K.; Kumar, S.; Bera, R.; Das, N. Chitosan grafted graphene oxide aerogel: Synthesis, characterization and carbon dioxide capture study. Int. J. Biol. Macromol. 2019, 125, 300–306. [Google Scholar] [CrossRef]
- Kumar, S.; Wani, M.Y.; Koh, J.; Gil, J.O.M.; Abilio, J.F.N.S. Carbon dioxide adsorption and cycloaddition reaction of epoxides using chitosan–graphene oxide nanocomposite as a catalyst. J. Environ. Sci. 2017, 69, 77–84. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Hosseini, S.S.; Vancaeyzeele, C. Graphene oxide nanocomposite hydrogel based on poly(acrylic acid) grafted onto salep: An adsorbent for the removal of noxious dyes from water. New J. Chem. 2019, 43, 3572–3582. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, W.; Liu, C.; He, S.; Zhang, F.; Shi, H.; Yang, M.; Wang, Z. Investigation of covalently grafted polyacrylate chains onto graphene oxide for epoxy composites with reinforced mechanical performance. J. Appl. Polym. Sci. 2019, 136, 47842–47851. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Ding, C.; Dai, Y.; Luo, C. Carboxylated carbon nanotubes-graphene oxide aerogels as ultralight and renewable high performance adsorbents for efficient adsorption of glyphosate. Environ. Chem. 2020, 17, 6–16. [Google Scholar] [CrossRef]

| Full Name | Abbreviation |
|---|---|
| GO, CNT and polystyrene blending composite | GOC/PS |
| polystyren | PS |
| CNT covalently bonded to KH550 | CNT-KH550 |
| CNT grafted GO aerogel | GO-CNTA |
| GO-CNT aerogel/polystyrene composite | GO-CNTA/PS |
| Sample | Tensile Strength (MPa) | Tensile Strain (%) | Flexural Strength (MPa) | Flexural Strain (%) | Impact Strength (KJ/m2) | Compressive Strength (MPa) | Compressive Strain (%) |
|---|---|---|---|---|---|---|---|
| PS | 8.29 | 0.858 | 18.74 | 0.95 | 2.09 | 33.45 | 7.56 |
| GOC/PS | 6.8 | 0.785 | 22.18 | 0.79 | 1.96 | 38.72 | 6.08 |
| GO-CNTA/PS | 9.037 | 1.074 | 27.525 | 1.405 | 2.168 | 53.441 | 10.853 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Xu, H.; Zhao, Z.; Zhang, L.; Ma, L.; Zhao, G.; Song, G.; Li, X. Investigation of Carbon Nanotube Grafted Graphene Oxide Hybrid Aerogel for Polystyrene Composites with Reinforced Mechanical Performance. Polymers 2021, 13, 735. https://doi.org/10.3390/polym13050735
Sun Y, Xu H, Zhao Z, Zhang L, Ma L, Zhao G, Song G, Li X. Investigation of Carbon Nanotube Grafted Graphene Oxide Hybrid Aerogel for Polystyrene Composites with Reinforced Mechanical Performance. Polymers. 2021; 13(5):735. https://doi.org/10.3390/polym13050735
Chicago/Turabian StyleSun, Yanzeng, Hui Xu, Zetian Zhao, Lina Zhang, Lichun Ma, Guozheng Zhao, Guojun Song, and Xiaoru Li. 2021. "Investigation of Carbon Nanotube Grafted Graphene Oxide Hybrid Aerogel for Polystyrene Composites with Reinforced Mechanical Performance" Polymers 13, no. 5: 735. https://doi.org/10.3390/polym13050735





