Carbon Nanomaterials Embedded in Conductive Polymers: A State of the Art
Abstract
:1. Introduction of Conductive Polymers
1.1. Polyaniline (PANI)
1.1.1. Synthesis
1.1.2. Characterization
1.2. Polypyrrole (PPy)
1.2.1. Synthesis
1.2.2. Characterization
1.3. Poly(3,4-ethylenedioxythiophene) PEDOT
1.3.1. Synthesis
1.3.2. Characterization
2. The Allotropic Nanoforms of Carbon
3. Carbon Dots and CP
3.1. Fabrication and Processing
3.1.1. Polymerization and Polymer Matrix Incorporation
3.1.2. Electrochemical Polymerization and Deposition
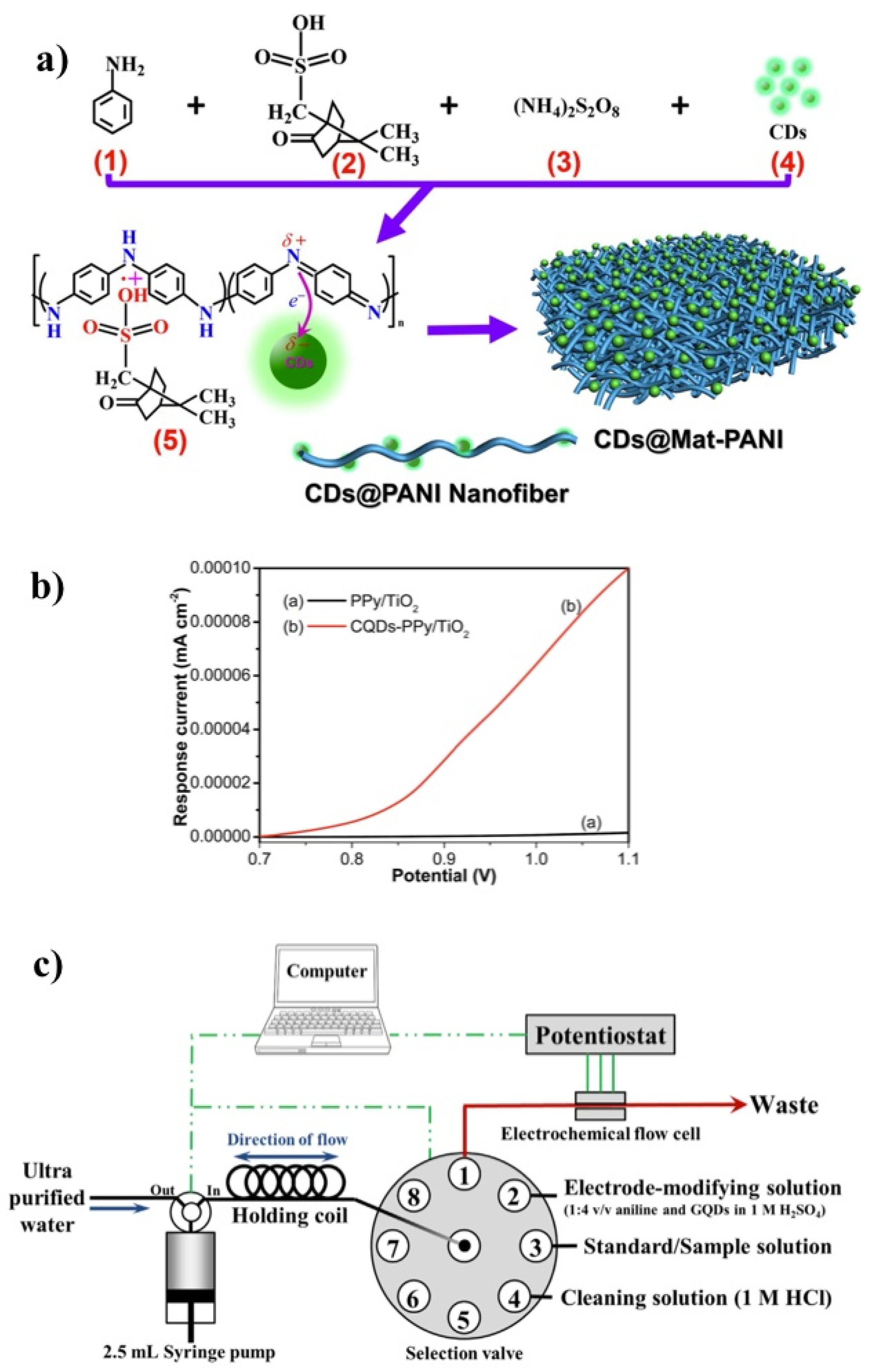
3.2. Characterization and Properties
3.2.1. Composition, Morphological and Physical Characterization and Properties
3.2.2. Optical Characterization and Properties
3.2.3. Electronic or Electrochemical and Thermoelectric Characterization and Properties
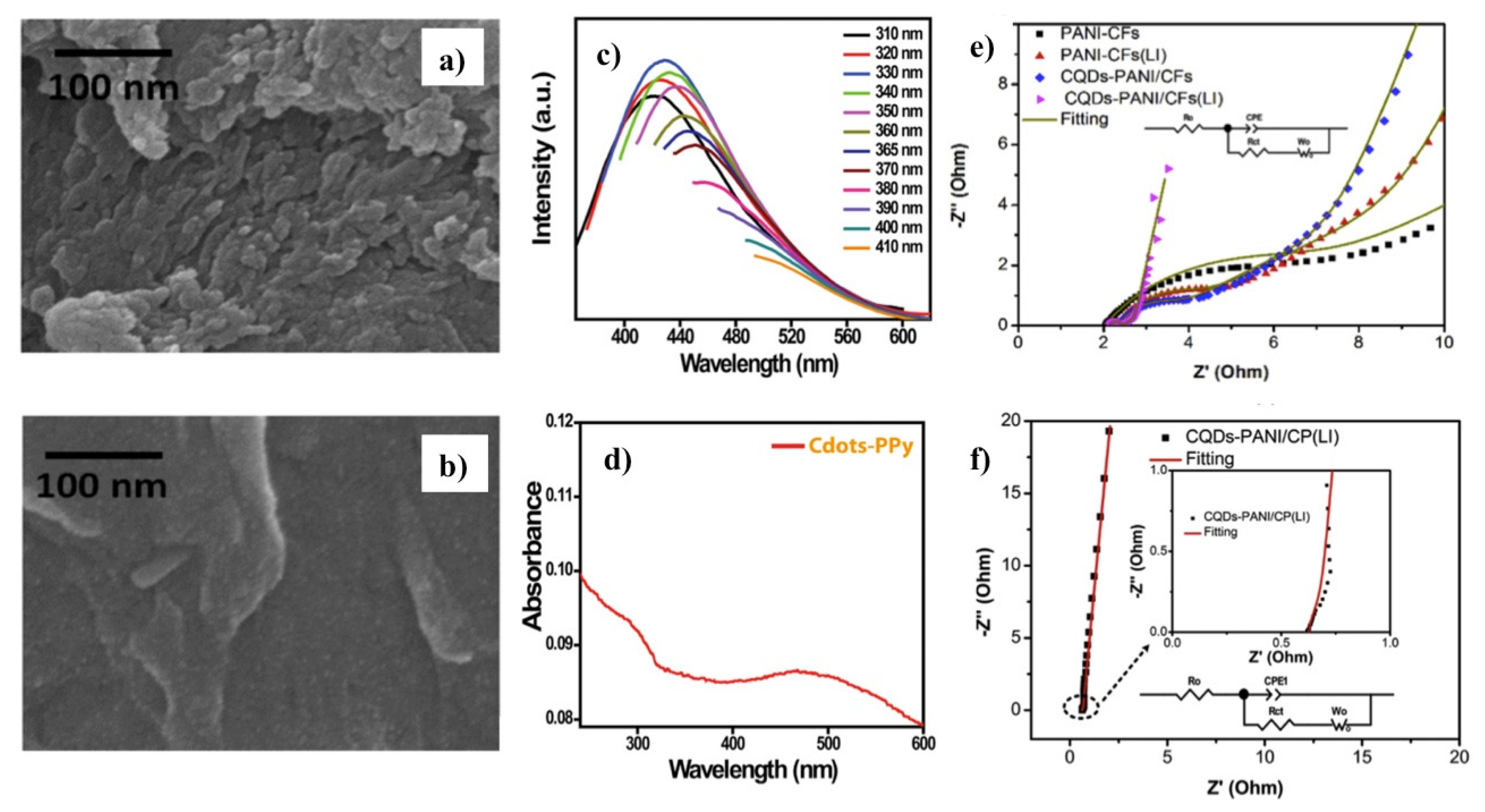
3.3. Applications
3.3.1. Supercapacitors for Energy Storage Applications
3.3.2. Dye-Sensitized Solar Cells
3.3.3. Sensing Applications
Fluorescence Biosensors
Electrical Conductivity Sensors
4. Graphene Mixed with CP
4.1. Fabrication and Processing
4.1.1. In Situ Production
4.1.2. Melt Processing
4.1.3. Solvent Mixing
4.1.4. Electrospinning
4.2. Characterization and Properties
4.3. Applications
4.3.1. Biomedical Applications
4.3.2. Electronic Applications
4.3.3. Sensing
5. CNT Embedded with Conjugated Polymers
5.1. Fabrication Approaches for Obtaining CNT/CP Materials
5.1.1. Coated Films
5.1.2. Fibers
5.1.3. Tridimensional Architectures
5.2. Applications of CNT/CP-Based Materials
5.2.1. Biomedical Purposes
5.2.2. Electronic Applications
5.2.3. Other Applications
6. Conclusions and Future Perspectives
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CD | carbon dots | CND | carbon nanodots |
| CNT | carbon nanotubes | CP | conductive polymers |
| CQD | carbon quantum dots | CVD | Chemical Vapour Deposition |
| DCM | dichloromethane | DMF | dimethylformamide |
| DSSC | Dye-Sensitized Solar Cells | ES | Electrospinning |
| FLG | few-layers graphene | FT-IR | Fourier-Transform Infrared Spectroscopy |
| G | graphene | GCE | Glassy Carbon Electrode |
| GO | graphene oxide | GPC | Gel Permeation Chromatography |
| GQD | graphene quantum dots | HA | hyaluronic acid |
| hfNSC | human fetal neural stem cells | hNSC | human neural stem cells |
| ITO | indium tin oxide | MALDI | Matrix-Assisted Laser Desorption/Ionization |
| MWCNT | multi-walled carbon nanotubes | NIPAM | N-isopropylacrylamide |
| PAA | poly(acrylamide) | PAN | polyacrylonitrile |
| PANI | polyaniline | PDMS | polydimethylsiloxane |
| PEDOT | poly(3,4-ethylenedioxythiophene) | PEG | polyethyleneglycol |
| PET | polyethylene terephthalate | pHEMA | poly(2-hydroxyethyl methacrylate) |
| PPy | polypyrrole | PSS | poly(styrenesulfonate) |
| PVA | polyvinyl alcohol | rGO | reduced graphene oxide |
| SEC | Size Exclusion Chromatography | SEM | Scanning Electron Microscopy |
| SPCE | Screen-Printed Carbon Electrode | SWCNT | single-walled carbon nanotubes |
| TCE | transparent conductive electrodes | TEM | Transmission Electron Microscopy |
| TGA | Thermogravimetric Analysis | UV-Vis-NIR | Ultraviolet-Visible-Near-IR Spectroscopy |
| VOC | volatile organic compounds | VPP | vapor phase polymerization |
| XPS | X-Ray Photoelectron Spectroscopy | YM | Young’s Modulus |
References
- Letheby, H. On the production of a blue substance by the electrolysis of sulphate of aniline. J. Chem. Soc. 1862, 15, 161–163. [Google Scholar] [CrossRef] [Green Version]
- De Surville, R.; Jozefowicz, M.; Yu, L.T.; Pepichon, J.; Buvet, R. Electrochemical chains using protolytic organic semiconductors. Electrochim. Acta 1968, 13, 1451–1458. [Google Scholar] [CrossRef]
- Diaz, A.F.; Logan, J.A. Electroactive polyaniline films. J. Electroanal. Chem. Interfacial Electrochem. 1980, 111, 111–114. [Google Scholar] [CrossRef]
- MacDiarmid, A.G.; Epstein, A.J. Polyanilines: A novel class of conducting polymers. Faraday Discuss. Chem. Soc. 1989, 88, 317. [Google Scholar] [CrossRef]
- Macdiarmid, A.G.; Chiang, J.C.; Richter, A.F.; Epstein, A.J. Polyaniline: A new concept in conducting polymers. Synth. Met. 1987, 18, 285–290. [Google Scholar] [CrossRef]
- Chiang, J.-C.; MacDiarmid, A.G. ‘Polyaniline’: Protonic acid doping of the emeraldine form to the metallic regime. Synth. Met. 1986, 13, 193–205. [Google Scholar] [CrossRef]
- Wu, K.; Xu, S.-z.; Zhou, X.-j.; Wu, H.-x. Graphene quantum dots enhanced electrochemical performance of polypyrrole as supercapacitor electrode. J. Electrochem. 2013, 19, 361–370. [Google Scholar]
- Xu, K.; Zhang, Q.; Hao, Z.; Tang, Y.; Wang, H.; Liu, J.; Yan, H. Integrated electrochromic supercapacitors with visual energy levels boosted by coating onto carbon nanotube conductive networks. Sol. Energy Mater. Sol. Cells 2020, 206. [Google Scholar] [CrossRef]
- Ben, J.; Song, Z.; Liu, X.; Lu, W.; Li, X. Fabrication and Electrochemical Performance of PVA/CNT/PANI Flexible Films as Electrodes for Supercapacitors. Nanoscale Res. Lett. 2020, 15. [Google Scholar] [CrossRef]
- Kazemi, F.; Naghib, S.M.; Zare, Y.; Rhee, K.Y. Biosensing Applications of Polyaniline (PANI)-Based Nanocomposites: A Review. Polym. Rev. 2020, 1–45. [Google Scholar] [CrossRef]
- Hu, L.; Tu, J.; Jiao, S.; Hou, J.; Zhu, H.; Fray, D.J. In situ electrochemical polymerization of a nanorod-PANI–Graphene composite in a reverse micelle electrolyte and its application in a supercapacitor. Phys. Chem. Chem. Phys. 2012, 14, 15652–15656. [Google Scholar] [CrossRef]
- Moayeri, A.; Ajji, A. Fabrication of polyaniline/poly (ethylene oxide)/non-covalently functionalized graphene nanofibers via electrospinning. Synth. Met. 2015, 200, 7–15. [Google Scholar] [CrossRef]
- Chauhan, N.P.S.; Mozafari, M. Synthetic route of PANI (II): Enzymatic method. In Fundamentals and Emerging Applications of Polyaniline; Elsevier: Amsterdam, The Netherlands, 2019; pp. 43–65. [Google Scholar] [CrossRef]
- Chauhan, N.P.S.; Milan, P.B.; Kargozar, S.; Mozafari, M. Synthetic route of PANI (III): Ultrasound-assisted polymerization. In Fundamentals and Emerging Applications of Polyaniline; Elsevier: Amsterdam, The Netherlands, 2019; pp. 67–89. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Saeb, M.R. Synthetic route of polyaniline (IV): Irradiation path. In Fundamentals and Emerging Applications of Polyaniline; Elsevier: Amsterdam, The Netherlands, 2019; pp. 91–103. [Google Scholar] [CrossRef]
- Dalmolin, C.; Canobre, S.C.; Biaggio, S.R.; Rocha-Filho, R.C.; Bocchi, N. Electropolymerization of polyaniline on high surface area carbon substrates. J. Electroanal. Chem. 2005, 578, 9–15. [Google Scholar] [CrossRef]
- Chen, W.C.; Wen, T.C.; Gopalan, A. Negative capacitance for polyaniline: An analysis via electrochemical impedance spectroscopy. Synth. Met. 2002, 128, 179–189. [Google Scholar] [CrossRef]
- Plesu, N.; Kellenberger, A.; Mihali, M.; Vaszilcsin, N. Effect of temperature on the electrochemical synthesis and properties of polyaniline films. J. Non-Cryst. Solids 2010, 356, 1081–1088. [Google Scholar] [CrossRef]
- Korent, A.; Soderžnik, K.Ž.; Šturm, S.; Rožman, K.Ž. A Correlative Study of Polyaniline Electropolymerization and its Electrochromic Behavior. J. Electrochem. Soc. 2020, 167, 106504. [Google Scholar] [CrossRef]
- Kaitsuka, Y.; Goto, H. UV Light Induces Dedoping of Polyaniline. Polymers 2016, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Rannou, P.; Pron, A.; Nechtschein, M. UV-vis-NIR studies of new PANI/dopant/solvent associations with metallic-like behaviour. Synth. Met. 1999, 101, 827–828. [Google Scholar] [CrossRef]
- Ibrahim, K.A. Synthesis and characterization of polyaniline and poly(aniline-co-o-nitroaniline) using vibrational spectroscopy. Arab. J. Chem. 2017, 10, S2668–S2674. [Google Scholar] [CrossRef] [Green Version]
- Karaoğlan, N.; Bindal, C. Synthesis and optical characterization of benzene sulfonic acid doped polyaniline. Eng. Sci. Technol. Int. J. 2018, 21, 1152–1158. [Google Scholar] [CrossRef]
- Šeděnková, I.; Trchová, M.; Stejskal, J. Thermal degradation of polyaniline films prepared in solutions of strong and weak acids and in water—FTIR and Raman spectroscopic studies. Polym. Degrad. Stab. 2008, 93, 2147–2157. [Google Scholar] [CrossRef]
- Yang, D.; Lu, W.; Goering, R.; Mattes, B.R. Investigation of polyaniline processibility using GPC/UV-vis analysis. Synth. Met. 2009, 159, 666–674. [Google Scholar] [CrossRef]
- Bláha, M.; Marek, F.; Morávkova, Z.; Svoboda, J.; Brus, J.; Dybal, J.; Prokes, J.; Varga, M.; Stejskal, J. Role of p-Benzoquinone in the Synthesis of a Conducting Polymer, Polyaniline. ACS Omega 2019, 4, 7128–7139. [Google Scholar] [CrossRef] [Green Version]
- Angeli, A.; Pieroni, A. Sopra un nuovo modo di formazione del nero di pirrolo. Gazz. Chim. Ital. 1919, 1, 154–158. [Google Scholar]
- Dall’ Olio, A.; Dascola, G.; Varacca, V.; Bocchi, V. Résonance paramagnétique électronique et conductivité d’un noir d’oxypyrrol électrolitique. CR. Acad. Sci. Paris 1968, 267, 433. [Google Scholar]
- Gardini, G.P. The Oxidation of Monocyclic Pyrroles. Adv. Heterocycl. Chem. 1973, 15, 67–98. [Google Scholar] [CrossRef]
- Diaz, A.F.; Kanazawa, K.K.; Gardini, G.P. Electrochemical polymerization of pyrrole. J. Chem. Soc. Chem. Commun. 1979, 635–636. [Google Scholar] [CrossRef]
- Jain, R.; Jadon, N.; Pawaiya, A. Polypyrrole based next generation electrochemical sensors and biosensors: A review. Trends Anal. Chem. 2017, 97, 363–373. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M. Conducting polypyrrole nanotubes: A review. Chem. Pap. 2018, 72, 1563–1595. [Google Scholar] [CrossRef]
- Vernitskaya, V.V.; Efimov, O.N. Polypyrrole: A conducting polymer; its synthesis, properties and applications. Russ. Chem. Rev. 1997, 66, 443–457. [Google Scholar] [CrossRef]
- Maksymiuk, K. Chemical reactivity of polypyrrole and its relevance to polypyrrole based electrochemical sensors. Electroanalysis 2006, 18, 1537–1551. [Google Scholar] [CrossRef]
- Watanabe, A.; Tanaka, M.; Tanaka, J. Electrical and Optical Properties of a Stable Synthetic Metallic Polymer: Polypyrrole. Bull. Chem. Soc. Jpn. 1981, 54, 2278–2281. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Drzal, L.T. Multilayered nanoarchitecture of graphene nanosheets and polypyrrole nanowires for high performance supercapacitor electrodes. Chem. Mater. 2010, 22, 5667–5671. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, P.; Wang, A.; Yu, C.; Qian, T.; Wu, S.; Shen, J. Dopamine fluorescent sensors based on polypyrrole/graphene quantum dots core/shell hybrids. Biosens. Bioelectron. 2015, 64, 404–410. [Google Scholar] [CrossRef]
- Bao, L.; Yao, J.; Zhao, S.; Lu, Y.; Su, Y.; Chen, L.; Zhao, C.; Wu, F. Densely Packed 3D Corrugated Papery Electrodes as Polysulfide Reservoirs for Lithium-Sulfur Battery with Ultrahigh Volumetric Capacity. ACS Sustain. Chem. Eng. 2020, 8, 5648–5661. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, Y.J.; Kim, D.W.; Cheruku, R.; Thogiti, S.; Ahn, K.-S.; Kim, J.H. Application of polypyrrole/sodium dodecyl sulfate/carbon nanotube counter electrode for solid-state dye-sensitized solar cells and dye-sensitized solar cells. Chem. Pap. 2019, 73, 2749–2755. [Google Scholar] [CrossRef]
- Duan, D.; Yang, H.; Ding, Y.; Li, L.; Ma, G. A three-dimensional conductive molecularly imprinted electrochemical sensor based on MOF derived porous carbon/carbon nanotubes composites and prussian blue nanocubes mediated amplification for chiral analysis of cysteine enantiomers. Electrochim. Acta 2019, 302, 137–144. [Google Scholar] [CrossRef]
- Deshmukh, K.; Ahamed, M.B.; Deshmukh, R.R.; Pasha, S.K.; Bhagat, P.R.; Chidambaram, K. Biopolymer Composites with High Dielectric Performance: Interface Engineering. In Biopolymer Composites in Electronics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–128. [Google Scholar] [CrossRef]
- Truong, V.T.; Ennis, B.C.; Forsyth, M. Ion exchange, anisotropic structure and thermal stability of polypyrrole films. Synth. Met. 1995, 69, 479–480. [Google Scholar] [CrossRef]
- Toshima, N.; Hara, S. Direct synthesis of conducting polymers from simple monomers. Prog. Polym. Sci. 1995, 20, 155–183. [Google Scholar] [CrossRef]
- Rapi, S.; Bocchi, V.; Gardini, G.P. Conducting polypyrrole by chemical synthesis in water. Synth. Met. 1988, 24, 217–221. [Google Scholar] [CrossRef]
- Kang, E.T.; Neoh, K.G.; Ong, Y.K.; Tan, K.L.; Tan, B.T.G. X-ray Photoelectron Spectroscopic Studies of Polypyrrole Synthesized with Oxidative Fe(III) Salts. Macromolecules 1991, 24, 2822–2828. [Google Scholar] [CrossRef]
- Qi, G.; Huang, L.; Wang, H. Highly conductive free standing polypyrrole films prepared by freezing interfacial polymerization. Chem. Commun. 2012, 48, 8246–8248. [Google Scholar] [CrossRef]
- Bloor, D.; Monkman, A.P.; Stevens, G.C.; Cheung, K.M.; Pugh, S. Structure-Property Relationships in Conductive Polymers. Mol. Cryst. Liq. Cryst. 1990, 187, 231–239. [Google Scholar] [CrossRef]
- Beck, F.; Oberst, M. Electrocatalytic deposition and transformation of polypyrrole layers. Synth. Met. 1989, 28, 43–50. [Google Scholar] [CrossRef]
- Debiemme-Chouvy, C.; Tran, T.T.M. An insight into the overoxidation of polypyrrole materials. Electrochem. Commun. 2008, 10, 947–950. [Google Scholar] [CrossRef]
- Shiigi, H.; Kishimoto, M.; Yakabe, H.; Deore, B.; Nagaoka, T. Highly Selective Molecularly Imprinted Overoxidized Polypyrrole Colloids: One-Step Preparation Technique. Anal. Sci. 2002, 18, 41–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Yu, N.; Zhang, L.; Liu, Z.; Wang, Z.; Chen, Z. Synthesis of polypyrrole nanoparticles for constructing full-polymer UV/NIR-shielding film. RSC Adv. 2015, 5, 96888–96895. [Google Scholar] [CrossRef]
- Song, M.K.; Kim, Y.T.; Kim, B.S.; Kim, J.; Char, K.; Rhee, H.W. Synthesis and characterization of soluble polypyrrole doped with alkylbenzenesulfonic acids. Synth. Met. 2004, 141, 315–319. [Google Scholar] [CrossRef]
- Omastová, M.; Mičušík, M. Polypyrrole coating of inorganic and organic materials by chemical oxidative polymerisation. Chem. Pap. 2012, 66, 392–414. [Google Scholar] [CrossRef]
- Liang, Y.; Goh, J.C.-H. Polypyrrole-Incorporated Conducting Constructs for Tissue Engineering Applications: A Review. Bioelectricity 2020, 2, 101–119. [Google Scholar] [CrossRef]
- Chougule, M.A.; Pawar, S.G.; Godse, P.R.; Mulik, R.N.; Sen, S.; Patil, V.B. Synthesis and Characterization of Polypyrrole (PPy) Thin Films. Soft Nanosci. Lett. 2011, 1, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Trchová, M.; Stejskal, J. Resonance Raman Spectroscopy of Conducting Polypyrrole Nanotubes: Disordered Surface versus Ordered Body. J. Phys. Chem. A 2018, 122, 9298–9306. [Google Scholar] [CrossRef]
- Bufon, C.C.B.; Vollmer, J.; Heinzel, T.; Espindola, P.; John, H.; Heinze, J. Relationship between chain length, disorder, and resistivity in polypyrrole films. J. Phys. Chem. B 2005, 109, 19191–19199. [Google Scholar] [CrossRef]
- Zotti, G.; Martina, S.; Wegner, G.; Schlüter, A.D.D. Well-defined pyrrole oligomers: Electrochemical and UV/vis studies. Adv. Mater. 1992, 4, 798–801. [Google Scholar] [CrossRef]
- Brédas, J.L.; Silbey, R.; Boudreaux, D.S.; Chance, R.R. Chain-Length Dependence of Electronic and Electrochemical Properties of Conjugated Systems: Polyacetylene, Polyphenylene, Polythiophene, and Polypyrrole. J. Am. Chem. Soc. 1983, 105, 6555–6559. [Google Scholar] [CrossRef]
- Mondal, S.; Sangaranarayanan, V.M. A novel, rapid synthetic protocol for controllable sizes, conductivities and monomer units of soluble polypyrrole. Eur. Polym. J. 2015, 71, 596–611. [Google Scholar] [CrossRef]
- Texidó, R.; Anguera, G.; Colominas, S.; Borrós, S.; Sánchez-García, D. Extended 2,2′-Bipyrroles: New Monomers for Conjugated Polymers with Tailored Processability. Polymers 2019, 11, 1068. [Google Scholar] [CrossRef] [Green Version]
- Andriukonis, E.; Ramanaviciene, A.; Ramanavicius, A. Synthesis of Polypyrrole Induced by [Fe(CN)6]3− and Redox Cycling of [Fe(CN)6]4−/[Fe(CN)6]3−. Polymers 2018, 10, 749. [Google Scholar] [CrossRef] [Green Version]
- Armour, M.; Davies, A.G.; Upadhyay, J.; Wassermann, A. Colored electrically conducting polymers from furan, pyrrole, and thiophene. J. Polym. Sci. Part A-1 Polym. Chem. 1967, 5, 1527–1538. [Google Scholar] [CrossRef]
- Tourillon, G.; Garnier, F. New electrochemically generated organic conducting polymers. J. Electroanal. Chem. Interfacial Electrochem. 1982, 135, 173–178. [Google Scholar] [CrossRef]
- McCullough, R.D.; Lowe, R.D.; Gu, H.B.; Yoshino, K.; Wudl, F.; Spinelli, D. Enhanced electrical conductivity in regioselectively synthesized poly(3-alkylthiophenes). J. Chem. Soc. Chem. Commun. 1992, 24, 70. [Google Scholar] [CrossRef]
- Chen, T.A.; Rieke, R.D. The first regioregular head-to-tail poly(3-hexylthiophene-2,5-diyl) and a regiorandom isopolymer: Nickel versus palladium catalysis of 2(5)-bromo-5(2)-(bromozincio)-3-hexylthiophene polymerization. J. Am. Chem. Soc. 1992, 114, 10087–10088. [Google Scholar] [CrossRef]
- Guillerez, S.; Bidan, G. New convenient synthesis of highly regioregular poly(3-octylthiophene) based on the Suzuki coupling reaction. Synth. Met. 1998, 93, 123–126. [Google Scholar] [CrossRef]
- Wegener, P.; Feldhues, M.; Litterer, H. Process for the Preparaton of Thiophene Ethers. Eur. Pat. Appl. 328984, 1988. [Google Scholar]
- Feldhues, M.; Kämpf, G.; Mecklenburg, T. Highly Soluble Electrically Conductive Polymers. Eur. Pat. Appl. 328983, 1988. [Google Scholar]
- Kämpf, G.; Feldhues, M. Electrically Conductive Coating Composition, Method for Its Manufacture and Its Use. Eur. Pat. Appl. 292905, 1987. [Google Scholar]
- Feldhues, M.; Mecklenburg, T.; Wegener, P.; Kämpf, G. Soluble Electrically Conductive Polymers, Method of Producing Them and Their Use. Eur. Pat. Appl. 257573, 1987. [Google Scholar]
- Feldhues, M.; Kämpf, G.; Mecklenburg, T. Modified Electrical Conductive Polymers. Eur. Pat. Appl. 313998, 1987. [Google Scholar]
- Kämpf, G.; Feldhues, M. Electrically Conducting Coating Compound, Process for Its Preparation and Its Use/Electrically Conductive Polymers and Their Production. Eur. Pat. Appl. 328981/328982, 1988. [Google Scholar]
- Heywang, G.; Jonas, F. Poly(alkylenedioxythiophene)s—New, very stable conducting polymers. Adv. Mater. 1992, 4, 116–118. [Google Scholar] [CrossRef]
- Mantione, D.; del Agua, I.; Sanchez-Sanchez, A.; Mecerreyes, D. Poly(3,4-ethylenedioxythiophene) (PEDOT) Derivatives: Innovative Conductive Polymers for Bioelectronics. Polymers 2017, 9, 354. [Google Scholar] [CrossRef]
- Ha, Y.H.; Nikolov, N.; Pollack, S.K.; Mastrangelo, J.; Martin, B.D.; Shashidhar, R. Towards a Transparent, Highly Conductive Poly(3,4-ethylenedioxythiophene). Adv. Funct. Mater. 2004, 14, 615–622. [Google Scholar] [CrossRef]
- Kirchmeyer, S.; Reuter, K. Scientific importance, properties and growing applications of poly(3,4-ethylenedioxythiophene). J. Mater. Chem. 2005, 15, 2077. [Google Scholar] [CrossRef]
- Zhang, X.; Lee, J.-S.; Lee, G.S.; Cha, D.-K.; Kim, M.J.; Yang, D.J.; Manohar, S.K. Chemical Synthesis of PEDOT Nanotubes. Macromolecules 2006, 39, 470–472. [Google Scholar] [CrossRef]
- Snaith, H.J.; Kenrick, H.; Chiesa, M.; Friend, R.H. Morphological and electronic consequences of modifications to the polymer anode ‘PEDOT:PSS’. Polymer 2005, 46, 2573–2578. [Google Scholar] [CrossRef]
- Wailes, E.M.; MacNeill, C.M.; McCabe, E.; Levi-Polyachenko, N.H. Shaping PEDOT nanoparticles for use in 3D tissue phantoms. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Xia, Y.; Ouyang, J. Significant different conductivities of the two grades of poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate), Clevios P and clevios PH1000, arising from different molecular weights. ACS Appl. Mater. Interfaces 2012, 4, 4131–4140. [Google Scholar] [CrossRef] [PubMed]
- Mantione, D.; Del Agua, I.; Schaafsma, W.; Diez-Garcia, J.; Castro, B.; Sardon, H.; Mecerreyes, D. Poly(3,4-ethylenedioxythiophene):GlycosAminoGlycan Aqueous Dispersions: Toward Electrically Conductive Bioactive Materials for Neural Interfaces. Macromol. Biosci. 2016, 16, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Sanyoto, B.; Park, W.-T.; Kim, S.; Mandal, S.; Lim, J.-C.; Noh, Y.-Y.; Kim, J.-H. Purification of PEDOT:PSS by Ultrafiltration for Highly Conductive Transparent Electrode of All-Printed Organic Devices. Adv. Mater. 2016, 28, 10149–10154. [Google Scholar] [CrossRef]
- Cho, H.; Cho, W.; Kim, Y.; Lee, J.G.; Kim, J.H. Influence of residual sodium ions on the structure and properties of poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate). RSC Adv. 2018, 8, 29044–29050. [Google Scholar] [CrossRef] [Green Version]
- Yoo, D.; Kim, J.; Kim, J.H. Direct synthesis of highly conductive poly(3,4-ethylenedioxythiophene):Poly(4-styrenesulfonate) (PEDOT:PSS)/graphene composites and their applications in energy harvesting systems. Nano Res. 2014, 7, 717–730. [Google Scholar] [CrossRef]
- Wijeratne, K.; Vagin, M.; Brooke, R.; Crispin, X. Poly(3,4-ethylenedioxythiophene)-tosylate (PEDOT-Tos) electrodes in thermogalvanic cells. J. Mater. Chem. A 2017, 5, 19619–19625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Petsagkourakis, I.; Spampinato, N.; Kuang, C.; Liu, X.; Brooke, R.; Kang, E.S.H.; Fahlman, M.; Crispin, X.; Pavlopoulou, E.; et al. Unraveling vertical inhomogeneity in vapour phase polymerized PEDOT:Tos films. J. Mater. Chem. A 2020, 8, 18726–18734. [Google Scholar] [CrossRef]
- Petsagkourakis, I.; Kim, N.; Tybrandt, K.; Zozoulenko, I.; Crispin, X. Poly(3,4-ethylenedioxythiophene): Chemical Synthesis, Transport Properties, and Thermoelectric Devices. Adv. Electron. Mater. 2019, 5, 1800918. [Google Scholar] [CrossRef]
- Seki, Y.; Takahashi, M.; Takashiri, M. Effects of different electrolytes and film thicknesses on structural and thermoelectric properties of electropolymerized poly(3,4-ethylenedioxythiophene) films. RSC Adv. 2019, 9, 15957–15965. [Google Scholar] [CrossRef] [Green Version]
- Massonnet, N.; Carella, A.; Jaudouin, O.; Rannou, P.; Laval, G.; Celle, C.; Simonato, J.-P.P. Improvement of the Seebeck coefficient of PEDOT:PSS by chemical reduction combined with a novel method for its transfer using free-standing thin films. J. Mater. Chem. C 2014, 2, 1278–1283. [Google Scholar] [CrossRef]
- Koromilas, N.D.; Lainioti, G.C.; Oikonomou, E.K.; Bokias, G.; Kallitsis, J.K. Synthesis and self-association in dilute aqueous solution of hydrophobically modified polycations and polyampholytes based on 4-vinylbenzyl chloride. Eur. Polym. J. 2014, 54, 39–51. [Google Scholar] [CrossRef]
- Sriprachuabwong, C.; Karuwan, C.; Wisitsorrat, A.; Phokharatkul, D.; Lomas, T.; Sritongkham, P.; Tuantranont, A. Inkjet-printed graphene-PEDOT:PSS modified screen printed carbon electrode for biochemical sensing. J. Mater. Chem. 2012, 22, 5478–5485. [Google Scholar] [CrossRef]
- Langford, E.G.; Shaughnessy, K.D.; Devore, T.C.; Lawrence, D.; Constantin, C. Analysis of PEDOT:PSS Films after Sulfuric Acid Treatment on Silicon and Fused Silica using FT-IR and UV-VIS. MRS Adv. 2016, 1, 465–469. [Google Scholar] [CrossRef]
- Naithani, S.; Schaubroeck, D.; Vercammen, Y.; Mandamparambil, R.; Yakimets, I.; Van Vaeck, L.; Van Steenberge, G. Excimer laser patterning of PEDOT:PSS thin-films on flexible barrier foils: A surface analysis study. Appl. Surf. Sci. 2013, 280, 504–511. [Google Scholar] [CrossRef]
- Casado, N.; Hernández, G.; Veloso, A.; Devaraj, S.; Mecerreyes, D.; Armand, M. PEDOT Radical Polymer with Synergetic Redox and Electrical Properties. ACS Macro Lett. 2016, 5, 59–64. [Google Scholar] [CrossRef]
- Kumar, A.; Reynolds, J.R. Soluble alkyl-substituted poly(ethylenedioxythiophenes) as electrochromic materials. Macromolecules 1996, 29, 7629–7630. [Google Scholar] [CrossRef]
- Chayer, M.; Faïd, K.; Leclerc, M. Highly Conducting Water-Soluble Polythiophene Derivatives. Chem. Mater. 1997, 9, 2902–2905. [Google Scholar] [CrossRef]
- Schottland, P.; Fichet, O.; Teyssié, D.; Chevrot, C. Langmuir-Blodgett films of an alkoxy derivative of poly(3,4-ethylenedioxythiophene). Synth. Met. 1999, 101, 7–8. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Li, X.; Rui, M.; Song, J.; Shen, Z.; Zeng, H. Carbon and Graphene Quantum Dots for Optoelectronic and Energy Devices: A Review. Adv. Funct. Mater. 2015, 25, 4929–4947. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, A.; Soriano, M.L.; Carrillo-Carrión, C.; Valcárcel, M. Semiconductor and carbon-based fluorescent nanodots: The need for consistency. Chem. Commun. 2016, 52, 1311–1326. [Google Scholar] [CrossRef]
- Liu, J.; Bi, H.; Cesar Morais, P.; Zhang, X.; Zhang, F.; Hu, L. Room-temperature magnetism in carbon dots and enhanced ferromagnetism in carbon dots-polyaniline nanocomposite. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Feast, W.J. Synthesis of conducting polymers. In Handbook of Conducting Polymers; CRC Press: New York, NY, USA, 1986; pp. 1–43. [Google Scholar]
- Pal, A.; Sk, M.P.; Chattopadhyay, A. Conducting Carbon Dot-Polypyrrole Nanocomposite for Sensitive Detection of Picric acid. ACS Appl. Mater. Interfaces 2016, 8, 5758–5762. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, M.; Kumar, V.B.; Porat, Z.E.; Gedanken, A. Novel polymerization of aniline and pyrrole by carbon dots. New J. Chem. 2018, 42, 535–540. [Google Scholar] [CrossRef]
- Chen, L.; Guo, C.X.; Zhang, Q.; Lei, Y.; Xie, J.; Ee, S.; Guai, G.; Song, Q.; Li, C.M. Graphene quantum-dot-doped polypyrrole counter electrode for high-performance dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2013, 5, 2047–2052. [Google Scholar] [CrossRef]
- Dinari, M.; Momeni, M.M.; Goudarzirad, M. Nanocomposite films of polyaniline/graphene quantum dots and its supercapacitor properties. Surf. Eng. 2016, 32, 535–540. [Google Scholar] [CrossRef]
- Xie, Y.; Du, H. Electrochemical capacitance of a carbon quantum dots-polypyrrole/titania nanotube hybrid. RSC Adv. 2015, 5, 89689–89697. [Google Scholar] [CrossRef]
- Punrat, E.; Maksuk, C.; Chuanuwatanakul, S.; Wonsawat, W.; Chailapakul, O. Polyaniline/graphene quantum dot-modified screen-printed carbon electrode for the rapid determination of Cr(VI) using stopped-flow analysis coupled with voltammetric technique. Talanta 2016, 150, 198–205. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Devadas, B.; Imae, T. Effect of Carbon Dots on Conducting Polymers for Energy Storage Applications. ACS Sustain. Chem. Eng. 2018, 6, 127–134. [Google Scholar] [CrossRef]
- Alaş, M.O.; Güngör, A.; Genç, R.; Erdem, E. Feeling the power: Robust supercapacitors from nanostructured conductive polymers fostered with Mn2+ and carbon dots. Nanoscale 2019, 11, 12804–12816. [Google Scholar] [CrossRef] [PubMed]
- Kepić, D.P.; Marković, Z.M.; Jovanović, S.P.; Peruško, D.B.; Budimir, M.D.; Holclajtner-Antunović, I.D.; Pavlović, V.B.; Todorović Marković, B.M. Preparation of PEDOT:PSS thin films doped with graphene and graphene quantum dots. Synth. Met. 2014, 198, 150–154. [Google Scholar] [CrossRef]
- Li, H.; Yuan, J.; Zha, L.; Wang, L.; Chen, H.; Che, J. Soft conducting polymer hydrogels in situ doped by sulfonated graphene quantum dots for enhanced electrochemical activity. J. Mater. Sci. Mater. Electron. 2020, 31, 2153–2161. [Google Scholar] [CrossRef]
- Zhou, Y.; Sharma, S.K.; Peng, Z.; Leblanc, R.M. Polymers in Carbon Dots: A Review. Polymers 2017, 9, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, F.P.; Cao, N.N.; Zhang, Y.F.; Fu, P.; Wu, Y.G.; Lin, Z.D.; Shi, R.; Amini, A.; Cheng, C. PEDOT:PSS/graphene quantum dots films with enhanced thermoelectric properties via strong interfacial interaction and phase separation. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.C.; Min, S.H.; Lee, E.; Jang, J.; Kim, S.H.; Hong, J.I. Self-assembled Poly(3,4-ethylene dioxythiophene):Poly(styrenesulfonate)/Graphene quantum dot organogels for efficient charge transport in photovoltaic devices. ACS Appl. Mater. Interfaces 2015, 7, 11069–11073. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.K.; Kwon, O.S.; Jang, J. Conducting polymer nanomaterials for biomedical applications: Cellular interfacing and biosensing. Polym. Rev. 2013, 53, 407–442. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 14, 6766–6843. [Google Scholar] [CrossRef]
- Zhao, Z.; Xie, Y. Enhanced electrochemical performance of carbon quantum dots-polyaniline hybrid. J. Power Sour. 2017, 337, 54–64. [Google Scholar] [CrossRef]
- Kausar, A. Polymer/carbon-based quantum dot nanocomposite: Forthcoming materials for technical application. J. Macromol. Sci. Part A 2019, 56, 341–356. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dy-sensitized colloidad TiO2 films. Nature 1991, 353, 737–740. [Google Scholar]
- Boschloo, G. Improving the performance of dye-sensitized solar cells. Front. Chem. 2019, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randviir, E.P.; Brownson, D.A.; Banks, C.E. A decade of graphene research: Production, applications and outlook. Mater. Today 2014, 17, 426–432. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Morozov, S.; Novoselov, K.; Katsnelson, M.; Schedin, F.; Elias, D.; Jaszczak, J.A.; Geim, A. Giant intrinsic carrier mobilities in graphene and its bilayer. Phys. Rev. Lett. 2008, 100, 016602. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [Green Version]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Schwierz, F. Graphene transistors. Nat. Nanotechnol. 2010, 5, 487. [Google Scholar] [CrossRef] [PubMed]
- Raju, A.P.A.; Lewis, A.; Derby, B.; Young, R.J.; Kinloch, I.A.; Zan, R.; Novoselov, K.S. Wide-area strain sensors based upon graphene-polymer composite coatings probed by Raman spectroscopy. Adv. Funct. Mater. 2014, 24, 2865–2874. [Google Scholar] [CrossRef]
- Yoo, J.J.; Balakrishnan, K.; Huang, J.; Meunier, V.; Sumpter, B.G.; Srivastava, A.; Conway, M.; Mohana Reddy, A.L.; Yu, J.; Vajtai, R. Ultrathin planar graphene supercapacitors. Nano Lett. 2011, 11, 1423–1427. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Bonaccorso, F.; Fal’Ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.; Palermo, V.; Pugno, N. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar]
- Patchkovskii, S.; John, S.T.; Yurchenko, S.N.; Zhechkov, L.; Heine, T.; Seifert, G. Graphene nanostructures as tunable storage media for molecular hydrogen. Proc. Natl. Acad. Sci. USA 2005, 102, 10439–10444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, A.; Cheng, H.-M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M. All in the graphene family–A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.A.; Salvati, A.; Vázquez, E.; Ballerini, L. Classification framework for graphene-based materials. Angew. Chem. Int. Ed. 2014, 53, 7714–7718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pumera, M. Graphene in biosensing. Mater. Today 2011, 14, 308–315. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef]
- Sarno, M.; Baldino, L.; Scudieri, C.; Cardea, S.; Ciambelli, P.; Reverchon, E. Supercritical CO2 processing to improve the electrochemical properties of graphene oxide. J. Supercrit. Fluids 2016, 118, 119–127. [Google Scholar] [CrossRef]
- Du, J.; Cheng, H.M. The fabrication, properties, and uses of graphene/polymer composites. Macromol. Chem. Phys. 2012, 213, 1060–1077. [Google Scholar] [CrossRef]
- Potts, J.R.; Lee, S.H.; Alam, T.M.; An, J.; Stoller, M.D.; Piner, R.D.; Ruoff, R.S. Thermomechanical properties of chemically modified graphene/poly (methyl methacrylate) composites made by in situ polymerization. Carbon 2011, 49, 2615–2623. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Yang, W.; Yuan, W.; Xu, J.; Jiang, Y. In situ polymerization deposition of porous conducting polymer on reduced graphene oxide for gas sensor. ACS Appl. Mater. Interfaces 2014, 6, 13807–13814. [Google Scholar] [CrossRef]
- Shi, Y.; Peng, L.; Ding, Y.; Zhao, Y.; Yu, G. Nanostructured conductive polymers for advanced energy storage. Chem. Soc. Rev. 2015, 44, 6684–6696. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Yang, X.; Wickramasinghe, D.; Sundhoro, M.; Orbey, N.; Chow, K.-F.; Yan, M. Functionalization of pristine graphene for the synthesis of covalent graphene–polyaniline nanocomposite. RSC Adv. 2020, 10, 26486–26493. [Google Scholar] [CrossRef]
- Kumari, P.; Khawas, K.; Nandy, S.; Kuila, B.K. A supramolecular approach to Polyaniline graphene nanohybrid with three dimensional pillar structures for high performing electrochemical supercapacitor applications. Electrochim. Acta 2016, 190, 596–604. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, E.; Liu, X.; Song, W.; Li, Y.; Wang, X.; Liu, C. Epoxy coating with in-situ synthesis of polypyrrole functionalized graphene oxide for enhanced anticorrosive performance. Prog. Org. Coat. 2020, 140, 105488. [Google Scholar] [CrossRef]
- Khan, R.; Nishina, Y. Grafting conductive polymers on graphene oxide through cross-linker: A stepwise approach. J. Mater. Chem. A 2020, 8, 13718–13724. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Uddin, M.E.; Kim, N.H.; Lau, A.K.; Lee, J.H. In-situ synthesis and characterization of electrically conductive polypyrrole/graphene nanocomposites. Polymer 2010, 51, 5921–5928. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, H.; Hu, W. Electrochemical polymerization for two-dimensional conjugated polymers. J. Mater. Chem. C 2018, 6, 10672–10686. [Google Scholar] [CrossRef]
- Österholm, A.; Lindfors, T.; Kauppila, J.; Damlin, P.; Kvarnström, C. Electrochemical incorporation of graphene oxide into conducting polymer films. Electrochim. Acta 2012, 83, 463–470. [Google Scholar] [CrossRef]
- Jiang, F.; Yao, Z.; Yue, R.; Du, Y.; Xu, J.; Yang, P.; Wang, C. Electrochemical fabrication of long-term stable Pt-loaded PEDOT/graphene composites for ethanol electrooxidation. Int. J. Hydrog. Energy 2012, 37, 14085–14093. [Google Scholar] [CrossRef]
- Luo, X.; Weaver, C.L.; Tan, S.; Cui, X.T. Pure graphene oxide doped conducting polymer nanocomposite for bio-interfacing. J. Mat. Chem. B 2013, 1, 1340–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.-W.; Li, F.; Zhao, J.; Ren, W.; Chen, Z.-G.; Tan, J.; Wu, Z.-S.; Gentle, I.; Lu, G.Q.; Cheng, H.-M. Fabrication of graphene/polyaniline composite paper via in situ anodic electropolymerization for high-performance flexible electrode. ACS Nano 2009, 3, 1745–1752. [Google Scholar] [CrossRef]
- Sanes, J.; Sánchez, C.; Pamies, R.; Avilés, M.-D.; Bermúdez, M.-D. Extrusion of polymer nanocomposites with graphene and graphene derivative nanofillers: An overview of recent developments. Materials 2020, 13, 549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garzón, C.; Palza, H. Electrical behavior of polypropylene composites melt mixed with carbon-based particles: Effect of the kind of particle and annealing process. Compos. Sci. Technol. 2014, 99, 117–123. [Google Scholar] [CrossRef]
- Zhang, H.-B.; Zheng, W.-G.; Yan, Q.; Yang, Y.; Wang, J.-W.; Lu, Z.-H.; Ji, G.-Y.; Yu, Z.-Z. Electrically conductive polyethylene terephthalate/graphene nanocomposites prepared by melt compounding. Polymer 2010, 51, 1191–1196. [Google Scholar] [CrossRef]
- Qiu, S.; Li, W.; Zheng, W.; Zhao, H.; Wang, L. Synergistic effect of polypyrrole-intercalated graphene for enhanced corrosion protection of aqueous coating in 3.5% NaCl solution. ACS Appl. Mater. Interfaces 2017, 9, 34294–34304. [Google Scholar] [CrossRef] [PubMed]
- Subtil, E.L.; Goncalves, J.; Lemos, H.G.; Venancio, E.C.; Mierzwa, J.C.; de Souza, J.d.S.; Alves, W.; Le-Clech, P. Preparation and characterization of a new composite conductive polyethersulfone membrane using polyaniline (PANI) and reduced graphene oxide (rGO). Chem. Eng. J. 2020, 390, 124612. [Google Scholar] [CrossRef]
- Yu, J.; Kan, C.-W. Review on fabrication of structurally colored fibers by electrospinning. Fibers 2018, 6, 70. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Barzegar, F.; Bello, A.; Fabiane, M.; Khamlich, S.; Momodu, D.; Taghizadeh, F.; Dangbegnon, J.; Manyala, N. Preparation and characterization of poly (vinyl alcohol)/graphene nanofibers synthesized by electrospinning. J. Phys. Chem. Solids 2015, 77, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Ceretti, E.; Ginestra, P.S.; Ghazinejad, M.; Fiorentino, A.; Madou, M. Electrospinning and characterization of polymer–graphene powder scaffolds. Cirp Ann. 2017, 66, 233–236. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Ratinac, K.R.; Ringer, S.P.; Thordarson, P.; Gooding, J.J.; Braet, F. Carbon nanomaterials in biosensors: Should you use nanotubes or graphene? Angew. Chem. Int. Ed. 2010, 49, 2114–2138. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, H.; Yang, J.x.; Wang, S.; Tang, D.Y.; Jose, R.; Ramakrishna, S.; Lim, C.T.; Loh, K.P. Graphene–polymer nanofiber membrane for ultrafast photonics. Adv. Funct. Mater. 2010, 20, 782–791. [Google Scholar] [CrossRef]
- Song, J.; Gao, H.; Zhu, G.; Cao, X.; Shi, X.; Wang, Y. The preparation and characterization of polycaprolactone/graphene oxide biocomposite nanofiber scaffolds and their application for directing cell behaviors. Carbon 2015, 95, 1039–1050. [Google Scholar] [CrossRef]
- Chu, C.-Y.; Tsai, J.-T.; Sun, C.-L. Synthesis of PEDOT-modified graphene composite materials as flexible electrodes for energy storage and conversion applications. Int. J. Hydrog. Energy 2012, 37, 13880–13886. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, X.; Ding, S.; Liu, Z. Solvent evaporation induced self-assembly of graphene foam for thermally conductive polymers. RSC Adv. 2017, 7, 15469–15474. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.-H.; Chang, C.-K.; Tsai, Y.-C.; Liao, C.-S. Electrochemically synthesized graphene/polypyrrole composites and their use in supercapacitor. Carbon 2012, 50, 2331–2336. [Google Scholar] [CrossRef]
- Yan, X.; Chen, J.; Yang, J.; Xue, Q.; Miele, P. Fabrication of free-standing, electrochemically active, and biocompatible graphene oxide− polyaniline and graphene− polyaniline hybrid papers. ACS Appl. Mater. Interfaces 2010, 2, 2521–2529. [Google Scholar] [CrossRef]
- Lyu, H. Triple layer tungsten trioxide, graphene, and polyaniline composite films for combined energy storage and electrochromic applications. Polymers 2020, 12, 49. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.-C.; Liu, J.-Q.; Wei, D.-X.; Kang, X.-Y.; Zhang, C.; Du, J.-C.; Yang, B.; Chen, X.; Zhu, H.-Y.; NuLi, Y.-N. Graphene oxide doped conducting polymer nanocomposite film for electrode-tissue interface. Biomaterials 2014, 35, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Luo, L.; Ding, Y.; Liu, B.; Xu, D. Simultaneous determination of purine and pyrimidine bases in DNA using poly (3, 4-ethylenedioxythiophene)/graphene composite film. J. Electroanal. Chem. 2014, 735, 51–56. [Google Scholar] [CrossRef]
- Lei, W.; Wu, L.; Huang, W.; Hao, Q.; Zhang, Y.; Xia, X. Microwave-assisted synthesis of hemin–graphene/poly (3, 4-ethylenedioxythiophene) nanocomposite for a biomimetic hydrogen peroxide biosensor. J. Mat. Chem. B 2014, 2, 4324–4330. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Mater. Mater. Sustain. Energy 2011, 129–137. [Google Scholar] [CrossRef]
- Zahid, M.; Papadopoulou, E.L.; Athanassiou, A.; Bayer, I.S. Strain-responsive mercerized conductive cotton fabrics based on PEDOT: PSS/graphene. Mater. Des. 2017, 135, 213–222. [Google Scholar] [CrossRef]
- Weaver, C.L.; LaRosa, J.M.; Luo, X.; Cui, X.T. Electrically controlled drug delivery from graphene oxide nanocomposite films. ACS Nano 2014, 8, 1834–1843. [Google Scholar] [CrossRef]
- Byun, K.-E.; Choi, D.S.; Kim, E.; Seo, D.H.; Yang, H.; Seo, S.; Hong, S. Graphene–polymer hybrid nanostructure-based bioenergy storage device for real-time control of biological motor activity. ACS Nano 2011, 5, 8656–8664. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene-family nanomaterials: An interdisciplinary review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Liao, N.; Zhang, M.; Xue, W. Theoretical approach to evaluate graphene/PANI composite as highly selective ammonia sensor. Appl. Surf. Sci. 2018, 453, 336–340. [Google Scholar] [CrossRef]
- Muthusankar, E.; Ragupathy, D. Graphene/Poly (aniline-co-diphenylamine) nanohybrid for ultrasensitive electrochemical glucose sensor. Nano-Struct. Nano-Objects 2019, 20, 100390. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Cao, J.; Liu, Y.; Zhou, Y.; Ouyang, J.-H.; Jia, D. Facile co-electrodeposition method for high-performance supercapacitor based on reduced graphene oxide/polypyrrole composite film. ACS Appl. Mater. Interfaces 2017, 9, 19831–19842. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Si, W.; Xu, Y.; Gu, Z.; Hao, Q. Conducting polymer composites with graphene for use in chemical sensors and biosensors. Microchim. Acta 2014, 181, 707–722. [Google Scholar] [CrossRef]
- Kinloch, I.A.; Suhr, J.; Lou, J.; Young, R.J.; Ajayan, P.M. Composites with carbon nanotubes and graphene: An outlook. Science 2018, 362, 547–553. [Google Scholar] [CrossRef] [Green Version]
- Hamada, N.; Sawada, S.-i.; Oshiyama, A. New one-dimensional conductors: Graphitic microtubules. Phys. Rev. Lett. 1992, 68, 1579–1581. [Google Scholar] [CrossRef]
- Mintmire, J.W.; Dunlap, B.I.; White, C.T. Are fullerene tubules metallic? Phys. Rev. Lett. 1992, 68, 631–634. [Google Scholar] [CrossRef]
- Walker, B.W.; Lara, R.P.; Mogadam, E.; Yu, C.H.; Kimball, W.; Annabi, N. Rational design of microfabricated electroconductive hydrogels for biomedical applications. Prog. Polym. Sci. 2019, 92, 135–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Luo, Y.; Wu, H.; Jiang, K.; Li, Q.; Fan, S.; Li, J.; Wang, J. Ultrastretchable carbon nanotube composite electrodes for flexible lithium-ion batteries. Nanoscale 2018, 10, 19972–19978. [Google Scholar] [CrossRef] [PubMed]
- Senokos, E.; Ou, Y.; Torres, J.J.; Sket, F.; González, C.; Marcilla, R.; Vilatela, J.J. Energy storage in structural composites by introducing CNT fiber/polymer electrolyte interleaves. Sci. Rep. 2018, 8, 3407. [Google Scholar] [CrossRef] [Green Version]
- Breuer, O.; Sundararaj, U. Big returns from small fibers: A review of polymer/carbon nanotube composites. Polym. Compos. 2004, 25, 630–645. [Google Scholar] [CrossRef]
- Tkalya, E.E.; Ghislandi, M.; de With, G.; Koning, C.E. The use of surfactants for dispersing carbon nanotubes and graphene to make conductive nanocomposites. Curr. Opin. Colloid Interface Sci. 2012, 17, 225–232. [Google Scholar] [CrossRef]
- Alegret, N.; Dominguez-Alfaro, A.; González-Domínguez, J.M.; Arnaiz, B.; Cossío, U.; Bosi, S.; Vázquez, E.; Ramos-Cabrer, P.; Mecerreyes, D.; Prato, M. Three-Dimensional Conductive Scaffolds as Neural Prostheses Based on Carbon Nanotubes and Polypyrrole. ACS Appl. Mater. Interfaces 2018, 10, 43904–43914. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Alfaro, A.; Alegret, N.; Arnaiz, B.; González-Domínguez, J.M.; Martin-Pacheco, A.; Cossío, U.; Porcarelli, L.; Bosi, S.; Vázquez, E.; Mecerreyes, D.; et al. Tailored Methodology Based on Vapor Phase Polymerization to Manufacture PEDOT/CNT Scaffolds for Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 1269–1278. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, Q.; Chang, J.; Chen, L. Enhanced thermoelectric properties of CNT/PANI composite nanofibers by highly orienting the arrangement of polymer chains. J. Mater. Chem. 2012, 22, 17612–17618. [Google Scholar] [CrossRef]
- Dominguez-Alfaro, A.; Gómez, I.J.; Alegret, N.; Mecerreyes, D.; Prato, M. 2D and 3D Immobilization of Carbon Nanomaterials into PEDOT via Electropolymerization of a Functional Bis-EDOT Monomer. Polymers 2021, 13, 436. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Alfaro, A.; Alegret, N.; Arnaiz, B.; Salsamendi, M.; Mecerreyes, D.; Prato, M. Toward Spontaneous Neuronal Differentiation of SH-SY5Y Cells Using Novel Three-Dimensional Electropolymerized Conductive Scaffolds. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, M.; Baniassadi, M.; Baghani, M.; Dadmun, M.; Tehrani, M. 3D reconstruction of carbon nanotube networks from neutron scattering experiments. Nanotechnology 2015, 26. [Google Scholar] [CrossRef] [PubMed]
- Goshi, N.; Castagnola, E.; Vomero, M.; Gueli, C.; Cea, C.; Zucchini, E.; Bjanes, D.; Maggiolini, E.; Moritz, C.; Kassegne, S.; et al. Glassy carbon MEMS for novel origami-styled 3D integrated intracortical and epicortical neural probes. J. Micromech. Microeng. 2018, 28, 12. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Liang, X.; Zhang, D.; Miao, M. A multifunctional supercapacitor based on 2D nanosheets on a flexible carbon nanotube film. Dalton Trans. 2020, 49, 9312–9321. [Google Scholar] [CrossRef]
- Markose, K.K.; Jasna, M.; Subha, P.P.; Antony, A.; Jayaraj, M.K. Performance enhancement of organic/Si solar cell using CNT embedded hole selective layer. Sol. Energy 2020, 211, 158–166. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, T.; Geng, H.-Z.; Etihraj, A.S.; Gu, Z.-Z.; Jing, L.-C.; Yuan, X.-T.; Zhao, H.; Wen, J.-G.; Xu, Z.-H. Improved resistance stability of transparent conducting films prepared by PEDOT: PSS hybrid CNTs treated by a two-step method. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Du, Y.; Shi, Y.; Meng, Q.; Shen, S.Z. Preparation and thermoelectric properties of flexible SWCNT/PEDOT:PSS composite film. Synth. Met. 2020, 261. [Google Scholar] [CrossRef]
- Chung, S.-H.; Kim, D.H.; Kim, H.; Kim, H.; Jeong, S.W. Thermoelectric properties of PEDOT: PSS and acid-treated SWCNT composite films. Mater. Today Commun. 2020, 23. [Google Scholar] [CrossRef]
- Lee, T.; Kwon, W.; Park, M. Highly conductive, transparent and metal-free electrodes with a PEDOT:PSS/SWNT bilayer for high-performance organic thin film transistors. Org. Electron. 2019, 67, 26–33. [Google Scholar] [CrossRef]
- Zhao, H.; Geng, W.; Cao, W.-W.; Wen, J.-G.; Wang, T.; Tian, Y.; Jing, L.-C.; Yuan, X.-T.; Zhu, Z.-R.; Geng, H.-Z. Highly stable and conductive PEDOT:PSS/GO-SWCNT bilayer transparent conductive films. New J. Chem. 2020, 44, 780–790. [Google Scholar] [CrossRef]
- Xu, L.; Xu, J.; Yang, Y.; Mao, X.; He, X.; Yang, W.; Zhao, Y.; Zhou, Y. A flexible fabric electrode with hierarchical carbon-polymer composite for functional supercapacitors. J. Mater. Sci. Mater. Electron. 2018, 29, 2322–2330. [Google Scholar] [CrossRef]
- Chang, Z.-H.; Feng, D.-Y.; Huang, Z.-H.; Liu, X.-X. Electrochemical deposition of highly loaded polypyrrole on individual carbon nanotubes in carbon nanotube film for supercapacitor. Chem. Eng. J. 2018, 337, 552–559. [Google Scholar] [CrossRef]
- Wang, T.; Jing, L.-C.; Zhu, Q.; Ethiraj, A.S.; Fan, X.; Liu, H.; Tian, Y.; Zhu, Z.; Meng, Z.; Geng, H.-Z. Tannic acid modified graphene/CNT three-dimensional conductive network for preparing high-performance transparent flexible heaters. J. Colloid Interface Sci. 2020, 577, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.-Z.; Tian, Y.; Geng, H.-Z.; Rhen, D.S.; Ethiraj, A.S.; Zhang, X.; Jing, L.-C.; Wang, T.; Xu, Z.-H.; Yuan, X.-T. Highly conductive sandwich-structured CNT/PEDOT:PSS/CNT transparent conductive films for OLED electrodes. Appl. Nanosci. 2019, 9, 1971–1979. [Google Scholar] [CrossRef]
- Kizildag, N.; Ucar, N. Electrospinning Functional Polyacrylonitrile Nanofibers with Polyaniline, Carbon Nanotubes, and Silver Nitrate as Additives. In Electrospinning—Material, Techniques, and Biomedical Applications; Intech Open: London, UK, 2016; pp. 25–43. [Google Scholar] [CrossRef] [Green Version]
- Im, J.S.; Yun, J.; Kim, J.G.; Bae, T.-S.; Lee, Y.-S. The effects of carbon nanotube addition and oxyfluorination on the glucose-sensing capabilities of glucose oxidase-coated carbon fiber electrodes. Appl. Surf. Sci. 2012, 258, 2219–2225. [Google Scholar] [CrossRef]
- Manesh, K.M.; Kim, H.T.; Santhosh, P.; Gopalan, A.I.; Lee, K.P. A novel glucose biosensor based on immobilization of glucose oxidase into multiwall carbon nanotubes-polyelectrolyte-loaded electrospun nanofibrous membrane. Biosens. Bioelectron. 2008, 23, 771–779. [Google Scholar] [CrossRef]
- Su, Z.; Ding, J.; Wei, G. Electrospinning: A facile technique for fabricating polymeric nanofibers doped with carbon nanotubes and metallic nanoparticles for sensor applications. RSC Adv. 2014, 4, 52598–52610. [Google Scholar] [CrossRef] [Green Version]
- Ayutsede, J.; Gandhi, M.; Sukigara, S.; Ye, H.; Hsu, C.M.; Gogotsi, Y.; Ko, F. Carbon nanotube reinforced Bombyx mori silk nanofibers by the electrospinning process. Biomacromolecules 2006, 7, 208–214. [Google Scholar] [CrossRef]
- Lewitus, D.Y.; Landers, J.; Branch, J.R.; Smith, K.L.; Callegari, G.; Kohn, J.; Neimark, A.V. Biohybrid Carbon Nanotube/Agarose Fibers for Neural Tissue Engineering. Adv. Funct. Mater. 2011, 21, 2624–2632. [Google Scholar] [CrossRef] [Green Version]
- Shao, S.; Zhou, S.; Li, L.; Li, J.; Luo, C.; Wang, J.; Li, X.; Weng, J. Osteoblast function on electrically conductive electrospun PLA/MWCNTs nanofibers. Biomaterials 2011, 32, 2821–2833. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Kong, H.; Han, Z.; Wang, C.; Zhu, G.; Xie, S.; Xu, H. Enhancement of nanofibrous scaffold of multiwalled carbon nanotubes/polyurethane composite to the fibroblasts growth and biosynthesis. J. Biomed. Mater. Res. Part A 2009, 88A, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Yardimci, A.I.; Aypek, H.; Ozturk, O.; Yilmaz, S.; Ozcivici, E.; Mese, G.; Selamet, Y. CNT Incorporated Polyacrilonitrile/Polypyrrole Nanofibers as Keratinocytes Scaffold. J. Biomim. Biomater. Biomed. Eng. 2019, 41, 69–81. [Google Scholar] [CrossRef]
- Roh, S.-H. Electricity Generation from Microbial Fuel Cell with Polypyrrole-Coated Carbon Nanofiber Composite. J. Nanosci. Nanotechnol. 2015, 15, 1700–1703. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-Y.; Roh, S.-H. Carbon Nanofiber/Polypyrrole Nanocomposite as Anode Material in Microbial Fuel Cells. J. Nanosci. Nanotechnol. 2017, 17, 5830–5833. [Google Scholar] [CrossRef]
- Ahmed, A.; Jia, Y.; Huang, Y.; Khoso, N.A.; Deb, H.; Fan, Q.; Shao, J. Preparation of PVDF-TrFE based electrospun nanofibers decorated with PEDOT-CNT/rGO composites for piezo-electric pressure sensor. J. Mater. Sci. Mater. Electron. 2019, 30, 14007–14021. [Google Scholar] [CrossRef]
- Hazarika, A.; Deka, B.K.; Kim, D.; Kong, K.; Park, Y.-B.; Park, H.W. Microwave-synthesized freestanding iron-carbon nanotubes on polyester composites of woven Kevlar fibre and silver nanoparticle-decorated graphene. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.S.; Griffith, A.Y.; Chang, E.; Looker, M.J.; Fisher, L.E.; Clapsaddle, B.; Cui, X.T. Evaluation of a conducting elastomeric composite material for intramuscular electrode application. Acta Biomater. 2020, 103, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Jie, W.; Song, F.; Li, X.; Li, W.; Wang, R.; Jiang, Y.; Zhao, L.; Fan, Z.; Wang, J.; Liu, B. Enhancing the proliferation of MC3T3-E1 cells on casein phosphopeptide-biofunctionalized 3D reduced-graphene oxide/polypyrrole scaffolds. RSC Adv. 2017, 7, 34415–34424. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.S.; Park, J.S.; Kim, B.; Lee, B.-T.; Yim, J.-H. In Vitro Biocompatibility of Vapour Phase Polymerised Conductive Scaffolds for Cell Lines. Polymer 2017, 124, 95–100. [Google Scholar] [CrossRef]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.P. 3D Cell Culture Systems: Advantages and Applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, B.; Gao, X.; Xu, D. A review of the interfacial characteristics of polymer nanocomposites containing carbon nanotubes. RSC Adv. 2018, 8, 28048–28085. [Google Scholar] [CrossRef] [Green Version]
- Gorain, B.; Choudhury, H.; Pandey, M.; Kesharwani, P.; Abeer, M.M.; Tekade, R.K.; Hussain, Z. Carbon nanotube scaffolds as emerging nanoplatform for myocardial tissue regeneration: A review of recent developments and therapeutic implications. Biomed. Pharmacother. 2018, 104, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Yeo, L.Y.; Friend, J.R. Electrospinning carbon nanotube polymer composite nanofibers. J. Exp. Nanosci. 2006, 1, 177–209. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ye, D.; Liu, W.; Shi, B.; Guo, R.; Pei, H.; Xie, J. A three-dimensional core-shell nanostructured composite of polypyrrole wrapped MnO(2)/reduced graphene oxide/carbon nanotube for high performance lithium ion batteries. J. Colloid Interface Sci. 2017, 493, 241–248. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, S.; Deng, S.; Wu, W.; Zeng, Y.; Xia, X.; Pan, G.; Tong, Y.; Lu, X. 3D CNTs Networks Enable MnO2 Cathodes with High Capacity and Superior Rate Capability for Flexible Rechargeable Zn-MnO2 Batteries. Small Methods 2019, 3. [Google Scholar] [CrossRef]
- Jyothibasu, J.P.; Kuo, D.-W.; Lee, R.-H. Flexible and freestanding electrodes based on polypyrrole/carbon nanotube/cellulose composites for supercapacitor application. Cellulose 2019, 26, 4495–4513. [Google Scholar] [CrossRef]
- Liu, J.; Wen, Y.; van Aken, P.A.; Maier, J.; Yu, Y. In situ reduction and coating of SnS2 nanobelts for free-standing SnS@polypyrrole-nanobelt/carbon-nanotube paper electrodes with superior Li-ion storage. J. Mater. Chem. A 2015, 3, 5259–5265. [Google Scholar] [CrossRef]
- Zhang, M.; Amin, K.; Cheng, M.; Yuan, H.; Mao, L.; Yan, W.; Wei, Z. A carbon foam-supported high sulfur loading composite as a self-supported cathode for flexible lithium-sulfur batteries. Nanoscale 2018, 10, 21790–21797. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, X.; Chen, Y.; Wen, Q.; Lin, C.; Zheng, J.; Li, W.; Xu, H.; Qi, L. A 3D porous nitrogen-doped carbon nanotube sponge anode modified with polypyrrole and carboxymethyl cellulose for high-performance microbial fuel cells. J. Appl. Electrochem. 2020, 50, 1281–1290. [Google Scholar] [CrossRef]
- Wu, X.; Lei, Y.; Li, S.; Huang, J.; Teng, L.; Chen, Z.; Lai, Y. Photothermal and Joule heating-assisted thermal management sponge for efficient cleanup of highly viscous crude oil. J. Hazard. Mater. 2021, 403, 124090. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, Y.; Wu, S.; Wang, D.; Zhao, X.; Xu, F.; Zou, M.; Zhang, H.; He, X.; Cao, A. Highly Stable Carbon Nanotube/Polyaniline Porous Network for Multifunctional Applications. ACS Appl. Mater. Interfaces 2016, 8, 34027–34033. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhen, Z.; Zhang, Z.; Lao, J.; Wei, J.; Wang, K.; Kang, F.; Zhu, H. In-situ synthesis of carbon nanotube/graphene composite sponge and its application as compressible supercapacitor electrode. Electrochim. Acta 2015, 157, 134–141. [Google Scholar] [CrossRef]
- Jiang, H.; Cai, X.; Qian, Y.; Zhang, C.; Zhou, L.; Liu, W.; Li, B.; Lai, L.; Huang, W. V2O5 embedded in vertically aligned carbon nanotube arrays as free-standing electrodes for flexible supercapacitors. J. Mater. Chem. A 2017, 5, 23727–23736. [Google Scholar] [CrossRef]
- Giffney, T.; Xie, M.; Sartelet, M.C.; Aw, K. Vapor phase polymerization of PEDOT on silicone rubber as flexible large strain sensor. Aims Mater. Sci. 2015, 2, 414–424. [Google Scholar] [CrossRef]
- Iandolo, D.; Ravichandran, A.; Liu, X.; Wen, F.; Chan, J.K.; Berggren, M.; Teoh, S.H.; Simon, D.T. Development and Characterization of Organic Electronic Scaffolds for Bone Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Tenhaeff, W.E.; Gleason, K.K. Initiated and Oxidative Chemical Vapor Deposition of Polymeric Thin Films: iCVD and oCVD. Adv. Funct. Mater. 2008, 18, 979–992. [Google Scholar] [CrossRef]
- Chang-Jian, C.-W.; Cho, E.-C.; Lee, K.-C.; Huang, J.-H.; Chen, P.-Y.; Ho, B.-C.; Hsiao, Y.-S. Thermally conductive polymeric composites incorporating 3D MWCNT/PEDOT:PSS scaffolds. Compos. Part B Eng. 2018, 136, 46–54. [Google Scholar] [CrossRef]
- Carayon, I.; Gaubert, A.; Mousli, Y.; Philippe, B. Electro-responsive hydrogels: Macromolecular and supramolecular approaches in the biomedical field. Biomater. Sci. 2020, 8, 5589–5600. [Google Scholar] [CrossRef]
- Cao, H.; Yang, Y.; Qi, Y.; Li, Y.; Sun, B.; Li, Y.; Cui, W.; Li, J.; Li, J. Intraparticle FRET for Enhanced Efficiency of Two-Photon Activated Photodynamic Therapy. Adv. Healthc. Mater. 2018, 7, 1701357. [Google Scholar] [CrossRef]
- Adewunmi, A.A.; Ismail, S.; Sultan, A.S. Carbon Nanotubes (CNTs) Nanocomposite Hydrogels Developed for Various Applications: A Critical Review. J. Inorg. Organomet. Polym. Mater. 2016, 26, 717–737. [Google Scholar] [CrossRef]
- Homenick, C.M.; Sheardown, H.; Adronov, A. Reinforcement of collagen with covalently-functionalized single-walled carbon nanotube crosslinkers. J. Mater. Chem. 2010, 20, 2887–2894. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, H.; Huang, C.; Du, Y. Mechanically and Electrically Enhanced CNT-Collagen Hydrogels As Potential Scaffolds for Engineered Cardiac Constructs. ACS Biomater. Sci. Eng. 2017, 3, 3017–3021. [Google Scholar] [CrossRef]
- Kolahchi, R.; Safari, M.; Esmailpour, M. Dynamic stability analysis of temperature-dependent functionally graded CNT-reinforced visco-plates resting on orthotropic elastomeric medium. Compos. Struct. 2016, 150, 255–265. [Google Scholar] [CrossRef]
- Rehman, H.U.; Chen, Y.; Guo, Y.; Du, Q.; Zhou, J.; Guo, Y.; Duan, H.; Li, H.; Liu, H. Stretchable, strong and self-healing hydrogel by oxidized CNT-polymer composite. Compos. Part A Appl. Sci. Manuf. 2016, 90, 250–260. [Google Scholar] [CrossRef]
- Castagnola, E.; Maggiolini, E.; Ceseracciu, L.; Ciarpella, F.; Zucchini, E.; De Faveri, S.; Fadiga, L.; Ricci, D. pHEMA Encapsulated PEDOT-PSS-CNT Microsphere Microelectrodes for Recording Single Unit Activity in the Brain. Front. Neurosci. 2016, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-R.; Qin, H.; Cong, H.-P.; Yu, S.-H. A Highly Stretchable and Real-Time Healable Supercapacitor. Adv. Mater. 2019, 31. [Google Scholar] [CrossRef]
- Deng, Z.; Guo, Y.; Zhao, X.; Ma, P.X.; Guo, B. Multifunctional Stimuli-Responsive Hydrogels with Self-Healing, High Conductivity, and Rapid Recovery through Host-Guest Interactions. Chem. Mater. 2018, 30, 1729–1742. [Google Scholar] [CrossRef]
- Shin, J.; Choi, E.J.; Cho, J.H.; Cho, A.N.; Jin, Y.; Yang, K.; Song, C.; Cho, S.W. Three-Dimensional Electroconductive Hyaluronic Acid Hydrogels Incorporated with Carbon Nanotubes and Polypyrrole by Catechol-Mediated Dispersion Enhance Neurogenesis of Human Neural Stem Cells. Biomacromolecules 2017, 18, 3060–3072. [Google Scholar] [CrossRef]
- MacDonald, R.A.; Laurenzi, B.F.; Viswanathan, G.; Ajayan, P.M.; Stegemann, J.P. Collagen-carbon nanotube composite materials as scaffolds in tissue engineering. J. Biomed. Mater. Res. A 2005, 74, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Pok, S.; Vitale, F.; Eichmann, S.L.; Benavides, O.M.; Pasquali, M.; Jacot, J.G. Biocompatible Carbon Nanotube–Chitosan Scaffold Matching the Electrical Conductivity of the Heart. ACS Nano 2014, 8, 9822–9832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosun, Z.; McFetridge, P.S. A composite SWNT-collagen matrix: Characterization and preliminary assessment as a conductive peripheral nerve regeneration matrix. J. Neural Eng. 2010, 7, 066002. [Google Scholar] [CrossRef] [PubMed]
- Peña, B.; Bosi, S.; Aguado, B.A.; Borin, D.; Farnsworth, N.L.; Dobrinskikh, E.; Rowland, T.J.; Martinelli, V.; Jeong, M.; Taylor, M.R.G.; et al. Injectable Carbon Nanotube-Functionalized Reverse Thermal Gel Promotes Cardiomyocytes Survival and Maturation. ACS Appl. Mater. Interfaces 2017, 9, 31645–31656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raphey, V.R.; Henna, T.K.; Nivitha, K.P.; Mufeedha, P.; Sabu, C.; Pramod, K. Advanced biomedical applications of carbon nanotube. Mater. Sci. Eng. C 2019, 100, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Malaiya, A.; Kesharwani, P.; Soni, D.; Jain, A. Biomedical applications and toxicities of carbon nanotubes. Drug Chem. Toxicol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.-H.; Yu, C. Sorting-free utilization of semiconducting carbon nanotubes for large thermoelectric responses. Nano Energy 2020, 67. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Zhuang, X.; Tomanec, O.; Zboril, R.; Yu, D.Y.W.; Rogach, A.L. Polypyrrole and Carbon Nanotube Co-Composited Titania Anodes with Enhanced Sodium Storage Performance in Ether-Based Electrolyte. Adv. Sustain. Syst. 2019, 3. [Google Scholar] [CrossRef]
- Rudramurthy, G.R.; Swamy, M.K. Potential applications of engineered nanoparticles in medicine and biology: An update. J. Biol. Inorg. Chem. 2018, 23, 1185–1204. [Google Scholar] [CrossRef]
- Gorjikhah, F.; Davaran, S.; Salehi, R.; Bakhtiari, M.; Hasanzadeh, A.; Panahi, Y.; Emamverdy, M.; Akbarzadeh, A. Improving “lab-on-a-chip” techniques using biomedical nanotechnology: A review. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1609–1614. [Google Scholar] [CrossRef]
- Silva, G.A. Neuroscience nanotechnology: Progress, opportunities and challenges. Nat. Rev. Neurosci. 2006, 7, 65–74. [Google Scholar] [CrossRef]
- Rauti, R.; Musto, M.; Bosi, S.; Prato, M.; Ballerini, L. Properties and Behavior of Carbon Nanomaterials when Interfacing Neuronal Cells: How Far Have We Come? Carbon 2019, 143, 430–446. [Google Scholar] [CrossRef]
- Mattson, M.P.; Haddon, R.C.; Rao, A.M. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J. Mol. Neurosci. 2000, 14, 175–182. [Google Scholar] [CrossRef]
- Fabbro, A.; Prato, M.; Ballerini, L. Carbon Nanotubes in Neuroregeneration and Repair. Adv. Drug Deliv. Rev. 2013, 65, 2034–2044. [Google Scholar] [CrossRef]
- Fabbro, A.; Bosi, S.; Ballerini, L.; Prato, M. Carbon Nanotubes: Artificial Nanomaterials to Engineer Single Neurons and Neuronal Networks. ACS Chem. Neurosci. 2012, 3, 611–618. [Google Scholar] [CrossRef] [Green Version]
- Pampaloni, N.P.; Scaini, D.; Perissinotto, F.; Bosi, S.; Prato, M.; Ballerini, L. Sculpting neurotransmission during synaptic development by 2D nanostructured interfaces. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2521–2532. [Google Scholar] [CrossRef] [Green Version]
- Fiorito, S.; Russier, J.; Salemme, A.; Soligo, M.; Manni, L.; Krasnowska, E.; Bonnamy, S.; Flahaut, E.; Serafino, A.; Togna, G.I.; et al. Switching on microglia with electro-conductive multi walled carbon nanotubes. Carbon 2018, 129, 572–584. [Google Scholar] [CrossRef] [Green Version]
- Lovat, V.; Pantarotto, D.; Lagostena, L.; Cacciari, B.; Grandolfo, M.; Righi, M.; Spalluto, G.; Prato, M.; Ballerini, L. Carbon Nanotube Substrates Boost Neuronal Electrical Signaling. Nano Lett. 2005, 5, 1107–1110. [Google Scholar] [CrossRef]
- Shao, H.; Li, T.T.; Zhu, R.; Xu, X.T.; Yu, J.D.; Chen, S.F.; Song, L.; Ramakrishna, S.; Lei, Z.G.; Ruan, Y.W.; et al. Carbon nanotube multilayered nanocomposites as multifunctional substrates for actuating neuronal differentiation and functions of neural stem cells. Biomaterials 2018, 175, 93–109. [Google Scholar] [CrossRef] [Green Version]
- Su, W.T.; Shih, Y.A. Nanofiber containing carbon nanotubes enhanced PC12 cell proliferation and neuritogenesis by electrical stimulation. Bio-Med. Mater. Eng. 2015, 26, S189–S195. [Google Scholar] [CrossRef] [Green Version]
- Mazzatenta, A.; Giugliano, M.; Campidelli, S.; Gambazzi, L.; Businaro, L.; Markram, H.; Prato, M.; Ballerini, L. Interfacing Neurons with Carbon Nanotubes: Electrical Signal Transfer and Synaptic Stimulation in Cultured Brain Circuits. J. Neurosci. 2007, 27, 6931–6936. [Google Scholar] [CrossRef]
- Fabbro, A.; Cellot, G.; Prato, M.; Ballerini, L. Interfacing neurons with carbon nanotubes: (re) engineering neuronal signaling. In Brain Machine Interfaces: Implications for Science, Clinical Practice and Society; Schouenborg, J., Garwicz, M., Danielsen, N., Eds.; Elsevier Science Bv: Amsterdam, The Netherlands, 2011; Volume 194, pp. 241–252. [Google Scholar]
- Cellot, G.; Cilia, E.; Cipollone, S.; Rancic, V.; Sucapane, A.; Giordani, S.; Gambazzi, L.; Markram, H.; Grandolfo, M.; Scaini, D.; et al. Carbon nanotubes might improve neuronal performance by favouring electrical shortcuts. Nat. Nanotechnol. 2009, 4, 126–133. [Google Scholar] [CrossRef]
- Cellot, G.; Toma, F.M.; Varley, Z.K.; Laishram, J.; Villari, A.; Quintana, M.; Cipollone, S.; Prato, M.; Ballerini, L. Carbon nanotube scaffolds tune synaptic strength in cultured neural circuits: Novel frontiers in nanomaterial-tissue interactions. J. Neurosci. 2011, 31, 12945–12953. [Google Scholar] [CrossRef] [Green Version]
- Fabbro, A.; Sucapane, A.; Toma, F.M.; Calura, E.; Rizzetto, L.; Carrieri, C.; Roncaglia, P.; Martinelli, V.; Scaini, D.; Masten, L.; et al. Adhesion to Carbon Nanotube Conductive Scaffolds Forces Action-Potential Appearance in Immature Rat Spinal Neurons. PLoS ONE 2013, 8, e73621. [Google Scholar] [CrossRef]
- Fabbro, A.; Villari, A.; Laishram, J.; Scaini, D.; Toma, F.M.; Turco, A.; Prato, M.; Ballerini, L. Spinal Cord Explants Use Carbon Nanotube Interfaces to Enhance Neurite Outgrowth and To Fortify Synaptic Inputs. ACS Nano 2012, 6, 2041–2055. [Google Scholar] [CrossRef]
- Lichtenstein, M.P.; Carretero, N.M.; Perez, E.; Pulido-Salgado, M.; Moral-Vico, J.; Sola, C.; Casan-Pastor, N.; Sunol, C. Biosafety assessment of conducting nanostructured materials by using co-cultures of neurons and astrocytes. Neurotoxicology 2018, 68, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Accardo, A.; Cirillo, C.; Lionnet, S.; Vieu, C.; Loubinoux, I. Interfacing cells with microengineered scaffolds for neural tissue reconstruction. Brain Res. Bull. 2019, 152, 202–211. [Google Scholar] [CrossRef]
- Papadimitriou, L.; Manganas, P.; Ranella, A.; Stratakis, E. Biofabrication for neural tissue engineering applications. Mater. Today Bio 2020, 6, 100043. [Google Scholar] [CrossRef]
- Roberts, M.J.; Leach, M.K.; Bedewy, M.; Meshot, E.R.; Copic, D.; Corey, J.M.; Hart, A.J. Growth of primary motor neurons on horizontally aligned carbon nanotube thin films and striped patterns. J. Neural Eng. 2014, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Cellot, G.; Lagonegro, P.; Tarabella, G.; Scaini, D.; Fabbri, F.; Iannotta, S.; Prato, M.; Salviati, G.; Ballerini, L. PEDOT:PSS Interfaces Support the Development of Neuronal Synaptic Networks with Reduced Neuroglia Response In Vitro. Front. Neurosci. 2015, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Zhu, W.; Nowicki, M.; Lee, G.; Heo, D.N.; Kim, J.; Zuo, Y.Y.; Zhang, L.G. 3D printing nano conductive multi-walled carbon nanotube scaffolds for nerve regeneration. J. Neural Eng. 2018, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Q.; Duan, B.; Lu, A.; Wang, Y.F.; Ye, Q.F.; Zhang, L.N. Biocompatible chitin/carbon nanotubes composite hydrogels as neuronal growth substrates. Carbohydr. Polym. 2017, 174, 830–840. [Google Scholar] [CrossRef]
- Liu, X.F.; Miller, A.L.; Park, S.; Waletzki, B.E.; Terzic, A.; Yaszemski, M.J.; Lu, L.C. Covalent crosslinking of graphene oxide and carbon nanotube into hydrogels enhances nerve cell responses. J. Mat. Chem. B 2016, 4, 6930–6941. [Google Scholar] [CrossRef]
- Rad, S.M.; Khorasani, M.T.; Joupari, M.D. Preparation of HMWCNT/PLLA nanocomposite scaffolds for application in nerve tissue engineering and evaluation of their physical, mechanical and cellular activity properties. Polym. Adv. Technol. 2016, 27, 325–338. [Google Scholar] [CrossRef]
- Tayi, A.S.; Pashuck, E.T.; Newcomb, C.J.; McClendon, M.T.; Stupp, S.I. Electrospinning Bioactive Supramolecular Polymers from Water. Biomacromolecules 2014, 15, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Holzwarth, J.M.; Ma, P.X. 3D nanofibrous scaffolds for tissue engineering. J. Mater. Chem. 2011, 21, 10243–10251. [Google Scholar] [CrossRef]
- Park, S.Y.; Kang, B.S.; Hong, S. Improved neural differentiation of human mesenchymal stem cells interfaced with carbon nanotube scaffolds. Nanomedicine 2013, 8, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, E.; Ebrahimi-Barough, S.; Mirzaei, E.; Azami, M.; Tavangar, S.M.; Mahmoodi, N.; Basiri, A.; Ai, J. Preparation of fibrin gel scaffolds containing MWCNT/PU nanofibers for neural tissue engineering. J. Biomed. Mater. Res. Part A 2019, 107, 802–814. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Electrical Stimulation: A Novel Tool for Tissue Engineering. Tissue Eng. Part B 2012, 19, 48–57. [Google Scholar] [CrossRef]
- Niu, X.; Rouabhia, M.; Chiffot, N.; King, M.W.; Zhang, Z. An electrically conductive 3D scaffold based on a nonwoven web of poly(L-lactic acid) and conductive poly(3,4-ethylenedioxythiophene). J. Biomed. Mater. Res. A 2015, 103, 2635–2644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, M.; Kang, E.-T.; Neoh, K.G. Electrical stimulation of adipose-derived mesenchymal stem cells in conductive scaffolds and the roles of voltage-gated ion channels. Acta Biomater. 2016, 32, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Inal, S.; Hama, A.; Ferro, M.; Pitsalidis, C.; Oziat, J.; Iandolo, D.; Pappa, A.-M.; Hadida, M.; Huerta, M.; Marchat, D.; et al. Conducting Polymer Scaffolds for Hosting and Monitoring 3D Cell Culture. Adv. Biosyst. 2017, 1. [Google Scholar] [CrossRef] [Green Version]
- Kolarcik, C.L.; Catt, K.; Rost, E.; Albrecht, I.N.; Bourbeau, D.; Du, Z.H.; Kozai, T.D.Y.; Luo, X.L.; Weber, D.J.; Cui, X.T. Evaluation of poly(3,4-ethylenedioxythiophene)/carbon nanotube neural electrode coatings for stimulation in the dorsal root ganglion. J. Neural Eng. 2015, 12, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Kim, B.S.; Song, H. An Integrated Approach of CNT Front-end Amplifier towards Spikes Monitoring for Neuro-prosthetic Diagnosis. Biochip J. 2018, 12, 332–339. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.J.; Xu, W.J.; Luo, W.H.; Li, M.; Chu, F.B.; Xu, L.; Cao, A.Y.; Guan, J.S.; Tang, S.M.; et al. Stretchable Transparent Electrode Arrays for Simultaneous Electrical and Optical Interrogation of Neural Circuits in Vivo. Nano Lett. 2018, 18, 2903–2911. [Google Scholar] [CrossRef] [PubMed]
- Abu-Saude, M.J.; Morshed, B.I. Patterned Vertical Carbon Nanotube Dry Electrodes for Impedimetric Sensing and Stimulation. IEEE Sens. J. 2015, 15, 5851–5858. [Google Scholar] [CrossRef]
- Su, J.Y.; Zhang, X.; Li, M.N.; Gao, T.; Wang, R.; Chai, X.Y.; Zhang, D.G.; Zhang, X.H.; Sui, X.H. Insulation of Carbon Nanotube Yarn Electrodes for Intrafascicular Neural Stimulation and Recording. In Proceedings of the 9th International IEEE/EMBS Conference on Neural Engineering, San Francisco, CA, USA, 20–23 March 2019; pp. 815–818. [Google Scholar]
- Pan, A.I.; Lin, M.H.; Chung, H.W.; Chen, H.; Yeh, S.R.; Chuang, Y.J.; Chang, Y.C.; Yew, T.R. Direct-growth carbon nanotubes on 3D structural microelectrodes for electrophysiological recording. Analyst 2016, 141, 279–284. [Google Scholar] [CrossRef]
- Liu, J.H.; Liu, M.L.; Bai, Y.; Zhang, J.H.; Liu, H.W.; Zhu, W.B. Recent Progress in Flexible Wearable Sensors for Vital Sign Monitoring. Sensors 2020, 20, 4009. [Google Scholar] [CrossRef]
- Massicotte, G.; Carrara, S.; Di Micheli, G.; Sawan, M. A CMOS Amperometric System for Multi-Neurotransmitter Detection. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Samba, R.; Fuchsberger, K.; Matiychyn, I.; Epple, S.; Kiesel, L.; Stett, A.; Schuhmann, W.; Stelzle, M. Application of PEDOT-CNT Microelectrodes for Neurotransmitter Sensing. Electroanalysis 2014, 26, 548–555. [Google Scholar] [CrossRef]
- Kim, K.; Kim, M.J.; Kim, W.; Kim, S.Y.; Park, S.; Park, C.B. Clinically accurate diagnosis of Alzheimer’s disease via multiplexed sensing of core biomarkers in human plasma. Nat. Commun. 2020, 11, 9. [Google Scholar] [CrossRef]
- Son, M.; Kim, D.; Park, K.S.; Hong, S.; Park, T.H. Detection of aquaporin-4 antibody using aquaporin-4 extracellular loop-based carbon nanotube biosensor for the diagnosis of neuromyelitis optica. Biosens. Bioelectron. 2016, 78, 87–91. [Google Scholar] [CrossRef]
- Afzal, A.; Abuilaiwi, F.A.; Habib, A.; Awais, M.; Waje, S.B.; Atieh, M.A. Polypyrrole/carbon nanotube supercapacitors: Technological advances and challenges. J. Power Sources 2017, 352, 174–186. [Google Scholar] [CrossRef]
- Singh, P. Composites Based on Conducting Polymers and Carbon Nanotubes for Supercapacitors. In Conducting Polymer Hybrids; Kumar, V., Kalia, S., Swart, H.C., Eds.; Springer: Cham, Germany, 2017; pp. 305–336. [Google Scholar] [CrossRef]
- Zeng, S.; Chen, H.; Cai, F.; Kang, Y.; Chen, M.; Li, Q. Electrochemical fabrication of carbon nanotube/polyaniline hydrogel film for all-solid-state flexible supercapacitor with high areal capacitance. J. Mater. Chem. A 2015, 3, 23864–23870. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, R.; Li, E.; Li, X.; Liu, Y.; Yuan, G. Graphene/Carbon Nanotube/Bacterial Cellulose assisted supporting for polypyrrole towards flexible supercapacitor applications. J. Alloy. Compd. 2019, 777, 524–530. [Google Scholar] [CrossRef]
- Yin, B.-S.; Zhang, S.-W.; Ren, Q.-Q.; Liu, C.; Ke, K.; Wang, Z.-B. Elastic soft hydrogel supercapacitor for energy storage. J. Mater. Chem. A 2017, 5, 24942–24950. [Google Scholar] [CrossRef]
- Song, C.; Yun, J.; Keum, K.; Jeong, Y.R.; Park, H.; Lee, H.; Lee, G.; Oh, S.Y.; Ha, J.S. High performance wire-type supercapacitor with Ppy/CNT-ionic liquid/AuNP/carbon fiber electrode and ionic liquid based electrolyte. Carbon 2019, 144, 639–648. [Google Scholar] [CrossRef]
- Ren, C.; Yan, Y.; Sun, B.; Gu, B.; Chou, T.-W. Wet-spinning assembly and in situ electrodeposition of carbon nanotube-based composite fibers for high energy density wire-shaped asymmetric supercapacitor. J. Colloid Interface Sci. 2020, 569, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Deng, Z.; Bi, S.; Ran, J.; Cheng, D.; Luo, L.; Cai, G.; Wang, X.; Tang, X. In situ polymerization of pyrrole on CNT/cotton multifunctional composite yarn for supercapacitors. Ionics 2020. [Google Scholar] [CrossRef]
- Hao, H.-Y.; Dai, L.; Li, Z.; Low, K.-H. Enhanced Conductivity and Color Neutrality of Transparent Conductive Electrodes Based on CNT/PEDOT: PSS Composite with a Layer-by-layer Structure. In Advanced Material Engineering: Proceedings of the 2015 International Conference on Advanced Material Engineering; World Scientific: Hackensack, NJ, USA, 2016; pp. 285–290. [Google Scholar]
- Mbuyise, X.G.; Arbab, E.A.A.; Kaviyarasu, K.; Pellicane, G.; Maaza, M.; Mola, G.T. Zinc oxide doped single wall carbon nanotubes in hole transport buffer layer. J. Alloy. Compd. 2017, 706, 344–350. [Google Scholar] [CrossRef]
- Fan, Q.; Zhang, Q.; Zhou, W.; Xia, X.; Yang, F.; Zhang, N.; Xiao, S.; Li, K.; Gu, X.; Xiao, Z.; et al. Novel approach to enhance efficiency of hybrid silicon-based solar cells via synergistic effects of polymer and carbon nanotube composite film. Nano Energy 2017, 33, 436–444. [Google Scholar] [CrossRef]
- Yoon, S.; Ha, S.R.; Moon, T.; Jeong, S.M.; Ha, T.-J.; Choi, H.; Kang, D.-W. Carbon nanotubes embedded poly(3,4-ethylenedioxythiophene):poly (styrenesulfonate) hybrid hole collector for inverted planar perovskite solar cells. J. Power Sour. 2019, 435. [Google Scholar] [CrossRef]
- Wang, X.; Ugur, A.; Goktas, H.; Chen, N.; Wang, M.; Lachman, N.; Kalfon-Cohen, E.; Fang, W.; Wardle, B.L.; Gleasont, K.K. Room Temperature Resistive Volatile Organic Compound Sensing Materials Based on a Hybrid Structure of Vertically Aligned Carbon Nanotubes and Conformal oCVD/iCVD Polymer Coatings. ACS Sens. 2016, 1, 374–383. [Google Scholar] [CrossRef]
- Chen, Y.; Owyeung, R.E.; Sonkusale, S.R. Combined optical and electronic paper-nose for detection of volatile gases. Anal. Chim. Acta 2018, 1034, 128–136. [Google Scholar] [CrossRef] [PubMed]
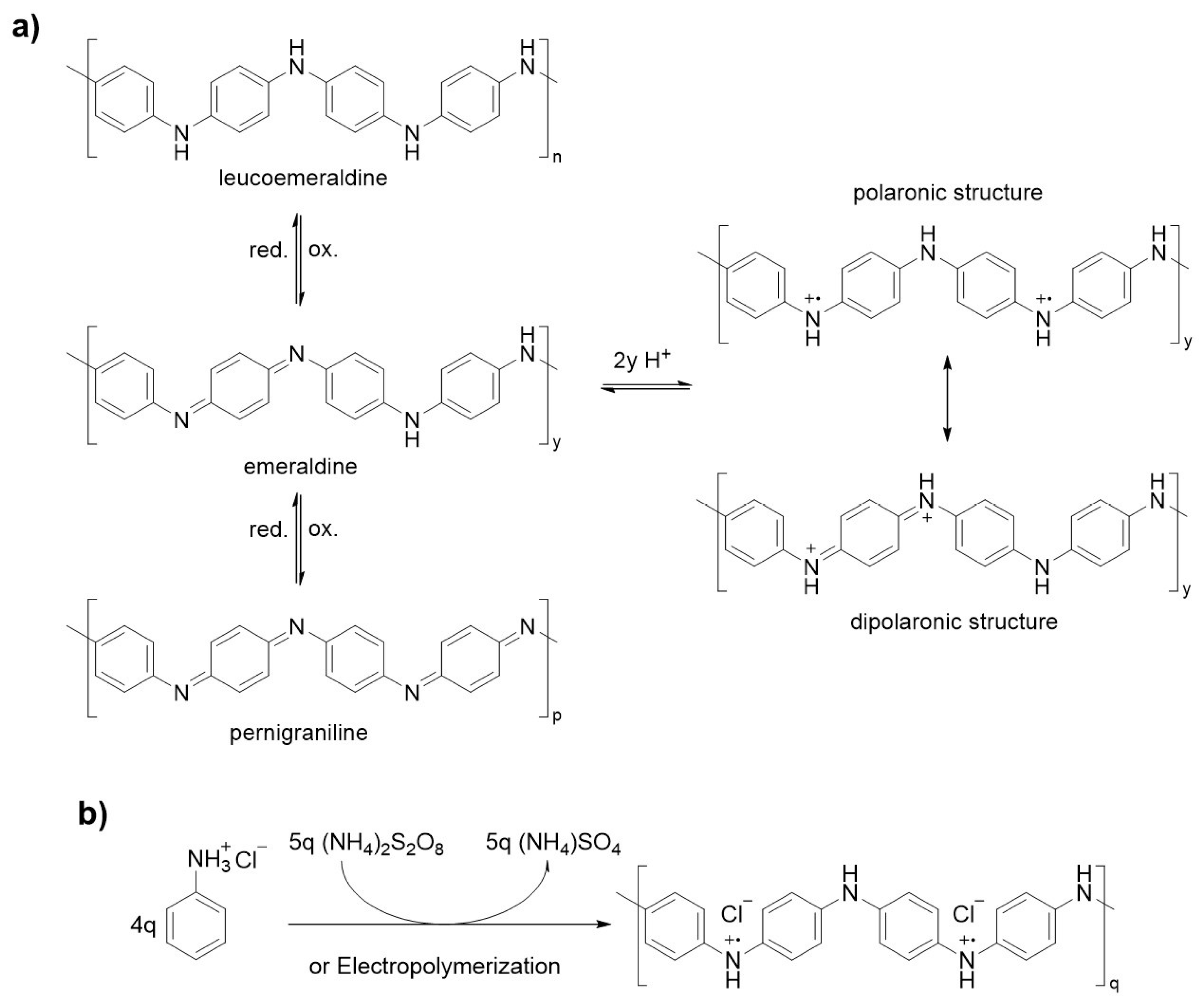
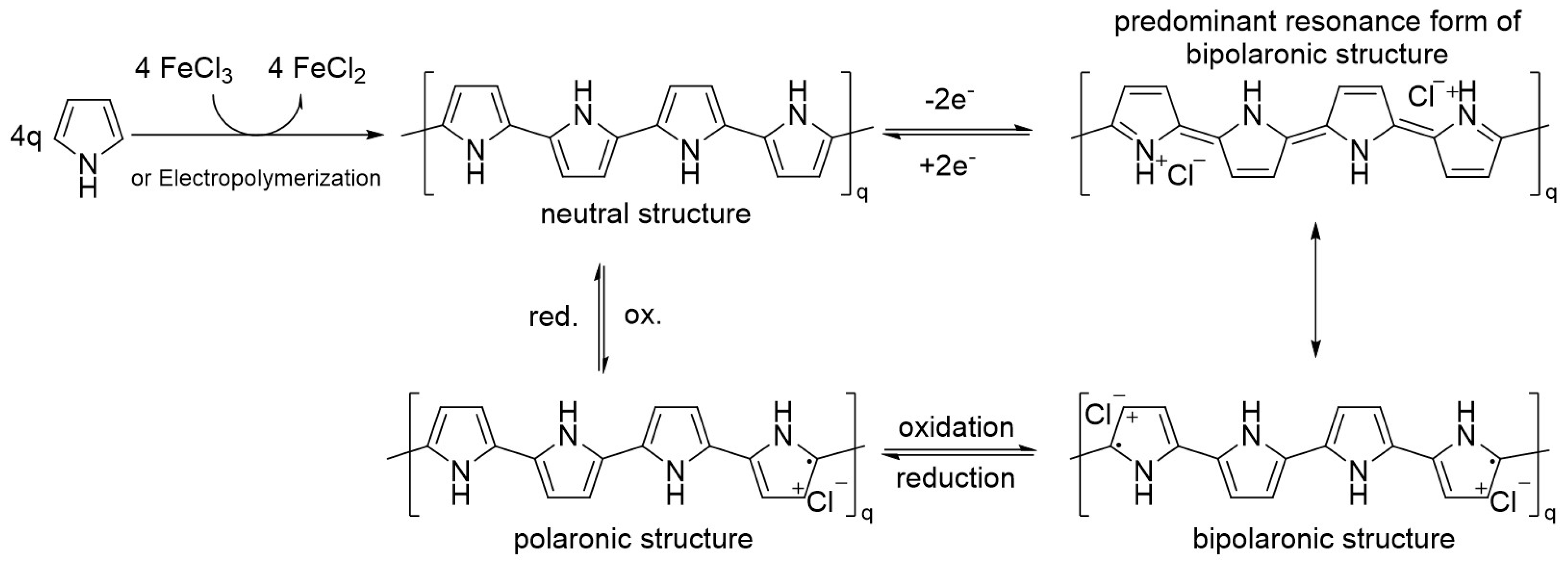
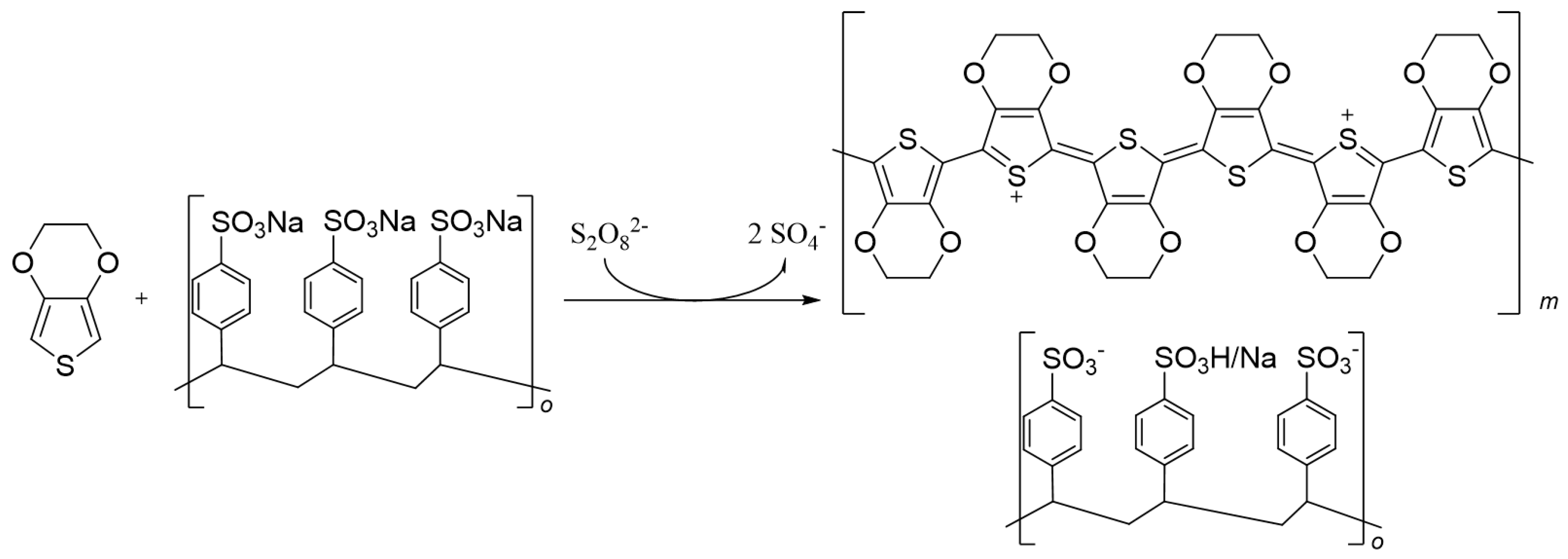
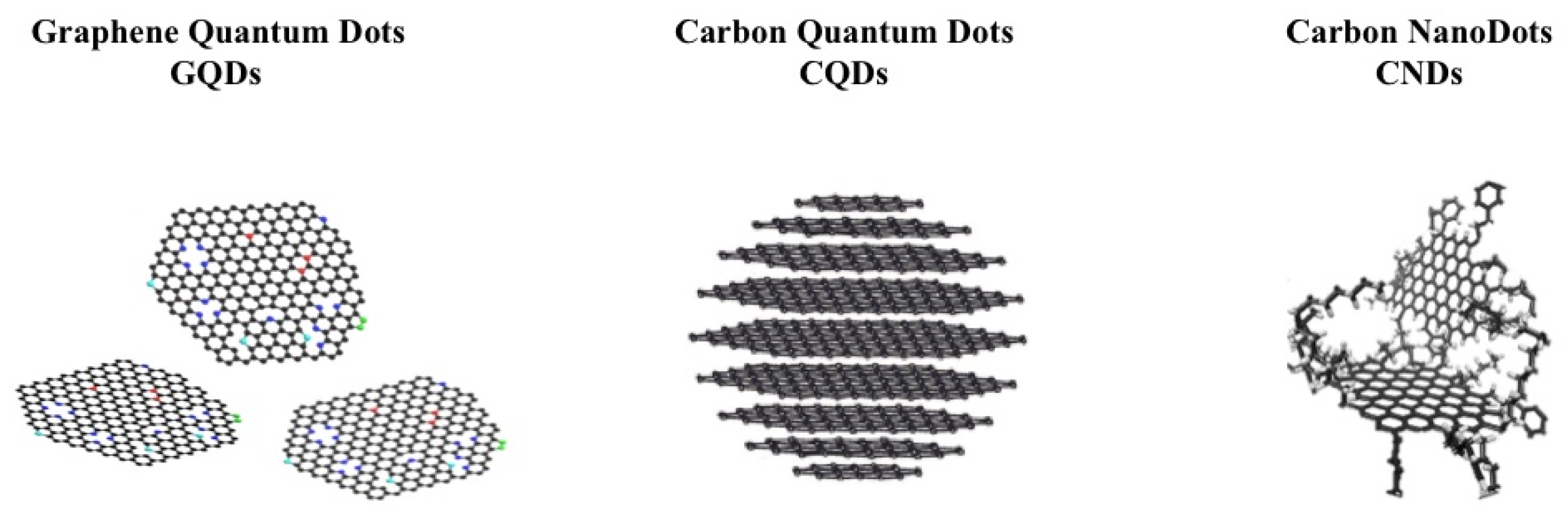
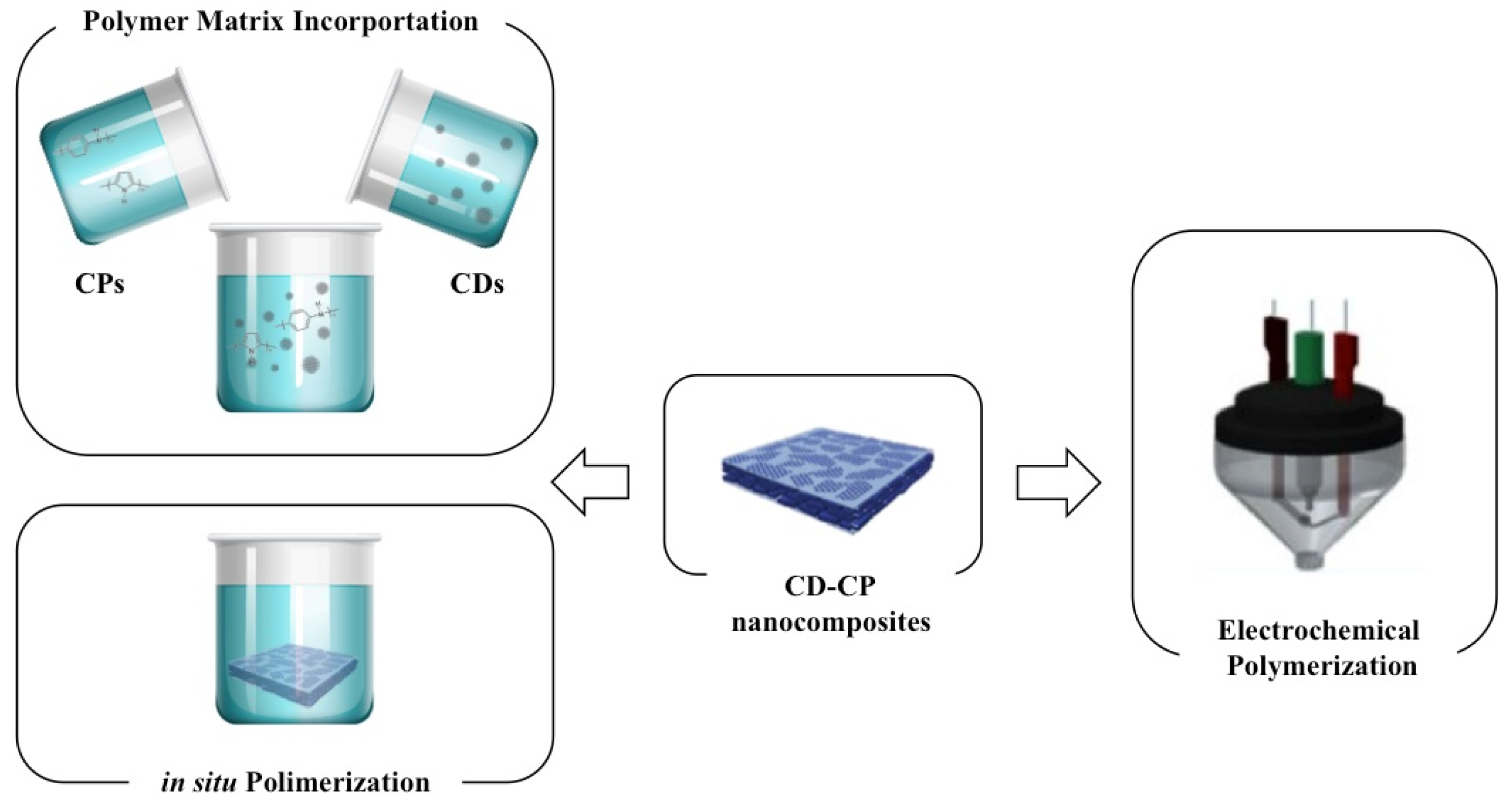
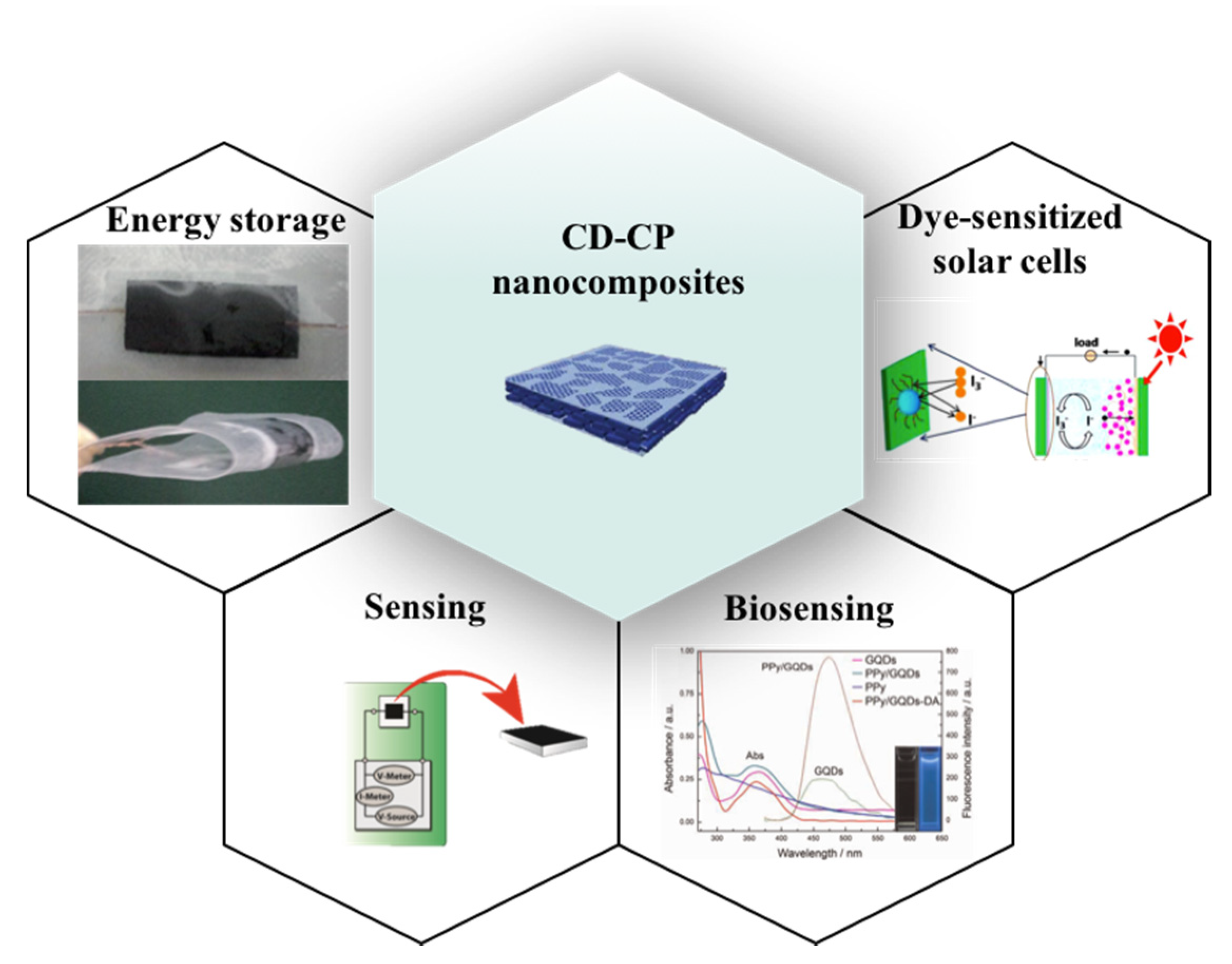
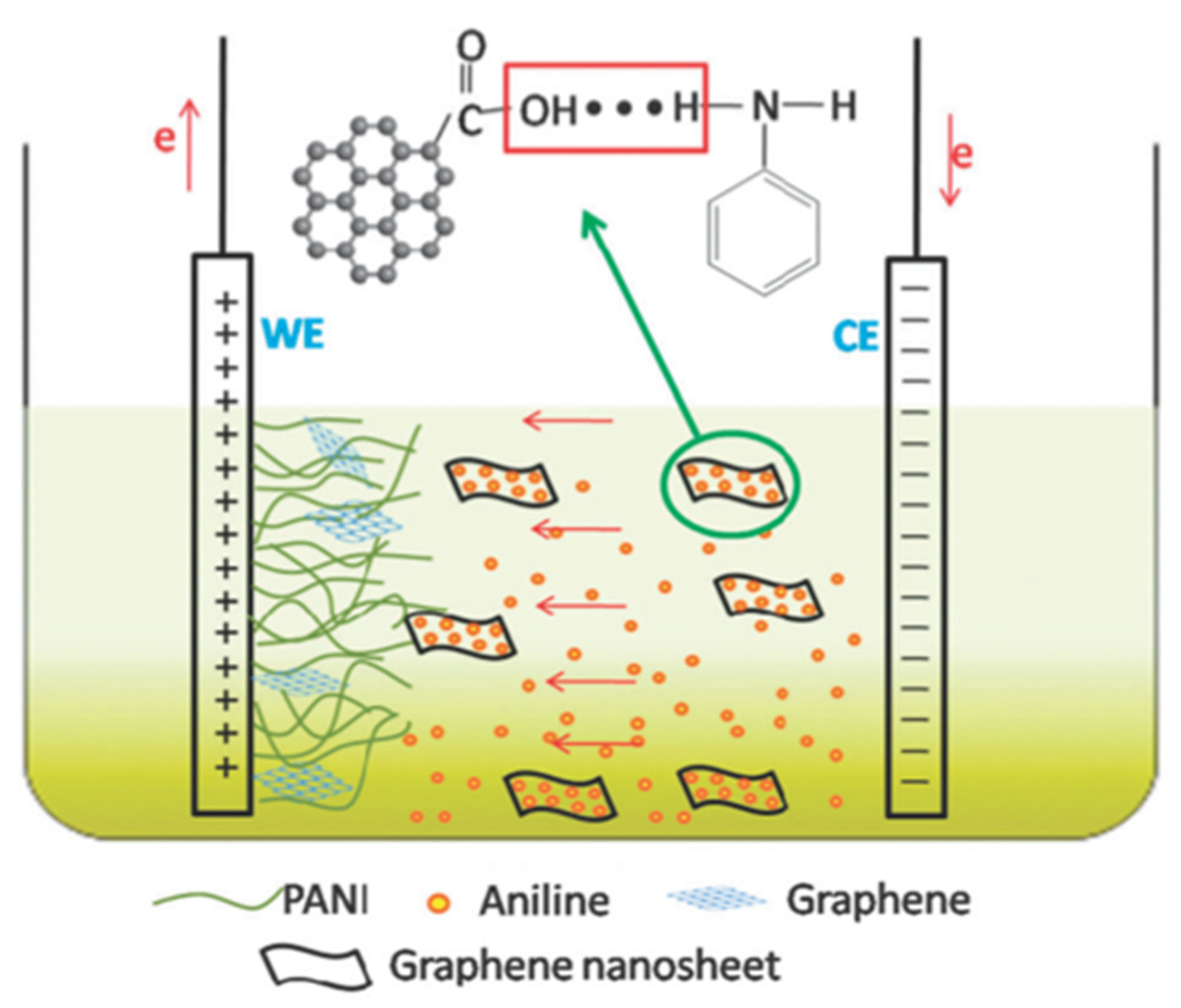
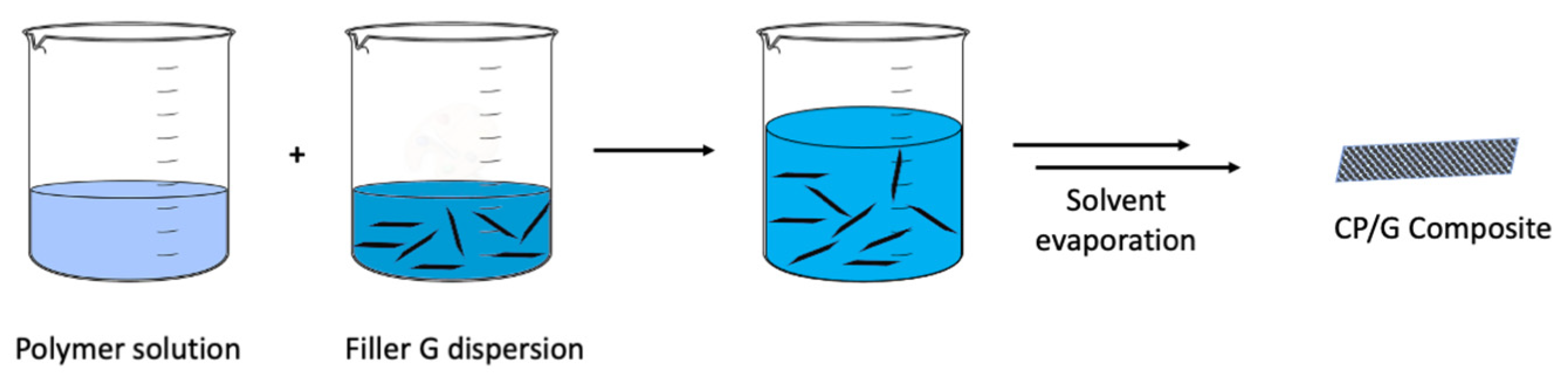
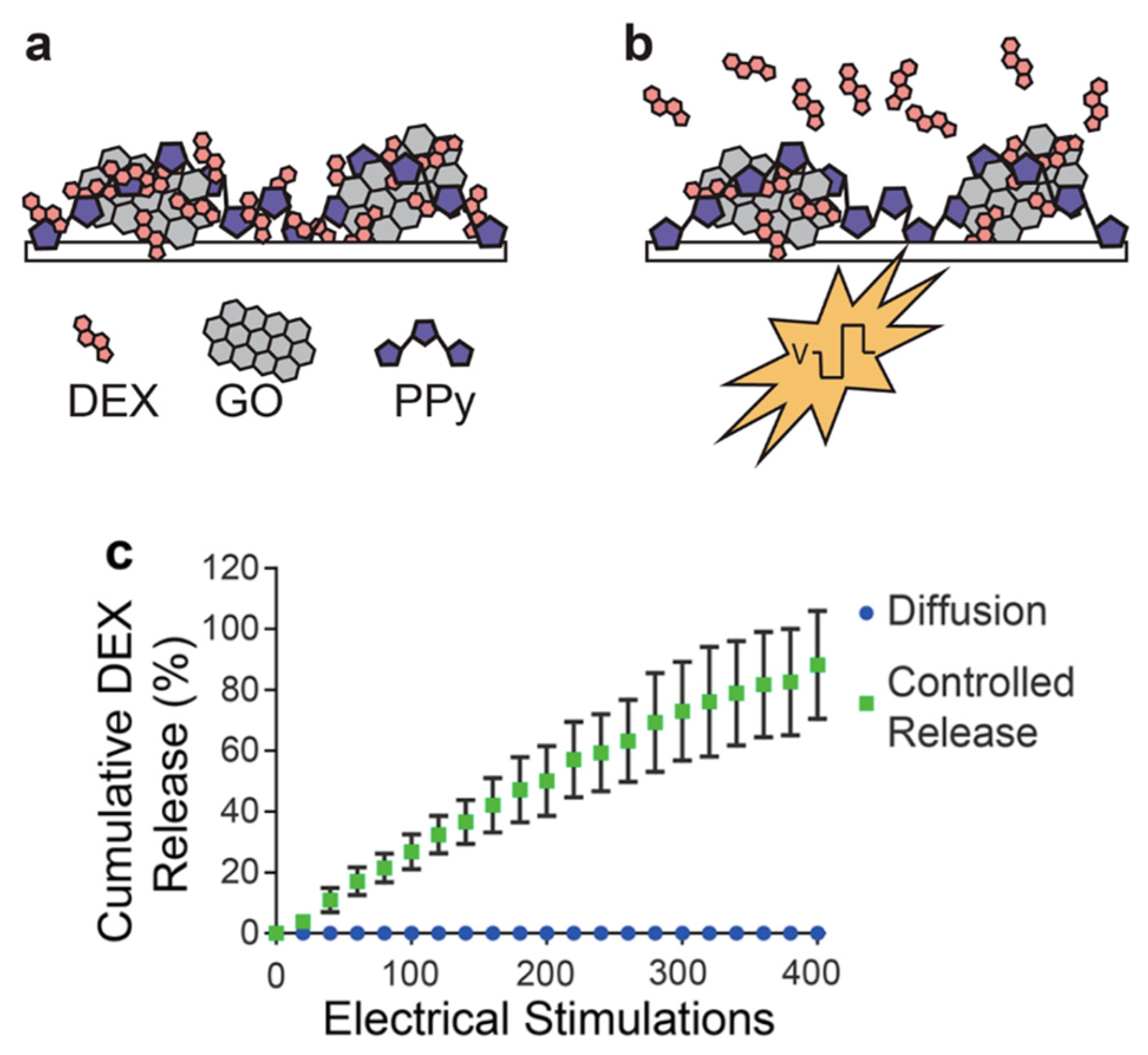
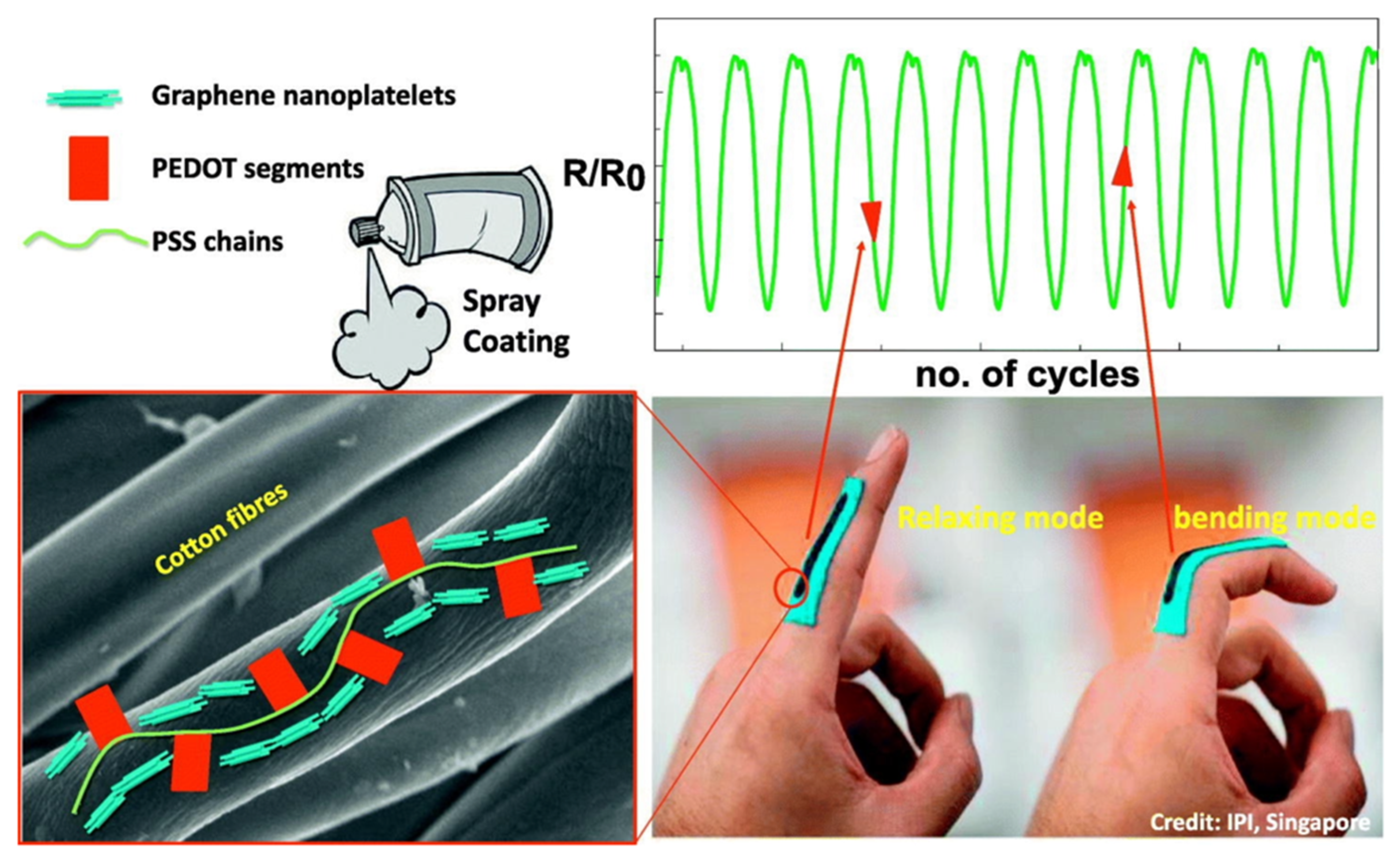
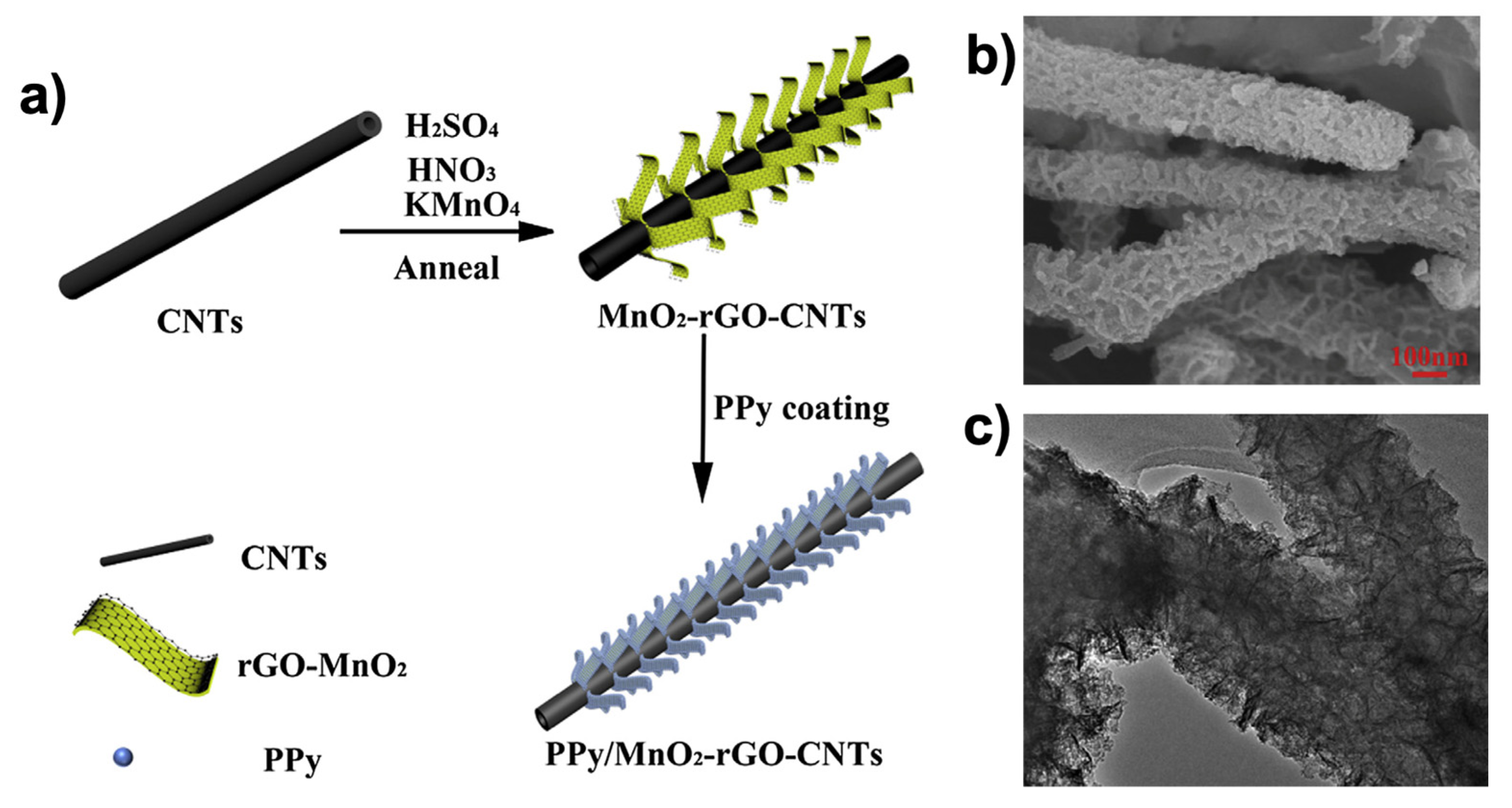
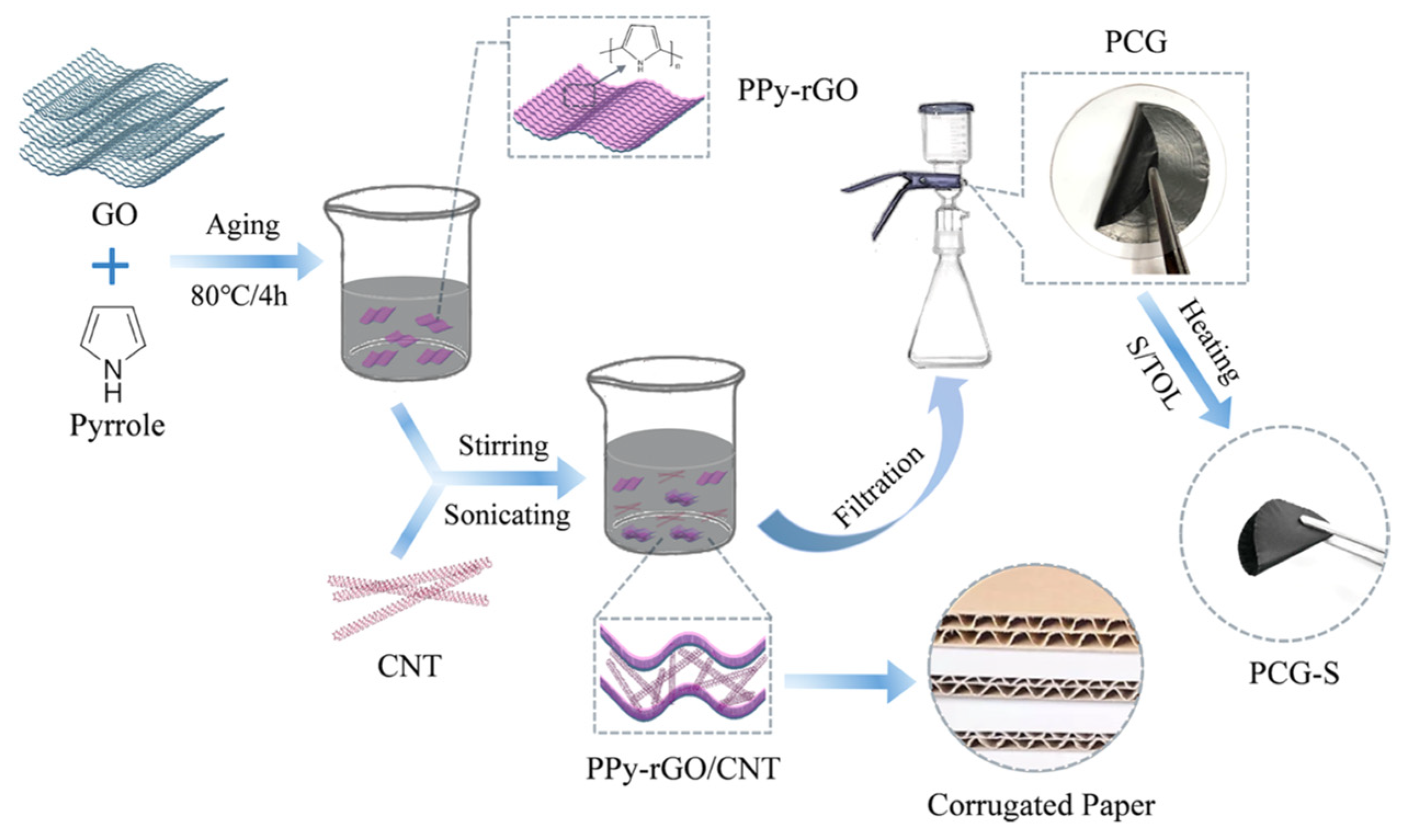
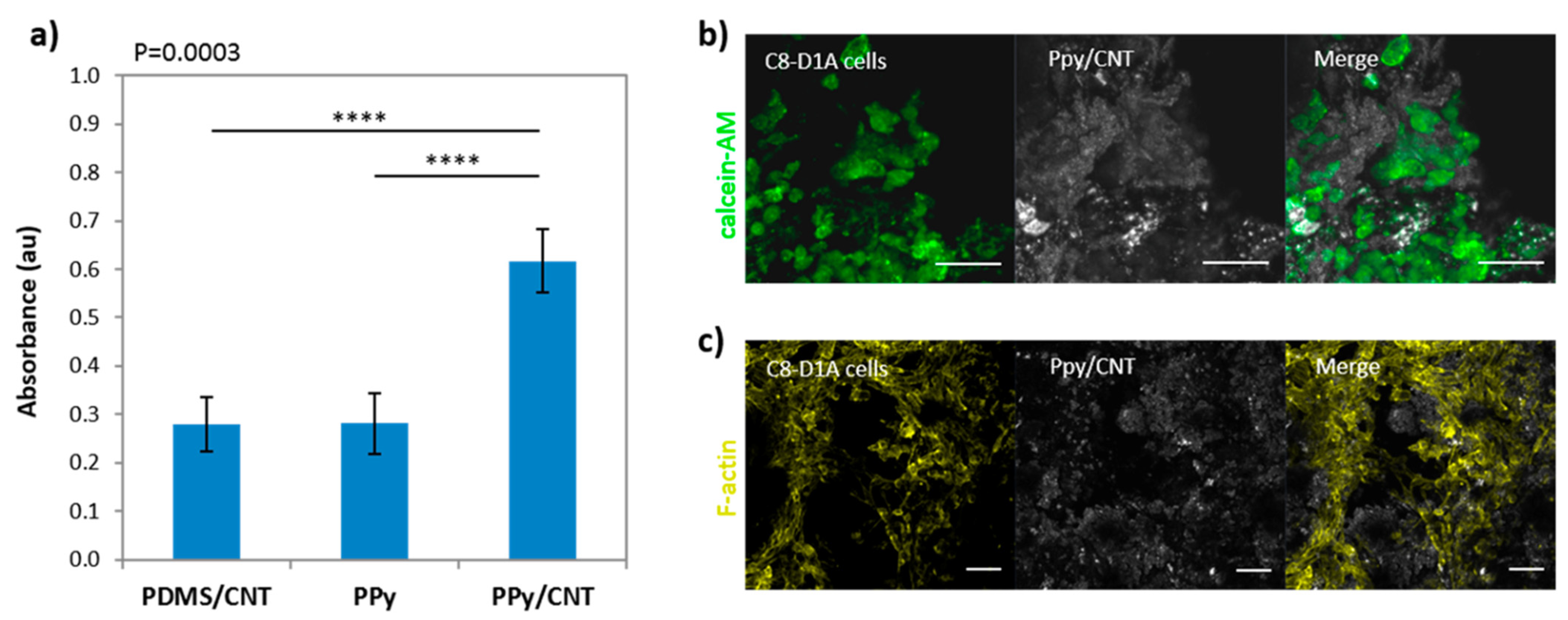
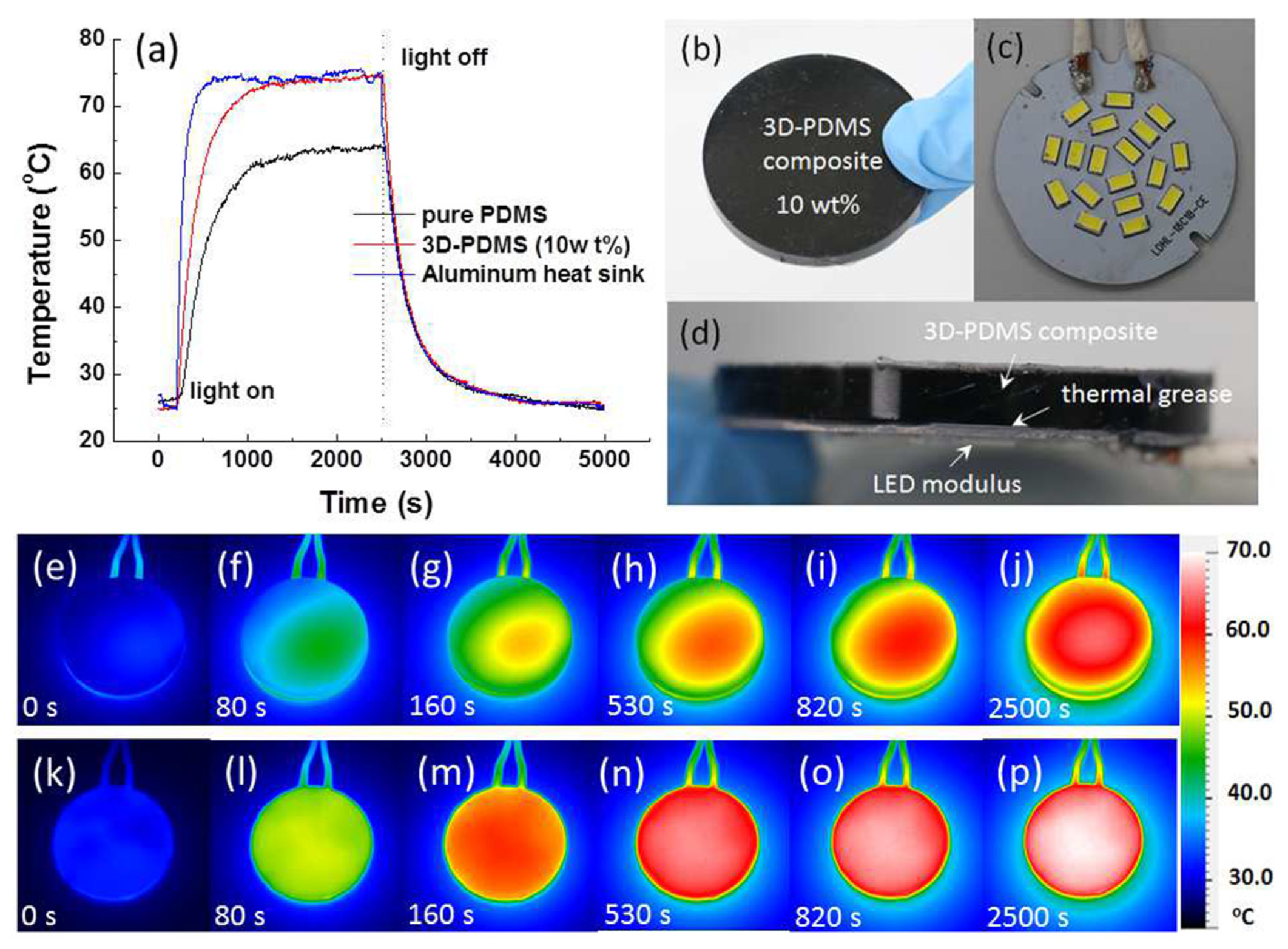

| CP | Graphene Material | Method | Application | Ref |
|---|---|---|---|---|
| PANI | FLG | in situ polymerization | Methodology | [152] |
| PANI | rGO | in situ polymerization | Supercapacitors | [153] |
| PANI | FLG | in situ polymerization | Supercapacitors | [11] |
| PANI/PES | rGO | Phase inversion | Conductive membranes | [166] |
| PANI | rGO | Electrospinning | Nanofibers | [12] |
| PANI | GO/FLG | in situ polymerization | Hybrid flexible papers | [177] |
| PANI | FLG | in situ polymerization | Electrochromic | [178] |
| PANI | GO | in situ polymerization | Flexible electrodes | [161] |
| PEDOT | GO | in situ polymerization | Bio-interfacing | [160] |
| PEDOT | GO | in situ polymerization | Tissue interface | [179] |
| PEDOT | FLG | in situ polymerization | Biosensor | [180] |
| PEDOT | GO | Microwave in situ polymerization | Biosensor | [181] |
| PEDOT | FLG | Electrochemical polymerization | Photocapacitor | [182] |
| PEDOT | G nanoplatelets | Solvent mixing | Sensor | [183] |
| PEDOT | rGO | in situ polymerization | Sensor | [150] |
| PPy | GO | in situ polymerization | Anticorrosive performance | [154] |
| PPy | FLG | Solvent mixing | Corrosion protection | [165] |
| PPy | GO | in situ polymerization | Drug release | [184] |
| PPy | CVDG | Electrochemical deposition | Bioenergy storage | [185] |
| PPy | GO | Electrochemical deposition | Supercapacitors | [176] |
| PPy | FLG | Solvent mixing/capillary force | Supercapacitors | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez, I.J.; Vázquez Sulleiro, M.; Mantione, D.; Alegret, N. Carbon Nanomaterials Embedded in Conductive Polymers: A State of the Art. Polymers 2021, 13, 745. https://doi.org/10.3390/polym13050745
Gómez IJ, Vázquez Sulleiro M, Mantione D, Alegret N. Carbon Nanomaterials Embedded in Conductive Polymers: A State of the Art. Polymers. 2021; 13(5):745. https://doi.org/10.3390/polym13050745
Chicago/Turabian StyleGómez, I. Jénnifer, Manuel Vázquez Sulleiro, Daniele Mantione, and Nuria Alegret. 2021. "Carbon Nanomaterials Embedded in Conductive Polymers: A State of the Art" Polymers 13, no. 5: 745. https://doi.org/10.3390/polym13050745
APA StyleGómez, I. J., Vázquez Sulleiro, M., Mantione, D., & Alegret, N. (2021). Carbon Nanomaterials Embedded in Conductive Polymers: A State of the Art. Polymers, 13(5), 745. https://doi.org/10.3390/polym13050745








