Sulfur-Modified Carbon Nanotubes for the Development of Advanced Elastomeric Materials
Abstract
:1. Introduction
2. Experimental Section
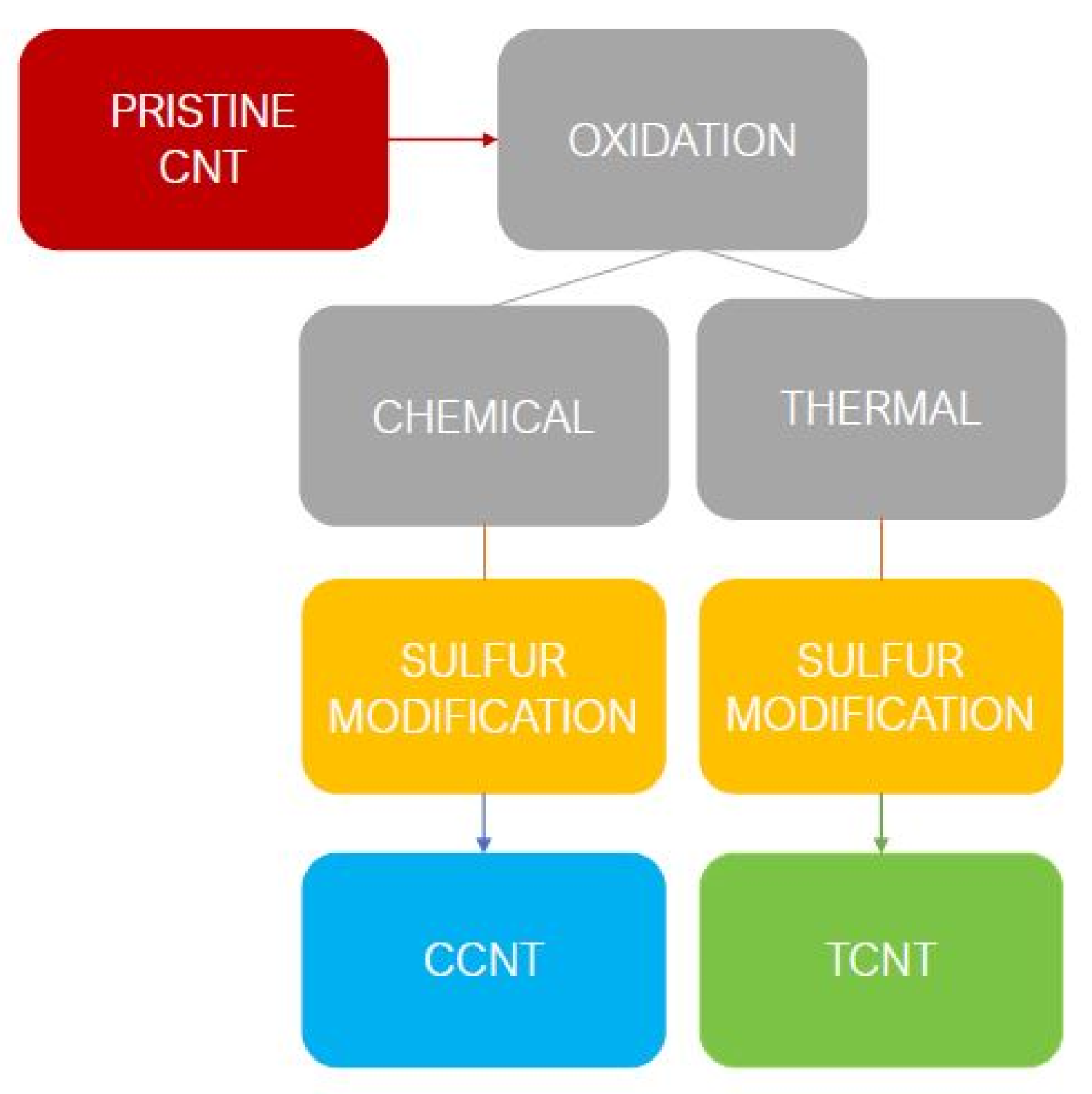
2.1. Functionalization of Carbon Nanotubes (CNTs)
2.1.1. Pristine CNT
2.1.2. Chemical Oxidation
2.1.3. Thermal Oxidation
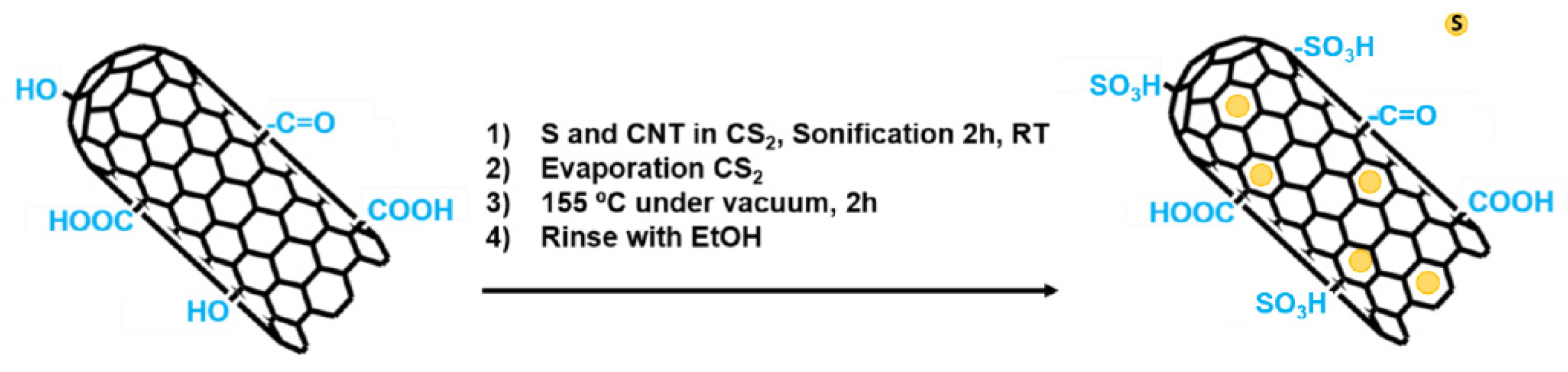
2.1.4. Functionalization with Elemental Sulfur
2.2. Materials and Preparation of Rubber Compounds
2.3. Characterization
2.3.1. Structure and Morphology of CNT
2.3.2. Rubber Compounds
3. Results
3.1. Characterization of Functionalized CNT
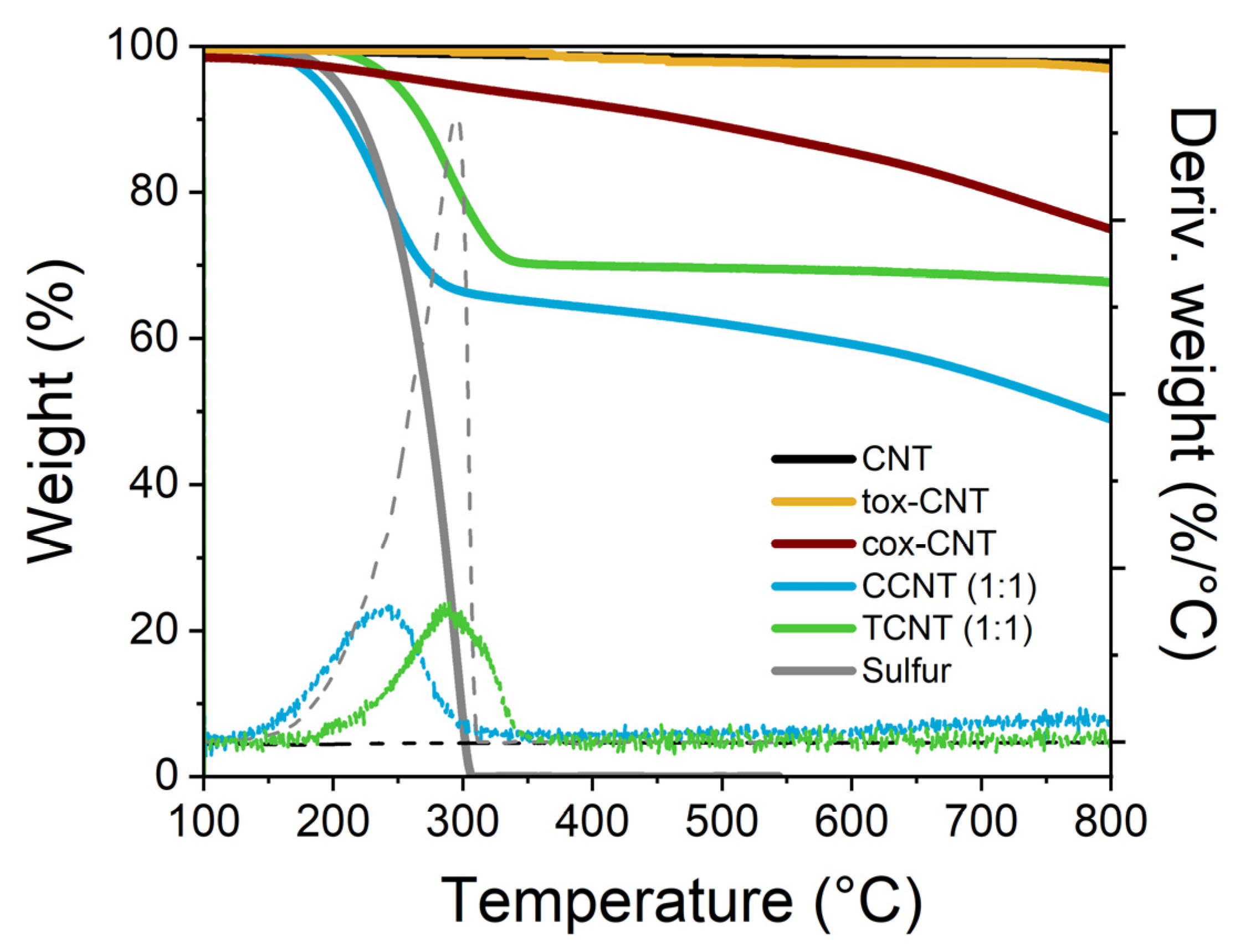
3.1.1. Thermogravimetric Analysis (TGA)
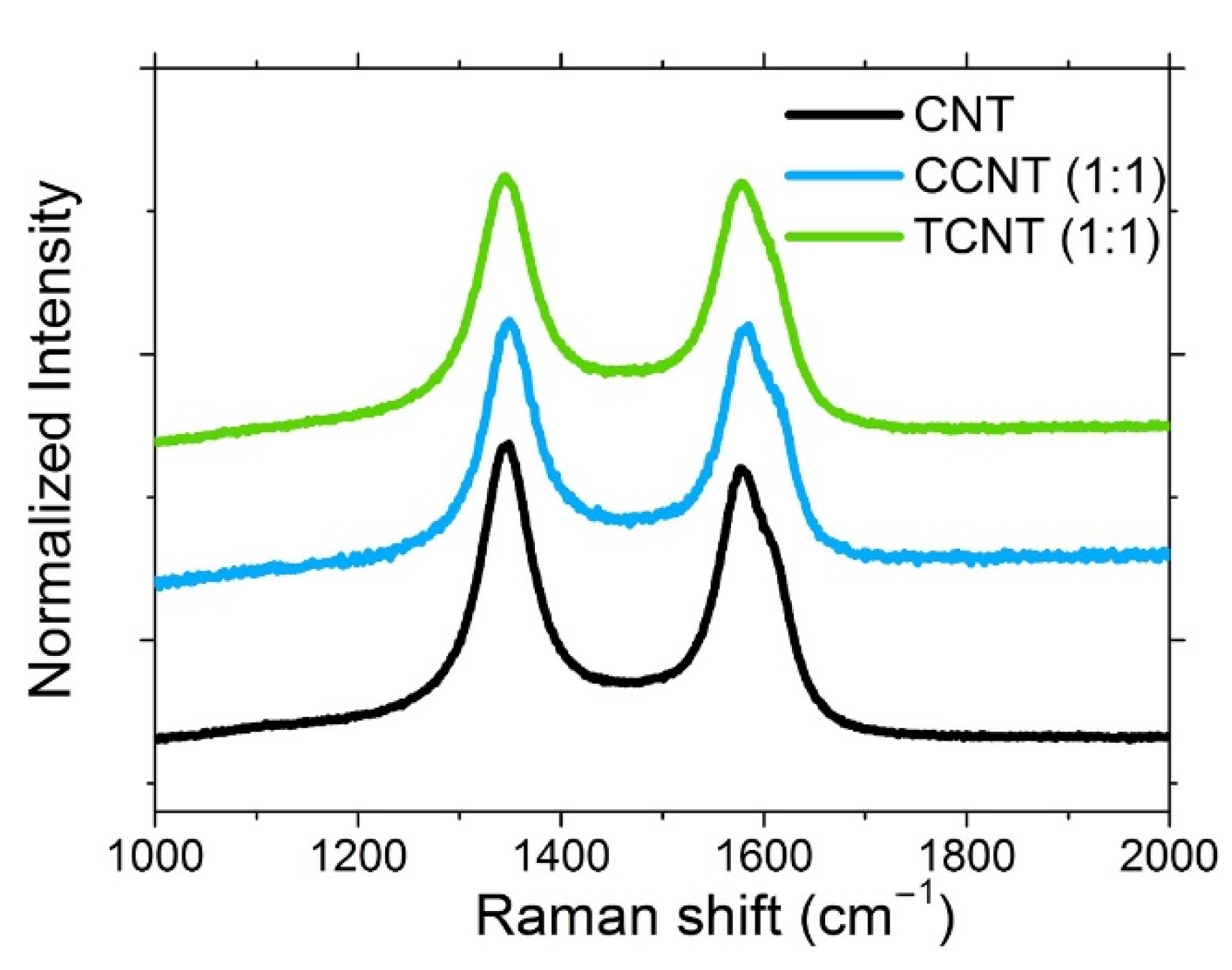
3.1.2. Raman Spectroscopy
3.1.3. X-ray Photoelectron Spectroscopy (XPS)
3.2. Characterization of Rubber Compounds
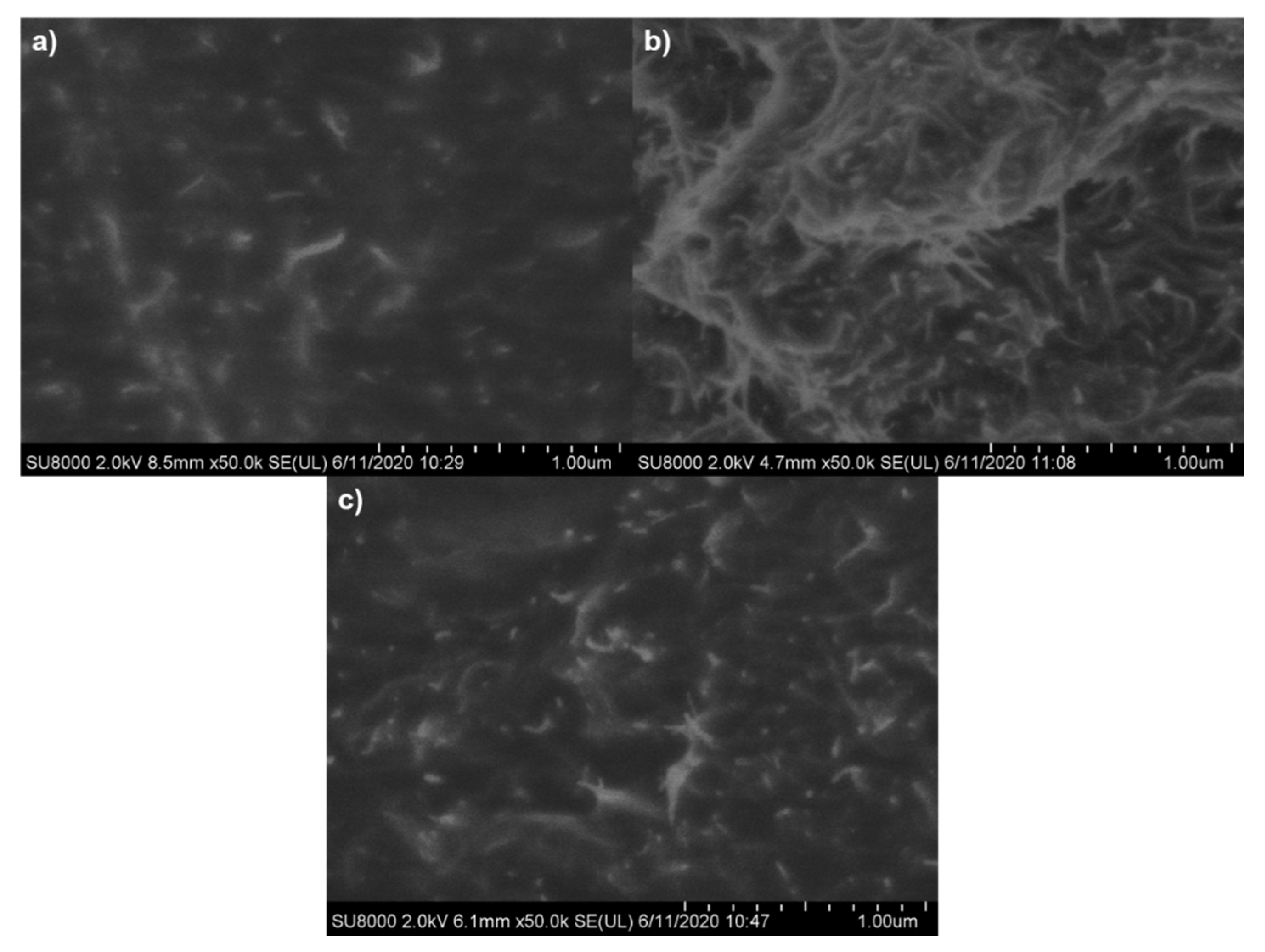
3.2.1. CNT Dispersion
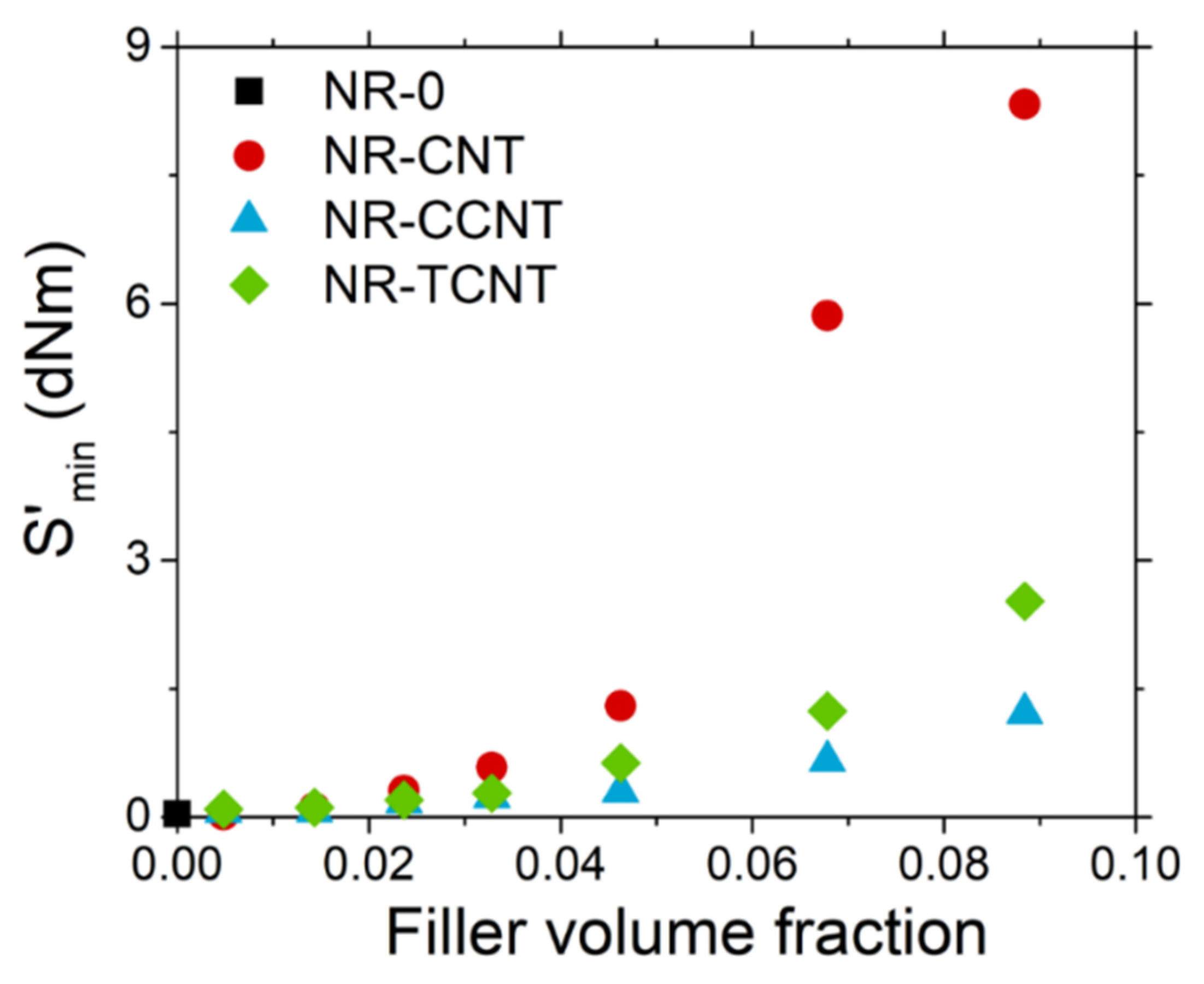
3.2.2. Vulcanization Process
3.2.3. Electrical Conductivity
3.2.4. Mechanical Properties
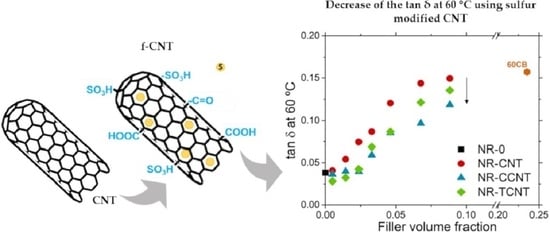
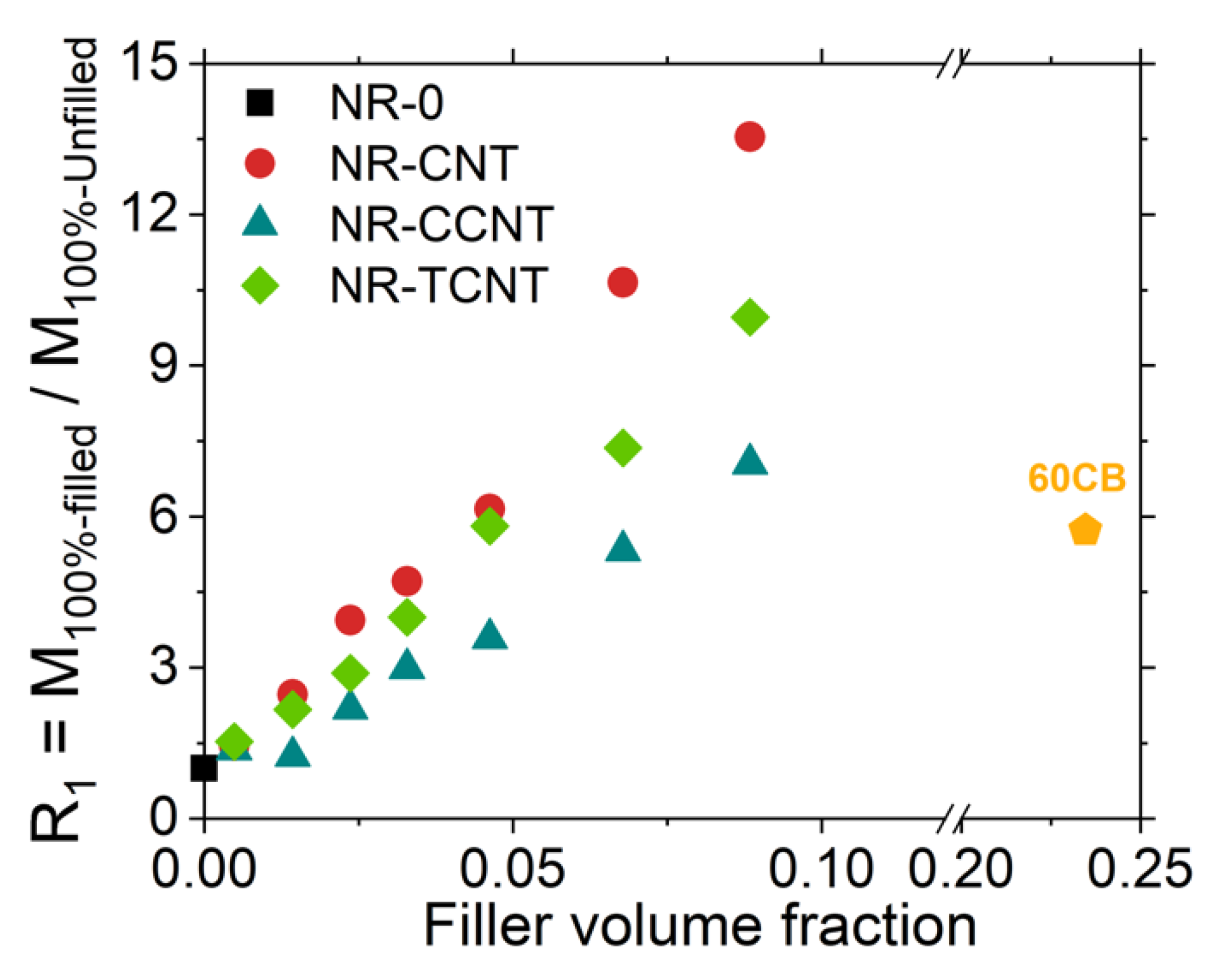
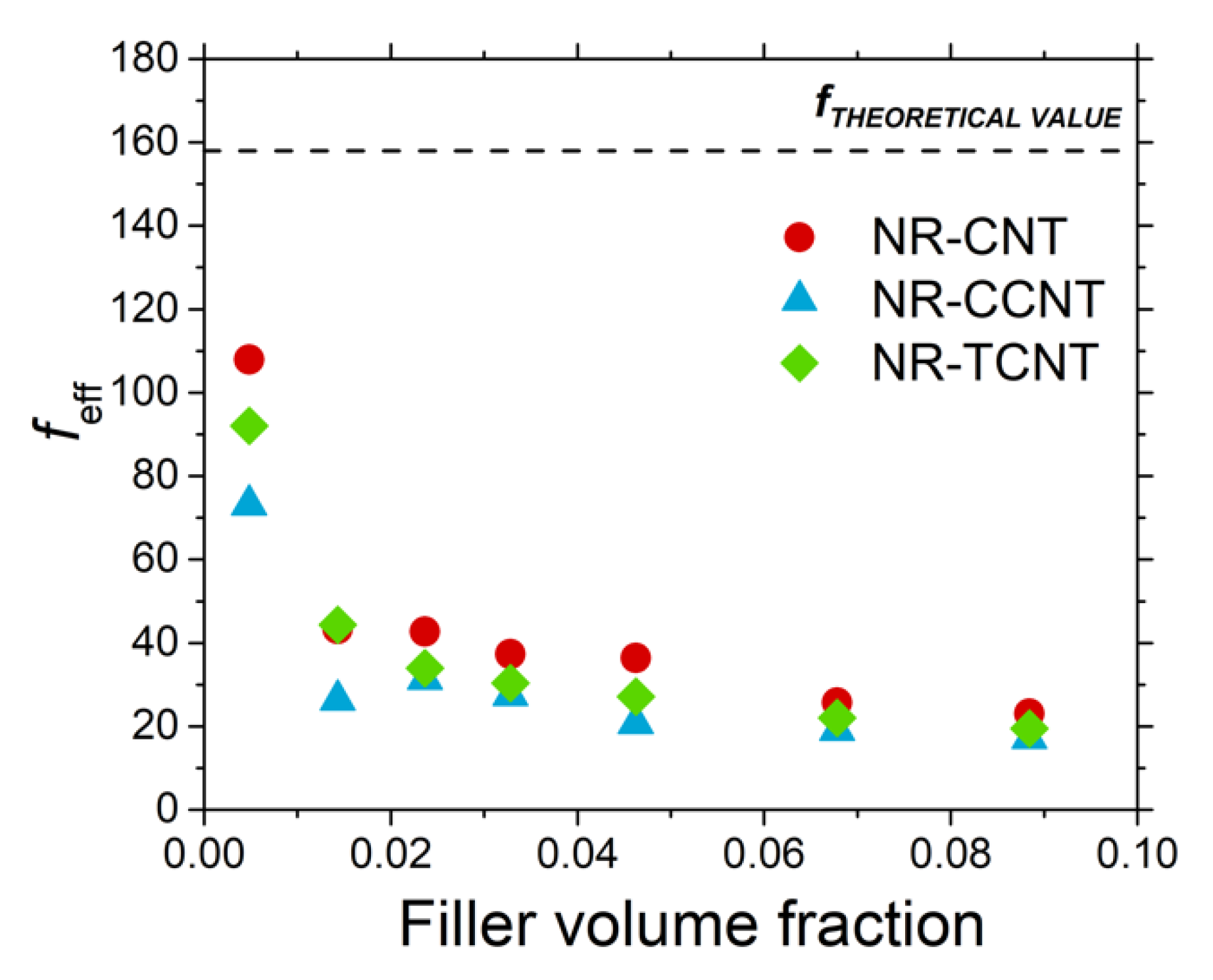
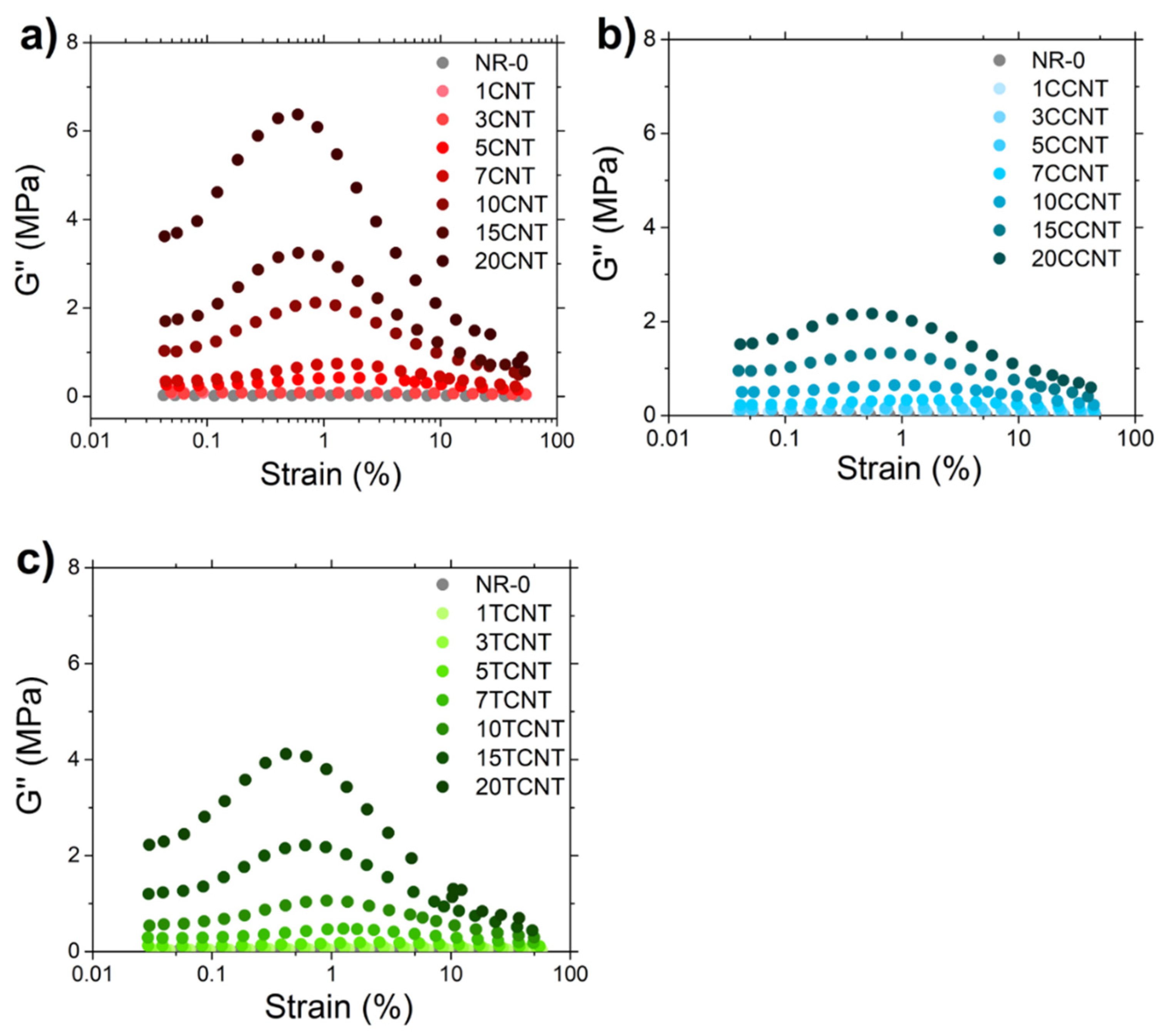
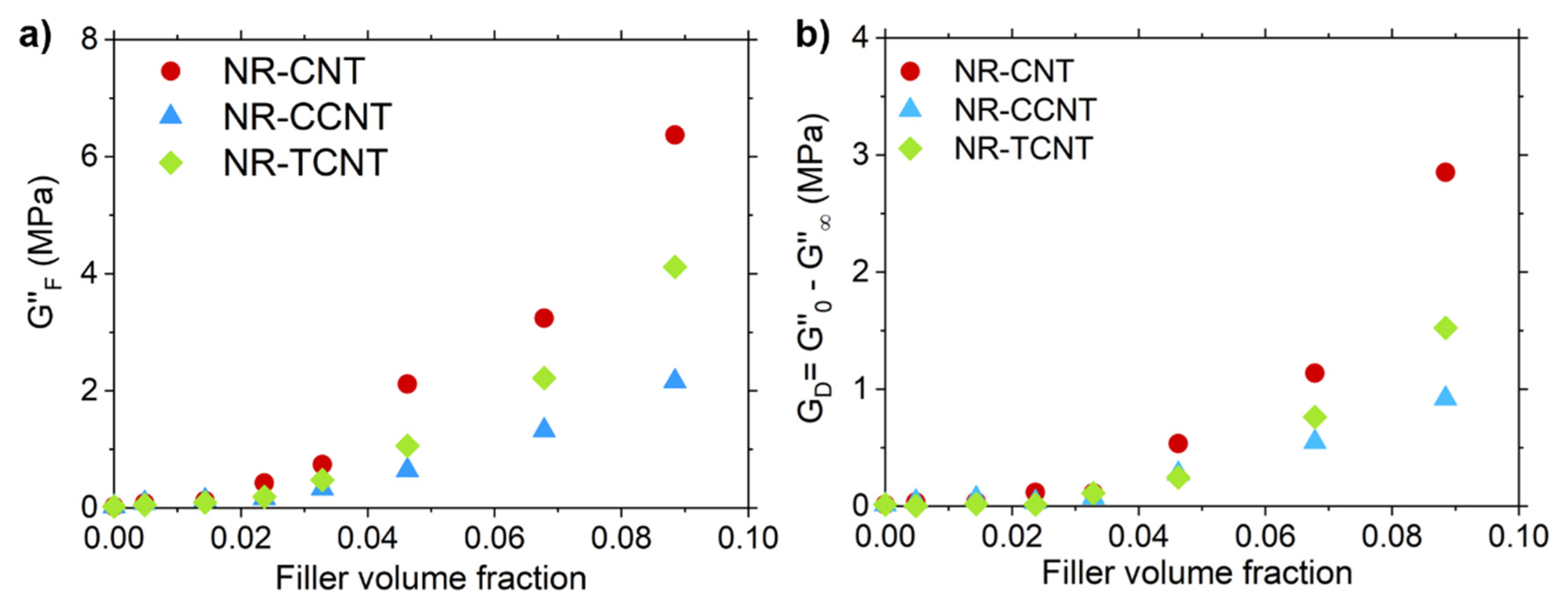
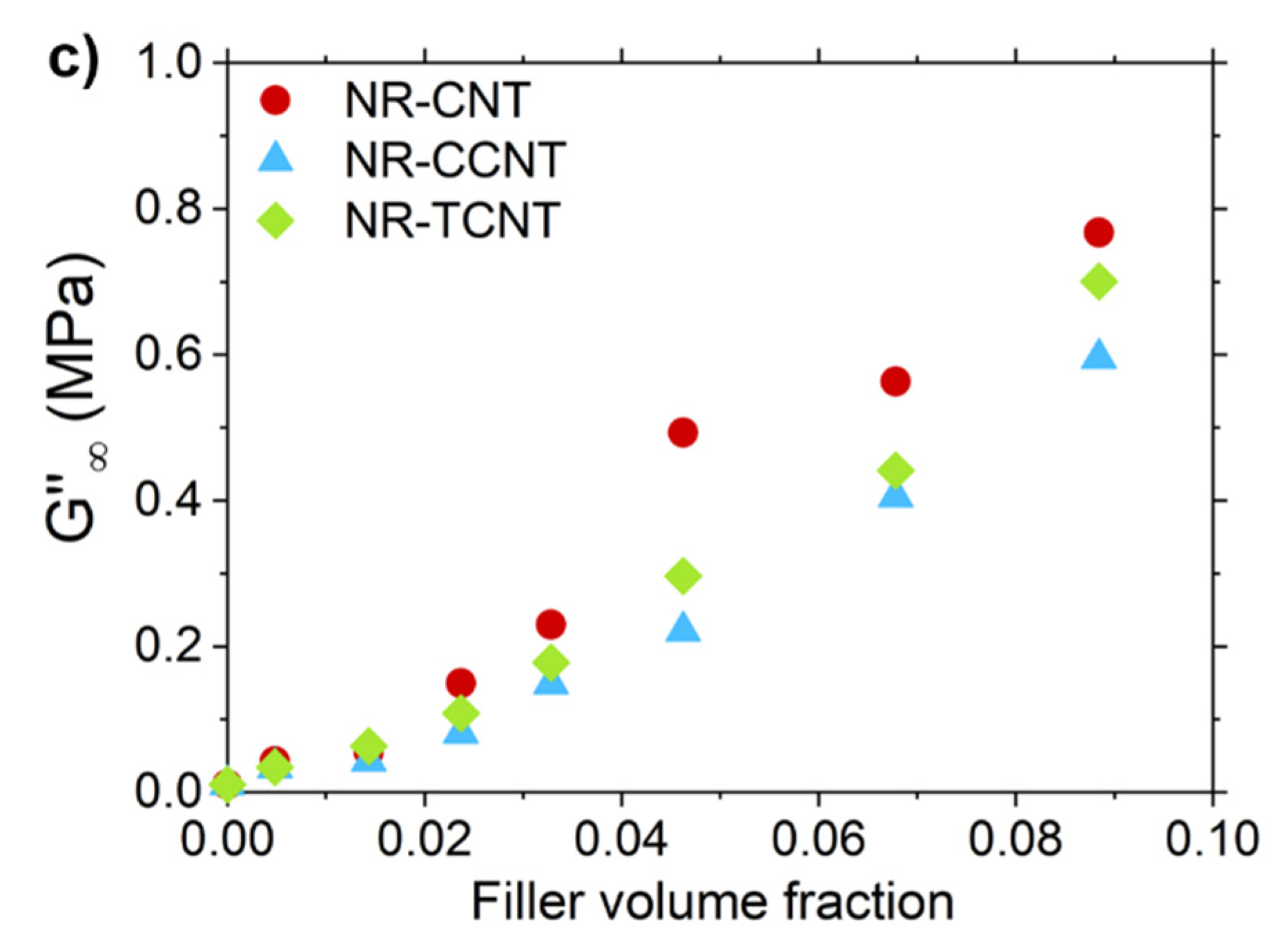
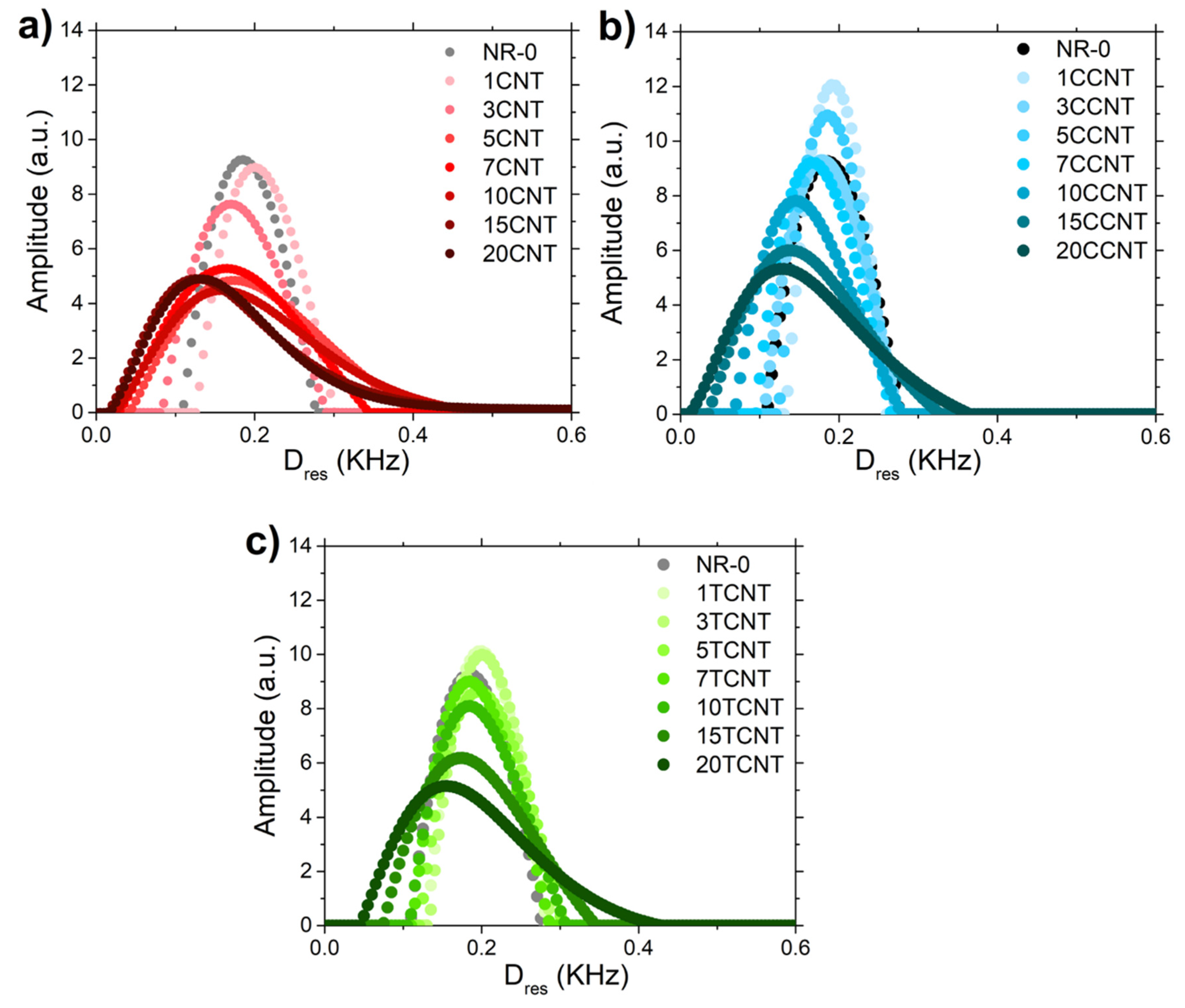
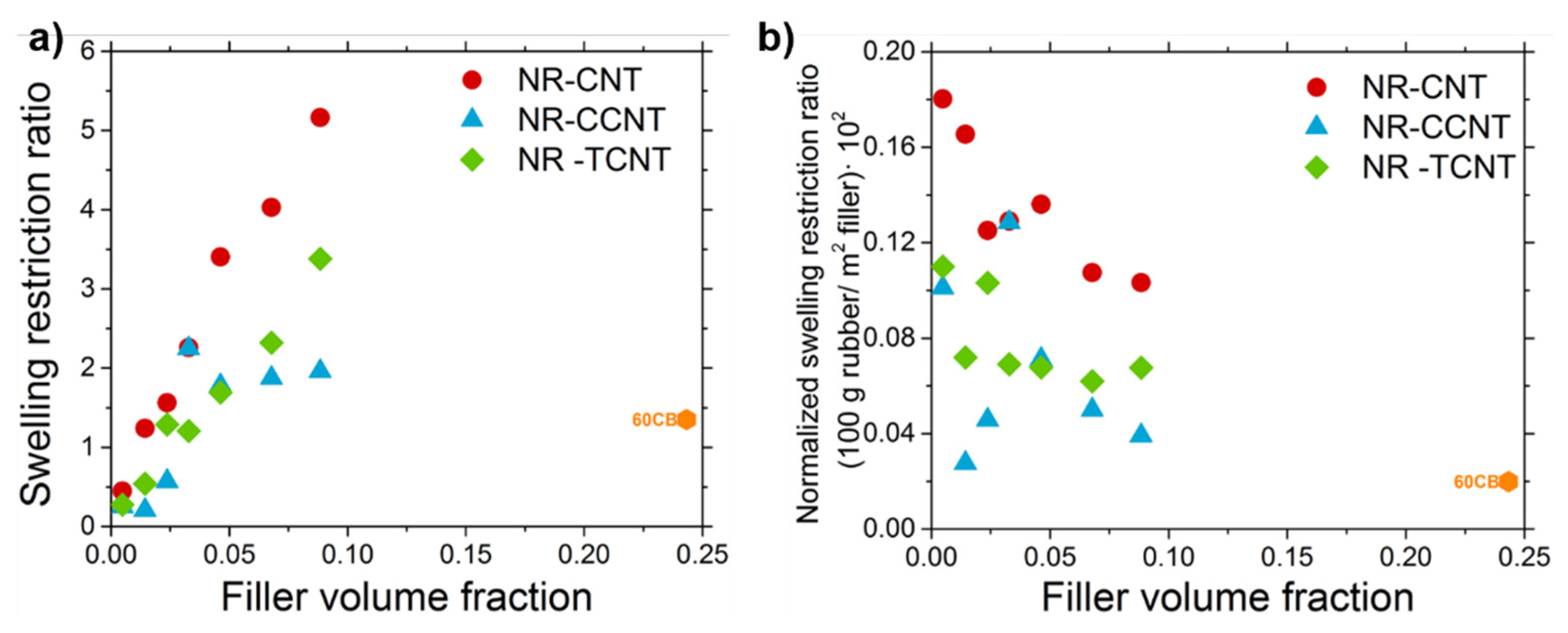
3.2.5. Rubber–CNT Network Structure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rafiee, R. Carbon Nanaotube-Reinforced Polymers. From Nanoscale to Macroscale, 1st ed.; Rafiee, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780323482219. [Google Scholar]
- Ponnamma, D.; Sadasivuni, K.K.; Grohens, Y.; Guo, Q.; Thomas, S. Carbon nanotube based elastomer composites-an approach towards multifunctional materials. J. Mater. Chem. C 2014, 2, 8446–8485. [Google Scholar] [CrossRef]
- Mayyas, A.; Qattawi, A.; Omar, M.; Shan, D. Design for sustainability in automotive industry: A comprehensive review. Renew. Sustain. Energy Rev. 2012, 16, 1845–1862. [Google Scholar] [CrossRef]
- Wenlong, S.; Xiaokai, C.; Lu, W. Analysis of energy saving and emission reduction of vehicles using light weight materials. Energy Procedia 2016, 88, 889–893. [Google Scholar] [CrossRef] [Green Version]
- Coelho, M.C.; Torräo, G.; Emami, N.; Grácio, J. Nanotechnology in automotive industry: Research strategy and trends for the future-small objects, big impacts. J. Nanosci. Nanotechnol. 2012, 12, 6621–6630. [Google Scholar] [CrossRef]
- Goede, M.; Stehlin, M.; Rafflenbeul, L.; Kopp, G.; Beeh, E. Super Light Car-lightweight construction thanks to a multi-material design and function integration. Eur. Transp. Res. Rev. 2009, 1, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Galimberti, M.; Cipolletti, V.; Kumar, V. Nanofillers in Natural Rubber. In Natural Rubber Materials: Volume 2: Composites and Nanocomposites; Thomas, S., Chan, C.H., Pothen, L., Joy, J., Maria, H., Eds.; Royal Society of Chemistry: London, UK, 2013. [Google Scholar]
- Ozden, S.; Tsafack, T.; Owuor, P.S.; Li, Y.; Jalilov, A.S.; Vajtai, R.; Tiwary, C.S.; Lou, J.; Tour, J.M.; Mohite, A.D.; et al. Chemically interconnected light-weight 3D-carbon nanotube solid network. Carbon N. Y. 2017, 119, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Ning, N.; Mi, T.; Chu, G.; Zhang, L.Q.; Liu, L.; Tian, M.; Yu, H.T.; Lu, Y.L. A quantitative approach to study the interface of carbon nanotubes/elastomer nanocomposites. Eur. Polym. J. 2018, 102, 10–18. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kumar, V.; Tang, X.W.; Lee, D.J. Mechanical and electrical behavior of rubber nanocomposites under static and cyclic strain. Compos. Sci. Technol. 2017, 142, 1–9. [Google Scholar] [CrossRef]
- Elango, N.; Gupta, N.S.; Jiun, Y.L.; Golshahr, A. The effect of high loaded multiwall carbon nanotubes in natural rubber and their nonlinear material constants. J. Nanomater. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Bolados, H.; Yazdani-Pedram, M.; Verdejo, R. Thermal, electrical, and sensing properties of rubber nanocomposites. High-Perform. Elastomeric Mater. Reinf. Nano-Carbons 2020, 149–175. [Google Scholar] [CrossRef]
- Zhan, Y.; Yan, N.; Fei, G.; Xia, H.; Meng, Y. Crack growth resistance of natural rubber reinforced with carbon nanotubes. J. Appl. Polym. Sci. 2020, 137, 48447. [Google Scholar] [CrossRef]
- Gumede, J.I.; Carson, J.; Hlangothi, S.P.; Bolo, L.L. Effect of single-walled carbon nanotubes on the cure and mechanical properties of reclaimed rubber/natural rubber blends. Mater. Today Commun. 2020, 23, 100852. [Google Scholar] [CrossRef]
- García, D.B.; Mansilla, M.A.; Crisnejo, M.; Farabollini, H.; Escobar, M.M. Effect of carbon nanotubes content on the vulcanization kinetic in styrene–butadiene rubber compounds. Polym. Eng. Sci. 2019, 59, E327–E336. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, W.; Jiang, N.; Futaba, D.N.; Xu, M. A general strategy for optimizing composite properties by evaluating the interfacial surface area of dispersed carbon nanotubes by fractal dimension. Carbon N. Y. 2019, 154, 457–465. [Google Scholar] [CrossRef]
- Tang, Y.; Ma, L.; He, Y.; Chen, H.; Jiang, Y.; Xu, J. Preparation and Performance Evaluation of Natural Rubber Composites with Aluminum Nitride and Aligned Carbon Nanotubes. Polym. Sci.-Ser. A 2019, 61, 366–374. [Google Scholar] [CrossRef]
- Bokobza, L. Natural Rubber Nanocomposites: A Review. Nanomaterials 2018, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Avilés, F.; Cauich-Rodríguez, J.V.; Toro-Estay, P.; Yazdani-Pedram, M.; Aguilar-Bolados, H. Improving Carbon Nanotube/Polymer Interactions in Nanocomposites; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780323482226. [Google Scholar]
- Bernal-Ortega, P.; Bernal, M.M.; González-Jiménez, A.; Posadas, P.; Navarro, R.; Valentín, J.L. New insight into structure-property relationships of natural rubber and styrene-butadiene rubber nanocomposites filled with MWCNT. Polymer 2020, 201. [Google Scholar] [CrossRef]
- Sreenath, P.R.; Mandal, S.; Singh, S.; Panigrahi, H.; Das, P.; Bhowmick, A.K.; Kumar, K.D. Unique approach to debundle carbon nanotubes in polymer matrix using carbon dots for enhanced properties. Eur. Polym. J. 2020, 123, 109454. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, T.; Kim, Y.S.; Choi, H.S.; Lim, H.J.; Yang, S.J.; Park, C.R. Surface modifications for the effective dispersion of carbon nanotubes in solvents and polymers. Carbon N. Y. 2012, 50, 3–33. [Google Scholar] [CrossRef]
- Szadkowski, B.; Marzec, A.; Zaborski, M. Effect of in situ silanization of multiwalled carbon nanotubes on the properties of NBR/MWCNT-OH composites. Polym.-Plast. Technol. Eng. 2018, 58, 1327–1341. [Google Scholar] [CrossRef]
- Walle, M.D.; Zhang, Z.; Zhang, M.; You, X.; Li, Y.; Liu, Y.N. Hierarchical 3D nitrogen and phosphorous codoped graphene/carbon nanotubes–sulfur composite with synergistic effect for high performance of lithium–sulfur batteries. J. Mater. Sci. 2018, 53, 2685–2696. [Google Scholar] [CrossRef]
- Mishra, K.; Singh, R.P. Effect of APTMS modification on multiwall carbon nanotube reinforced epoxy nanocomposites. Compos. Part B Eng. 2019, 162, 425–432. [Google Scholar] [CrossRef]
- Kondrashov, S.V.; Soldatov, M.A.; Gunyaeva, A.G.; Shashkeev, K.A.; Komarova, O.A.; Barinov, D.Y.; Yurkov, G.Y.; Shevchenko, V.G.; Muzafarov, A.M. The use of noncovalently modified carbon nanotubes for preparation of hybrid polymeric composite materials with electrically conductive and lightning resistant properties. J. Appl. Polym. Sci. 2018, 135, 2–9. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, Q.; Zhang, Y.; Liu, F.; Han, J.; Wu, C. High-performance natural rubber latex composites developed by a green approach using ionic liquid-modified multiwalled carbon nanotubes. J. Appl. Polym. Sci. 2018, 135, 46588. [Google Scholar] [CrossRef]
- Bai, L.; Bai, Y.; Zheng, J. Improving the filler dispersion and performance of silicone rubber/multi-walled carbon nanotube composites by noncovalent functionalization of polymethylphenylsiloxane. J. Mater. Sci. 2017, 52, 7516–7529. [Google Scholar] [CrossRef]
- Li, M.; Tu, W.; Chen, X.; Wang, H.; Chen, J. NR/SBR composites reinforced with organically functionalized MWCNTs: Simultaneous improvement of tensile strength and elongation and enhanced thermal stability. J. Polym. Eng. 2016, 36, 813–818. [Google Scholar] [CrossRef]
- Nakaramontri, Y.; Nakason, C.; Kummerlöwe, C.; Vennemann, N. Enhancement of Electrical Conductivity and Filler Dispersion of Carbon Nanotube Filled Natural Rubber Composites By Latex Mixing and in Situ Silanization. Rubber Chem. Technol. 2016, 89, 272–291. [Google Scholar] [CrossRef]
- Velasco-Santos, C.; Martinez-Hernandez, A.L.; Castano, V.M. Silanization of Carbon Nanotubes: Surface Modification and Polymer Nanocomposites. Carbon Nanotub.-Polym. Nanocomposites 2011. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Hemraj-Benny, T.; Wong, S.S. Covalent surface chemistry of single-walled carbon nanotubes. Adv. Mater. 2005, 17, 17–29. [Google Scholar] [CrossRef]
- Ma, P.C.; Kim, J.K.; Tang, B.Z. Functionalization of carbon nanotubes using a silane coupling agent. Carbon N. Y. 2006, 44, 3232–3238. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanian, K.; Burghard, M. Chemically Functionalized Carbon Nanotubes. Small 2005, 1, 180–192. [Google Scholar] [CrossRef]
- Velasco-Santos, C.; Martínez-Hernández, A.L.; Lozada-Cassou, M.; Alvarez-Castillo, A.; Castaño, V.M. Chemical functionalization of carbon nanotubes through an organosilane. Nanotechnology 2002, 13, 495–498. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon N. Y. 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Shanmugharaj, A.M.; Hun Ryu, S. Influence of aminosilane-functionalized carbon nanotubes on the rheometric, mechanical, electrical and thermal degradation properties of epoxidized natural rubber nanocomposites. Polym. Int. 2013, 62, 1433–1441. [Google Scholar] [CrossRef]
- Wang, C.; Chen, H.; Dong, W.; Ge, J.; Lu, W.; Wu, X.; Guo, L.; Chen, L. Sulfur-amine chemistry-based synthesis of multi-walled carbon nanotube-sulfur composites for high performance Li-S batteries. Chem. Commun. 2014, 50, 1202–1204. [Google Scholar] [CrossRef]
- Galimberti, M.; Barbera, V.; Sironi, A. Functionalized sp2 Carbon Allotropes as Fillers for Rubber Nanocomposites; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128161982. [Google Scholar]
- Park, T.; Banerjee, S.; Hemraj-benny, T.; Wong, S.S.; Park, T. Purification strategies and purity visualization techniques for single-walled carbon nanotubes. J. Mater. Chem. 2006, 16, 141–154. [Google Scholar] [CrossRef]
- Avilés, F.; Cauich-Rodríguez, J.V.; Rodríguez-González, J.A.; May-Pat, A. Oxidation and silanization of MWCNTs for MWCNT/vinyl ester composites. Express Polym. Lett. 2011, 5, 766–776. [Google Scholar] [CrossRef]
- Karousis, N.; Tagmatarchis, N. Current Progress on the Chemical Modification of Carbon Nanotubes. Chem. Rev. 2010, 110, 5366–5397. [Google Scholar] [CrossRef]
- Dementev, N.; Osswald, S.; Gogotsi, Y.; Borguet, E. Purification of carbon nanotubes by dynamic oxidation in air. J. Mater. Chem. 2009, 19, 7904–7908. [Google Scholar] [CrossRef]
- Hou, P.; Liu, C.; Cheng, H. Purification of carbon nanotubes. Carbon N. Y. 2008, 2003–2025. [Google Scholar] [CrossRef]
- Yang, K.; Han, H.; Pan, X.; Chen, N.; Gu, M. The effect of chemical treatment on the crystallinity of multi-walled carbon nanotubes. J. Phys. Chem. Solids 2008, 69, 222–229. [Google Scholar] [CrossRef]
- Su, S.H.; Chiang, W.T.; Lin, C.C.; Yokoyama, M. Multi-wall carbon nanotubes: Purification, morphology and field emission performance. Phys. E Low-Dimens. Syst. Nanostructures 2008, 40, 2322–2326. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon N. Y. 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Xu, T.; Yang, J.; Liu, J.; Fu, Q. Surface modification of multi-walled carbon nanotubes by O 2 plasma. Appl. Surf. Sci. 2007, 253, 8945–8951. [Google Scholar] [CrossRef]
- Blume, A.; Gatti, L.; Luginsland, H.D.; Maschke, D.; Moser, R.; Nian, J.C.; Röben, C.; Wehmeier, A. Silica and silanes. Rubber Compd. Chem. Appl. Second Ed. 2015, 251–332. [Google Scholar] [CrossRef]
- Unger, E.; Graham, A.; Kreupl, F.; Liebau, M.; Hoenlein, W. Electrochemical functionalization of multi-walled carbon nanotubes for solvation and purification. Curr. Appl. Phys. 2002, 2, 107–111. [Google Scholar] [CrossRef]
- Kelly, K.F.; Chiang, I.W.; Mickelson, E.T.; Hauge, R.H.; Margrave, J.L.; Wang, X.; Scuseria, G.E.; Radloff, C.; Halas, N.J. Insight into the mechanism of sidewall functionalization of single-walled nanotubes: An STM study. Chem. Phys. Lett. 1999, 313, 445–450. [Google Scholar] [CrossRef]
- Khabashesku, V.N.; Billups, W.E.; Margrave, J.L. Fluorination of single-wall carbon nanotubes and subsequent derivatization reactions. Acc. Chem. Res. 2002, 35, 1087–1095. [Google Scholar] [CrossRef]
- Adamska, M.; Narkiewicz, U. Fluorination of Carbon Nanotubes—A Review. J. Fluor. Chem. 2017, 200, 179–189. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, H.; Qing, Q.; Yang, Y.; Li, Q.; Liu, Z. Effect of Chemical Oxidation on the Structure of Single-Walled Carbon Nanotubes. J. Phys. Chem. 2003, 3712–3718. [Google Scholar] [CrossRef]
- Callejas, M.A.; Benito, A.M.; Cochet, M.; Seeger, T.; Anson, A.; Maser, W.K. Sensitivity of single wall carbon nanotubes to oxidative processing: Structural modification, intercalation and functionalisation. Carbon N. Y. 2003, 41, 2247–2256. [Google Scholar]
- Chiang, I.W.; Brinson, B.E.; Smalley, R.E.; Margrave, J.L.; Hauge, R.H. Purification and Characterization of Single-Wall Carbon Nanotubes. J. Phys. Chem. 2001, 105, 1157–1161. [Google Scholar] [CrossRef]
- Jin, K.; Zhou, X.; Zhang, L.; Xin, X.; Wang, G.; Liu, Z. Sulfur/carbon nanotube composite film as a flexible cathode for lithium-sulfur batteries. J. Phys. Chem. C 2013, 117, 21112–21119. [Google Scholar] [CrossRef]
- Yan, J.; Liu, X.; Wang, X.; Li, B. Long-life, high-efficiency lithium/sulfur batteries from sulfurized carbon nanotube cathodes. J. Mater. Chem. A 2015, 3, 10127–10133. [Google Scholar] [CrossRef]
- Bernal, M.M.; Di Pierro, A.; Novara, C.; Giorgis, F.; Mortazavi, B.; Saracco, G.; Fina, A. Edge-Grafted Molecular Junctions between Graphene Nanoplatelets: Applied Chemistry to Enhance Heat Transfer in Nanomaterials. Adv. Funct. Mater. 2018, 28, 1706954. [Google Scholar] [CrossRef] [Green Version]
- Saalwächter, K. Proton multiple-quantum NMR for the study of chain dynamics and structural constraints in polymeric soft materials. Prog. NMR Spectrosc. 2007, 51, 1–35. [Google Scholar] [CrossRef]
- Saalwächter, K.; Ziegler, P.; Spyckerelle, O.; Haidar, B.; Vidal, A.; Sommer, J.U. 1H multiple-quantum nuclear magnetic resonance investigations of molecular order distributions in poly (dimethylsiloxane) networks: Evidence for a linear mixing law in bimodal systems. J. Chem. Phys. 2003, 119, 3468–3482. [Google Scholar] [CrossRef] [Green Version]
- Valentín, J.L.; Posadas, P.; Fernández-Torres, A.; Malmierca, M.A.; González, L.; Chassé, W.; Saalwächter, K. Inhomogeneities and chain dynamics in diene rubbers vulcanized with different cure systems. Macromolecules 2010, 43, 4210–4222. [Google Scholar] [CrossRef]
- Chassé, W.; Lang, M.; Sommer, J.-U.; Saalwächter, K. Cross-Link Density Estimation of PDMS Networks with Precise Consideration of Networks Defects. Macromolecules 2012, 45, 899–912. [Google Scholar] [CrossRef]
- Chassé, W.; Valentn, J.L.; Genesky, G.D.; Cohen, C.; Saalwächter, K. Precise dipolar coupling constant distribution analysis in proton multiple-quantum NMR of elastomers. J. Chem. Phys. 2011, 134, 044907. [Google Scholar] [CrossRef] [PubMed]
- Basterra-Beroiz, B.; Rommel, R.; Kayser, F.; Valentín, J.L.; Westermann, S.; Heinrich, G. Revisiting Segmental Order: A Simplified Approach for Sulfur-Cured Rubbers Considering Junction Fluctuations and Entanglements. Macromolecules 2018, 51, 2076–2088. [Google Scholar] [CrossRef]
- Shi, S.; Liang, J. Thermal Decomposition Behavior of Silica-Phenolic Composite Exposed to One-Sided Radiant Heating. Polym. Compos. 2008, 16, 101–113. [Google Scholar] [CrossRef]
- Avilés, F.; Cauich-Rodríguez, J.V.; Moo-Tah, L.; May-Pat, A.; Vargas-Coronado, R. Evaluation of mild acid oxidation treatments for MWCNT functionalization. Carbon N. Y. 2009, 47, 2970–2975. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Jorio, A. Raman spectroscopy of carbon nanotubes in 1997 and 2007. J. Phys. Chem. C 2007, 111, 17887–17893. [Google Scholar] [CrossRef]
- Bokobza, L.; Zhang, J. Raman spectroscopic characterization of multiwall carbon nanotubes and of composites. Express Polym. Lett. 2012, 6, 601–608. [Google Scholar] [CrossRef]
- Payne, B.P.; Biesinger, M.C.; McIntyre, N.S. X-ray photoelectron spectroscopy studies of reactions on chromium metal and chromium oxide surfaces. J. Electron Spectros. Relat. Phenom. 2011, 184, 29–37. [Google Scholar] [CrossRef]
- Fujimori, T.; Morelos-Gómez, A.; Zhu, Z.; Muramatsu, H.; Futamura, R.; Urita, K.; Terrones, M.; Hayashi, T.; Endo, M.; Young Hong, S.; et al. Conducting linear chains of sulphur inside carbon nanotubes. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, S.; Gu, L.; Zhao, N.; Yin, Y.; Zhou, L.; Guo, Y.; Wan, L. Smaller Sulfur Molecules Promise Better Lithium-Sulfur Batteries. J. Am. Chem. Soc. 2012, 134, 18510–18513. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xu, Y.; Wang, C. Sulfur-Impregnated Disordered Carbon Nanotubes Cathode for Lithium-Sulfur Batteries. Nano Lett. 2011, 11, 4288–4294. [Google Scholar] [CrossRef] [PubMed]
- Marzocca, J.; Goyanes, S. An analysis of the influence of the accelerator/sulfur ratio in the cure reaction and the uniaxial stress-strain behavior of SBR. J. Appl. Polym. Sci. 2004, 91, 2601–2609. [Google Scholar] [CrossRef]
- Salgueiro, W.; Marzocca, A.; Somoza, A.; Consolati, G.; Cerveny, S.; Quasso, F.; Goyanes, S. Dependence of the network structure of cured styrene butadiene rubber on the sulphur content. Polymer 2004, 45, 6037–6044. [Google Scholar] [CrossRef]
- Roland, C.M. Electrical and dielectric properties of rubber. Rubber Chem. Technol. 2016, 89, 32–53. [Google Scholar] [CrossRef]
- Karásek, L.; Meissner, B.; Asai, S.; Sumita, M. Percolation concept: Polymer-filler gel formation, electrical conductivity and dynamic electrical properties of carbon-black-filled rubbers. Polym. J. 1996, 28, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Endo, M.; Noguchi, T.; Ito, M.; Takeuchi, K.; Hayashi, T.; Kim, Y.A.; Wanibuchi, T.; Jinnai, H.; Terrones, M.; Dresselhaus, M.S. Extreme-performance rubber nanocomposites for probing and excavating deep oil resources using multi-walled carbon nanotubes. Adv. Funct. Mater. 2008, 18, 3403–3409. [Google Scholar] [CrossRef]
- Bokobza, L. Enhanced electrical and mechanical properties of multiwall carbon nanotube rubber composites. Polym. Adv. Technol. 2012, 23, 1543–1549. [Google Scholar] [CrossRef]
- Heinrich, G.; Kluppel, M.; Vilgis, T.A. Reinforcement of elastomers. Solid State Mater. Sci. 2002, 6, 195–203. [Google Scholar] [CrossRef]
- Voet, A. Reinforcement of Elastomers By Fillers: Review of Period 1967–1976. J. Polym. Sci. Macromol. Rev. 1980, 15, 327–373. [Google Scholar] [CrossRef]
- Kraus, G. Reinforcement of elastomers by carbon black. Die Angew. Makromol. Chem. 1977, 60, 215–248. [Google Scholar] [CrossRef]
- Antal Huba, G.L. Reinforcement of elastomers by silica. Rubber Chem. Technol. 2014, 50, 342–355. [Google Scholar]
- Bokobza, L.; Rapoport, O. Reinforcement of natural rubber. J. Appl. Polym. Sci. 2002, 85, 2301–2316. [Google Scholar] [CrossRef]
- Rattanasom, N.; Saowapark, T.; Deeprasertkul, C. Reinforcement of natural rubber with silica/carbon black hybrid filler. Polym. Test. 2007, 26, 369–377. [Google Scholar] [CrossRef]
- Payne, A.R. The dynamic properties of carbon black loaded natural rubber vulcanizates. Part I. J. Appl. Polym. Sci. 1962, 6, 57–63. [Google Scholar] [CrossRef]
- Payne, A.R. The dynamic properties of carbon black loaded natural rubber vulcanizates. Part II. J. Appl. Polym. Sci. 1962, 6, 368–372. [Google Scholar] [CrossRef]
- Payne, A.R.; Whittaker, R.E. Low strain dynamic properties of filled rubbers. Rubber Chem. Technol. 1971, 44, 440–478. [Google Scholar] [CrossRef]
- Wang, M.-J. Effect of Polymer-Filler and Filler-Filler Interactions on Dynamic Properties of Filled Vulcanizates. Rubber Chem. Technol. 1998, 71, 520–589. [Google Scholar] [CrossRef]
- Wolff, S.; Wang, M.J.; Tan, E.H. Filler-elastomer interactions. Part VII. Study on bound rubber. Rubber Chem. Technol. 1993, 66, 163–177. [Google Scholar] [CrossRef]
- Bandzierz, K.; Reuvekamp, L.; Dryzek, J.; Dierkes, W.; Blume, A.; Bielinski, D. Influence of network structure on glass transition temperature of elastomers. Materials 2016, 9, 607. [Google Scholar] [CrossRef] [Green Version]
- Galimberti, M.; Infortuna, G.; Guerra, S.; Barbera, V.; Agnelli, S.; Pandini, S. sp2carbon allotropes in elastomer matrix: From master curves for the mechanical reinforcement to lightweight materials. Express Polym. Lett. 2018, 12, 265–283. [Google Scholar] [CrossRef]
- Merabia, S.; Sotta, P.; Long, D.R. A microscopic model for the reinforcement and the nonlinear behavior of filled elastomers and thermoplastic elastomers (Payne and Mullins Effects). Macromolecules 2008, 41, 8252–8266. [Google Scholar] [CrossRef]
- Basterra-Beroiz, B.; Rommel, R.; Kayser, F.; Westermann, S.; Valentín, J.L.; Heinrich, G. New insights into rubber network structure by a combination of experimental techniques. Rubber Chem. Technol. 2016, 90, 347–366. [Google Scholar] [CrossRef]
- Nagaraja, S.M.; Mujtaba, A.; Beiner, M. Quantification of different contributions to dissipation in elastomer nanoparticle composites. Polymer 2017, 111, 48–52. [Google Scholar] [CrossRef]
- Williams, M.L.; Landel, R.F.; Ferry, J.D. The Temperature Dependence of Relaxation Mechanisms in Amorphous Polymers and Other Glass-forming Liquids. J. Am. Chem. Soc. 1955, 77, 3701–3707. [Google Scholar] [CrossRef]
- Schallamach, K.A.G.A. Tire friction on wet roads. Rubber Chem. Technol. 1976, 49, 862–908. [Google Scholar]
- Hall, D.E.; Moreland, J.C. Fundamentals of rolling resistance. Rubber Chem. Technol. 2001, 74, 525–539. [Google Scholar] [CrossRef]
- Zhong, M.; Wang, R.; Kawamoto, K.; Olsen, B.D.; Johnson, J.A. Quantifying the impact of molecular defects on polymer network elasticity. Science 2016, 353, 1264–1268. [Google Scholar] [CrossRef] [Green Version]
- Campise, F.; Agudelo, D.C.; Acosta, R.H.; Villar, M.A.; Vallés, E.M.; Monti, G.A.; Vega, D.A. Contribution of Entanglements to Polymer Network Elasticity. Macromolecules 2017, 50, 2964–2972. [Google Scholar] [CrossRef]
- Valentín, J.L.; Mora-Barrantes, I.; Carretero-González, J.; López-Manchado, M.A.; Sotta, P.; Long, D.R.; Saalwächter, K. Novel experimental approach to evaluate Filler-Elastomer interactions. Macromolecules 2010, 43, 334–346. [Google Scholar] [CrossRef]
- Vieyres, A.; Pérez-Aparicio, R.; Albouy, P.A.; Sanseau, O.; Saalwächter, K.; Long, D.R.; Sotta, P. Sulfur-cured natural rubber elastomer networks: Correlating cross-link density, chain orientation, and mechanical response by combined techniques. Macromolecules 2013, 46, 889–899. [Google Scholar] [CrossRef]
- Mujtaba, A.; Keller, M.; Ilisch, S.; Radusch, H.J.; Beiner, M.; Thurn-Albrecht, T.; Saalwächter, K. Detection of surface-immobilized components and their role in viscoelastic reinforcement of rubber-silica nanocomposites. ACS Macro Lett. 2014, 3, 481–485. [Google Scholar] [CrossRef]
- Malmierca, M.A.; González-Jiménez, A.; Mora-Barrantes, I.; Posadas, P.; Rodríguez, A.; Ibarra, L.; Nogales, A.; Saalwächter, K.; Valentín, J.L. Characterization of network structure and chain dynamics of elastomeric ionomers by means of 1H Low-Field NMR. Macromolecules 2014, 47, 5655–5667. [Google Scholar] [CrossRef]
- Valentín, J.L.; Carretero-González, J.; Mora-Barrantes, I.; Chassé, W.; Saalwächter, K. Uncertainties in the determination of cross-link density by equilibrium swelling experiments in natural rubber. Macromolecules 2008, 41, 4717–4729. [Google Scholar] [CrossRef]









| Ingredient | phr | |||
|---|---|---|---|---|
| NR | 100 | 100 | 100 | 100 |
| ZnO | 3 | 3 | 3 | 3 |
| Stearic Acid | 3 | 3 | 3 | 3 |
| CBS | 2 | 2 | 2 | 2 |
| S | 1 | a | a | 1 |
| CNT | 1–20 | - | - | - |
| CCNT | - | 1–20 b | - | - |
| TCNT | - | - | 1–20 b | - |
| CB | - | - | - | 60 |
| CCNTs | TCNTs | |||||
|---|---|---|---|---|---|---|
| S/CNT Weight Ratio | Grafted Functional Groups (% w/w) | f-Filler Added to Rubber Compounds (phr) | Free Sulfur Added to Rubber Compounds (phr) | Grafted Functional Groups (% w/w) | f-Filler Added to Rubber Compounds (phr) | Free Sulfur Added to Rubber Compounds (phr) |
| 1:1 | 29.0 | 1.41 | 0.59 | 25.5 | 1.34 | 0.66 |
| 1:3 | 11.3 | 3.38 | 0.62 | 3.8 | 3.12 | 0.88 |
| 1:5 | 11.0 | 5.62 | 0.38 | 2.7 | 5.14 | 0.86 |
| 1:7 | 9.5 | 7.73 | 0.27 | 4.3 | 7.32 | 0.68 |
| 1:10 | 9.5 | 11.05 | --- | 3.7 | 10.38 | 0.62 |
| 1:15 | 10.9 | 16.83 | --- | 4.5 | 15.70 | 0.30 |
| 1:20 | 10.8 | 22.43 | --- | 4.8 | 21.01 | --- |
| Sample | ID/IG Ratio |
|---|---|
| CNTs | 1.1 ± 0.1 |
| CCNTs | 1.2 ± 0.1 |
| TCNTs | 1.16 ± 0.1 |
| NR-CNT | NR-CCNT | NR-TCNT | ||||
|---|---|---|---|---|---|---|
| phr | t0 | t97 | t0 | t97 | t0 | t97 |
| 0 | 5.92 | 13.91 | 5.92 | 13.91 | 5.92 | 13.91 |
| 1 | 4.43 | 13.01 | 5.50 | 11.92 | 4.76 | 13.16 |
| 3 | 3.97 | 10.65 | 5.33 | 10.66 | 4.04 | 8.37 |
| 5 | 3.36 | 9.66 | 3.76 | 10.61 | 3.42 | 8.03 |
| 7 | 2.99 | 8.6 | 3.64 | 10.45 | 3.15 | 7.58 |
| 10 | 2.89 | 8.15 | 3.32 | 7.59 | 2.79 | 7.43 |
| 15 | 2.55 | 7.5 | 2.62 | 6.83 | 1.89 | 7.05 |
| 20 | 2.12 | 7.32 | 1.96 | 6.78 | 0.37 | 5.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal-Ortega, P.; Bernal, M.M.; Blume, A.; González-Jiménez, A.; Posadas, P.; Navarro, R.; Valentín, J.L. Sulfur-Modified Carbon Nanotubes for the Development of Advanced Elastomeric Materials. Polymers 2021, 13, 821. https://doi.org/10.3390/polym13050821
Bernal-Ortega P, Bernal MM, Blume A, González-Jiménez A, Posadas P, Navarro R, Valentín JL. Sulfur-Modified Carbon Nanotubes for the Development of Advanced Elastomeric Materials. Polymers. 2021; 13(5):821. https://doi.org/10.3390/polym13050821
Chicago/Turabian StyleBernal-Ortega, Pilar, M. Mar Bernal, Anke Blume, Antonio González-Jiménez, Pilar Posadas, Rodrigo Navarro, and Juan L. Valentín. 2021. "Sulfur-Modified Carbon Nanotubes for the Development of Advanced Elastomeric Materials" Polymers 13, no. 5: 821. https://doi.org/10.3390/polym13050821