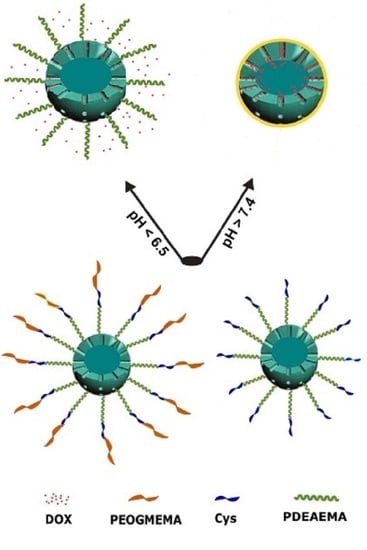
Poly(oligo(ethylene glycol) methyl ether methacrylate) Capped pH-Responsive Poly(2-(diethylamino)ethyl methacrylate) Brushes Grafted on Mesoporous Silica Nanoparticles as Nanocarrier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
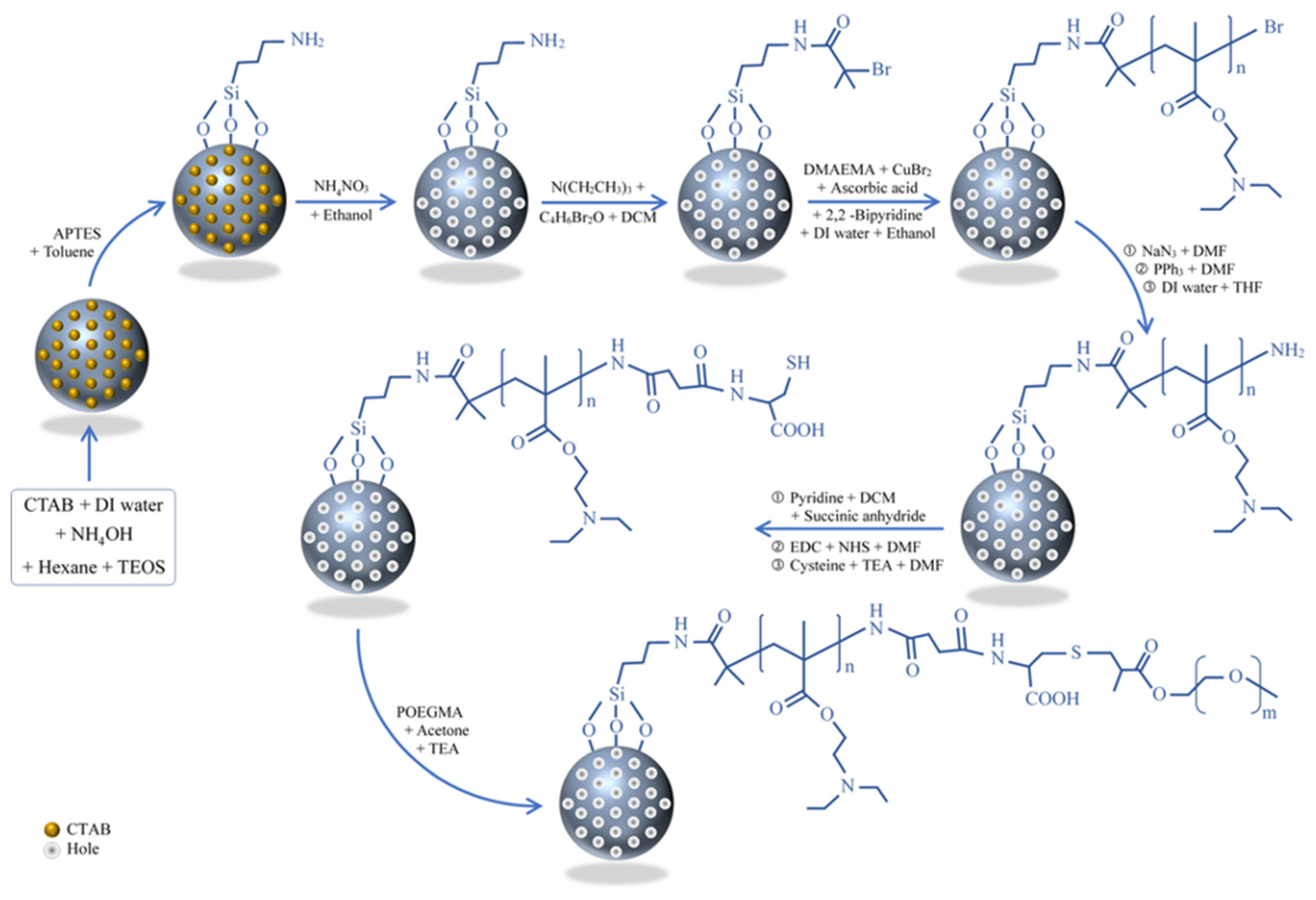
2.2.1. Synthesis of Mesoporous Silica Nanoparticles
2.2.2. The Modification of Mesoporous Silica Nanoparticles with an Amine Group (MSN–NH2)
2.2.3. Immobilization of BIBB Initiator on MSNs (MSN–Br) and Channel Formation
2.2.4. The Modification of MSNs with Poly(2-(diethylamino) ethyl methacrylate) Brushes (MSN–PDEAEMA)
2.2.5. Coating the MSNs Surface with Amine Groups (MSN–PDEAEMA–NH2)
2.2.6. Immobilization of Cysteine on the Surface of MSNs (MSN–PDEAEMA–Cys)
2.2.7. Immobilization of POEGMEMA on the Surface of MSN–PDEAEMA (MSN–PDEAEMA-Cys-POEGMEMA)
2.3. Measurement and Characterization
2.4. Drug Loading and Release
2.5. Cell Culture and Exposure Protocol
MTT Cell Viability Assay
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Estimated Age-Standardized Incidence Rates (World) in 2020, All cancers, Both Sexes, All Ages. 2020. Available online: https://www.uicc.org/news/globocan-2020-new-global-cancer-data (accessed on 26 January 2021).
- Li, Q.; Sun, A.; Si, Y.; Chen, M.; Wu, L. One-Pot Synthesis of Polysaccharide-Diphenylalanine Ensemble with Gold Nanoparticles and Dye for Highly Efficient Detection of Glutathione. Chem. Mater. 2017, 29, 6758–6765. [Google Scholar] [CrossRef]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef]
- Khan, R.U.; Yu, H.; Wang, L.; Zhang, Q.; Xiong, W.; Zain-ul-Abdin, W.; Nazir, A.; Fahad, S.; Chen, X.; Elsharaarani, T. Synthesis of polyorganophosphazenes and preparation of their polymersomes for reductive/acidic dual-responsive anticancer drugs release. J. Mater. Sci. 2020, 55, 8264–8284. [Google Scholar] [CrossRef]
- Yi, P.; Wang, Y.; Zhang, S.; Zhan, Y.; Zhang, Y.; Sun, Z.; Li, Y.; He, P. Stimulative nanogels with enhanced thermosensitivity for therapeutic delivery via β-cyclodextrin-induced formation of inclusion complexes. Carbohydr. Polym. 2017, 166, 219–227. [Google Scholar] [CrossRef]
- Bae, Y.H.; Park, K. Advanced drug delivery 2020 and beyond: Perspectives on the future. Adv. Drug Deliv. Rev. 2020, 158, 4–16. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J. Control. Release 2012, 161, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Bin, H.; Xin, S.; Bing, Y.; Song, W.; Youqing, S.; Hailin, C. Recent advances in drug delivery systems for enhancing drug penetration into tumors. Drug Deliv. 2021, 27, 1474–1490. [Google Scholar] [CrossRef]
- Sanadgol, N.; Wackerlig, J. Developments of Smart Drug-Delivery Systems Based on Magnetic Molecularly Imprinted Polymers for Targeted Cancer Therapy: A Short Review. Pharmaceutics 2020, 12, 831. [Google Scholar] [CrossRef] [PubMed]
- Descalzo, A.B.; Martínez-Máñez, R.; Sancenón, F.; Hoffmann, K.; Rurack, K. The Supramolecular Chemistry of Organic–Inorganic Hybrid Materials. Angew. Chem. Int. Ed. 2006, 45, 5924–5948. [Google Scholar] [CrossRef]
- He, Q.; Zhang, J.; Shi, J.; Zhu, Z.; Zhang, L.; Bu, W.; Guo, L.; Chen, Y. The effect of PEGylation of mesoporous silica nanoparticles on nonspecific binding of serum proteins and cellular responses. Biomaterials 2010, 31, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Trewyn, B.G.; Lin, V.S. Mesoporous silica nanomaterial-based biotechnological and biomedical delivery systems. Nanomedicine 2007, 2, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Trewyn, B.G.; Giri, S.; Lin, V.S.Y. Mesoporous Silica Nanoparticles for Drug Delivery and Biosensing Applications. Adv. Funct. Mater. 2007, 17, 1225–1236. [Google Scholar] [CrossRef]
- Carvalho, G.C.; Sábio, R.M.; Ribeiro, T.C.; Monteiro, A.S.; Pereira, D.V.; Ribeiro, S.J.L.; Chorilli, M. Highlights in Mesoporous Silica Nanoparticles as a Multifunctional Controlled Drug Delivery Nanoplatform for Infectious Diseases Treatment. Pharm. Res. 2020, 37, 1–30. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Ito, A.; Yoshiyuki, K.; Sogo, Y.; Watanabe, Y.; Yamazaki, A.; Ohno, T.; Tsuji, N.M. Silica Nanospheres: Hollow Structure Improved Anti-Cancer Immunity of Mesoporous Silica Nanospheres In Vivo. Small 2016, 12, 3602. [Google Scholar] [CrossRef]
- Song, N.; Yang, Y.-W. Molecular and supramolecular switches on mesoporous silica nanoparticles. Chem. Soc. Rev. 2015, 44, 3474–3504. [Google Scholar] [CrossRef]
- Silveira, C.P.; Apolinário, L.M.; Fávaro, W.J.; Paula, A.J.; Durán, N. Doxorubicin-Functionalized Silica Nanoparticles Incorporated into a Thermoreversible Hydrogel and Intraperitoneally Administered Result in High Prostate Antitumor Activity and Reduced Cardiotoxicity of Doxorubicin. ACS Biomater. Sci. Eng. 2016, 2, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Pandele, A.M.; Andronescu, C.; Ghebaur, A.; Garea, S.A.; Iovu, H. New Biocompatible Mesoporous Silica/Polysaccharide Hybrid Materials as Possible Drug Delivery Systems. Materials 2019, 12, 15. [Google Scholar] [CrossRef] [Green Version]
- Keshavarz, H.; Khavandi, A.; Alamolhoda, S.; Naimi-Jamal, M.R. pH-Sensitive magnetite mesoporous silica nanocomposites for controlled drug delivery and hyperthermia. RSC Adv. 2020, 10, 39008–39016. [Google Scholar] [CrossRef]
- Nie, D.; Dai, Z.; Li, J.; Yang, Y.; Xi, Z.; Wang, J.; Li, Y.; Yu, M.; Zhang, X.; Shi, X.; et al. Cancer-Cell-Membrane-Coated Nanoparticles with a Yolk-Shell Structure Augment Cancer Chemotherapy. Nano Lett. 2020, 20, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Beagan, A.; Lahmadi, S.; Alghamdi, A.; Halwani, M.; Almeataq, M.; Alhazaa, A.; Alotaibi, K.; Alswieleh, A. Glucosamine Modified the Surface of pH-Responsive Poly(2-(diethylamino)ethyl Methacrylate) Brushes Grafted on Hollow Mesoporous Silica Nanoparticles as Smart Nanocarrier. Polymers 2020, 12, 2749. [Google Scholar] [CrossRef] [PubMed]
- Alswieleh, A.M.; Alshahrani, M.M.; Alzahrani, K.E.; Alghamdi, H.S.; Niazy, A.A.; Alsilme, A.S.; Beagan, A.M.; Alsheheri, B.M.; Alghamdi, A.A.; Almeataqet, M.S. Surface modification of pH-responsive poly(2-(tert-butylamino)ethyl methacrylate) brushes grafted on mesoporous silica nanoparticles. Des. Monomers Polym. 2019, 22, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous Materials for Drug Delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhao, D.; Li, D.; Wang, X.; Jin, Z.; Zhao, K. Polymer-Based Nanomaterials and Applications for Vaccines and Drugs. Polymers 2018, 10, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, A.M.; Amaral, C.; Veiga, F.; Figueiras, A. Chapter 8. Polymeric micelles as a versatile tool in oral chemotherapy. In Design and Development of New Nanocarriers; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Nabar, G.; Mahajan, K.; Calhoun, M.; Duong, A.; Souva, M.; Xu, J.; Czeisler, C.; Puduvalli, V.K.; Otero, J.J.; Wyslouzilet, B.E. Micelle-templated, poly(lactic-co-glycolic acid) nanoparticles for hydrophobic drug delivery. Int. J. Nanomed. 2018, 13, 351–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rață, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Bacaita, S.E.; Mihalache, C.; Daraba, O.M.; Gherghel, D.; Popa, M. “In vitro” behaviour of aptamer-functionalized polymeric nanocapsules loaded with 5-fluorouracil for targeted therapy. Mater. Sci. Eng. C 2019, 103, 109828. [Google Scholar] [CrossRef]
- Chen, M.; Hu, J.; Wang, L.; Li, Y.; Zhu, C.; Chen, C.; Shi, M.; Ju, Z.; Cao, X.; Zhang, Z. Targeted and redox-responsive drug delivery systems based on carbonic anhydrase IX-decorated mesoporous silica nanoparticles for cancer therapy. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Iurciuc-Tincu, C.-E.; Cretan, M.S.; Purcar, V.; Popa, M.; Daraba, O.M.; Atanase, L.I.; Ochiuz, L. Drug Delivery System Based on pH-Sensitive Biocompatible Poly(2-vinyl pyridine)-b-poly(ethylene oxide) Nanomicelles Loaded with Curcumin and 5-Fluorouracil. Polymers 2020, 12, 1450. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Xia, F.; Jiang, L. Constructing Tunable Nanopores and Their Application in Drug Delivery. ACS Nano 2013, 7, 8344–8349. [Google Scholar] [CrossRef] [PubMed]
- Khorsand, B.; Lapointe, G.; Brett, C.; Oh, J.K. Intracellular Drug Delivery Nanocarriers of Glutathione-Responsive Degradable Block Copolymers Having Pendant Disulfide Linkages. Biomacromolecules 2013, 14, 2103–2111. [Google Scholar] [CrossRef]
- Mo, R.; Jiang, T.; DiSanto, R.; Tai, W.; Gu, Z. ATP-triggered anticancer drug delivery. Nat. Commun. 2019, 5, 1–10. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nature Mater. 2013, 12, 1–13. [Google Scholar] [CrossRef]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar] [PubMed]
- Alswieleh, A.M.; Beagan, A.M.; Alsheheri, B.M.; Alotaibi, K.M.; Alharthi, M.D.; Almeataq, M.S. Hybrid Mesoporous Silica Nanoparticles Grafted with 2-(tert-butylamino)ethyl Methacrylate-b-poly(ethylene Glycol) Methyl Ether Methacrylate Diblock Brushes as Drug Nanocarrier. Molecules 2020, 25, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beagan, A.M.; Alghamdi, A.A.; Lahmadi, S.S.; Halwani, M.A.; Almeataq, M.S.; Alhazaa, A.N.; Alotaibi, K.M.; Alswieleh, A.M. Folic Acid-Terminated Poly(2-Diethyl Amino Ethyl Methacrylate) Brush-Gated Magnetic Mesoporous Nanoparticles as a Smart Drug Delivery System. Polymers 2021, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Nayak, B.; Dey, R.K. PEGylation in anti-cancer therapy: An overview. Asian J. Pharm. Sci. 2016, 11, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Hatakeyama, H.; Sakurai, Y.; Hyodo, M.; Akita, H.; Harashima, H. A pH-sensitive cationic lipid facilitates the delivery of liposomal siRNA and gene silencing activity in vitro and in vivo. J. Control. Release 2012, 163, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Xiao, J.; Xiao, W.; Lin, W.; Chen, J.; Chen, Q.; Zhang, L.; Zhang, C.; Guo, J. Fabrication of PDEAEMA-based pH-responsive mixed micelles for application in controlled doxorubicin release. RSC Adv. 2017, 7, 27564–27573. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Wen, W.; Jia, Y.-G.; Liu, S.; Guo, A.J. pH-Responsive Micelles Assembled by Three-Armed Degradable Block Copolymers with a Cholic Acid Core for Drug Controlled-Release. Polymers 2019, 11, 511. [Google Scholar] [CrossRef] [Green Version]
- Kang, N.; Perron, M.-È.; Prud’homme, R.E.; Zhang, Y.; Gaucher, G.; Leroux, J.-C. Stereocomplex Block Copolymer Micelles: Core−Shell Nanostructures with Enhanced Stability. Nano Lett. 2005, 5, 315–319. [Google Scholar] [CrossRef]
- Nik, A.B.; Zare, H.; Razavi, S.; Mohammadi, H.; Ahmadi, P.T.; Yazdani, N.; Bayandorif, M.; Rabiee, N.; Mobarakeh, J.I. Smart drug delivery: Capping strategies for mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2020, 299, 110115. [Google Scholar] [CrossRef]
- Atanase, L.I. Micellar Drug Delivery Systems Based on Natural Biopolymers. Polymers 2021, 13, 477. [Google Scholar] [CrossRef]
- Bilalis, P.; Tziveleka, L.-A.; Varlas, S.; Iatrou, H. pH-Sensitive nanogates based on poly(l-histidine) for controlled drug release from mesoporous silica nanoparticles. Polym. Chem. 2016, 7, 1475–1485. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Siddiqui, M.A.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; AlSalhi, M.S.; Alrokayan, S.A. Oxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicology 2011, 283, 101–108. [Google Scholar] [CrossRef]
- Alenazi, N.; Hussein, M.; Alamry, K.; Asiri, A. Nanocomposite-Based Aminated Polyethersulfone and Carboxylate Activated Carbon for Environmental Application. A Real Sample Analysis. C J. Carbon Res. 2018, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, I.A.; Mustapha, A. Synthesis of New Azo Compounds Based on N-(4-Hydroxypheneyl)maleimide and N-(4-Methylpheneyl)maleimide. Molecules 2010, 15, 7498–7508. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, K.M.; Almethen, A.A.; Beagan, A.M.; Alfhaid, L.H.; Ahamed, M.; El-Toni, A.M.; Alswieleh, A.M. Poly(oligo(ethylene glycol) methyl ether methacrylate) Capped pH-Responsive Poly(2-(diethylamino)ethyl methacrylate) Brushes Grafted on Mesoporous Silica Nanoparticles as Nanocarrier. Polymers 2021, 13, 823. https://doi.org/10.3390/polym13050823
Alotaibi KM, Almethen AA, Beagan AM, Alfhaid LH, Ahamed M, El-Toni AM, Alswieleh AM. Poly(oligo(ethylene glycol) methyl ether methacrylate) Capped pH-Responsive Poly(2-(diethylamino)ethyl methacrylate) Brushes Grafted on Mesoporous Silica Nanoparticles as Nanocarrier. Polymers. 2021; 13(5):823. https://doi.org/10.3390/polym13050823
Chicago/Turabian StyleAlotaibi, Khalid M., Abdurrahman A. Almethen, Abeer M. Beagan, Latifah H. Alfhaid, Maqusood Ahamed, Ahmed M. El-Toni, and Abdullah M. Alswieleh. 2021. "Poly(oligo(ethylene glycol) methyl ether methacrylate) Capped pH-Responsive Poly(2-(diethylamino)ethyl methacrylate) Brushes Grafted on Mesoporous Silica Nanoparticles as Nanocarrier" Polymers 13, no. 5: 823. https://doi.org/10.3390/polym13050823