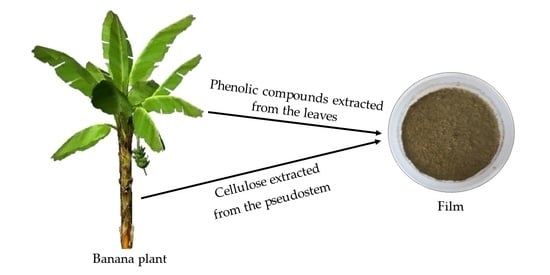
Rendering Banana Plant Residues into a Potentially Commercial Byproduct by Doping Cellulose Films with Phenolic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Phenolic Compounds Extraction
2.2.1.1. Batch Solid-Liquid Extraction
2.2.1.2. Soxhlet Extraction
2.2.1.3. Extracts Analysis
- Determining Total Phenolic Content (TPC) Using Folin Ciocalteau Method
- Antioxidant Activity Determination by DPPH Method
2.2.2. Cellulose Extraction and Analysis
- (1)
- Fiber preparation: banana plant pseudostem was cut into small pieces (2 × 2 cm) and immersed in continually stirred deionized water, at 80 °C for 1 h. This enables the removal of the non-cellulosic compounds. Fibers were washed in tap water, to remove soluble sugars and impurities and then dried at 100 °C for 3 h in a cabinet dryer (Venticell, MMM Group, München, Germany).
- (2)
- Fibers mercerization: 1 g fibers were treated with 20 mL 5% NaOH (40%, AkzoNobel Fine Chemicals, Amsterdam, The Netherlands), at 90 °C for 1 h, under stirring. Afterwards, samples were cooled at room temperature, filtrated and washed with deionized water, until pH = 7. The fibers were then dried, at 60 °C, in a cabinet dryer (Venticell, MMM Group), until a constant mass value was reached.
- (3)
- Fibers bleaching: 1 g of fibers were treated with 40% [16% (v/v) H2O2 (9.0%, Alifar) + 5% NaOH (40%, AkzoNobel Fine Chemicals, Amsterdam, The Netherlands)], at 55 °C, for 90 min, under stirring. This step allows the removal of residual hemicellulose and lignin. The mixture was cooled at room temperature and filtered under vacuum. The resulting fibers were washed with deionized water until pH = 5 and dried at 60 °C, until constant mass was reached.
- (4)
- Acid-catalyzed hydrolysis: dried pseudostem fibers were slowly added into the 60 wt% H2SO4 (95.0–97.0%, Honeywell, Charlotte, NC, USA) acid solution, previously cooled in an ice bath, under vigorous stirring and heated until 45 °C, for 1 h. To stop the reaction, the mixture was immediately cooled in an ice bath and 500 mL deionized ice water was added. The addition of deionized water dilutes the acid solution, resulting in a turbid supernatant. The resulting solution was dialyzed (12.0 S, Carl ROTH, Karlsruhe, Germany) against deionized water until pH = 7 was reached. This step allows the removal of free acid molecules from the suspension. Afterwards, the neutral solution was lyophilized (CoolSafe, ScanVac, Allerod, Denmark) and stored in a desiccator.
2.2.3. Films Development
2.2.3.1. Cellulose-Based Films
2.2.3.2. Films Doped with Phenolic Compounds
2.2.3.3. Films Characterization
- Scanning Electron Microscopy (SEM)
- X-ray Diffraction (XRD)
- Thermogravimetric Analysis (TGA)
- Fourier Transform Infrared (FTIR) Spectroscopy
- Contact Angel
- Mechanical Properties
3. Results and Discussion
3.1. Phenolic Compounds Extracts Analysis
3.1.1. Total Phenolic Content (TPC) Determination by the Folin Ciocalteau Method
3.1.2. Antioxidant Activity Determination by DPPH Method
3.2. Cellulose Extraction and Analysis
3.3. Films Analysis
3.3.1. Scanning Electron Microscopy (SEM)
3.3.2. X-ray Diffraction (XRD)
3.3.3. Thermogravimetric Analysis (TGA)
3.3.4. Fourier Transform Infrared (FTIR) Spectroscopy
3.3.5. Contact Angle
3.3.6. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hussain, S.; Malik, F.; Khalid, N.; Qayyum, M.A.; Riaz, H. Alternative and Traditional Medicines Systems in Pakistan: History, Regulation, Trends, Usefulness, Challenges, Prospects and Limitations. Intechopen 2012, 4, 89–108. [Google Scholar]
- Aziz, N.A.A.; Ho, L.-H.; Azahari, B.; Bhat, R.; Cheng, L.-H.; Ibrahim, M.N.M. Chemical and Functional Properties of the Native Banana (Musa Acuminata×balbisiana Colla Cv. Awak) Pseudo-Stem and Pseudo-Stem Tender Core Flours. Food Chem. 2011, 128, 748–753. [Google Scholar] [CrossRef]
- Forster, M.P.; Rodríguez Rodríguez, E.; Díaz Romero, C. Differential Characteristics in the Chemical Composition of Bananas from Tenerife (Canary Islands) and Ecuador. J. Agric. Food Chem. 2002, 50, 7586–7592. [Google Scholar] [CrossRef]
- Thompson, A.K. Banana (Musa spp.); Woodhead Publishing Limited: Sawston, Cambridge, UK, 2011. [Google Scholar]
- Simmonds, N.W.; Weatherup, S.T.C. Numerical Taxonomy of the Wild Bananas (Musa). New Phytol. 1990, 115, 567–571. [Google Scholar] [CrossRef]
- Qamar, S.; Shaikh, A. Therapeutic Potentials and Compositional Changes of Valuable Compounds from Banana—A Review. Trends Food Sci. Technol. 2018, 79, 1–9. [Google Scholar] [CrossRef]
- FAO—Food and Agriculture Organization of the United Nations. Banana Market Review. Available online: fao.org/3/ca9212en/ca9212en.pdf (accessed on 8 January 2021).
- Observador. Madeira Island Production. 2017. Available online: https://observador.pt/2018/02/01/madeira-aumentou-producao-de-banana-e-o-mercado-nacional-absorveu-85/ (accessed on 1 March 2019).
- Happi Emaga, T.; Robert, C.; Ronkart, S.N.; Wathelet, B.; Paquot, M. Dietary Fibre Components and Pectin Chemical Features of Peels during Ripening in Banana and Plantain Varieties. Bioresour. Technol. 2008, 99, 4346–4354. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.C.; Samman, S. Flavonoids-Chemistry, Metabolism, Carbdioprotective Effects, and Dietary Sources. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Quantitative Analysis, in Vitro Assessment of Bioavailability and Antioxidant Activity of Food Carotenoids—A Review. J. Food Compos. Anal. 2010, 23, 726–740. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.E.; Park, Y.; Subar, A.F.; Freedman, N.D.; Albanes, D.; Hollenbeck, A.; Leitzmann, M.F.; Schatzkin, A. Intakes of Fruit, Vegetables, and Specific Botanical Groups in Relation to Lung Cancer Risk in the NIH-AARP Diet and Health Study. Am. J. Epidemiol. 2008, 168, 1024–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant Properties of Several Tropical Fruits: A Comparative Study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- Prior, R.L.; Wang, H.; Cao, G.; Prior, R.L. Oxygen Radical Adsorbing Capacity of Anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar]
- Zia-ur-Rehman. Citrus Peel Extract—A Natural Source of Antioxidant. Food Chem. 2006, 99, 450–454. [Google Scholar] [CrossRef]
- Del Mar Verde Méndez, C.; Forster, M.P.; Rodríguez-Delgado, M.Á.; Rodríguez-Rodríguez, E.M.; Romero, C.D. Content of Free Phenolic Compounds in Bananas from Tenerife (Canary Islands) and Ecuador. Eur. Food Res. Technol. 2003, 217, 287–290. [Google Scholar] [CrossRef]
- Someya, S.; Yoshiki, Y.; Okubo, K. Antioxidant Compounds from Bananas (Musa Cavendish). Food Chem. 2002, 79, 351–354. [Google Scholar] [CrossRef]
- Mathew, N.S.; Negi, P.S. Traditional Uses, Phytochemistry and Pharmacology of Wild Banana (Musa Acuminata Colla): A Review. J. Ethnopharmacol. 2017, 196, 124–140. [Google Scholar] [CrossRef]
- Duvivier, P.; Hsieh, P.C.; Lai, P.Y.; Charles, A.L. Retention of Phenolics, Carotenoids, and Antioxidant Activity in the Taiwanese Sweet Potato. Afr. J. Food Agric. Nutr. Dev. 2010, 10, 4413–4429. [Google Scholar]
- Sagrin, M.S.; Chong, G.H. Effects of Drying Temperature on the Chemical and Physical Properties of Musa Acuminata Colla (AAA Group) Leaves. Ind. Crop Prod. 2013, 45, 430–434. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, C.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Pais, J.; Costa, N.; Oliveira, C.; Mafra, L.; Hilliou, L.; Oliveira, R.; Reis, M.A.M. Characterization of an Extracellular Polysaccharide Produced by a Pseudomonas Strain Grown on Glycerol. Bioresour. Technol. 2009, 100, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Prado, K.; Spinacé, M. Isolation and Characterization of Cellulose Nanocrystals from Pineapple Crown Waste and Their Potential Uses. Int. J. Biol. Macromol. 2019, 122, 410–416. [Google Scholar] [CrossRef]
- Lim, D.B.K.; Gong, H. Highly Stretchable and Transparent Films Based on Cellulose. Carbohydr. Polym. 2018, 201, 446–453. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Martin, R.M. Quantum-Mechanical Theory of Stress and Force. Phys. Rev. B 1985, 32, 3780–3791. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Pan, Z.; Ma, H. Extraction Modeling and Activities of Antioxidants from Pomegranate Marc. J. Food Eng. 2010, 99, 16–23. [Google Scholar] [CrossRef]
- Karki, S.; Kim, H.; Na, S.; Shin, D.; Jo, K.; Lee, J. Thin Films as an Emerging Platform for Drug Delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.; Wang, G.; Du, X.; Wang, Z.; Xu, S.; Zhang, Y. Extraction and Characterization of Cellulose Nanofibers and Nanocrystals from Liquefied Banana Pseudo-Stem Residue. Compos. Part B Eng. 2019, 160, 341–347. [Google Scholar] [CrossRef]
- Faradilla, R.H.F.; Lee, G.; Arns, J.Y.; Roberts, J.; Martens, P.; Stenzel, M.H.; Arcot, J. Characteristics of a Free-Standing Film from Banana Pseudostem Nanocellulose Generated from TEMPO-Mediated Oxidation. Carbohydr. Polym. 2017, 174, 1156–1163. [Google Scholar] [CrossRef]
- Patle, T.K.; Shrivas, K.; Kurrey, R.; Upadhyay, S.; Jangde, R.; Chauhan, R. Phytochemical Screening and Determination of Phenolics and Flavonoids in Dillenia Pentagyna Using UV–VIS and FTIR Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 242, 118717. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, D.; Sakkara, S.; Meenakshi, H.N.; Reddy, N. Properties and Applications of Citric Acid Crosslinked Banana Fiber-wheat Gluten Films. Ind. Crops Prod. 2018, 124, 265–272. [Google Scholar] [CrossRef]






| Film | Thickness (μm) | Normalized Tensile Strength (MPa/mm) | ||||
|---|---|---|---|---|---|---|
| 0.577 aw | 0.753 aw | 0.843 aw | 0.577 aw | 0.753 aw | 0.843 aw | |
| HEC | 116.00 | 117.33 | 101.33 | 37.10 ± 0.04 | 12.47 ± 2.78 | 13.44 ± 0.65 |
| HEC + L | 56.67 | 66.00 | - | 52.62 ± 0.50 | 6.52 ± 1.90 | - |
| PS | 112.00 | 157.33 | 70.67 | 2.13 ± 0.03 | 4.27 ± 0.09 | 2.87 ± 0.13 |
| PS + L | 82.00 | 120.00 | - | 1.89 ± 0.10 | 1.09 ± 0.07 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, R.E.A.; Monte, J.; Cadima, M.; Alves, V.D.; Neves, L.A. Rendering Banana Plant Residues into a Potentially Commercial Byproduct by Doping Cellulose Films with Phenolic Compounds. Polymers 2021, 13, 843. https://doi.org/10.3390/polym13050843
Nascimento REA, Monte J, Cadima M, Alves VD, Neves LA. Rendering Banana Plant Residues into a Potentially Commercial Byproduct by Doping Cellulose Films with Phenolic Compounds. Polymers. 2021; 13(5):843. https://doi.org/10.3390/polym13050843
Chicago/Turabian StyleNascimento, Rosa E. A., Joana Monte, Mafalda Cadima, Vítor D. Alves, and Luísa A. Neves. 2021. "Rendering Banana Plant Residues into a Potentially Commercial Byproduct by Doping Cellulose Films with Phenolic Compounds" Polymers 13, no. 5: 843. https://doi.org/10.3390/polym13050843
APA StyleNascimento, R. E. A., Monte, J., Cadima, M., Alves, V. D., & Neves, L. A. (2021). Rendering Banana Plant Residues into a Potentially Commercial Byproduct by Doping Cellulose Films with Phenolic Compounds. Polymers, 13(5), 843. https://doi.org/10.3390/polym13050843