Nanofibrillated Cellulose-Based Aerogels Functionalized with Tajuva (Maclura tinctoria) Heartwood Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Production and Characterization of the Tajuva Extracts
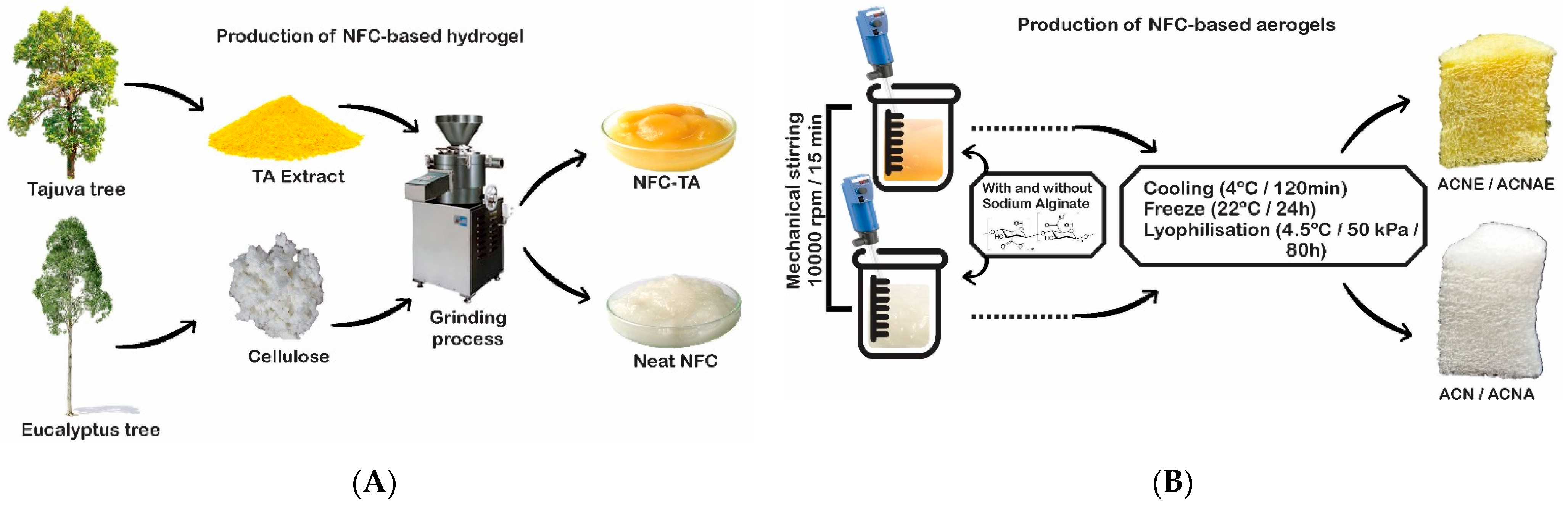
2.2. Production and Characterization of the NFC-Based Hydrogels
2.3. Production and Characterization of the NFC-Based Aerogels
3. Results
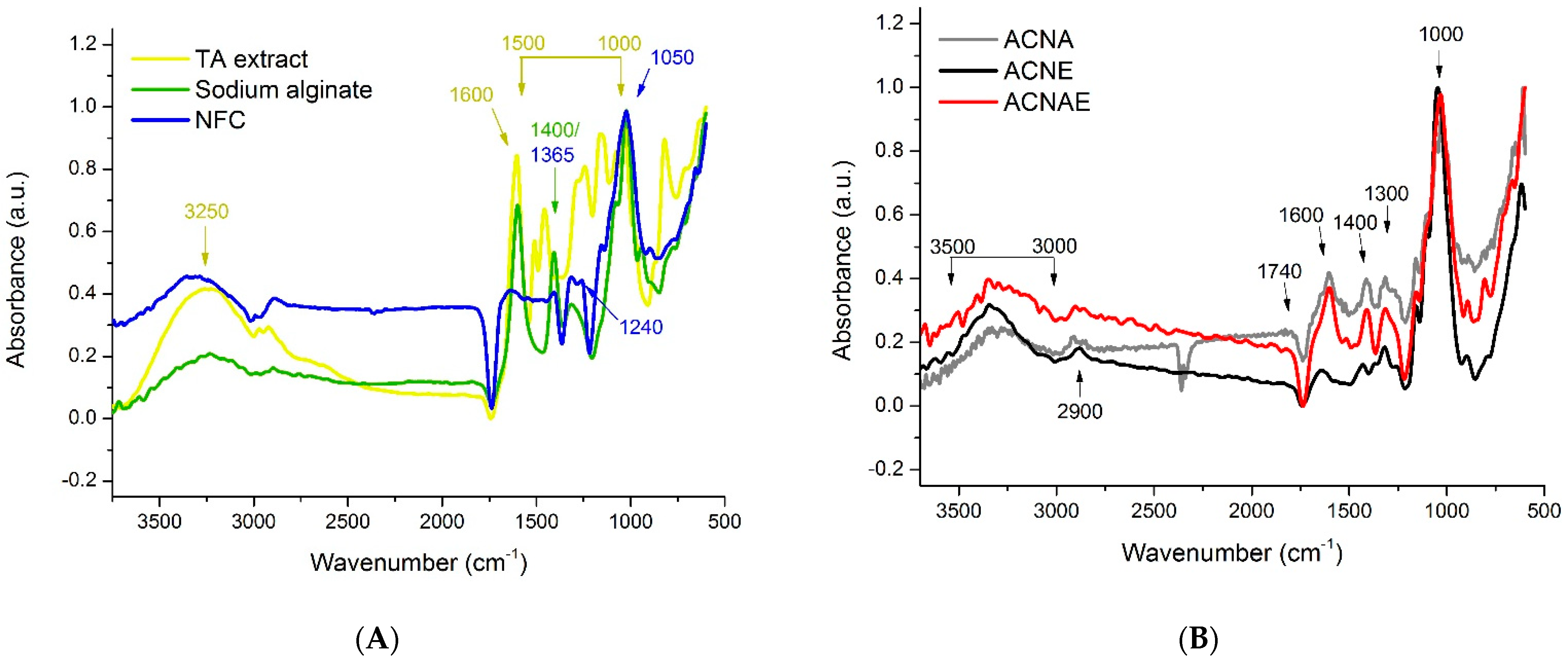
3.1. TA Extracts
3.2. NFC-Based Hydrogels
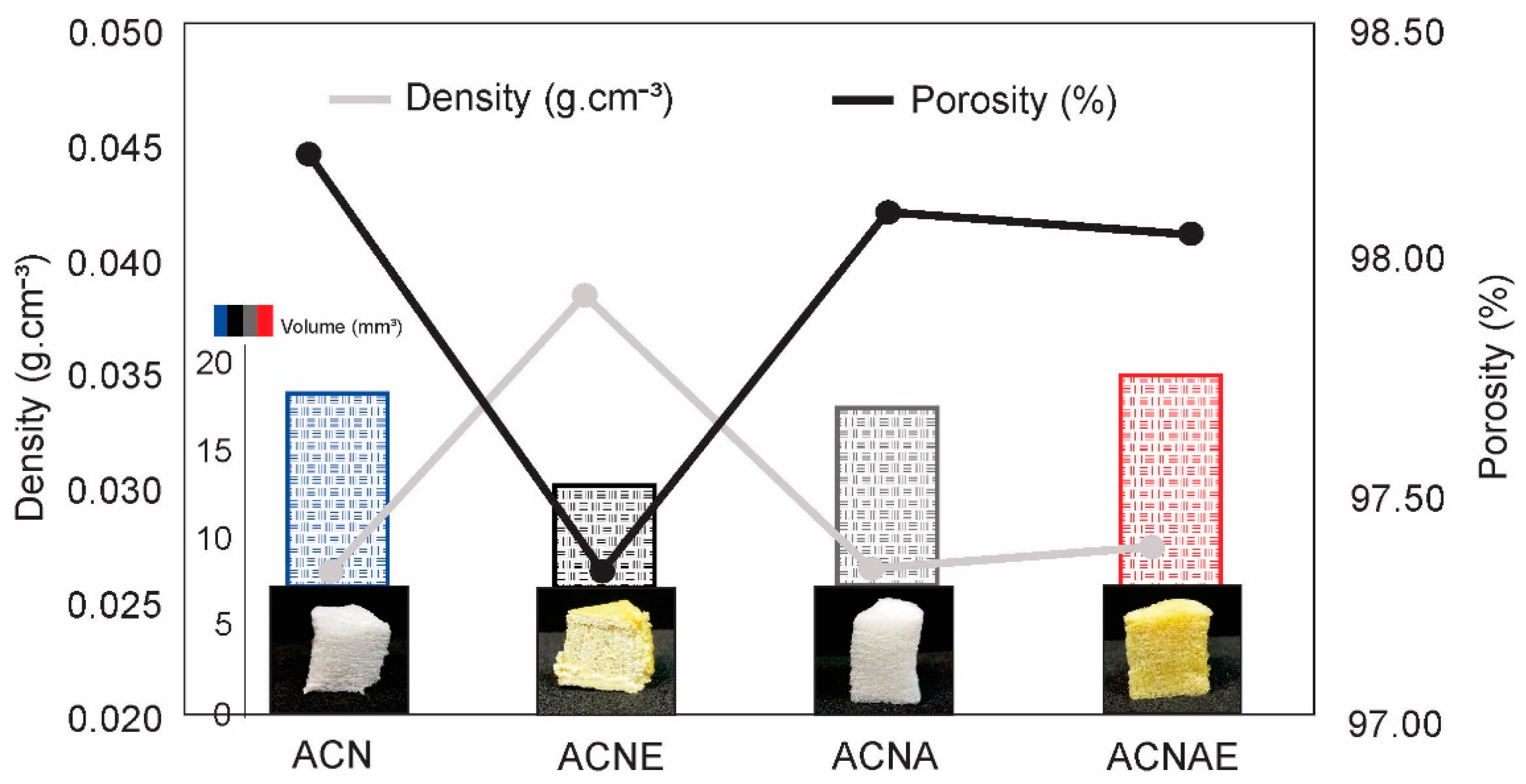
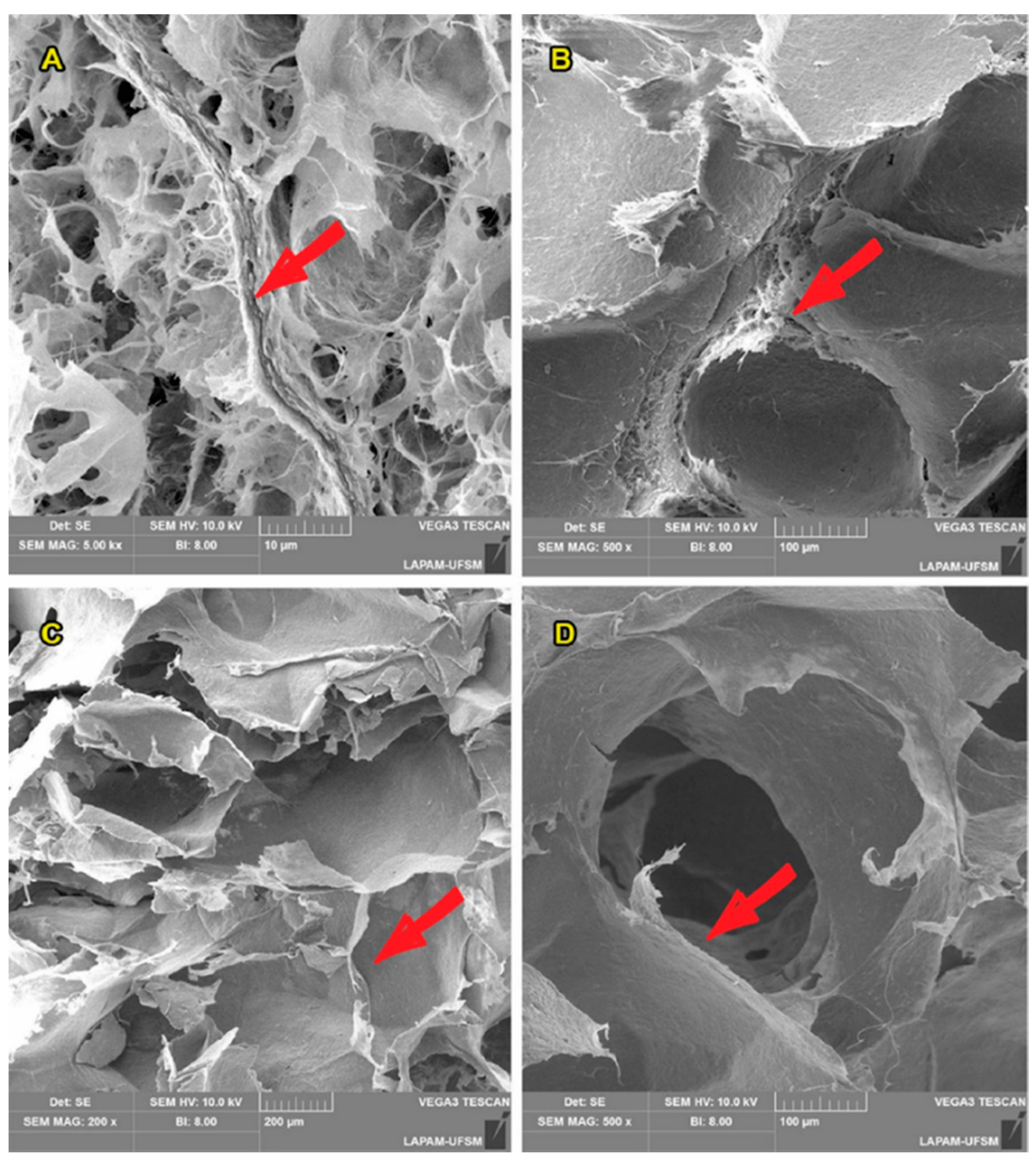
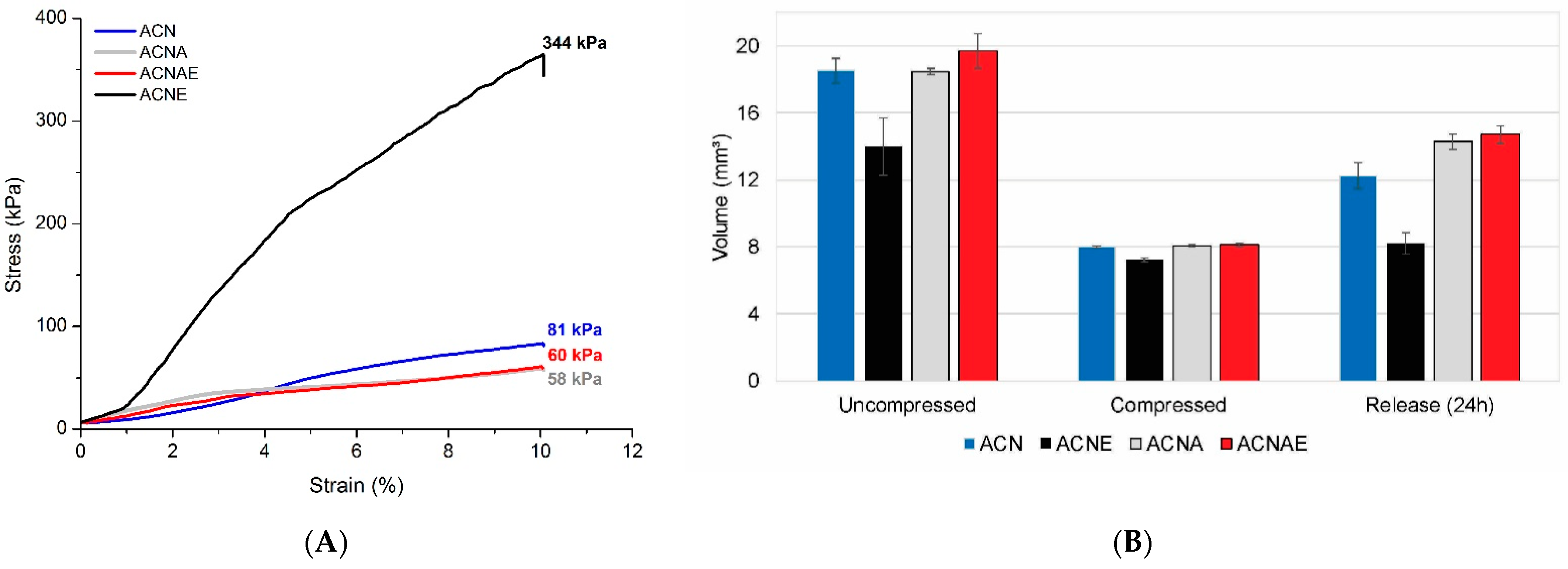
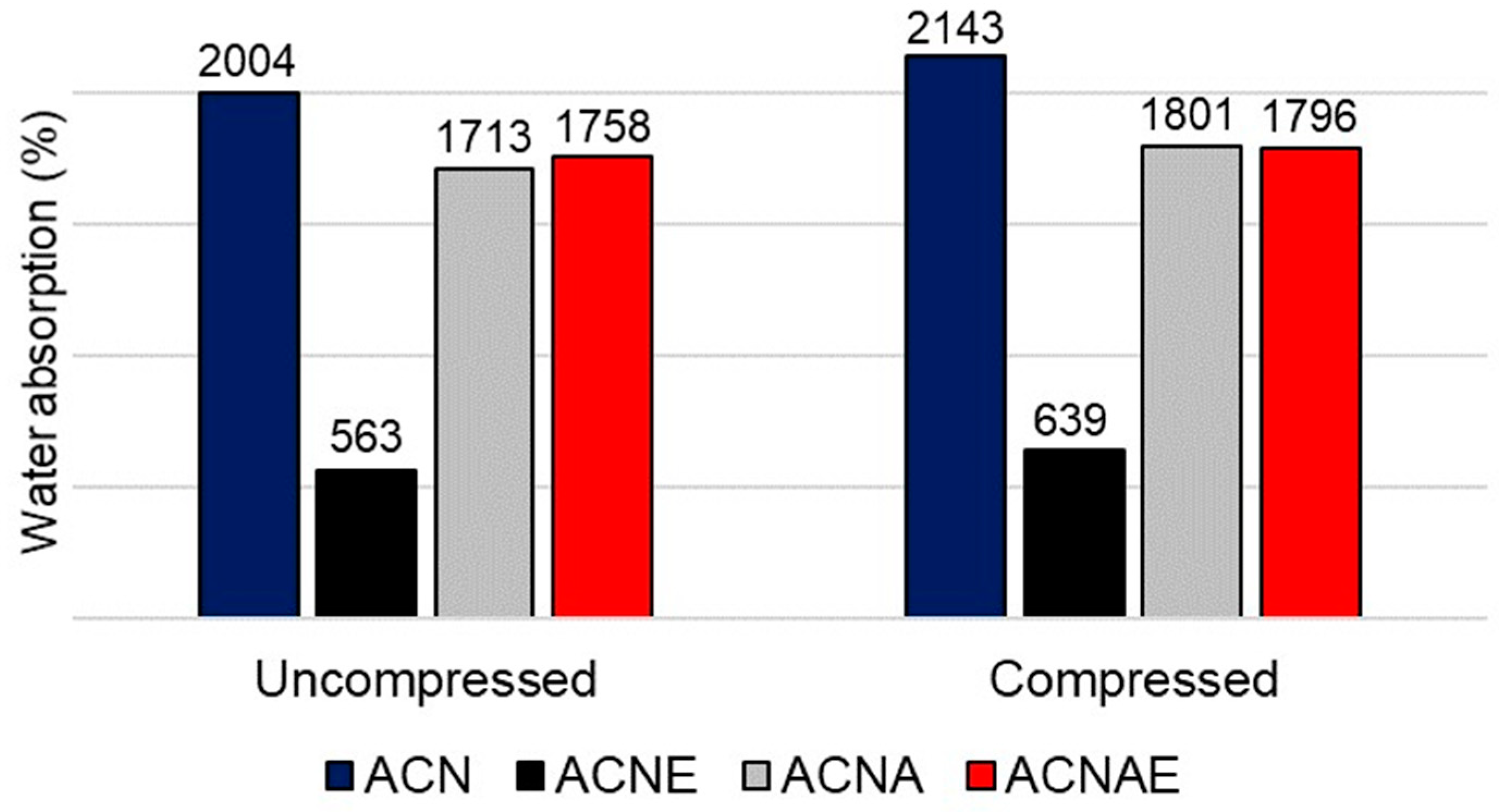
3.3. NFC-Based Aerogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gustaite, S.; Kazlauske, J.; Bobokalonov, J.; Perni, S.; Dutschk, V.; Liesiene, J.; Prokopovich, P. Characterization of cellulose based sponges for wound dressings. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 336–342. [Google Scholar] [CrossRef]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Michalska-Pożoga, I.; Thakur, V.K. Biopolymers for Biomedical and Pharmaceutical Applications: Recent Advances and Overview of Alginate Electrospinning. Nanomaterials 2019, 9, 404. [Google Scholar] [CrossRef]
- Athamneh, A.T.; Amin, A.; Benke, E.; Leopold, C.S.; Gurikov, P.; Smirnova, I. Alginate and hybrid alginate-hyaluronic acid aerogel microspheres as potential carrier for pulmonary drug delivery. J. Supercrit. Fluids 2019, 150, 49–55. [Google Scholar] [CrossRef]
- Liang, L.; Bhagia, S.; Li, M.; Huang, C.; Ragauskas, A.J. Cross-Linked Nanocellulosic Materials and Their Applications. ChemSusChem 2020, 13, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Mokhena, T.C.; John, M.J. Cellulose Nanomaterials: New Generation Materials for Solving Global Issues. Cellulose 2020, 27, 1149–1194. [Google Scholar] [CrossRef]
- Nair, S.S.; Zhu, J.Y.; Deng, Y.; Ragauskas, A.J. Hydrogels Prepared from Cross-Linked Nanofibrillated Cellulose. ACS Sustain. Chem. Eng. 2014, 2, 772–780. [Google Scholar] [CrossRef]
- Beh, J.H.; Lim, T.H.; Lew, J.H.; Lai, J.C. Cellulose nanofibril-based aerogel derived from sago pith waste and its application on methylene blue removal. Int. J. Biol. Macromol. 2020, 160, 836–845. [Google Scholar] [CrossRef]
- Long, L.; Weng, Y.; Wang, Y. Cellulose Aerogels: Synthesis, Applications, and Prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef] [PubMed]
- Geng, H. A facile approach to light weight, high porosity cellulose aerogels. Int. J. Biol. Macromol. 2018, 118, 921–931. [Google Scholar] [CrossRef]
- Salas, C.; Nypelö, T.; Rodriguez-abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose Properties and Applications in Colloids and Interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Edwards, J.; Fontenot, K.; Prevost, N.; Pircher, N.; Liebner, F.; Condon, B. Preparation, Characterization and Activity of a Peptide-Cellulosic Aerogel Protease Sensor from Cotton. Sensors 2016, 16, 1789. [Google Scholar] [CrossRef] [PubMed]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crop. Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Lin, N.; Bruzzese, C.; Dufresne, A. TEMPO-Oxidized Nanocellulose Participating as Crosslinking Aid for Alginate-Based Sponges. ACS Appl. Mater. Interfaces 2012, 4, 4948–4959. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, W.; Huang, R.; Zhang, Y.; Jia, C.; Zhao, H.; Chen, W.; Xue, Y. Fabrication and Characterization of Cellulose Nanofiber Aerogels Prepared via Two Different Drying Techniques. Polymers 2020, 12, 2583. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Gardner, D.J.; Han, Y. Drying cellulose nanofibrils: In search of a suitable method. Cellulose 2012, 19, 91–102. [Google Scholar] [CrossRef]
- Srinivasa, P.; Kulachenko, A.; Aulin, C. Experimental characterisation of nanofibrillated cellulose foams. Cellulose 2015, 22, 3739–3753. [Google Scholar] [CrossRef]
- Gordeyeva, K.S.; Fall, A.B.; Hall, S.; Wicklein, B.; Bergström, L. Stabilizing nanocellulose-nonionic surfactant composite foams by delayed Ca-induced gelation. J. Colloid Interface Sci. 2016, 472, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Munier, P.; Gordeyeva, K.; Bergström, L.; Fall, A.B. Directional Freezing of Nanocellulose Dispersions Aligns the Rod-Like Particles and Produces Low-Density and Robust Particle Networks. Biomacromolecules 2016, 17, 1875–1881. [Google Scholar] [CrossRef]
- Tang, W.; Gu, X.; Jiang, Y.; Zhao, J.; Ma, W.; Jiang, P.; Zhang, S. Flammability and thermal behaviors of polypropylene composite containing modified kaolinite. J. Appl. Polym. Sci. 2015, 132, 41761. [Google Scholar] [CrossRef]
- Udeni Gunathilake, T.; Ching, Y.; Chuah, C. Enhancement of Curcumin Bioavailability Using Nanocellulose Reinforced Chitosan Hydrogel. Polymers 2017, 9, 64. [Google Scholar] [CrossRef]
- Castro-Ceseña, A.B.; Camacho-Villegas, T.A.; Lugo-Fabres, P.H.; Novitskaya, E.E.; McKittrick, J.; Licea-Navarro, A. Effect of starch on the mechanical and in vitro properties of collagen-hydroxyapatite sponges for applications in dentistry. Carbohydr. Polym. 2016, 148, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Liu, Z.; Chen, Y.; Cao, Y.; Wang, Z. Effect of lignin on the performance of biodegradable cellulose aerogels made from wheat straw pulp-LiCl/DMSO solution. Cellulose 2020, 27, 879–894. [Google Scholar] [CrossRef]
- Gonçalves, V.S.S.; Gurikov, P.; Poejo, J.; Matias, A.A.; Heinrich, S.; Duarte, C.M.M.; Smirnova, I. Alginate-based hybrid aerogel microparticles for mucosal drug delivery. Eur. J. Pharm. Biopharm. 2016, 107, 160–170. [Google Scholar] [CrossRef]
- Picolotto, A.; Pergher, D.; Pereira, G.P.; Machado, K.G.; da Silva Barud, H.; Roesch-Ely, M.; Gonzalez, M.H.; Tasso, L.; Figueiredo, J.G.; Moura, S. Bacterial cellulose membrane associated with red propolis as phytomodulator: Improved healing effects in experimental models of diabetes mellitus. Biomed. Pharmacother. 2019, 112, 108640. [Google Scholar] [CrossRef] [PubMed]
- Voon, L.K.; Pang, S.C.; Chin, S.F. Porous Cellulose Beads Fabricated from Regenerated Cellulose as Potential Drug Delivery Carriers. J. Chem. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Andlinger, D.J.; Bornkeßel, A.C.; Jung, I.; Schröter, B.; Smirnova, I.; Kulozik, U. Microstructures of potato protein hydrogels and aerogels produced by thermal crosslinking and supercritical drying. Food Hydrocolloids 2021, 112, 106305. [Google Scholar] [CrossRef]
- Oprea, M.; Brito, D.; Vieira, T.B.; Mendes, P.; Lopes, S.R.; Fonseca, R.M.; Coutinho, R.Z.; Ditchfield, A.D. A note on the diet and foraging behavior of Artibeus lituratus (Chiroptera, Phyllostomidae) in an urban park in southeastern Brazil. Biota Neotrop. 2007, 7, 297–300. [Google Scholar] [CrossRef]
- Hajdu, Z.; Hohmann, J. An ethnopharmacological survey of the traditional medicine utilized in the community of Porvenir, Bajo Paraguá Indian Reservation, Bolivia. J. Ethnopharmacol. 2012, 139, 838–857. [Google Scholar] [CrossRef]
- Lamounier, K.C.; Cunha, L.C.S.; de Morais, S.A.L.; de Aquino, F.J.T.; Chang, R.; do Nascimento, E.A.; de Souza, M.G.M.; Martins, C.H.G.; Cunha, W.R. Chemical Analysis and Study of Phenolics, Antioxidant Activity, and Antibacterial Effect of the Wood and Bark of Maclura tinctoria (L.) D. Don ex Steud. Evidence-Based Complement. Altern. Med. 2012, 2012, 1–7. [Google Scholar] [CrossRef][Green Version]
- Nguyen, D.; Hägg, D.A.; Forsman, A.; Ekholm, J.; Nimkingratana, P.; Brantsing, C.; Kalogeropoulos, T.; Zaunz, S.; Concaro, S.; Brittberg, M.; et al. Cartilage Tissue Engineering by the 3D Bioprinting of iPS Cells in a Nanocellulose/Alginate Bioink. Sci. Rep. 2017, 7, 658. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef]
- Missio, A.L.; Mattos, B.D.; Otoni, C.G.; Gentil, M.; Coldebella, R.; Khakalo, A.; Gatto, D.A.; Rojas, O.J. Cogrinding Wood Fibers and Tannins: Surfactant Effects on the Interactions and Properties of Functional Films for Sustainable Packaging Materials. Biomacromolecules 2020, 21, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Machado, G.H.A.; Marques, T.R.; de Carvalho, T.C.L.; Duarte, A.C.; de Oliveira, F.C.; Gonçalves, M.C.; Piccoli, R.H.; Corrêa, A.D. Antibacterial activity and in vivo wound healing potential of phenolic extracts from jaboticaba skin. Chem. Biol. Drug Des. 2018, 92, 1333–1343. [Google Scholar] [CrossRef]
- Liu, C.H.; Wu, C.T. Optimization of nanostructured lipid carriers for lutein delivery. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 149–156. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 1–17. [Google Scholar] [CrossRef]
- Claro, F.C.; Matos, M.; Jordão, C.; Avelino, F.; Lomonaco, D.; Magalhães, W.L.E. Enhanced microfibrillated cellulose-based film by controlling the hemicellulose content and MFC rheology. Carbohydr. Polym. 2019, 218, 307–314. [Google Scholar] [CrossRef]
- Claro, F.C.; Jordão, C.; de Viveiros, B.M.; Isaka, L.J.E.; Villanova Junior, J.A.; Magalhães, W.L.E. Low cost membrane of wood nanocellulose obtained by mechanical defibrillation for potential applications as wound dressing. Cellulose 2020, 27, 10765–10779. [Google Scholar] [CrossRef]
- Tondi, G.; Link, M.; Oo, C.W.; Petutschnigg, A. A simple approach to distinguish classic and formaldehyde-free tannin based rigid foams by ATR FT-IR. J. Spectrosc. 2015, 2015, 902340. [Google Scholar] [CrossRef]
- Fuzlin, A.F.; Saadiah, M.A.; Yao, Y.; Nagao, Y.; Samsudin, A.S. Enhancing proton conductivity of sodium alginate doped with glycolic acid in bio-based polymer electrolytes system. J. Polym. Res. 2020, 27, 1–16. [Google Scholar] [CrossRef]
- Lazzari, L.K.; Perondi, D.; Zampieri, V.B.; Zattera, A.J.; Santana, R.M.C. Cellulose/biochar aerogels with excellent mechanical and thermal insulation properties. Cellulose 2019, 26, 9071–9083. [Google Scholar] [CrossRef]
- Fauziyah, M.; Widiyastuti, W.; Balgis, R.; Setyawan, H. Production of cellulose aerogels from coir fibers via an alkali–urea method for sorption applications. Cellulose 2019, 26, 9583–9598. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Jiang, Z.; Wang, H. The effect of freezing speed and hydrogel concentration on the microstructure and compressive performance of bamboo-based cellulose aerogel. J. Wood Sci. 2015, 61, 595–601. [Google Scholar] [CrossRef]
- Lu, T.; Li, Q.; Chen, W.; Yu, H. Composite aerogels based on dialdehyde nanocellulose and collagen for potential applications as wound dressing and tissue engineering scaffold. Compos. Sci. Technol. 2014, 94, 132–138. [Google Scholar] [CrossRef]
- Sehaqui, H.; Zhou, Q.; Berglund, L.A. High-porosity aerogels of high specific surface area prepared from nanofibrillated cellulose (NFC). Compos. Sci. Technol. 2011, 71, 1593–1599. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, J.; Xia, J.; Dai, H.; Cao, Y.; Wang, Z. Industrial Crops & Products Characterization of lignocellulose aerogels fabricated using a LiCl/DMSO solution. Ind. Crop. Prod. 2019, 131, 293–300. [Google Scholar]
- Elufioye, T.O.; Chinaka, C.G.; Oyedeji, A.O. Antioxidant and Anticholinesterase Activities of Macrosphyra Longistyla (DC) Hiern Relevant in the Management of Alzheimer’s Disease. Antioxidants 2019, 8, 400. [Google Scholar] [CrossRef]
- Sadeer, N.B.; Rocchetti, G.; Senizza, B.; Montesano, D.; Zengin, G.; Uysal, A.; Jeewon, R.; Lucini, L.; Mahomoodally, M.F. Untargeted Metabolomic Profiling, Multivariate Analysis and Biological Evaluation of the True Mangrove (Rhizophora mucronata Lam.). Antioxidants 2019, 8, 489. [Google Scholar] [CrossRef]
- Tenuta, M.C.; Deguin, B.; Loizzo, M.R.; Dugay, A.; Acquaviva, R.; Malfa, G.A.; Bonesi, M.; Bouzidi, C.; Tundis, R. Contribution of Flavonoids and Iridoids to the Hypoglycaemic, Antioxidant, and Nitric Oxide (NO) Inhibitory Activities of Arbutus unedo L. Antioxidants 2020, 9, 184. [Google Scholar] [CrossRef] [PubMed]
| Materials | Chromo-bacterium violaceum (μg) | Staphylo-coccus aureus (μg) | Strepto-coccus oralis (μg) | Entero-coccus feacalis (μg) | Escheri-chia coli (μg) |
|---|---|---|---|---|---|
| Aqueous extract | 3125 | na | 1525 | 25,000 | 12,500 |
| Hydroacetic extract | 781 | 12,500 | 781 | na | 6250 |
| Hydroethanolic extract | 781 | 12,500 | 782 | 12,500 | 12,500 |
| Alginate | na | na | na | na | na |
| NFC | na | na | na | na | na |
| NFC + alginate | na | na | na | na | na |
| Hydrogels | Mean Diameter (nm) | IPD | Zeta Potential (mV) |
|---|---|---|---|
| NFC | 265.3 ± 51 | 0.597 ± 0.11 | −7.05 ± 0.9 |
| NFC + TA extract | 221.3 ± 29 | 0.575 ± 0.15 | −11.05 ± 1.3 |
| Treatments | Total phenolic Content | Total Flavonoid Content | DPPH (mM Trolox L−1) | Percentage Inhibition (%) |
|---|---|---|---|---|
| TA extract | 43.80 ± 0.15 * | 108.74 ± 3.93 * | 1.74 ± 0.04 * | 78.81 ± 1.91 |
| Alginate | 0.27 ± 0.02 | 0.45 ± 0.03 | nd | nd |
| ACN | 0.27 ± 0.02 | 0.60 ± 0.03 | nd | nd |
| ACNE | 4.28 ± 0.01 | 4.90 ± 0.05 | 0.13 ± 0.002 | 29.35 ± 0.43 |
| ACNA | 0.32 ± 0.01 | 0.55 ± 0.05 | nd | nd |
| ACNAE | 4.20 ± 0.01 | 2.81 ± 0.01 | 0.07 ± 0.001 | 17.65 ± 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coldebella, R.; Gentil, M.; Berger, C.; Dalla Costa, H.W.; Pedrazzi, C.; Labidi, J.; Delucis, R.A.; Missio, A.L. Nanofibrillated Cellulose-Based Aerogels Functionalized with Tajuva (Maclura tinctoria) Heartwood Extract. Polymers 2021, 13, 908. https://doi.org/10.3390/polym13060908
Coldebella R, Gentil M, Berger C, Dalla Costa HW, Pedrazzi C, Labidi J, Delucis RA, Missio AL. Nanofibrillated Cellulose-Based Aerogels Functionalized with Tajuva (Maclura tinctoria) Heartwood Extract. Polymers. 2021; 13(6):908. https://doi.org/10.3390/polym13060908
Chicago/Turabian StyleColdebella, Rodrigo, Marina Gentil, Camila Berger, Henrique W. Dalla Costa, Cristiane Pedrazzi, Jalel Labidi, Rafael A. Delucis, and André L. Missio. 2021. "Nanofibrillated Cellulose-Based Aerogels Functionalized with Tajuva (Maclura tinctoria) Heartwood Extract" Polymers 13, no. 6: 908. https://doi.org/10.3390/polym13060908
APA StyleColdebella, R., Gentil, M., Berger, C., Dalla Costa, H. W., Pedrazzi, C., Labidi, J., Delucis, R. A., & Missio, A. L. (2021). Nanofibrillated Cellulose-Based Aerogels Functionalized with Tajuva (Maclura tinctoria) Heartwood Extract. Polymers, 13(6), 908. https://doi.org/10.3390/polym13060908











