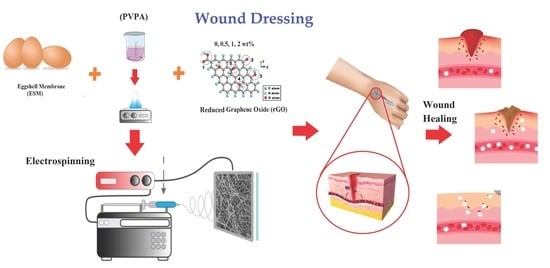
Fabrication and Characterization of Polyvinylpyrrolidone-Eggshell Membrane-Reduced Graphene Oxide Nanofibers for Tissue Engineering Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
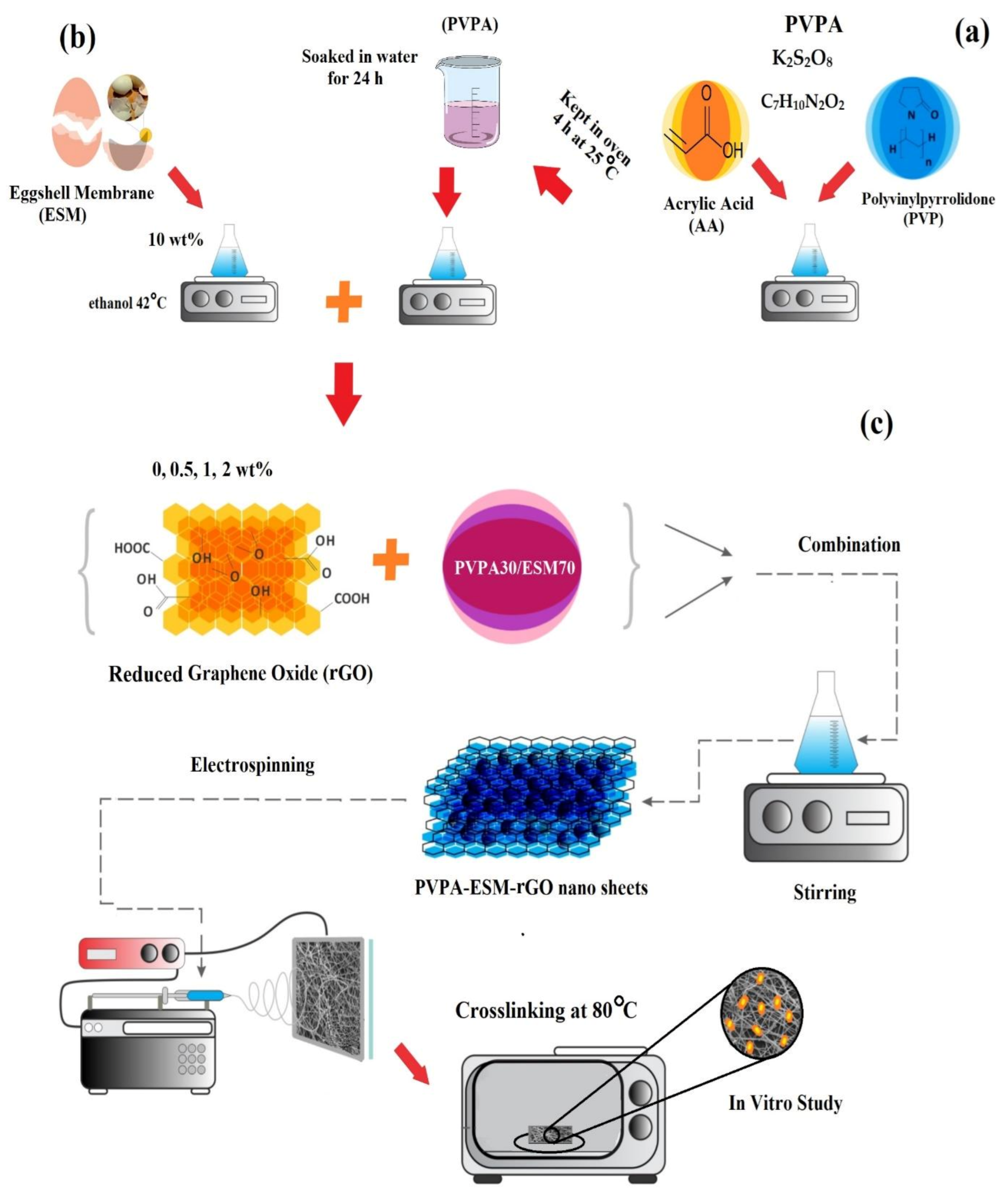
2.2. Preparation of the Eggshell Membrane (ESM)
2.3. Fabrication and Crosslinking Procedure of PVP/Acrylic Acid-Based Hydrogel
2.4. Synthesis of Reduced Graphene Oxide (rGO)
2.5. Fabrication Procedure of PVPA–ESM/rGO Nanofibers
2.6. Measurements and Characterizations of PVPA–ESM–rGO Nanofibers
2.7. Cell Culture
2.8. Statistical Analysis
3. Results and Discussion
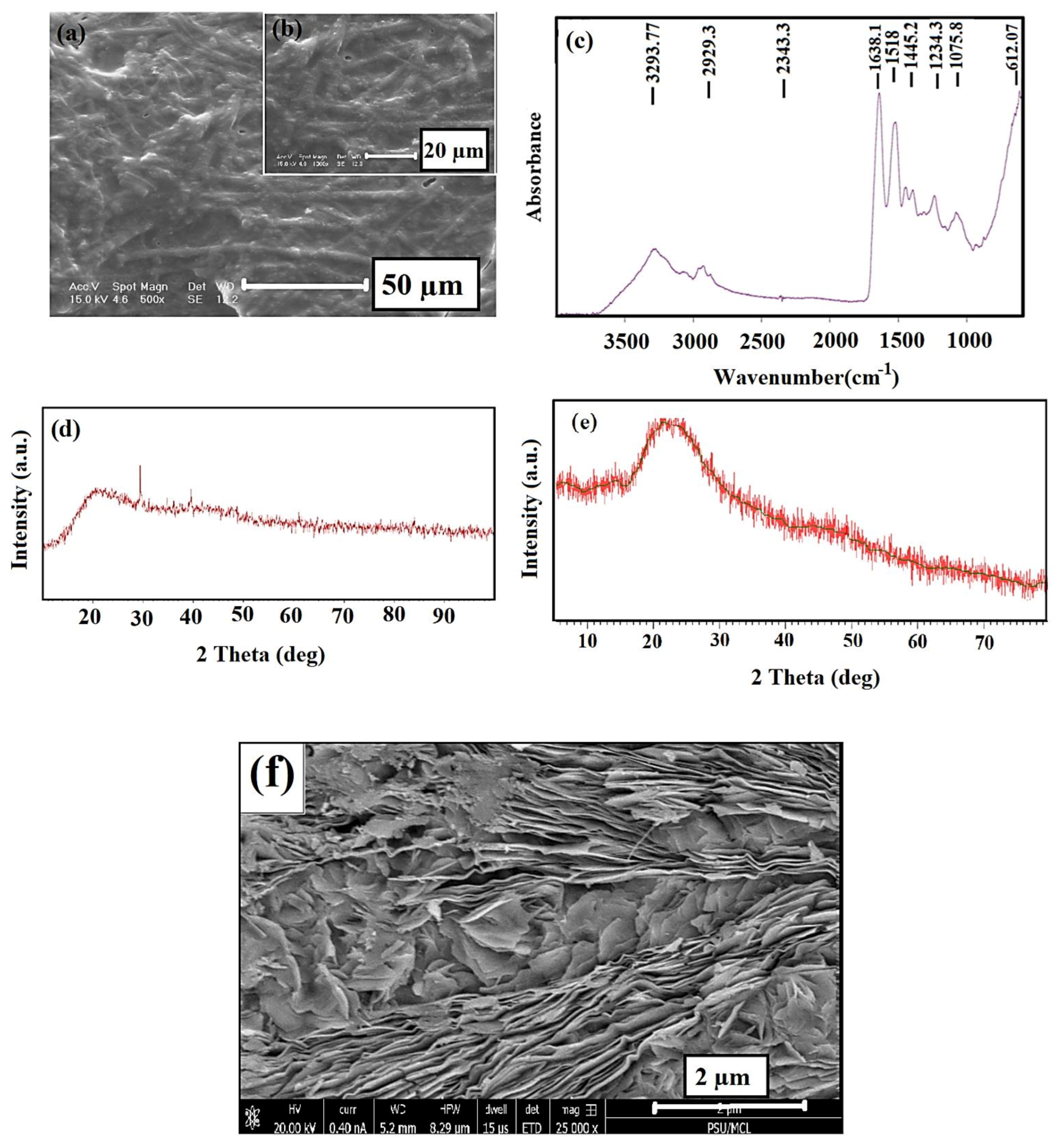
3.1. Eggshell Membrane Characterization
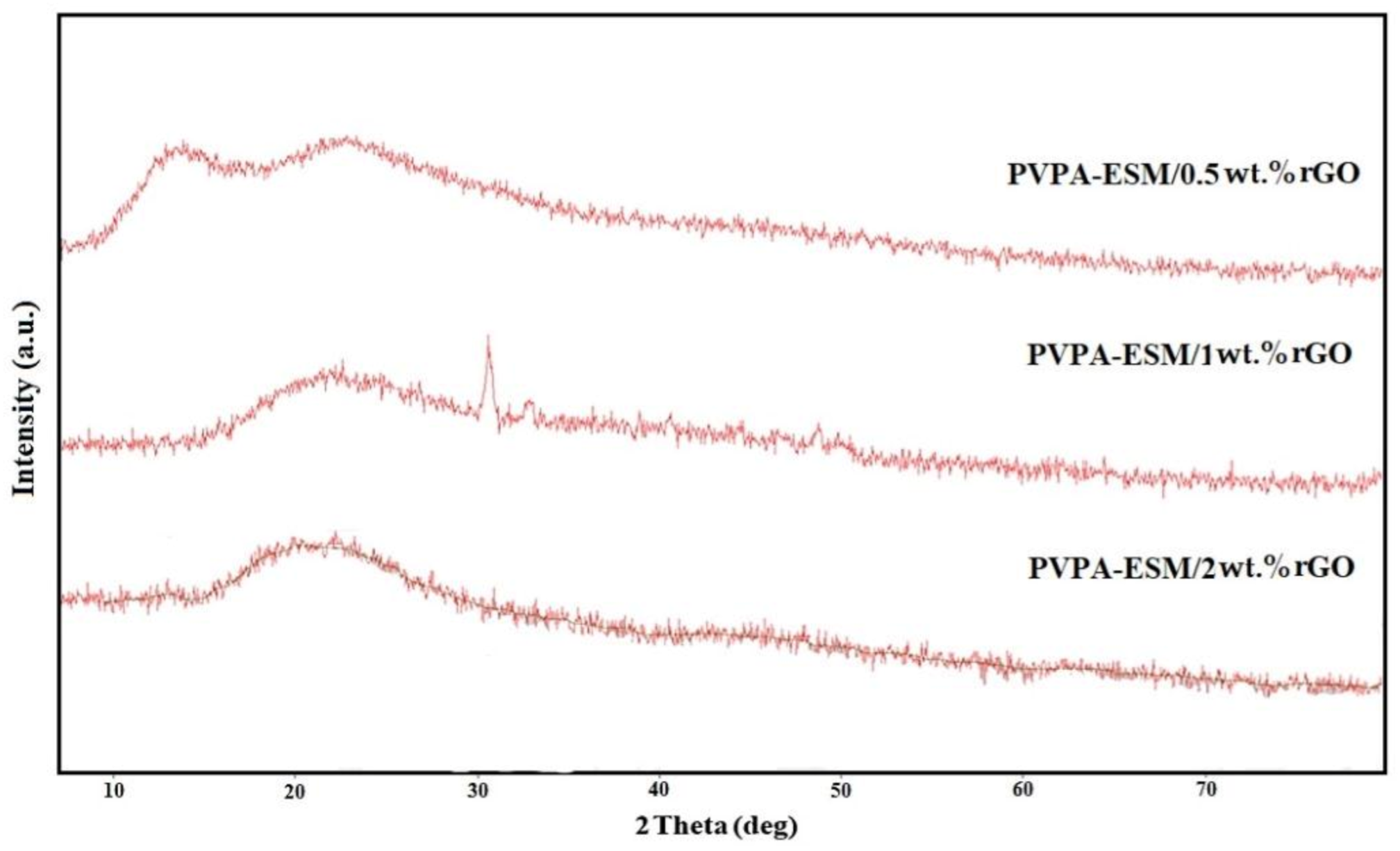
3.2. Morphology of the PVP–ESM–rGO Nanofibers
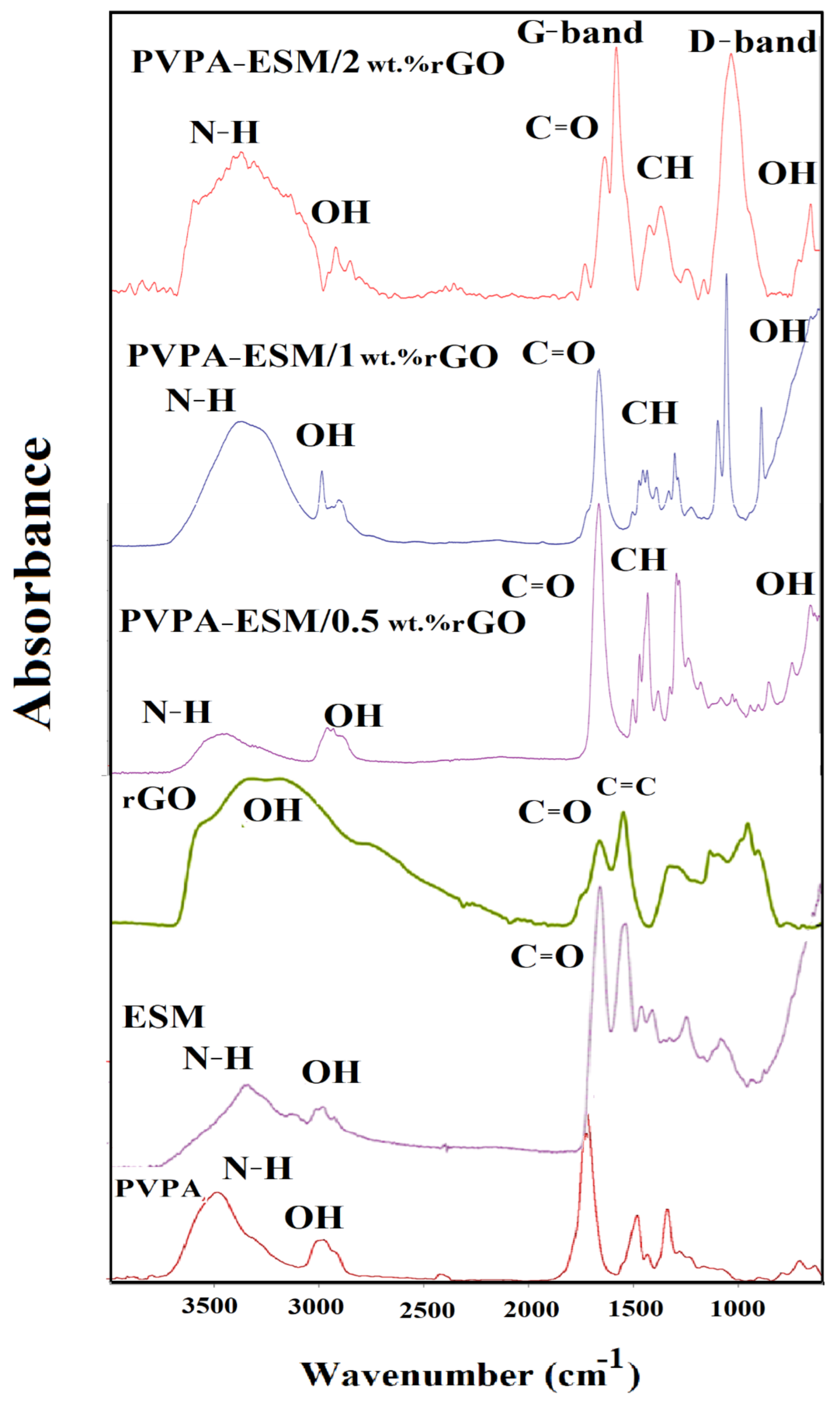
3.3. FTIR Analysis
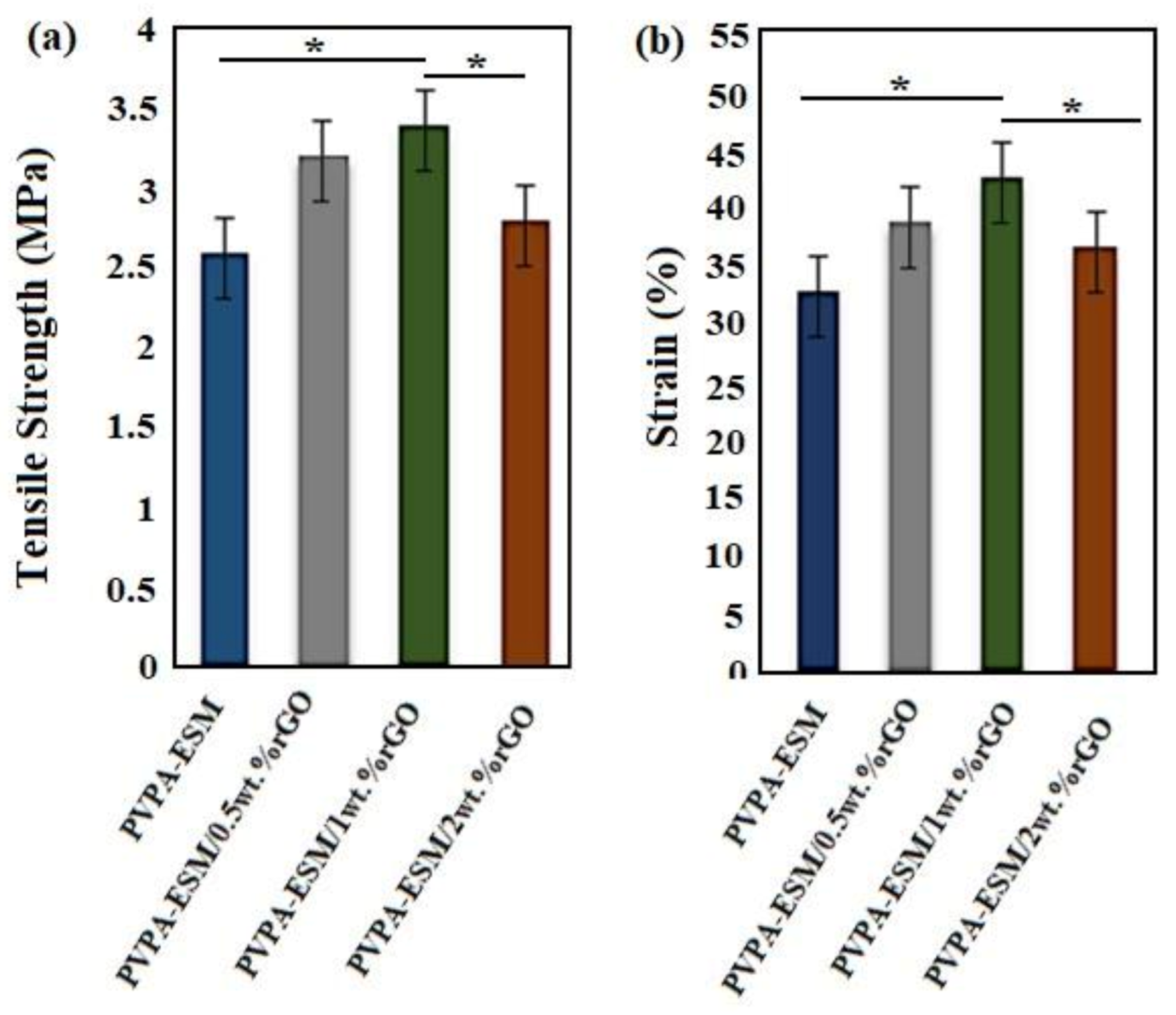
3.4. Mechanical Properties
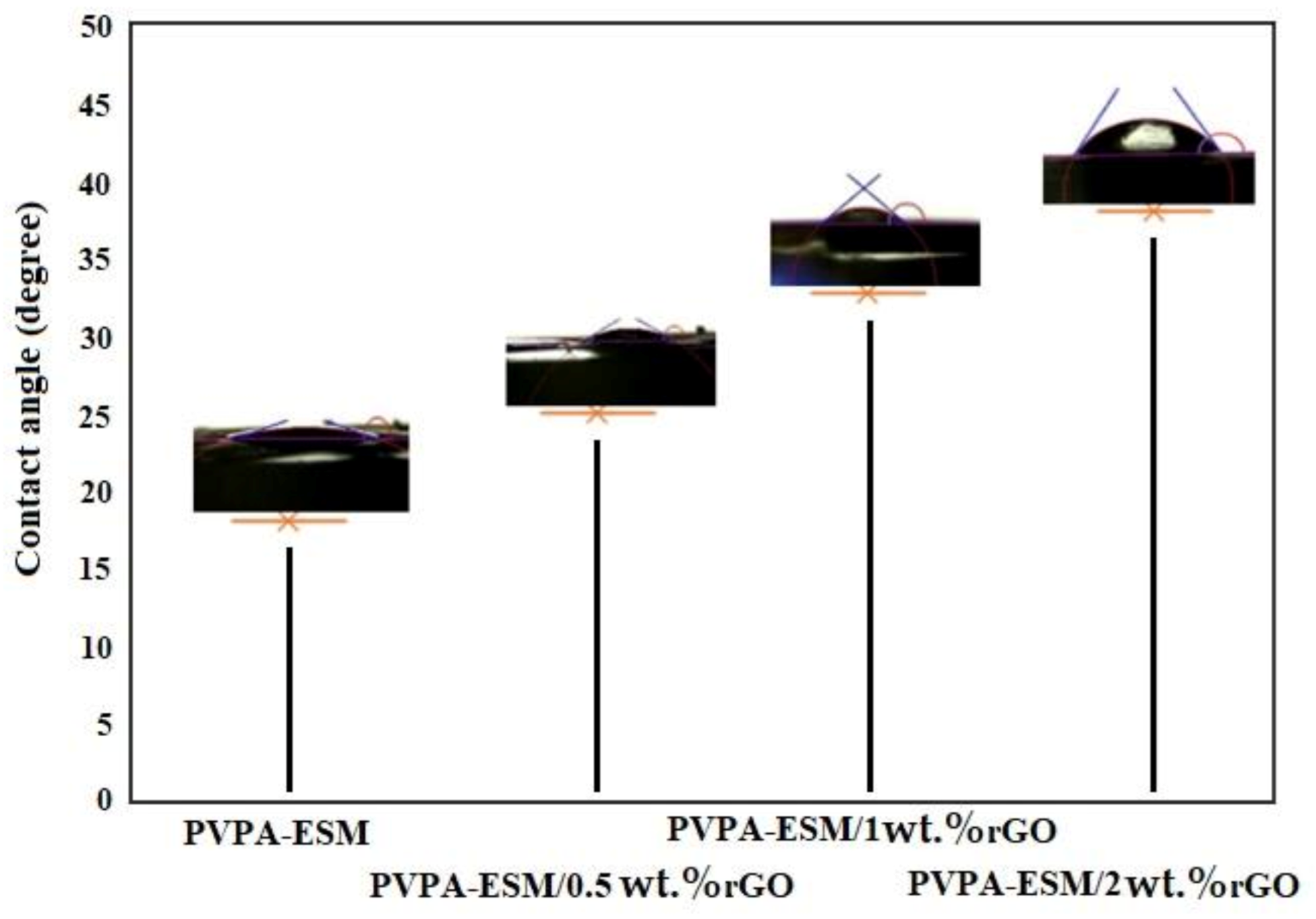
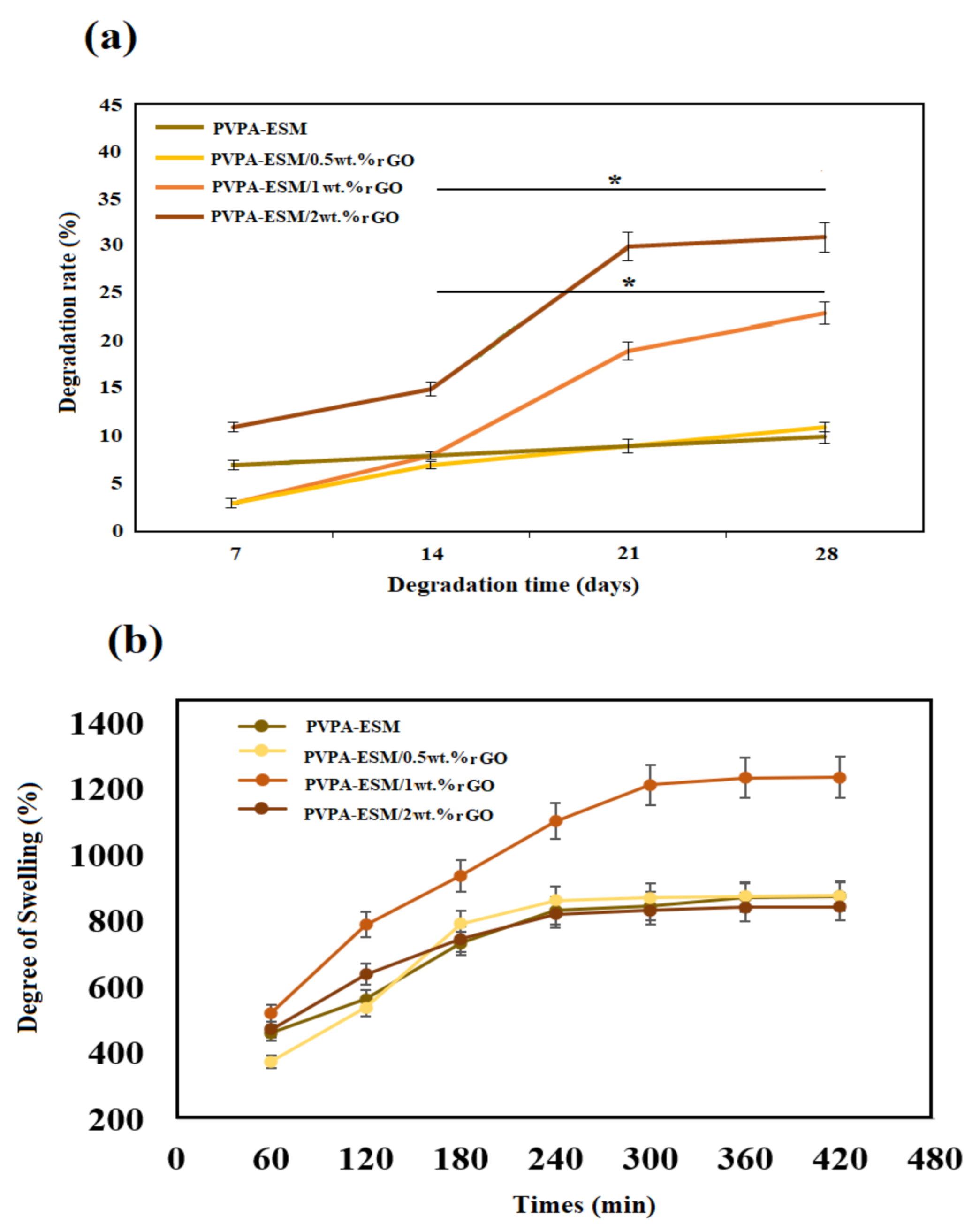
3.5. Contact Angle, Degradation Behavior and Swelling Results
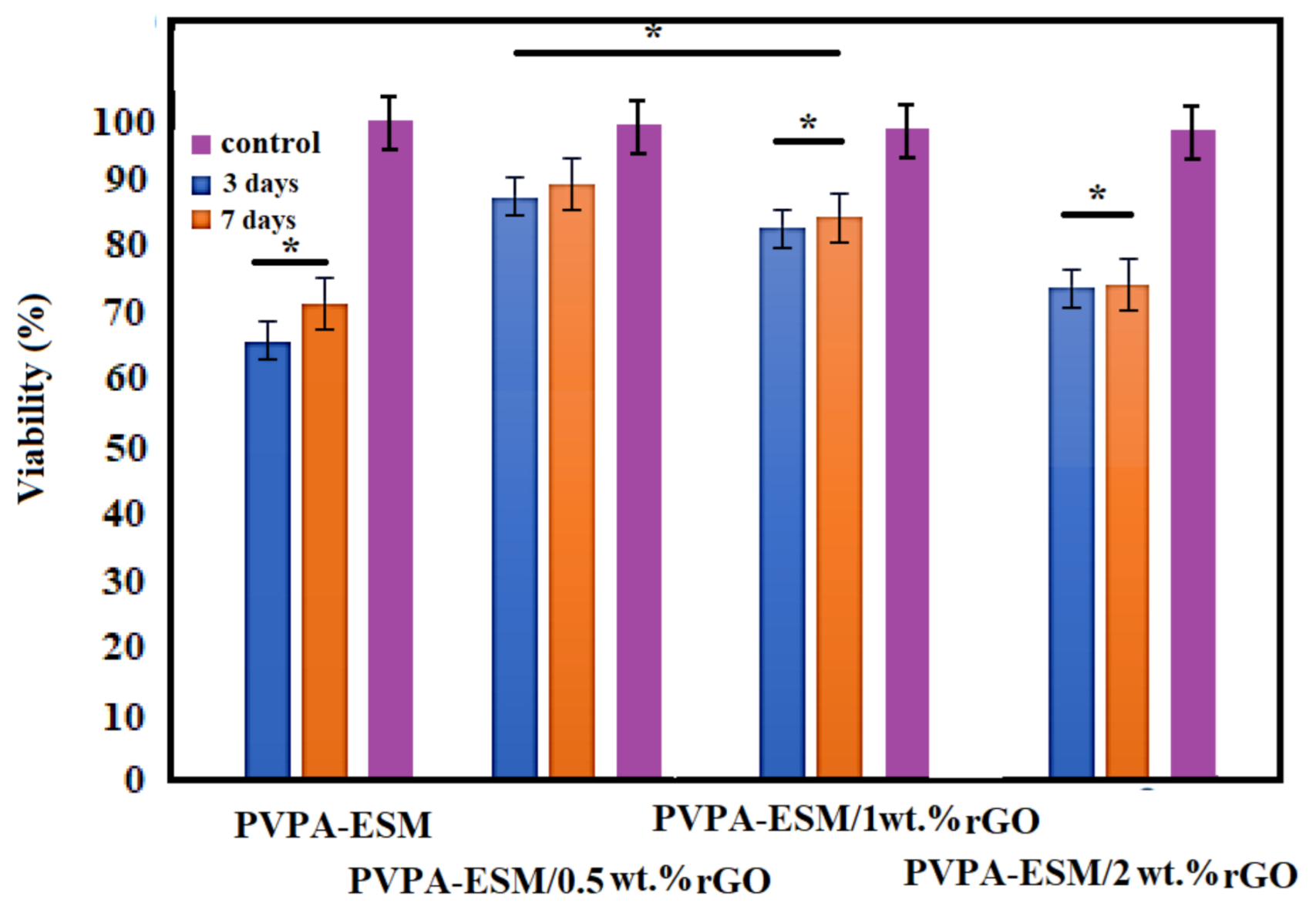
3.6. In Vitro Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chrit, L.; Bastien, P.; Biatry, B.; Simonnet, J.; Potter, A.; Minondo, A.M.; Flament, F.; Bazin, R.; Sockalingum, G.D.; Leroy, F. In vitro and in vivo confocal Raman study of human skin hydration: Assessment of a new moisturizing agent, pMPC. Biopolym. Orig. Res. Biomol. 2007, 85, 359–369. [Google Scholar] [CrossRef]
- Sarvari, R.; Sattari, S.; Massoumi, B.; Agbolaghi, S.; Beygi-Khosrowshahi, Y.; Kahaie-Khosrowshahi, A. Composite electrospun nanofibers of reduced graphene oxide grafted with poly (3-dodecylthiophene) and poly (3-thiophene ethanol) and blended with polycaprolactone. J. Biomater. Sci. Polym. Ed. 2017, 28, 1740–1761. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Sun, L.; Huang, Z.; Chen, Z.; Xu, Z.; Ruan, G.; Zhao, C. Electrospun reduced graphene oxide/TiO2/poly (acrylonitrile-co-maleic acid) composite nanofibers for efficient adsorption and photocatalytic removal of malachite green and leucomalachite green. Chemosphere 2020, 239, 124764. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhang, F.; Wang, Q.; Wu, X. Fabrication of chitosan/graphene oxide polymer nanofiber and its biocompatibility for cartilage tissue engineering. Mater. Sci. Eng. C 2017, 79, 697–701. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Ghayour, H.; Ismail, A.F.; Nur, H.; Berto, F. Electrospun Nano-Fibers for Biomedical and Tissue Engineering Applications: A Comprehensive Review. Materials 2020, 13, 2153. [Google Scholar] [CrossRef] [PubMed]
- Golafshan, N.; Kharaziha, M.; Fathi, M. Tough and conductive hybrid graphene-PVA: Alginate fibrous scaffolds for engineering neural construct. Carbon N. Y. 2017, 111, 752–763. [Google Scholar] [CrossRef]
- Li, J.H.; Zhang, H.; Zhang, W.; Liu, W. Nanofiber membrane of graphene oxide/polyacrylonitrile with highly efficient antibacterial activity. J. Biomater. Sci. Polym. Ed. 2019, 30, 1620–1635. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, X.; Cai, J.; Ye, D.; Wu, Y.; Liu, P. Dual controlled release nanomicelle-in-nanofiber system for long-term antibacterial medical dressings. J. Biomater. Sci. Polym. Ed. 2019, 30, 64–76. [Google Scholar] [CrossRef]
- Li, T.; Liu, L.; Wang, L.; Ding, X. Solid drug particles encapsulated bead-on-string nanofibers: The control of bead number and its corresponding release profile. J. Biomater. Sci. Polym. Ed. 2019, 30, 1454–1469. [Google Scholar] [CrossRef]
- Zhan, J.; Morsi, Y.; EI-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Preparation and characterization of electrospun in-situ cross-linked gelatin-graphite oxide nanofibers. J. Biomater. Sci. Polym. Ed. 2016, 27, 385–402. [Google Scholar] [CrossRef]
- Ardekani, N.T.; Khorram, M.; Zomorodian, K.; Yazdanpanah, S.; Veisi, H.; Veisi, H. Evaluation of electrospun poly (vinyl alcohol)-based nanofiber mats incorporated with Zataria multiflora essential oil as potential wound dressing. Int. J. Biol. Macromol. 2019, 125, 743–750. [Google Scholar] [CrossRef]
- Zha, Z.; Teng, W.; Markle, V.; Dai, Z.; Wu, X. Fabrication of gelatin nanofibrous scaffolds using ethanol/phosphate buffer saline as a benign solvent. Biopolymers 2012, 97, 1026–1036. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Omidi, M.; Chen, X. Development of the PVA/CS nanofibers containing silk protein sericin as a wound dressing: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2020, 149, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cicek, N.; Levin, D.B.; Logsetty, S.; Liu, S. Bacteria-triggered release of a potent biocide from core-shell polyhydroxyalkanoate (PHA)-based nanofibers for wound dressing applications. J. Biomater. Sci. Polym. Ed. 2020, 31, 394–406. [Google Scholar] [CrossRef]
- Yu, H.; Yang, P.; Jia, Y.; Zhang, Y.; Ye, Q.; Zeng, S. Regulation of biphasic drug release behavior by graphene oxide in polyvinyl pyrrolidone/poly (ε-caprolactone) core/sheath nanofiber mats. Colloids Surf. B Biointerfaces 2016, 146, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Kundu, B.; Naskar, D.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Nanofibrous nonmulberry silk/PVA scaffold for osteoinduction and osseointegration. Biopolymers 2015, 103, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Ranjha, N.M.; Shahzad, Y. Swelling and controlled release of tramadol hydrochloride from a pH-sensitive hydrogel. Des. Monomers Polym. 2011, 14, 233–249. [Google Scholar] [CrossRef]
- Naghdeali, M.H.; Miri, T.; Adimi, M.; Eskandari, H.; Nemati, N. Experimental investigation and modeling of release of anti-asthmatic drug amino phylline from hydrogels based on PVP. Int. J. Biosci. 2014, 5, 275–280. [Google Scholar]
- Maswal, M.; Chat, O.A.; Dar, A.A. Rheological characterization of multi-component hydrogel based on carboxymethyl cellulose: Insight into its encapsulation capacity and release kinetics towards ibuprofen. Colloid. Polym. Sci. 2015, 293, 1723–1735. [Google Scholar] [CrossRef]
- Anwar, M.; Pervaiz, F.; Shoukat, H.; Noreen, S.; Shabbir, K.; Majeed, A.; Ijaz, S. Formulation and evaluation of interpenetrating network of xanthan gum and polyvinylpyrrolidone as a hydrophilic matrix for controlled drug delivery system. Polym. Bull. 2020, 7, 1–22. [Google Scholar] [CrossRef]
- Muresan-Pop, M.; Magyari, K.; Vulpoi, A. PVA and PVP Hydrogel Blends for Wound dressing: Synthesis and characterisation. Adv. Mater. Res. 2019, 1151, 9–14. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Emadi, R.; Soleimani, B.; Tavangarian, F. Two-step modification process to improve mechanical properties and bioactivity of hydroxyfluorapatite scaffolds. Ceram. Int. 2018, 44, 19756–19763. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Emadi, R.; Tavangarian, F.; Doostmohammadi, A. In vitro evaluation of diopside/baghdadite bioceramic scaffolds modified by polycaprolactone fumarate polymer coating. Mater. Sci. Eng. C 2020, 106, 110176. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Galindo-Alfaro, D.; Sánchez-Ballester, S.; Teruel-Juanes, R.; Badia, J.D.; Ribes-Greus, A. Crosslinked sulfonated poly (vinyl alcohol)/graphene oxide electrospun nanofibers as polyelectrolytes. Nanomaterials 2019, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jeong, H.-K. Highly H2O Permeable Ionic Liquid Encapsulated Metal-Organic Framework Membranes for Energy-efficient Air-Dehumidification. J. Mater. Chem. A 2020, 8, 23645–23653. [Google Scholar] [CrossRef]
- Yang, C.; Yan, Z.; Lian, Y.; Wang, J.; Zhang, K. Graphene oxide coated shell-core structured chitosan/PLLA nanofibrous scaffolds for wound dressing. J. Biomater. Sci. Polym. Ed. 2020, 31, 622–641. [Google Scholar] [CrossRef]
- Liang, J.; Yuan, C.; Li, H.; Fan, K.; Wei, Z.; Sun, H.; Ma, J. Growth of SnO 2 nanoflowers on N-doped carbon nanofibers as anode for Li-and Na-ion batteries. Nano-Micro Lett. 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Chen, F.; Zhang, C.; Zhi, X.; Wang, K.; Cui, D. The antifungal activity of graphene oxide–silver nanocomposites. Biomaterials 2013, 34, 3882–3890. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Gong, J.; Yao, Y. Electrospun nanofiber regulates assembly of keratin and vimentin intermediate filaments of PANC-1 pancreatic carcinoma cells. Mater. Sci. Eng. C 2019, 96, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Golafshan, N.; Kharaziha, M.; Fathi, M.; Larson, B.L.; Giatsidis, G.; Masoumi, N. Anisotropic architecture and electrical stimulation enhance neuron cell behaviour on a tough graphene embedded PVA: Alginate fibrous scaffold. RSC Adv. 2018, 8, 6381–6389. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Khoshnava, S.M.; Pagan, E.; Chen, X. Co-incorporation of graphene oxide/silver nanoparticle into poly-L-lactic acid fibrous: A route toward the development of cytocompatible and antibacterial coating layer on magnesium implants. Mater. Sci. Eng. C 2020, 111, 110812. [Google Scholar] [CrossRef]
- Pant, H.R.; Park, C.H.; Tijing, L.D.; Amarjargal, A.; Lee, D.-H.; Kim, C.S. Bimodal fiber diameter distributed graphene oxide/nylon-6 composite nanofibrous mats via electrospinning. Colloids Surf. A Physicochem. Eng. Asp. 2012, 407, 121–125. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, C.; Wang, L.; Chen, H.; He, J.; Chen, Y. Novel Poly (l-lactide)/graphene oxide films with improved mechanical flexibility and antibacterial activity. J. Colloid Interface Sci. 2017, 507, 344–352. [Google Scholar] [CrossRef]
- Bharathi, B.S.; Stalin, T. Cerium oxide and peppermint oil loaded polyethylene oxide/graphene oxide electrospun nanofibrous mats as antibacterial wound dressings. Mater. Today Commun. 2019, 21, 100664. [Google Scholar] [CrossRef]
- Liu, Y.; Park, M.; Shin, H.K.; Pant, B.; Choi, J.; Park, Y.W.; Lee, J.Y.; Park, S.-J.; Kim, H.-Y. Facile preparation and characterization of poly (vinyl alcohol)/chitosan/graphene oxide biocomposite nanofibers. J. Ind. Eng. Chem. 2014, 20, 4415–4420. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, L.; Wen, W.; Lu, L.; Luo, B.; Zhou, C. Biomimetic mineralisation of eggshell membrane featuring natural nanofiber network structure for improving its osteogenic activity. Colloids Surf. B Biointerfaces 2019, 179, 299–308. [Google Scholar] [CrossRef]
- Saeb, M.R.; Ghaffari, M.; Rastin, H.; Khonakdar, H.A.; Simon, F.; Najafi, F.; Goodarzi, V.; Puglia, D.; Asl, F.H.; Formela, K. Biowaste chicken eggshell powder as a potential cure modifier for epoxy/anhydride systems: Competitiveness with terpolymer-modified calcium carbonate at low loading levels. RSC Adv. 2017, 7, 2218–2230. [Google Scholar] [CrossRef]
- Abdulrahman, I.; Tijani, H.I.; Mohammed, B.A.; Saidu, H.; Yusuf, H.; Jibrin, M.N.; Mohammed, S. From garbage to biomaterials: An overview on egg shell based hydroxyapatite. J. Mater. 2014, 2014, 802467. [Google Scholar] [CrossRef]
- Saeb, M.R.; Rastin, H.; Nonahal, M.; Paran, S.M.R.; Khonakdar, H.A.; Puglia, D. Cure kinetics of epoxy/chicken eggshell biowaste composites: Isothermal calorimetric and chemorheological analyses. Prog. Org. Coat. 2018, 114, 208–215. [Google Scholar] [CrossRef]
- Bhagavatheswaran, E.S.; Das, A.; Rastin, H.; Saeidi, H.; Jafari, S.H.; Vahabi, H.; Najafi, F.; Khonakdar, H.A.; Formela, K.; Jouyandeh, M.; et al. The taste of waste: The edge of eggshell over calcium carbonate in acrylonitrile butadiene rubber. J. Polym. Environ. 2019, 27, 2478–2489. [Google Scholar] [CrossRef]
- Ishak, N.; Chan, M.; Ang, S.C.; Cheung, C.; Teoh, S.H. Bioengineered three-dimensional transparent eggshell as a chicken embryo experimentation platform for biomedical research. Eng. Rep. 2020, 2, 12092. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Ahmed, T.A.; Wu, L.; Diep, T.; Hincke, M.T. A novel eco-friendly green approach to produce particalized eggshell membrane (PEM) for skin health applications. Biomater. Sci. 2020, 8, 5346–5361. [Google Scholar] [CrossRef]
- Baláž, M. Eggshell membrane biomaterial as a platform for applications in materials science. Acta Biomater. 2014, 10, 3827–3843. [Google Scholar] [CrossRef]
- Yan, S.; Napiwocki, B.; Xu, Y.; Zhang, J.; Zhang, X.; Wang, X.; Crone, W.C.; Li, Q.; Turng, L.-S. Wavy small-diameter vascular graft made of eggshell membrane and thermoplastic polyurethane. Mater. Sci. Eng. C 2020, 107, 110311. [Google Scholar] [CrossRef] [PubMed]
- Yasin, G.; Arif, M.; Shakeel, M.; Dun, Y.; Zuo, Y.; Khan, W.Q.; Tang, Y.; Khan, A.; Nadeem, M. Exploring the Nickel–Graphene Nanocomposite Coatings for Superior Corrosion Resistance: Manipulating the Effect of Deposition Current Density on its Morphology, Mechanical Properties, and Erosion-Corrosion Performance. Adv. Eng. Mater. 2018, 20, 1701166. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, M.; Ren, J.; Wang, J.; Fan, L.; Xu, Q. Preparation and swelling properties of graphene oxide/poly (acrylic acid-co-acrylamide) super-absorbent hydrogel nanocomposites. Colloids Surf. A 2012, 401, 97–106. [Google Scholar] [CrossRef]
- Choi, J.; Pant, B.; Lee, C.; Park, M.; Park, S.-J.; Kim, H.-Y. Preparation and characterization of eggshell membrane/PVA hydrogel via electron beam irradiation technique. J. Ind. Eng. Chem. 2017, 47, 41–45. [Google Scholar] [CrossRef]
- Kang, J.; Kotaki, M.; Okubayashi, S.; Sukigara, S. Fabrication of electrospun eggshell membrane nanofibers by treatment with catechin. J. Appl. Polym. Sci. 2010, 117, 2042–2049. [Google Scholar] [CrossRef]
- Da Silva, R.J.; Lima, R.M.A.P.; de Oliveira, M.C.A.; Alcaraz-Espinoza, J.J.; de Melo, C.P.; de Oliveira, H.P. Supercapacitors based on (carbon nanostructure)/PEDOT/(eggshell membrane) electrodes. J. Electroanal. Chem. 2020, 856, 113658. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Emadi, R.; Tavangarian, F.; Naderi, M. Fabrication and evaluation of silica-based ceramic scaffolds for hard tissue engineering applications. Mater. Sci. Eng. C 2017, 71, 431–438. [Google Scholar] [CrossRef]
- Soleymani, F.; Emadi, R.; Sadeghzade, S.; Tavangarian, F. Applying Baghdadite/PCL/Chitosan Nanocomposite Coating on AZ91 Magnesium Alloy to Improve Corrosion Behavior, Bioactivity, and Biodegradability. Coatings 2019, 9, 789. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Daroonparvar, M.; Chen, X.B. Antibacterial activity and in vivo wound healing evaluation of polycaprolactone-gelatin methacryloyl-cephalexin electrospun nanofibrous. Mater. Lett. 2019, 256, 126618. [Google Scholar] [CrossRef]
- Torres, D.; Pérez-Rodríguez, S.; Sebastián, D.; Pinilla, J.L.; Lázaro, M.J.; Suelves, I. Graphene oxide nanofibers: A nanocarbon material with tuneable electrochemical properties. Appl. Surf. Sci. 2020, 509, 144774. [Google Scholar] [CrossRef]
- Golafshan, N.; Rezahasani, R.; Esfahani, M.T.; Kharaziha, M.; Khorasani, S.N. Nanohybrid hydrogels of laponite: PVA-Alginate as a potential wound healing material. Carbohydr. Polym. 2017, 176, 392–401. [Google Scholar] [CrossRef]
- Shuai, C.; Guo, W.; Wu, P.; Yang, W.; Hu, S.; Xia, Y.; Feng, P. A graphene oxide-Ag co-dispersing nanosystem: Dual synergistic effects on antibacterial activities and mechanical properties of polymer scaffolds. Chem. Eng. J. 2018, 347, 322–333. [Google Scholar] [CrossRef]
- Zhao, R.; Kong, W.; Sun, M.; Yang, Y.; Liu, W.; Lv, M.; Song, S.; Wang, L.; Song, H.; Hao, R. Highly stable graphene-based nanocomposite (GO–PEI–Ag) with broad-spectrum, long-term antimicrobial activity and antibiofilm effects. ACS Appl. Mater. Interfaces 2018, 10, 17617–17629. [Google Scholar] [CrossRef]
- Roy, N.; Saha, N.; Kitano, T.; Saha, P. Novel hydrogels of PVP–CMC and their swelling effect on viscoelastic properties. J. Appl. Polym. Sci. 2010, 117, 1703–1710. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Emadi, R.; Tavangarian, F.; Doostmohammadi, A. The influence of polycaporolacton fumarate coating on mechanical properties and in vitro behavior of porous diopside-hardystonite nano-composite scaffold. J. Mech. Behav. Biomed. Mater. 2020, 101, 103445. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Peng, J.; Xu, H.; Cheng, Q. Glycera-Inspired Synergistic Interfacial Interactions for Constructing Ultrastrong Graphene-Based Nanocomposites. Adv. Funct. Mater. 2018, 28, 1800924. [Google Scholar] [CrossRef]
- Ling, L.; Du, Y.; Ismail, M.; He, R.; Hou, Y.; Fu, Z.; Zhang, Y.; Yao, C.; Li, X. Self-assembled liposomes of dual paclitaxel-phospholipid prodrug for anticancer therapy. Int. J. Pharm. 2017, 526, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-T.; Choi, M.-K.; Sim, Y.; Lim, J.-T.; Kim, G.-S.; Seong, M.-J.; Hyung, J.-H.; Kim, K.S.; Umar, A.; Lee, S.-K. Effect of graphene oxide ratio on the cell adhesion and growth behavior on a graphene oxide-coated silicon substrate. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghorbanzadeh Sheish, S.; Emadi, R.; Ahmadian, M.; Sadeghzade, S.; Tavangarian, F. Fabrication and Characterization of Polyvinylpyrrolidone-Eggshell Membrane-Reduced Graphene Oxide Nanofibers for Tissue Engineering Applications. Polymers 2021, 13, 913. https://doi.org/10.3390/polym13060913
Ghorbanzadeh Sheish S, Emadi R, Ahmadian M, Sadeghzade S, Tavangarian F. Fabrication and Characterization of Polyvinylpyrrolidone-Eggshell Membrane-Reduced Graphene Oxide Nanofibers for Tissue Engineering Applications. Polymers. 2021; 13(6):913. https://doi.org/10.3390/polym13060913
Chicago/Turabian StyleGhorbanzadeh Sheish, Shahnaz, Rahmatollah Emadi, Mehdi Ahmadian, Sorour Sadeghzade, and Fariborz Tavangarian. 2021. "Fabrication and Characterization of Polyvinylpyrrolidone-Eggshell Membrane-Reduced Graphene Oxide Nanofibers for Tissue Engineering Applications" Polymers 13, no. 6: 913. https://doi.org/10.3390/polym13060913
APA StyleGhorbanzadeh Sheish, S., Emadi, R., Ahmadian, M., Sadeghzade, S., & Tavangarian, F. (2021). Fabrication and Characterization of Polyvinylpyrrolidone-Eggshell Membrane-Reduced Graphene Oxide Nanofibers for Tissue Engineering Applications. Polymers, 13(6), 913. https://doi.org/10.3390/polym13060913