Enhancement of Mechanical and Barrier Property of Hemicellulose Film via Crosslinking with Sodium Trimetaphosphate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction of Poplar Hemicellulose
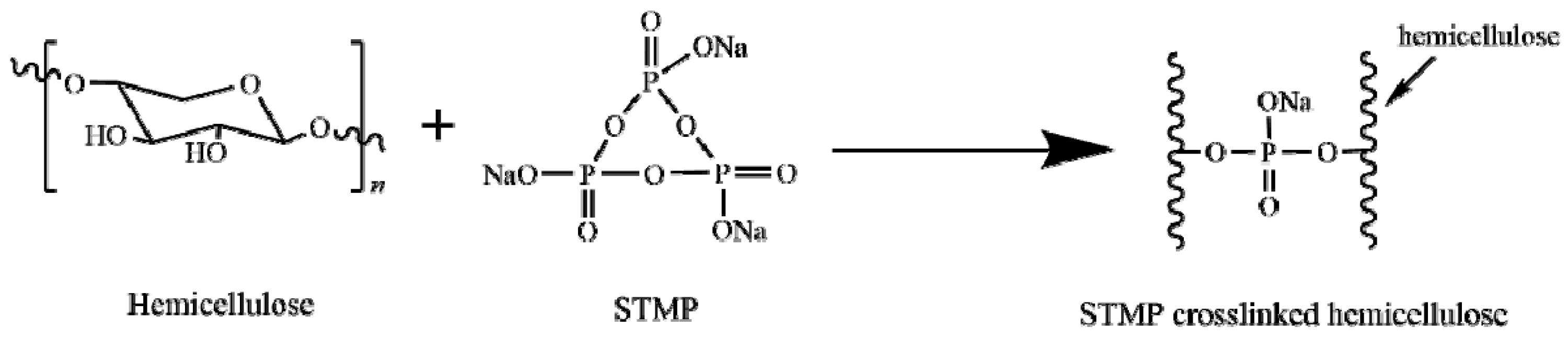
2.3. Preparation of STMP Crosslinked Hemicellulose−Based Film
2.4. Analytical Methods
3. Results and Discussion
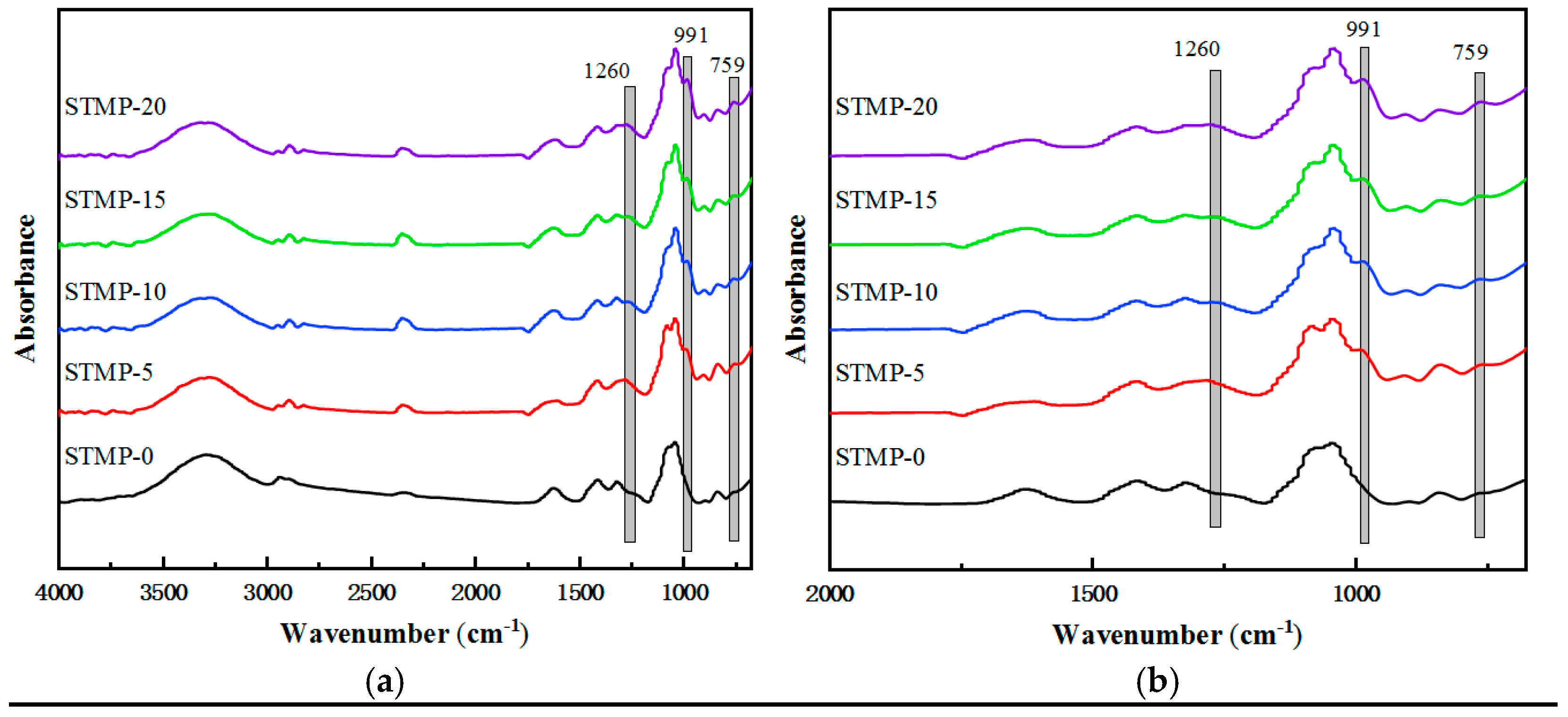
3.1. Structure Analysis of STMP Crosslinked Hemicellulose Films
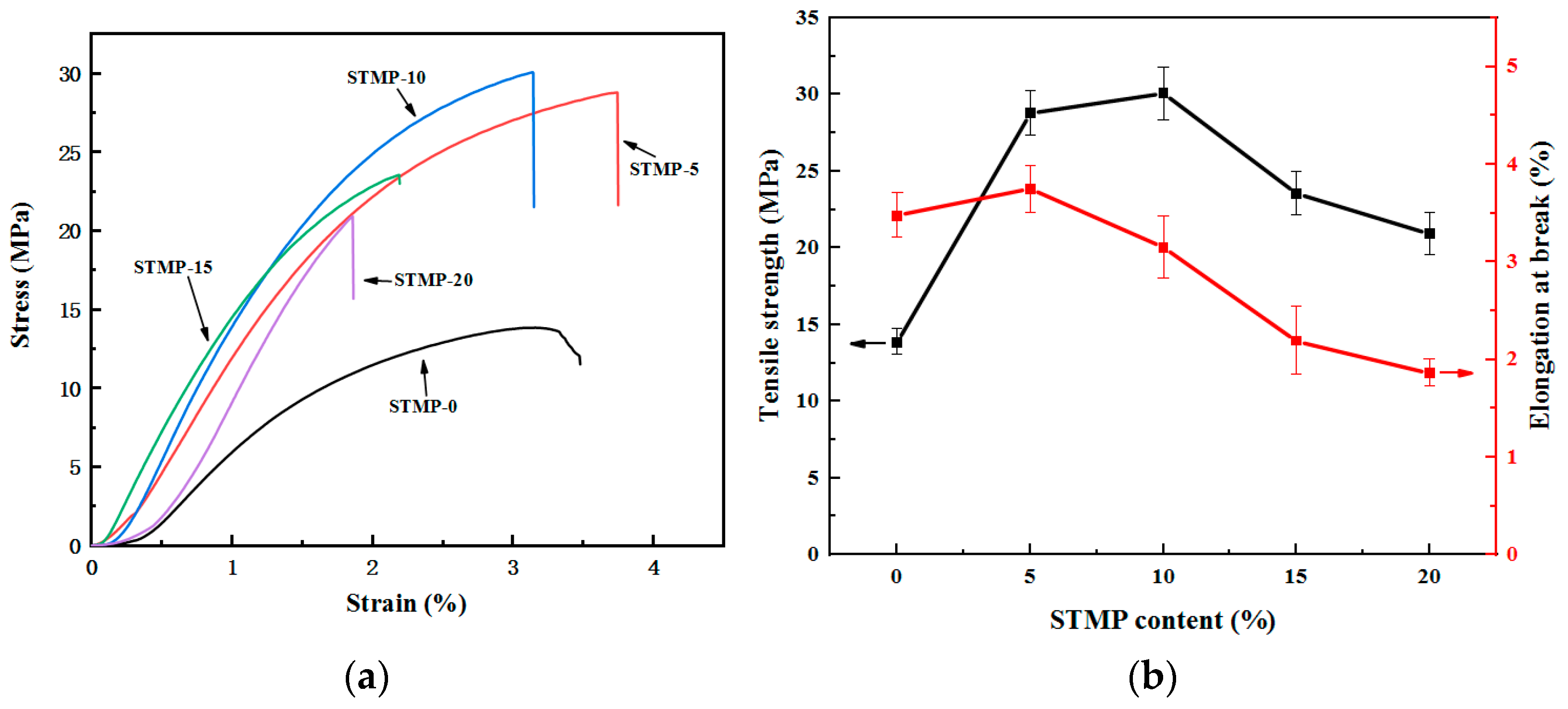
3.2. Mechanical Properties of STMP Crosslinked Hemicellulose Film
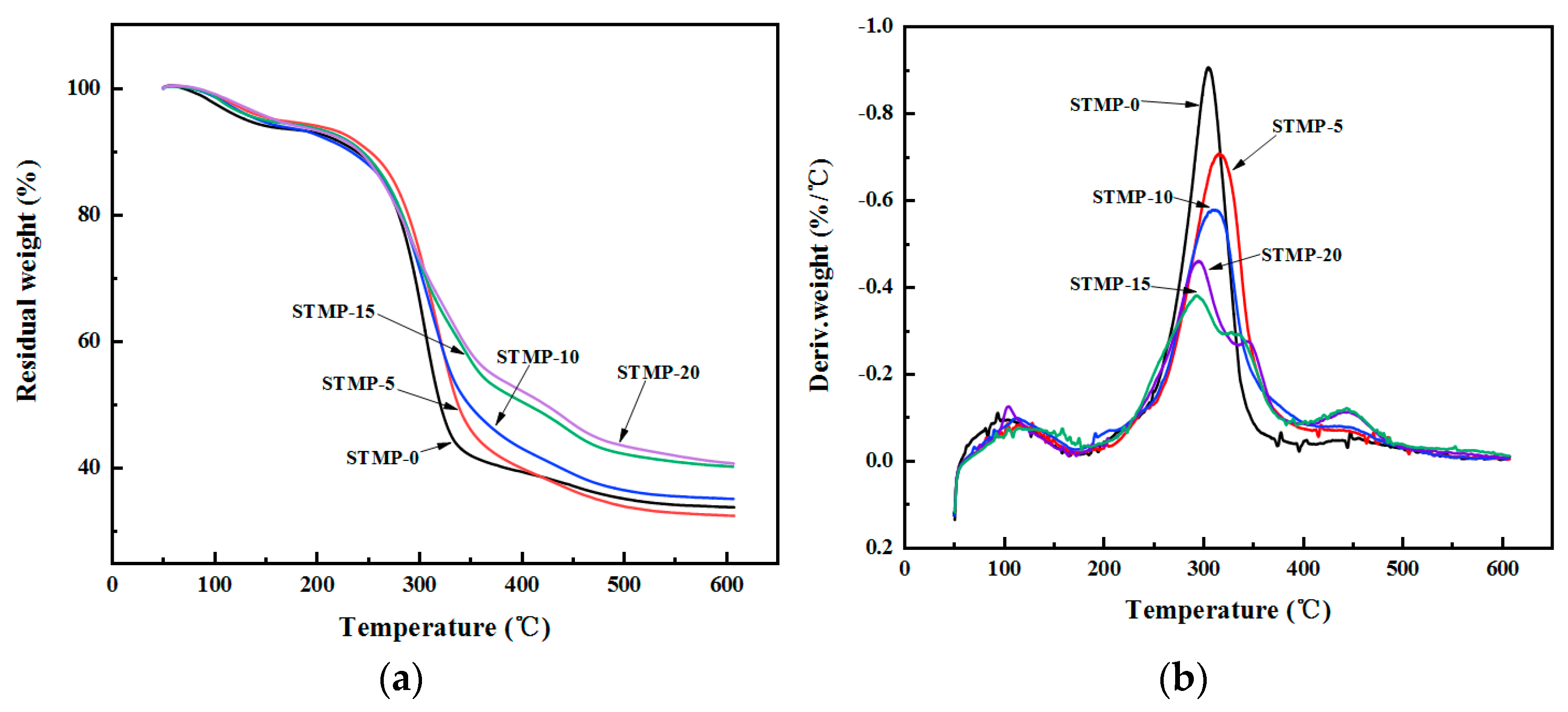
3.3. Thermal Stability Analysis of STMP Crosslinked Hemicellulose Films
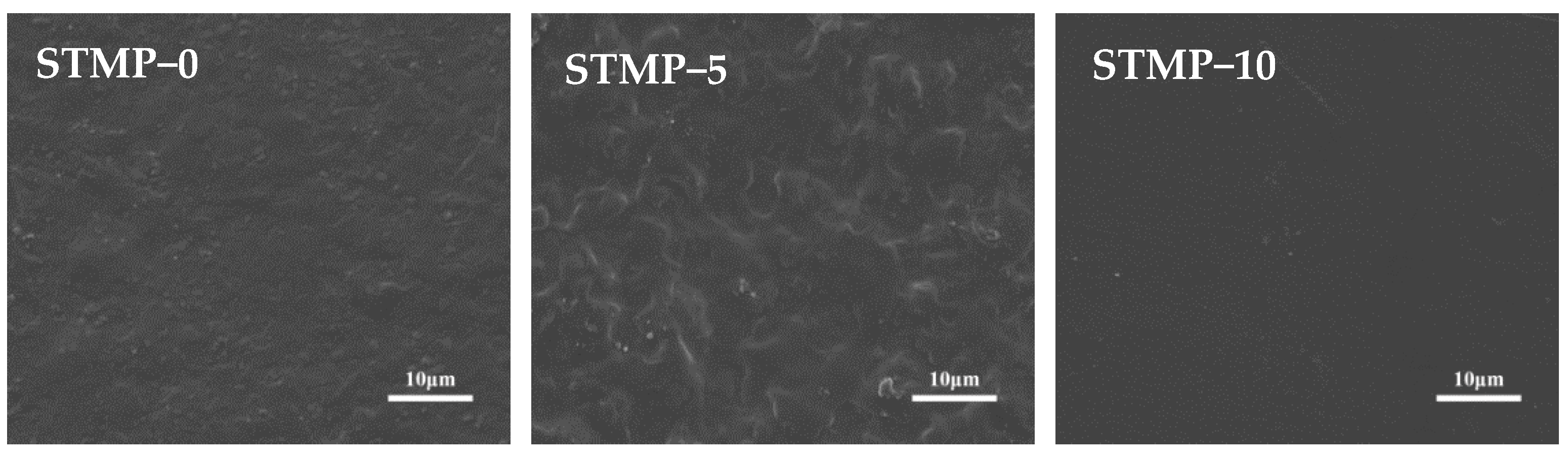
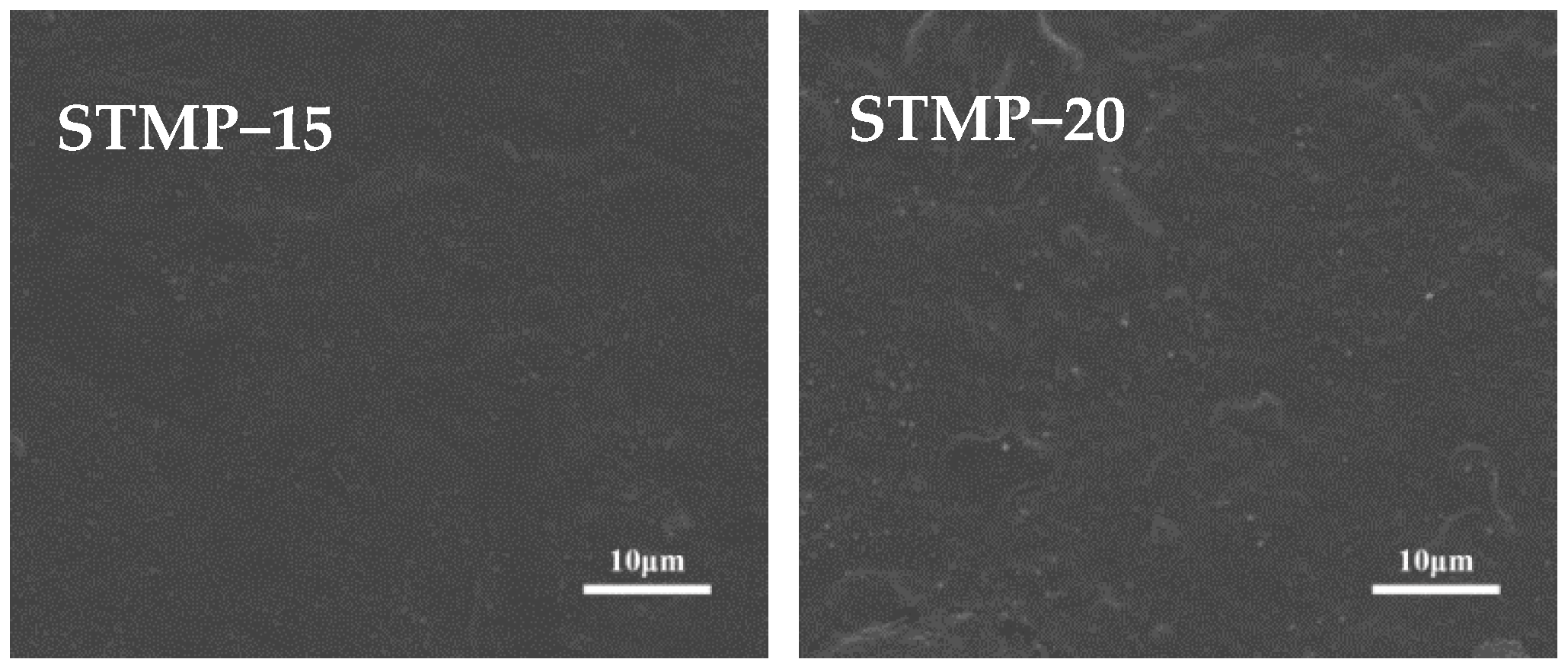
3.4. Surface Morphology Analysis of STMP Crosslinked Hemicellulose Film
3.5. Surface Wettability Analysis of STMP Crosslinked Hemicellulose Film
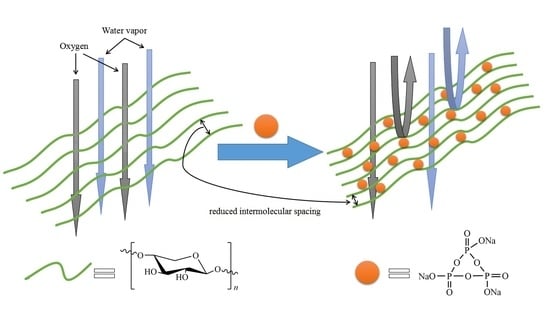
3.6. Oxygen Barrier Properties of STMP Crosslinked Hemicellulose Film
3.7. Water Vapor Barrier Properties of STMP Crosslinked Hemicellulose Film
3.8. Test of STMP Crosslinked Hemicellulose Film for Apple Preservation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Arrieta, M.P.; Peponi, L.; López, D.; López, J.; Kenny, J.M. An overview of nanoparticles role in the improvement of barrier properties of bioplastics for food packaging applications. Food Packag. 2017, 391–424. [Google Scholar]
- Huang, S.; Liu, X.; Chang, C.; Wang, Y. Recent developments and prospective food-related applications of cellulose nanocrystals: A review. Cellulose 2020, 27, 2991–3011. [Google Scholar] [CrossRef]
- Mendes, F.R.S.; Bastos, M.S.R.; Mendes, L.G.; Silva, A.R.A.; Sousa, F.D.; Monteiro-Moreira, A.C.O.; Cheng, H.N.; Biswas, A.; Moreira, R.A. Preparation and evaluation of hemicellulose films and their blends. Food Hydrocoll. 2017, 70, 181–190. [Google Scholar] [CrossRef]
- Tang, X.Z.; Kumar, P.; Alavi, S.; Sandeep, K. Recent Advances in Biopolymers and Biopolymer-Based Nanocomposites for Food Packaging Materials. Crit. Rev. Food Sci. Nutr. 2012, 52, 426–442. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, Protein and lipid-based natural edible films in food packaging: A review. Carbohydr. Polym. 2020, 238, 116–178. [Google Scholar] [CrossRef]
- Perez, J.; Muñoz-Dorado, J.; De La Rubia, T.; Martinez, J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: An overview. Int. Microbiol. 2002, 5, 53–63. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant. Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.M.L.; Plackett, D. Sustainable Films and Coatings from Hemicelluloses: A Review. Biomacromolecules 2008, 9, 1493–1505. [Google Scholar] [CrossRef]
- Huang, J.Z.; Liu, Y.X.; Sun, B.; Li, J.Y.; Zhang, R.F.; Nie, S.X. Laccase Pretreatment for Enhancing Microwave-assisted Alkaline Extraction of Hemicellulose from Bagasse. Bioresources 2019, 14, 931–942. [Google Scholar]
- Braga, R.D.; Poletto, M. Preparation and Characterization of Hemicellulose Films from Sugarcane Bagasse. Materials 2020, 13, 941. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Sun, B.; Wang, Z.L.; Ni, Y.H. Mechanical and Water Vapor Barrier Properties of Bagasse Hemicellulose-based Films. BioResources 2016, 11, 4226–4236. [Google Scholar] [CrossRef]
- Hirose, D.; Kusuma, S.B.W.; Nomura, S.; Yamaguchi, M.; Yasaka, Y.; Kakuchi, R.; Takahashi, K. Effect of anion in carboxylate-based ionic liquids on catalytic activity of transesterification with vinyl esters and the solubility of cellulose. RSC Adv. 2019, 9, 4048–4053. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, N.; Chen, M.; Wei, Y.; Liu, C. Functional packaging films originating from hemicelluloses laurate by direct transesterification in ionic liquid. Carbohydr. Polym. 2019, 229, 115336. [Google Scholar] [CrossRef] [PubMed]
- Gröndahl, M.; Gustafsson, A.; Gatenholm, P. Gas-Phase Surface Fluorination of Arabinoxylan Films. Macromolecules 2006, 39, 2718–2721. [Google Scholar] [CrossRef]
- Härdelin, L.; Bernin, D.; Börjesson, M.; Strom, A.; Larsson, A. Altered Thermal and Mechanical Properties of Spruce Galactoglucomannan Films Modified with an Etherification Reaction. Biomacromolecules 2020, 21, 1832–1840. [Google Scholar] [CrossRef]
- Ren, J.; Xinwen, P.; Linxin, Z.; Feng, P.; Sun, R. Novel hydrophobic hemicelluloses: Synthesis and characteristic. Carbohydr. Polym. 2012, 89, 152–157. [Google Scholar]
- Du, J.; Li, C.; Zhao, Y.; Wang, H. Hemicellulose isolated from waste liquor of viscose fiber mill for preparation of polyacrylamide-hemicellulose hybrid films. Int. J. Boil. Macromol. 2018, 108, 1255–1260. [Google Scholar] [CrossRef]
- Farhat, W.; Venditti, R.; Ayoub, A.; Prochazka, F.; Fernandez-De-Alba, C.; Mignard, N.; Taha, M.; Becquart, F. Towards thermoplastic hemicellulose: Chemistry and characteristics of poly-(ε-caprolactone) grafting onto hemicellulose backbones. Mater. Des. 2018, 153, 298–307. [Google Scholar] [CrossRef]
- Börjesson, M.; Westman, G. Branching of hemicelluloses through an azetidinium salt ring-opening reaction. Carbohydr. Res. 2016, 428, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Sun, H.; Yang, B.; Zhang, H.; Hu, Y. Facile and green preparation of hemicellulose-based film with elevated hydrophobicity via cross-linking with citric acid. RSC Adv. 2019, 9, 2395–2401. [Google Scholar] [CrossRef]
- Huang, C.; Fang, G.; Deng, Y.; Bhagia, S.; Meng, X.; Tao, Y.; Yong, Q.; Ragauskas, A.J. Robust galactomannan/graphene oxide film with ultra-flexible, gas barrier and self-clean properties. Compos. Part. A Appl. Sci. Manuf. 2020, 131, 105780. [Google Scholar] [CrossRef]
- Azeredo, H.M.; Kontou-Vrettou, C.; Moates, G.K.; Wellner, N.; Cross, K.; Pereira, P.H.; Waldron, K.W. Wheat straw hemicellulose films as affected by citric acid. Food Hydrocoll. 2015, 50, 1–6. [Google Scholar] [CrossRef]
- Wang, S.; Ren, J.; Li, W.; Sun, R.; Liu, S. Properties of polyvinyl alcohol/xylan composite films with citric acid. Carbohydr. Polym. 2014, 103, 94–99. [Google Scholar] [CrossRef]
- Chen, G.-G.; Qi, X.-M.; Li, M.-P.; Guan, Y.; Bian, J.; Peng, F.; Yao, C.-L.; Sun, R.-C. Hemicelluloses/montmorillonite hybrid films with improved mechanical and barrier properties. Sci. Rep. 2015, 5, 16405. [Google Scholar] [CrossRef] [PubMed]
- Kasemsuwan, T.; Bailey, T.; Jane, J. Preparation of clear noodles with mixtures of tapioca and high-amylose starches. Carbohydr. Polym. 1998, 36, 301–312. [Google Scholar] [CrossRef]
- Stéphane, L.; Virginie, D.; Didier, L.C.; Luc, P.; Jean, F.A.; Guy, M. Hydrogels Based on Pullulan Crosslinked with sodium trimetaphosphate (STMP): Rheological study. Polym. Bull. 2004, 52, 429–436. [Google Scholar]
- Gemma, L.; Paola, T.; Roberto, G.; Rolando, B. New phosphorylated derivatives of carboxymethylcellulose with osteogenic activity. Polym. Adv. Technol. 2008, 19, 824–830. [Google Scholar]
- Xu, J.Y.; Xia, R.R.; Zheng, L.; Yuan, T.Q.; Sun, R.C. Plasticized hemicelluloses /chitosan-based edible films reinforced by cellulose nanofiber with enhanced mechanical properties. Carbohydr. Polym. 2019, 224, 115164. [Google Scholar] [CrossRef]
- Shao, H.; Hu, Y.; Sun, H.; Yang, B.; Fan, B.M.; Zhang, H.J. Response Surface Optimization of Alkali Extraction and Characterization of Poplar Hemicellulose. Bioresources 2019, 14, 3844–3859. [Google Scholar]
- Zhao, T.Y.; Jiang, L. Contact angle measurement of natural materials. Colloids Surf. B: Biointerfaces. 2018, 161, 324–330. [Google Scholar] [CrossRef]
- Kurek, M.; Garofulic, I.E.; Bakic, M.T.; Scetar, M.; Uzelac, V.D.; Galic, K. Development and evaluation of a novel antioxidant and pH indicator film based on chitosan and food waste sources of antioxidants. Food Hydrocoll. 2018, 84, 238–246. [Google Scholar] [CrossRef]
- Chen, G.-G.; Qi, X.-M.; Guan, Y.; Peng, F.; Yao, C.-L.; Sun, R.-C. High Strength Hemicellulose-Based Nanocomposite Film for Food Packaging Applications. Acs Sustain. Chem. Eng. 2016, 4, 1985–1993. [Google Scholar] [CrossRef]
- Ribeiro, F.W.M.; Laurentino, L.D.; Alves, C.R.; Bastos, M.D.; Costa, J.M.C.; Canuto, K.M.; Furtado, R.F. Chemical modification of gum arabic and its application in the encapsulation of Cymbopogon citratus essential oil. J. Appl. Polym. Sci. 2015, 132, 1–7. [Google Scholar] [CrossRef]
- Prezotti, F.G.; Meneguin, A.B.; Evangelista, R.C.; Ferreira, C.; Beatriz, S. Preparation and characterization of free films of high amylose/pectin mixtures cross-linked with sodium trimetaphosphate. Drug Dev. Ind Pharm. 2012, 38, 1354–1359. [Google Scholar] [CrossRef]
- Racksanti, A.; Janhom, S.; Punyanitya, S.; Watanesk, R.; Watanesk, S. An approach for preparing an absorbable porous film of silk fibroin-rice starch modified with trisodium trimetaphosphate. J. Appl. Polym. Sci. 2015, 132, 1–7. [Google Scholar] [CrossRef]
- Sreedhar, B.; Sairam, M.; Chattopadhyay, D.K.; Syamala Rathnam, P.A.; Mohan Rao, D.V. Thermal, mechanical, and surface characterization of starch–poly(vinyl alcohol) blends and borax-crosslinked films. J. Appl. Polym. Sci. 2005, 96, 1313–1322. [Google Scholar] [CrossRef]
- Nikoliæ, V.M.; Žugiæ, D.L.; Maksiæ, A.D.; Šaponjiæ, D.P.; Kaninski, M.P.M. Performance comparison of modified poly(vinyl alcohol) based membranes in alkaline fuel cells. Int. J. Hydrog. Energy. 2011, 36, 11004–11010. [Google Scholar] [CrossRef]
- Das, G.; Deka, B.K.; Lee, S.H.; Park, Y.B.; Yoon, Y.S. Poly(vinyl alcohol)/silica nanoparticles based anion-conducting nanocomposite membrane for fuel-cell applications. Macromol. Res. 2015, 23, 256–264. [Google Scholar] [CrossRef]
- Hartman, J.; Albertsson, A.C.; Sjöberg, J. Surface- and Bulk-Modified Galactoglucomannan Hemicellulose Films and Film Laminates for Versatile Oxygen Barriers. Biomacromolecules 2006, 7, 1983–1989. [Google Scholar] [CrossRef]
- Svärd, A.; Brännvall, E.; Edlund, U. Modified and thermoplastic rapeseed straw xylan: A renewable additive in PCL biocomposites. Ind. Crops Prod. 2018, 119, 73–82. [Google Scholar] [CrossRef]
- Peter, Z.; Kenyo, C.; Renner, K.; Krohnke, C.; Pukanszky, B. Decreased oxygen permeability of EVOH through molecular interactions. Express Polym. Lett. 2014, 8, 756–766. [Google Scholar] [CrossRef][Green Version]
- Weber, C.J. Biobased Packaging Materials for the Food Industry–Status and Perspectives; Weber, C.J., Ed.; KVL Department of Dairy and Food Science: Frederiksberg, Denmark, 2000. [Google Scholar]
- Olivato, J.B.; Grossmann, M.V.E.; Bilck, A.P.; Yamashita, F. Effect of organic acids as additives on the performance of thermoplastic starch/polyester blown films. Carbohydr. Polym. 2012, 90, 159–164. [Google Scholar] [CrossRef] [PubMed]



| Sample | STMP Mass Fraction (%) | Mass of Hemicellulose (g) | Mass of PVA (g) | Mass of Sorbitol (g) |
|---|---|---|---|---|
| STMP−0 | 0 | 0.90 | 0.30 | 0.30 |
| STMP−5 | 5 | 0.90 | 0.30 | 0.30 |
| STMP−10 | 10 | 0.90 | 0.30 | 0.30 |
| STMP−15 | 15 | 0.90 | 0.30 | 0.30 |
| STMP−20 | 20 | 0.90 | 0.30 | 0.30 |
| Sample | Thickness (μm) | Tensile Strength (MPa) | Elongation at Break (%) | Modulus of Elasticity (MPa) |
|---|---|---|---|---|
| STMP−0 | 52 ± 3 | 13.85 ± 0.84 | 3.48 ± 0.23 | 827 ± 85 |
| STMP−5 | 56 ± 2 | 28.79 ± 1.43 | 3.75 ± 0.24 | 929 ± 93 |
| STMP−10 | 61 ± 2 | 30.08 ± 1.72 | 3.15 ± 0.32 | 1512 ± 116 |
| STMP−15 | 62 ± 3 | 23.56 ± 1.38 | 2.19 ± 0.35 | 1473 ± 133 |
| STMP−20 | 64 ± 1 | 20.92 ± 1.35 | 1.86 ± 0.14 | 958 ± 79 |
| Sample | Tmax (°C) | Carbon Residue Rate at 600 °C (%) | |||
|---|---|---|---|---|---|
| Tmax1 (°C) | Tmax2 (°C) | Tmax3 (°C) | Tmax4 (°C) | ||
| STMP−0 | 103.59 | − | 304.41 | 446.96 | 33.84 |
| STMP−5 | 114.41 | − | 316.25 | 441.48 | 32.51 |
| STMP−10 | 113.37 | − | 311.96 | 442.52 | 35.17 |
| STMP−15 | 115.45 | 293.58 | 334.63 | 443.70 | 40.27 |
| STMP−20 | 107.89 | 294.78 | 345.45 | 442.85 | 40.78 |
| Sample | Oxygen Permeability 1 (cm3 × μm × m−2 × d−1 × kPa−1) |
|---|---|
| STMP−0 | 10.46 ±0.38 |
| STMP−5 | 6.71 ± 0.29 |
| STMP−10 | 3.72 ± 0.11 |
| STMP−15 | 3.76 ± 0.20 |
| STMP−20 | 3.98 ± 0.08 |
| Sample | Water Vapor Permeability 1 (10−10 g × m−1 × s−1 × Pa−1) |
|---|---|
| STMP−0 | 4.82 ± 0.63 |
| STMP−5 | 3.51 ± 0.25 |
| STMP−10 | 2.85 ± 0.50 |
| STMP−15 | 2.94 ± 0.47 |
| STMP−20 | 3.19 ± 0.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Sun, H.; Yang, B.; Fan, B.; Zhang, H.; Weng, Y. Enhancement of Mechanical and Barrier Property of Hemicellulose Film via Crosslinking with Sodium Trimetaphosphate. Polymers 2021, 13, 927. https://doi.org/10.3390/polym13060927
Zhao Y, Sun H, Yang B, Fan B, Zhang H, Weng Y. Enhancement of Mechanical and Barrier Property of Hemicellulose Film via Crosslinking with Sodium Trimetaphosphate. Polymers. 2021; 13(6):927. https://doi.org/10.3390/polym13060927
Chicago/Turabian StyleZhao, Yuelong, Hui Sun, Biao Yang, Baomin Fan, Huijuan Zhang, and Yunxuan Weng. 2021. "Enhancement of Mechanical and Barrier Property of Hemicellulose Film via Crosslinking with Sodium Trimetaphosphate" Polymers 13, no. 6: 927. https://doi.org/10.3390/polym13060927
APA StyleZhao, Y., Sun, H., Yang, B., Fan, B., Zhang, H., & Weng, Y. (2021). Enhancement of Mechanical and Barrier Property of Hemicellulose Film via Crosslinking with Sodium Trimetaphosphate. Polymers, 13(6), 927. https://doi.org/10.3390/polym13060927