Effects of Counter Anions on AC and DC Electrical Conductivity in Poly(Dimethylsiloxane) Crosslinked by Metal-Ligand Coordination
Abstract
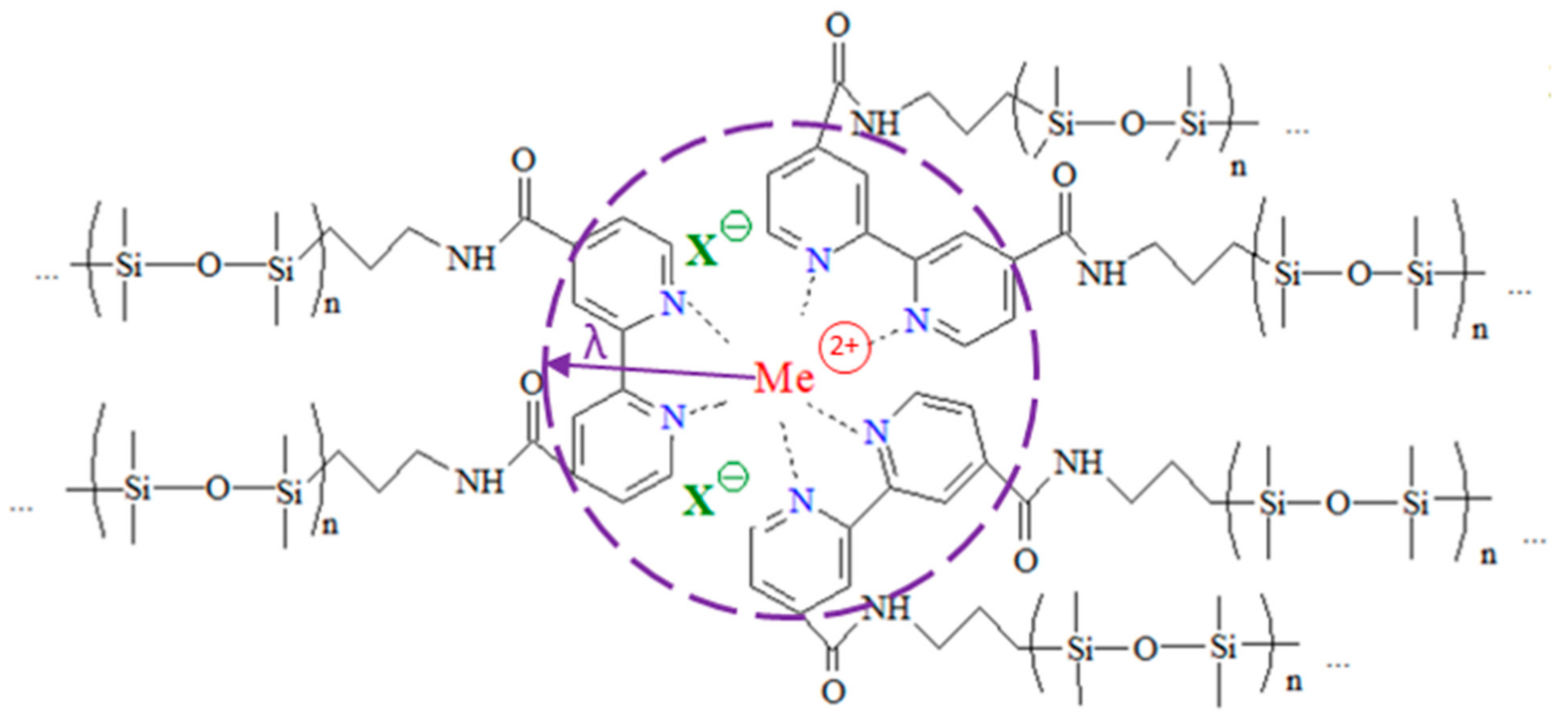
1. Introduction
2. Materials and Methods
2.1. Differential Scanning Calorimetry (DSC)
2.2. Broadband Dielectric Spectroscopy (BDS)
3. Results and Discussion
3.1. Differential Scanning Calorimetry
3.2. Broadband Dielectric Spectroscopy
3.2.1. Isothermal Representations of the Dielectric Responses of the Studied Systems
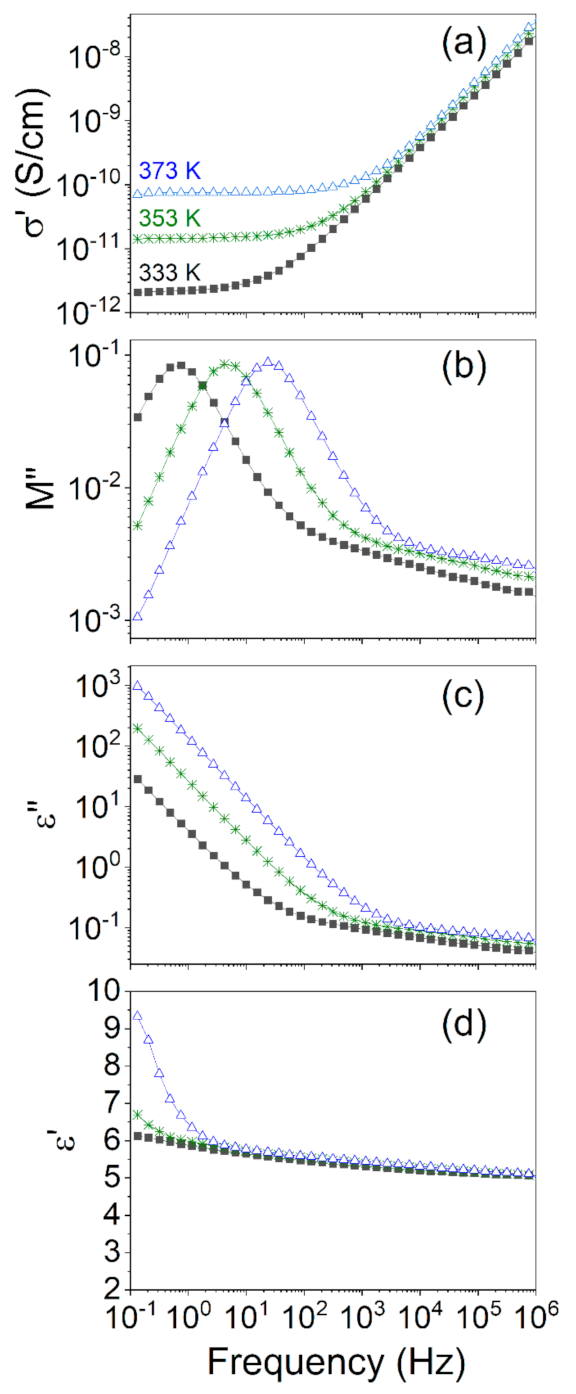
3.2.2. Descriptions of DC and AC Electrical Conductivity Contributions in the Complex Conductivity of the Studied Systems
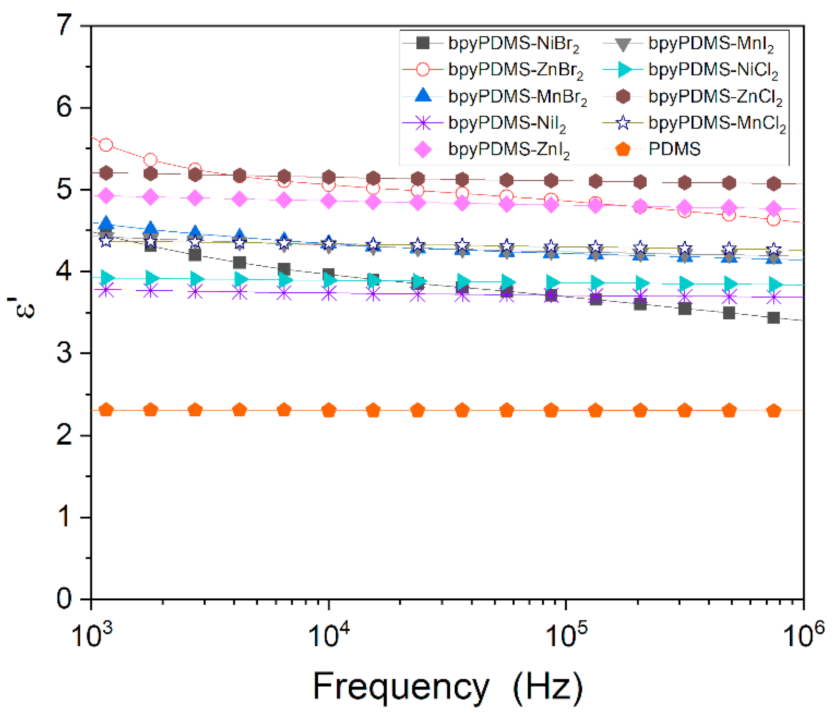
3.2.3. Comparison of Conductivity in Neat PDMS and Its Metalloorganic Complexes
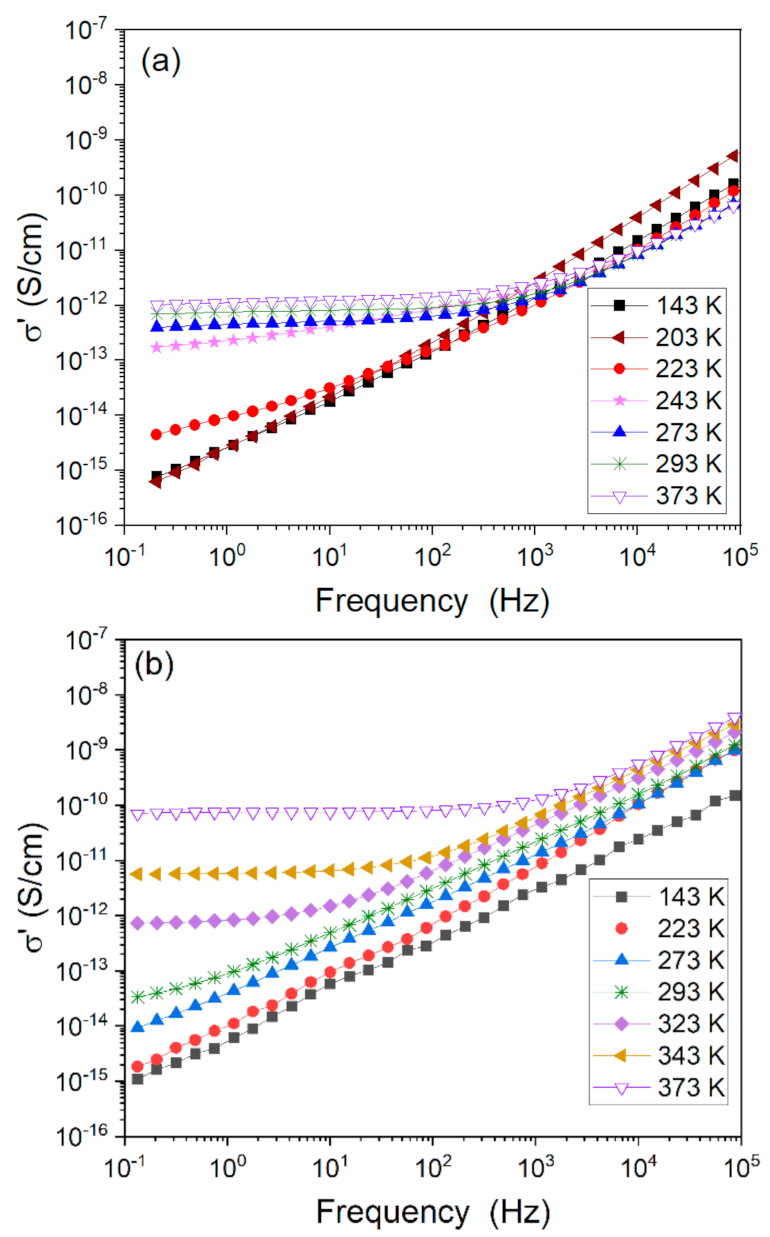
3.2.4. Influences of Counter Anions on DC Conductivity in Metalloorganic Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, J.; Pietrasik, J.; Wypych-Puszkarz, A.; Ciekanska, M.; Matyjaszewski, K. Synthesis of High k Nanoparticles by Controlled Radical Polymerization. In Solution-Processable Components for Organic Electronic Devices; Ulanski, J., Luszczynska, B., Matyjaszewski, K., Eds.; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2019; pp. 181–226. ISBN 978-3-527-34442-0. [Google Scholar]
- Goswami, K.; Daugaard, A.E.; Skov, A.L. Dielectric properties of ultraviolet cured poly(dimethyl siloxane) sub-percolative composites containing percolative amounts of multi-walled carbon nanotubes. RSC Adv. 2015, 5, 12792–12799. [Google Scholar] [CrossRef]
- Sun, H.; Liu, X.; Yu, B.; Feng, Z.; Ning, N.; Hu, G.H.; Tian, M.; Zhang, L. Simultaneously improved dielectric and mechanical properties of silicone elastomer by designing a dual crosslinking network. Polym. Chem. 2019, 10, 633–645. [Google Scholar] [CrossRef]
- Bele, A.; Cazacu, M.; Stiubianu, G.; Vlad, S.; Ignat, M. Polydimethylsiloxane-barium titanate composites: Preparation and evaluation of the morphology, moisture, thermal, mechanical and dielectric behavior. Compos. Part B Eng. 2015, 68, 237–245. [Google Scholar] [CrossRef]
- Budzalek, K.; Ding, H.; Janasz, L.; Wypych-Puszkarz, A.; Cetinkaya, O.; Pietrasik, J.; Kozanecki, M.; Ulanski, J.; Matyjaszewski, K. Star polymer–TiO2 nanohybrids to effectively modify the surface of PMMA dielectric layers for solution processable OFETs. J. Mater. Chem. C 2021. [Google Scholar] [CrossRef]
- Dünki, S.J.; Tress, M.; Kremer, F.; Ko, S.Y.; Nüesch, F.A.; Varganici, C.D.; Racles, C.; Opris, D.M. Fine-tuning of the dielectric properties of polysiloxanes by chemical modification. RSC Adv. 2015, 5, 50054–50062. [Google Scholar] [CrossRef]
- Kussmaul, B.; Risse, S.; Kofod, G.; Waché, R.; Wegener, M.; McCarthy, D.N.; Krüger, H.; Gerhard, R. Enhancement of dielectric permittivity and electromechanical response in silicone elastomers: Molecular grafting of organic dipoles to the macromolecular network. Adv. Funct. Mater. 2011, 21, 4589–4594. [Google Scholar] [CrossRef]
- Rao, Y.L.; Chortos, A.; Pfattner, R.; Lissel, F.; Chiu, Y.C.; Feig, V.; Xu, J.; Kurosawa, T.; Gu, X.; Wang, C.; et al. Stretchable self-healing polymeric dielectrics cross-linked through metal-ligand coordination. J. Am. Chem. Soc. 2016, 138, 6020–6027. [Google Scholar] [CrossRef]
- Oh, J.Y.; Rondeau-Gagné, S.; Chiu, Y.C.; Chortos, A.; Lissel, F.; Wang, G.J.N.; Schroeder, B.C.; Kurosawa, T.; Lopez, J.; Katsumata, T.; et al. Intrinsically stretchable and healable semiconducting polymer for organic transistors. Nature 2016, 539, 411–415. [Google Scholar] [CrossRef]
- Wrzesińska, A.; Bobowska, I.; Maczugowska, P.; Małolepsza, J.; Błazewska, K.M.; Wypych-Puszkarz, A. Effect of metal-ligand coordination complexes on molecular dynamics and structure of cross-linked poly(dimethylosiloxane). Polymers 2020, 12, 1680. [Google Scholar] [CrossRef]
- Burnworth, M.; Tang, L.; Kumpfer, J.R.; Duncan, A.J.; Beyer, F.L.; Fiore, G.L.; Rowan, S.J.; Weder, C. Optically healable supramolecular polymers. Nature 2011, 472, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Zuo, J.L. Self-Healing Polymers Based on Coordination Bonds. Adv. Mater. 2020, 32, 1–29. [Google Scholar] [CrossRef]
- Li, C.H.; Wang, C.; Keplinger, C.; Zuo, J.L.; Jin, L.; Sun, Y.; Zheng, P.; Cao, Y.; Lissel, F.; Linder, C.; et al. A highly stretchable autonomous self-healing elastomer. Nat. Chem. 2016, 8, 618–624. [Google Scholar] [CrossRef]
- Oh, I.; Jeon, S.I.; Chung, I.J.; Ahn, C.H. Self-Healable Dielectric Polydimethylsiloxane Composite Based on Zinc-Imidazole Coordination Bond. Macromol. Res. 2019, 27, 435–443. [Google Scholar] [CrossRef]
- Williams, Z.H.; Burwell, E.D.; Chiomento, A.E.; Demsko, K.J.; Pawlik, J.T.; Harris, S.O.; Yarolimek, M.R.; Whitney, M.B.; Hambourger, M.; Schwab, A.D. Rubber-elasticity and electrochemical activity of iron(II) tris(bipyridine) crosslinked poly(dimethylsiloxane) networks. Soft Matter 2017, 13, 6542–6554. [Google Scholar] [CrossRef]
- Gainaru, C.; Stacy, E.W.; Bocharova, V.; Gobet, M.; Holt, A.P.; Saito, T.; Greenbaum, S.; Sokolov, A.P. Mechanism of Conductivity Relaxation in Liquid and Polymeric Electrolytes: Direct Link between Conductivity and Diffusivity. J. Phys. Chem. B 2016, 120, 11074–11083. [Google Scholar] [CrossRef] [PubMed]
- Geirhos, K.; Lunkenheimer, P.; Michl, M.; Reuter, D.; Loidl, A. Communication: Conductivity enhancement in plastic-crystalline solid-state electrolytes. J. Chem. Phys. 2015, 143. [Google Scholar] [CrossRef] [PubMed]
- von Hippel, A.R. Dielectrics and Waves; Artech: Boston, MA, USA, 1995. [Google Scholar]
- Schrøder, T.B.; Dyre, J.C. Scaling and universality of ac conduction in disordered solids. Phys. Rev. Lett. 2000, 84, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Dyre, J.C.; Schrøder, T.B. Hopping models and ac universality. Phys. Status Solidi Basic Res. 2002, 230, 5–13. [Google Scholar] [CrossRef]
- Jonscher, A.K. Universal Relaxation Law, Chapter 5; Chelsea Dielectrics Press: London, UK, 1992. [Google Scholar]
- Dyre, J.C. The random free-energy barrier model for ac conduction in disordered solids. J. Appl. Phys. 1988, 64, 2456–2468. [Google Scholar] [CrossRef]
- Samukaite-Bubniene, U.; Valiūnienė, A.; Bucinskas, V.; Genys, P.; Ratautaite, V.; Ramanaviciene, A.; Aksun, E.; Tereshchenko, A.; Zeybek, B.; Ramanavicius, A. Towards supercapacitors: Cyclic voltammetry and fast Fourier transform electrochemical impedance spectroscopy based evaluation of polypyrrole electrochemically deposited on the pencil graphite electrode. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 610. [Google Scholar] [CrossRef]
- Stacy, E.W.; Gainaru, C.P.; Gobet, M.; Wojnarowska, Z.; Bocharova, V.; Greenbaum, S.G.; Sokolov, A.P. Fundamental Limitations of Ionic Conductivity in Polymerized Ionic Liquids. Macromolecules 2018, 51, 8637–8645. [Google Scholar] [CrossRef]
- Böttger Harald, B.V. V No Title. In Hopping Conduction in Solids; Verlag Akademie: Berlin, Germany, 1985; pp. 169–213. [Google Scholar]
- Mauri, M.; Peterson, A.; Senol, A.; Elamin, K.; Gitsas, A.; Hjertberg, T.; Matic, A.; Gkourmpis, T.; Prieto, O.; Müller, C. Byproduct-free curing of a highly insulating polyethylene copolymer blend: An alternative to peroxide crosslinking. J. Mater. Chem. C 2018, 6, 11292–11302. [Google Scholar] [CrossRef]
- Roling, B. Conductivity spectra of disordered ionic conductors: Calculating the time-dependent mean square displacement of the mobile ions introduction. Dielectr. Newsl. 2002, pp. 1–8. Available online: https://www.novocontrol.de/newsletter/DNL17.PDF (accessed on 18 March 2021).
- Dyre, J.C.; Maass, P.; Roling, B.; Sidebottom, D.L. Fundamental questions relating to ion conduction in disordered solids. Rep. Prog. Phys. 2009, 72. [Google Scholar] [CrossRef]
- Ngai, K.L. Relaxation and diffusion in Complex Systems; Springer: New York, NY, USA, 2011. [Google Scholar]
- Galindo, B.; Benedito, A.; Gimenez, E.; Compañ, V. Comparative study between the microwave heating efficiency of carbon nanotubes versus multilayer graphene in polypropylene nanocomposites. Compos. Part B Eng. 2016, 98, 330–338. [Google Scholar] [CrossRef]
- Lee, S.H. Molecular dynamics simulation of ion mobility. 2. alkali metal and halide ions using the spc/e model for water at 25 °C. J. Phys. Chem. 1996, 100, 1420–1425. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wrzesińska, A.; Wypych-Puszkarz, A.; Bobowska, I.; Ulański, J. Effects of Counter Anions on AC and DC Electrical Conductivity in Poly(Dimethylsiloxane) Crosslinked by Metal-Ligand Coordination. Polymers 2021, 13, 956. https://doi.org/10.3390/polym13060956
Wrzesińska A, Wypych-Puszkarz A, Bobowska I, Ulański J. Effects of Counter Anions on AC and DC Electrical Conductivity in Poly(Dimethylsiloxane) Crosslinked by Metal-Ligand Coordination. Polymers. 2021; 13(6):956. https://doi.org/10.3390/polym13060956
Chicago/Turabian StyleWrzesińska, Angelika, Aleksandra Wypych-Puszkarz, Izabela Bobowska, and Jacek Ulański. 2021. "Effects of Counter Anions on AC and DC Electrical Conductivity in Poly(Dimethylsiloxane) Crosslinked by Metal-Ligand Coordination" Polymers 13, no. 6: 956. https://doi.org/10.3390/polym13060956
APA StyleWrzesińska, A., Wypych-Puszkarz, A., Bobowska, I., & Ulański, J. (2021). Effects of Counter Anions on AC and DC Electrical Conductivity in Poly(Dimethylsiloxane) Crosslinked by Metal-Ligand Coordination. Polymers, 13(6), 956. https://doi.org/10.3390/polym13060956







