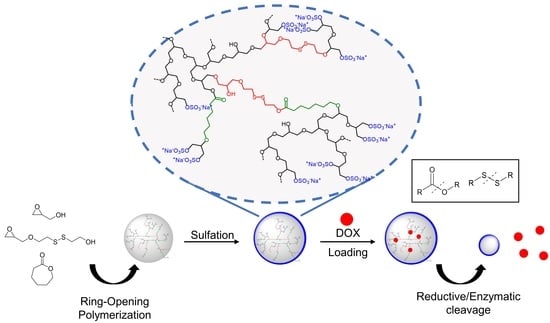
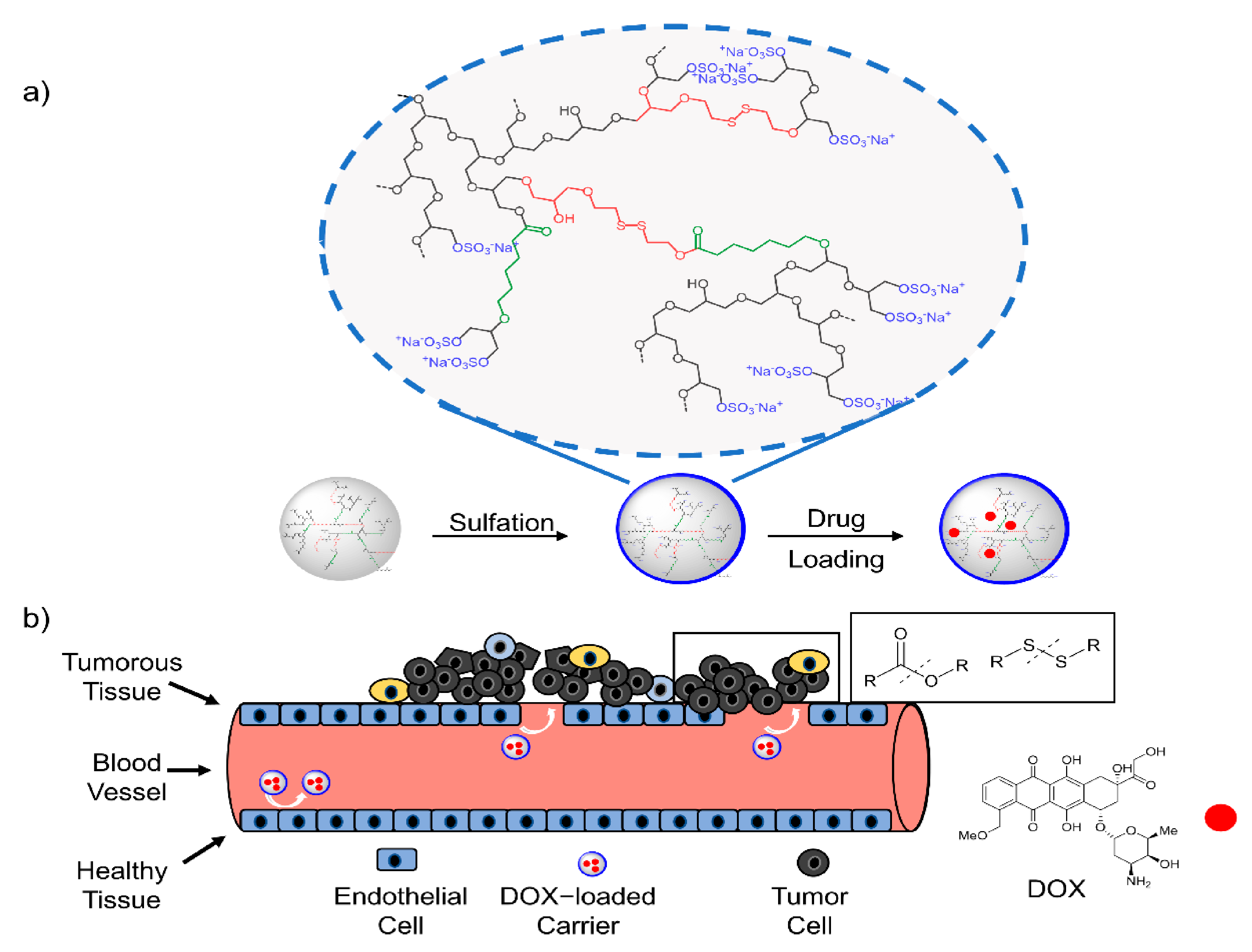
Gram Scale Synthesis of Dual-Responsive Dendritic Polyglycerol Sulfate as Drug Delivery System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Synthesis
2.2.1. 2-((2-(oxiran-2-ylmethoxy)ethyl)disulfanyl)ethan-1-ol (SSG Monomer)
2.2.2. Polymerization Procedure
2.2.3. Sulfation of Polymers
2.2.4. Preparation of Doxorubicin (Free Base)
2.3. Drug Encapsulation
2.4. Degradation Study with Reducing Agents
2.5. Degradation Study with Lipase B
2.6. Release Study of Doxorubicin
2.7. Cytotoxicity Studies
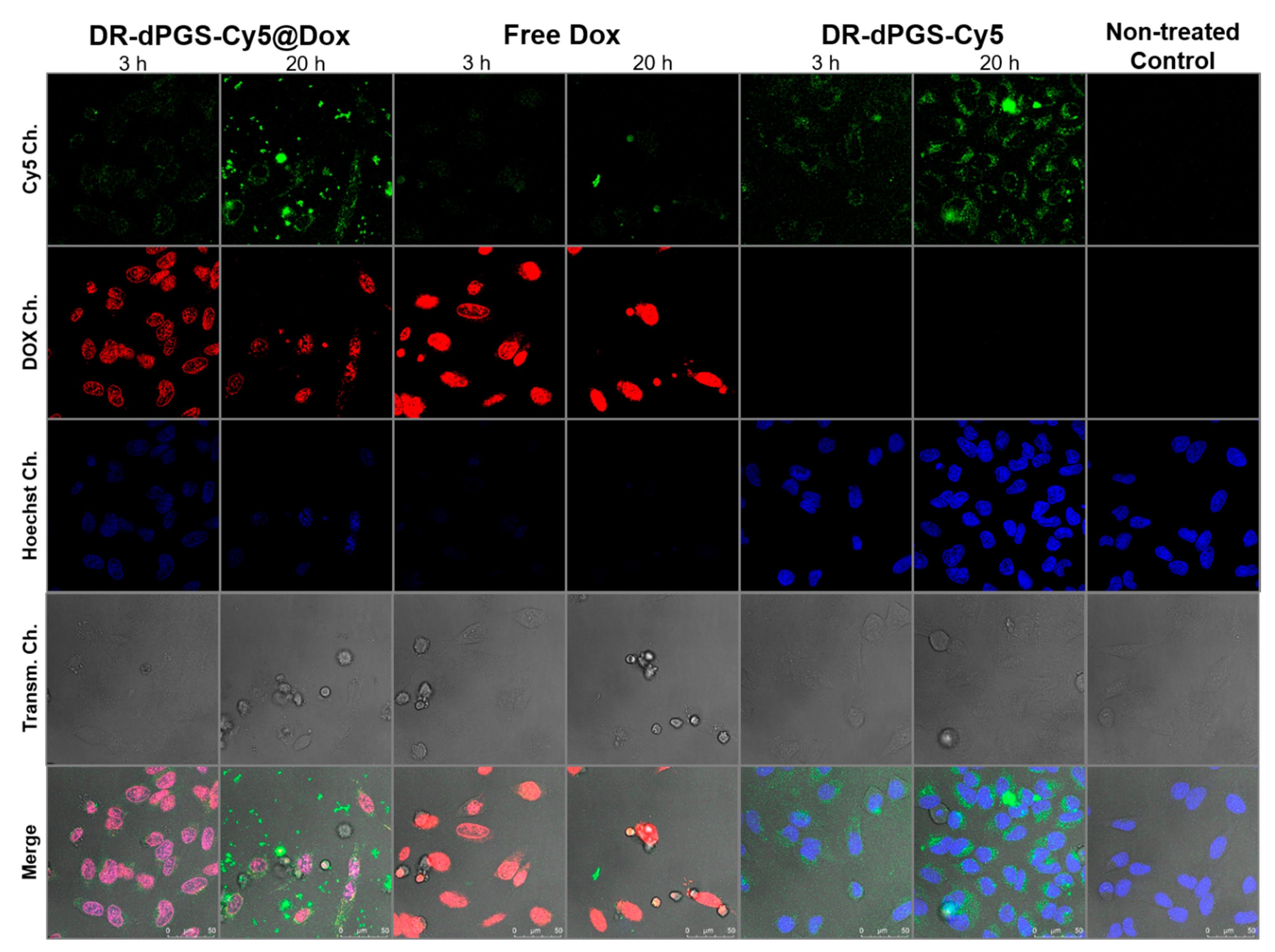
2.8. Confocal Laser Scanning Microscopy (CLSM)
3. Results
3.1. Polymer Synthesis and Analysis
3.2. Degradation Study
3.3. Sulfation
3.4. Drug-Loading and Release Study
3.5. Cytotoxicity and Cell Uptake
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karabasz, A.; Bzowska, M.; Szczepanowicz, K. Biomedical Applications of Multifunctional Polymeric Nanocarriers: A Review of Current Literature. Int. J. Nanomed. 2020, 15, 8673–8696. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, T.; Jiang, C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharma. Sin. B 2018, 8, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Abbina, S.; Vappala, S.; Kumar, P.; Siren, E.M.J.; La, C.C.; Abbasi, U.; Brooks, D.E.; Kizhakkedathu, J.N. Hyperbranched polyglycerols: Recent advances in synthesis, biocompatibility and biomedical applications. J. Mater. Chem. B 2017, 5, 9249–9277. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef] [PubMed]
- Vlakh, E.; Ananyan, A.; Zashikhina, N.; Hubina, A.; Pogodaev, A.; Volokitina, M.; Sharoyko, V.; Tennikova, T. Preparation, Characterization, and Biological Evaluation of Poly(Glutamic Acid)-b-Polyphenylalanine Polymersomes. Polymers 2016, 8, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Pang, L.; Cong, H.; Shen, Y.; Yu, B. Application and design of esterase-responsive nanoparticles for cancer therapy. Drug Deliv. 2019, 26, 416–432. [Google Scholar] [CrossRef] [Green Version]
- Rades, N.; Licha, K.; Haag, R. Dendritic Polyglycerol Sulfate for Therapy and Diagnostics. Polymers 2018, 10, 595. [Google Scholar] [CrossRef] [Green Version]
- Khandare, J.; Calderón, M.; Dagia, N.M.; Haag, R. Multifunctional dendritic polymers in nanomedicine: Opportunities and challenges. Chem. Soc. Rev. 2012, 41, 2824–2848. [Google Scholar] [CrossRef] [Green Version]
- Türk, H.; Haag, R.; Alban, S. Dendritic Polyglycerol Sulfates as New Heparin Analogues and Potent Inhibitors of the Complement System. Bioconjug. Chem. 2004, 15, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Dernedde, J.; Rausch, A.; Weinhart, M.; Enders, S.; Tauber, R.; Licha, K.; Schirner, M.; Zügel, U.; von Bonin, A.; Haag, R. Dendritic polyglycerol sulfates as multivalent inhibitors of inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 19679–19684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, T.; Welker, P.; Licha, K.; Haag, R.; Schulze-Tanzil, G. Influence of dendritic polyglycerol sulfates on knee osteoarthritis: An experimental study in the rat osteoarthritis model. BMC Musculoskelet. Disord. 2015, 16, 387. [Google Scholar] [CrossRef] [Green Version]
- Maysinger, D.; Ji, J.; Moquin, A.; Hossain, S.; Hancock, M.A.; Zhang, I.; Chang, P.K.Y.; Rigby, M.; Anthonisen, M.; Grütter, P.; et al. Dendritic Polyglycerol Sulfates in the Prevention of Synaptic Loss and Mechanism of Action on Glia. ACS Chem. Neurosci. 2018, 9, 260–271. [Google Scholar] [CrossRef]
- Maysinger, D.; Gröger, D.; Lake, A.; Licha, K.; Weinhart, M.; Chang, P.K.Y.; Mulvey, R.; Haag, R.; McKinney, R.A. Dendritic Polyglycerol Sulfate Inhibits Microglial Activation and Reduces Hippocampal CA1 Dendritic Spine Morphology Deficits. Biomacromolecules 2015, 16, 3073–3082. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Dimde, M.; Stöbener, D.; Meng, F.; Deng, C.; Zhong, Z.; Haag, R. Micelles with Sheddable Dendritic Polyglycerol Sulfate Shells Show Extraordinary Tumor Targetability and Chemotherapy in Vivo. ACS Appl. Mater. Interfaces 2016, 8, 27530–27538. [Google Scholar] [CrossRef]
- Ferber, S.; Tiram, G.; Sousa-Herves, A.; Eldar-Boock, A.; Krivitsky, A.; Scomparin, A.; Yeini, E.; Ofek, P.; Ben-Shushan, D.; Vossen, L.I.; et al. Co-targeting the tumor endothelium and P-selectin-expressing glioblastoma cells leads to a remarkable therapeutic outcome. eLife 2017, 6, e25281. [Google Scholar] [CrossRef] [PubMed]
- Weinhart, M.; Gröger, D.; Enders, S.; Riese, S.B.; Dernedde, J.; Kainthan, R.K.; Brooks, D.E.; Haag, R. The Role of Dimension in Multivalent Binding Events: Structure–Activity Relationship of Dendritic Polyglycerol Sulfate Binding to L-Selectin in Correlation with Size and Surface Charge Density. Macromol. Biosci. 2011, 11, 1088–1098. [Google Scholar] [CrossRef]
- Zabihi, F.; Graff, P.; Schumacher, F.; Kleuser, B.; Hedtrich, S.; Haag, R. Synthesis of poly(lactide-co-glycerol) as a biodegradable and biocompatible polymer with high loading capacity for dermal drug delivery. Nanoscale 2018, 10, 16848–16856. [Google Scholar] [CrossRef]
- Adeli, M.; Namazi, H.; Du, F.; Hönzke, S.; Hedtrich, S.; Keilitz, J.; Haag, R. Synthesis of multiarm star copolymers based on polyglycerol cores with polylactide arms and their application as nanocarriers. RSC Adv. 2015, 5, 14958–14966. [Google Scholar] [CrossRef] [Green Version]
- Kainthan, R.K.; Janzen, J.; Kizhakkedathu, J.N.; Devine, D.V.; Brooks, D.E. Hydrophobically derivatized hyperbranched polyglycerol as a human serum albumin substitute. Biomaterials 2008, 29, 1693–1704. [Google Scholar] [CrossRef]
- Kurniasih, I.N.; Liang, H.; Rabe, J.P.; Haag, R. Supramolecular Aggregates of Water Soluble Dendritic Polyglycerol Architectures for the Solubilization of Hydrophobic Compounds. Macromol. Rapid Commun. 2010, 31, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Shin, E.; Kim, B.-S. Redox-Degradable Biocompatible Hyperbranched Polyglycerols: Synthesis, Copolymerization Kinetics, Degradation, and Biocompatibility. Macromolecules 2015, 48, 600–609. [Google Scholar] [CrossRef]
- Ferraro, M.; Silberreis, K.; Mohammadifar, E.; Neumann, F.; Dernedde, J.; Haag, R. Biodegradable Polyglycerol Sulfates Exhibit Promising Features for Anti-inflammatory Applications. Biomacromolecules 2018, 19, 4524–4533. [Google Scholar] [CrossRef]
- Stefani, S.; Sharma, S.K.; Haag, R.; Servin, P. Core-shell nanocarriers based on PEGylated hydrophobic hyperbranched polyesters. Eur. Polym. J. 2016, 80, 158–168. [Google Scholar] [CrossRef]
- Shenoi, R.A.; Chafeeva, I.; Lai, B.F.L.; Horte, S.; Kizhakkedathu, J.N. Bioreducible hyperbranched polyglycerols with disulfide linkages: Synthesis and biocompatibility evaluation. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2104–2115. [Google Scholar] [CrossRef]
- Kamber, N.E.; Jeong, W.; Waymouth, R.M.; Pratt, R.C.; Lohmeijer, B.G.G.; Hedrick, J.L. Organocatalytic Ring-Opening Polymerization. Chem. Rev. 2007, 107, 5813–5840. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Wittek, K.I.; Lee, Y. Synthesis of poly(disulfide)s with narrow molecular weight distributions via lactone ring-opening polymerization. Chem. Sci. 2020, 11, 4882–4886. [Google Scholar] [CrossRef] [Green Version]
- Bandelli, D.; Weber, C.; Schubert, U.S. Strontium Isopropoxide: A Highly Active Catalyst for the Ring-Opening Polymerization of Lactide and Various Lactones. Macromol. Rapid Commun. 2019, 40, 1900306. [Google Scholar] [CrossRef]
- Mohammadifar, E.; Zabihi, F.; Tu, Z.; Hedtrich, S.; Nemati Kharat, A.; Adeli, M.; Haag, R. One-pot and gram-scale synthesis of biodegradable polyglycerols under ambient conditions: Nanocarriers for intradermal drug delivery. Polym. Chem. 2017, 8, 7375–7383. [Google Scholar] [CrossRef]
- Hölter, D.; Burgath, A.; Frey, H. Degree of branching in hyperbranched polymers. Acta Polym. 1997, 48, 30–35. [Google Scholar] [CrossRef]
- Hernández, B.; Pflüger, F.; López-Tobar, E.; Kruglik, S.G.; Garcia-Ramos, J.V.; Sanchez-Cortes, S.; Ghomi, M. Disulfide linkage Raman markers: A reconsideration attempt. J. Raman Spectrosc. 2014, 45, 657–664. [Google Scholar] [CrossRef] [Green Version]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Cheng, R.; Feng, F.; Meng, F.; Deng, C.; Feijen, J.; Zhong, Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J. Control. Release 2011, 152, 2–12. [Google Scholar] [CrossRef]
- Kepsutlu, B.; Wycisk, V.; Achazi, K.; Kapishnikov, S.; Pérez-Berná, A.J.; Guttmann, P.; Cossmer, A.; Pereiro, E.; Ewers, H.; Ballauff, M.; et al. Cells Undergo Major Changes in the Quantity of Cytoplasmic Organelles after Uptake of Gold Nanoparticles with Biologically Relevant Surface Coatings. ACS Nano 2020, 14, 2248–2264. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Elgart, V.; Lin, J.-R.; Loscalzo, J. Determinants of drug-target interactions at the single cell level. PLoS Comput. Biol. 2018, 14, e1006601. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Catalyst | Mn (a) [kDa] | Mw (a) [kDa] | Đ | SS Content (b) [%] | SS Content (c) [%] | CL Content (b) [%] | DB (b) [%] | Yield [g] |
|---|---|---|---|---|---|---|---|---|
| Sn(Oct)2 | 12.8 | 17.1 | 1.3 | 2.3 | 2.6 | 5.8 | 27 | 1.2 |
| Sr(OiPr)2 | 23.4 | 38.8 | 1.6 | 4.2 | 3.5 | 5.2 | 56 | 1.8 |
| DBU | 1.2 | 3.4 | 2.8 | 4.2 | 3.6 | 3.5 | 56 | 1.0 |
| TBD | 0.4 | 3.1 | 7.8 | 3.7 | 3.9 | 6.8 | 50 | 2.0 |
| Mg(HMDS)2 | <0.5 | N/D | N/D | 3.6 | N/D | N/D | 2.9 | |
| DPP | <0.5 | N/D | N/D | N/D | N/D | N/D | 0 | |
| IC50 DR-dPGS@DOX (µg/mL) | IC50 DOX (µg/mL) | |
|---|---|---|
| HeLa | 0.036 | 0.117 |
| A549 | 0.039 | 0.2549 |
| MCF-7 | 0.444 | 6.163 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reisbeck, F.; Ozimkovski, A.; Cherri, M.; Dimde, M.; Quaas, E.; Mohammadifar, E.; Achazi, K.; Haag, R. Gram Scale Synthesis of Dual-Responsive Dendritic Polyglycerol Sulfate as Drug Delivery System. Polymers 2021, 13, 982. https://doi.org/10.3390/polym13060982
Reisbeck F, Ozimkovski A, Cherri M, Dimde M, Quaas E, Mohammadifar E, Achazi K, Haag R. Gram Scale Synthesis of Dual-Responsive Dendritic Polyglycerol Sulfate as Drug Delivery System. Polymers. 2021; 13(6):982. https://doi.org/10.3390/polym13060982
Chicago/Turabian StyleReisbeck, Felix, Alexander Ozimkovski, Mariam Cherri, Mathias Dimde, Elisa Quaas, Ehsan Mohammadifar, Katharina Achazi, and Rainer Haag. 2021. "Gram Scale Synthesis of Dual-Responsive Dendritic Polyglycerol Sulfate as Drug Delivery System" Polymers 13, no. 6: 982. https://doi.org/10.3390/polym13060982
APA StyleReisbeck, F., Ozimkovski, A., Cherri, M., Dimde, M., Quaas, E., Mohammadifar, E., Achazi, K., & Haag, R. (2021). Gram Scale Synthesis of Dual-Responsive Dendritic Polyglycerol Sulfate as Drug Delivery System. Polymers, 13(6), 982. https://doi.org/10.3390/polym13060982