Effect of Chitosan on the Activity of Water-Soluble and Hydrophobic Porphyrin Photosensitizers Solubilized by Amphiphilic Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Kinetics of Tryptophan Photo-Oxidation
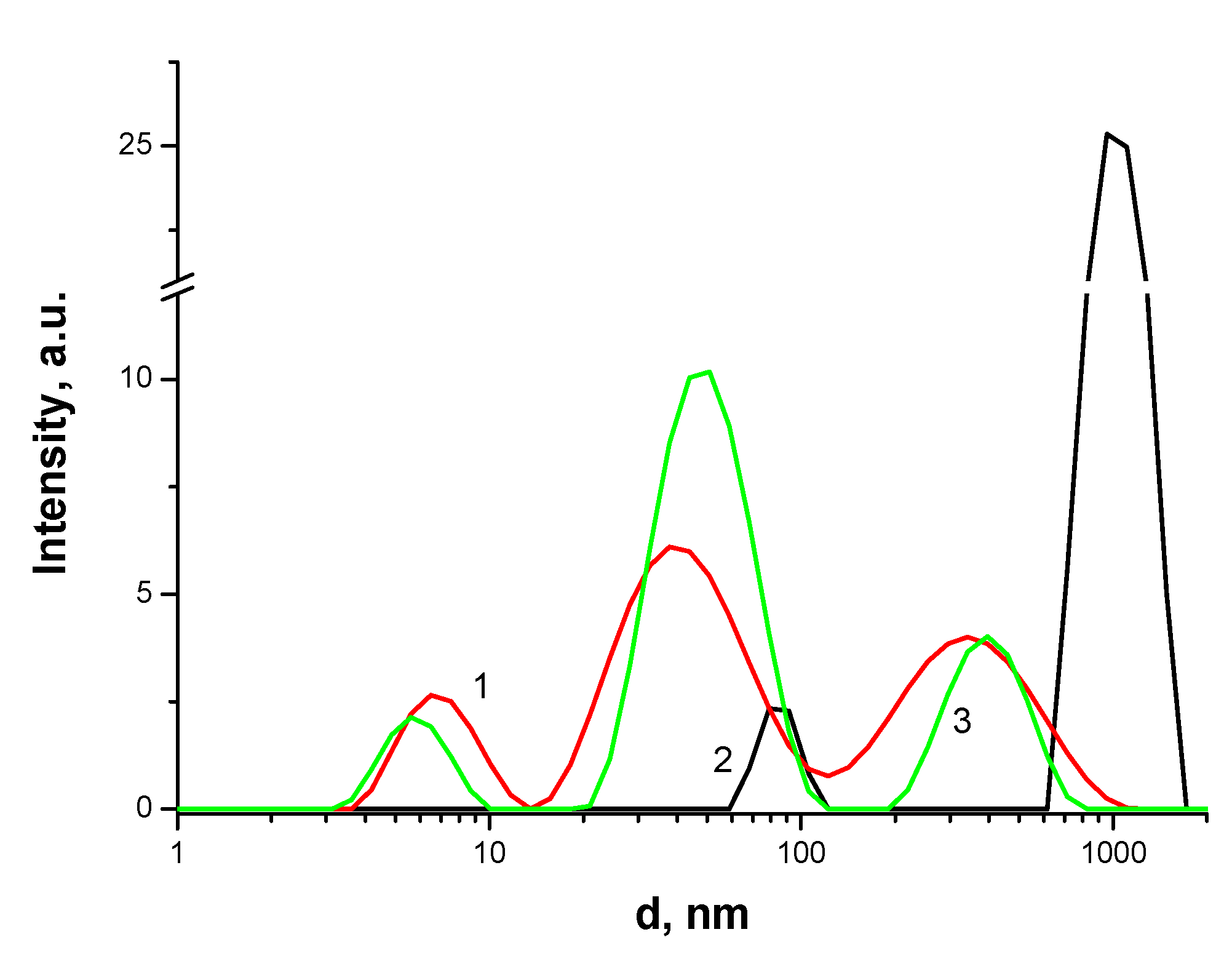
2.3. Dynamic Light Scattering
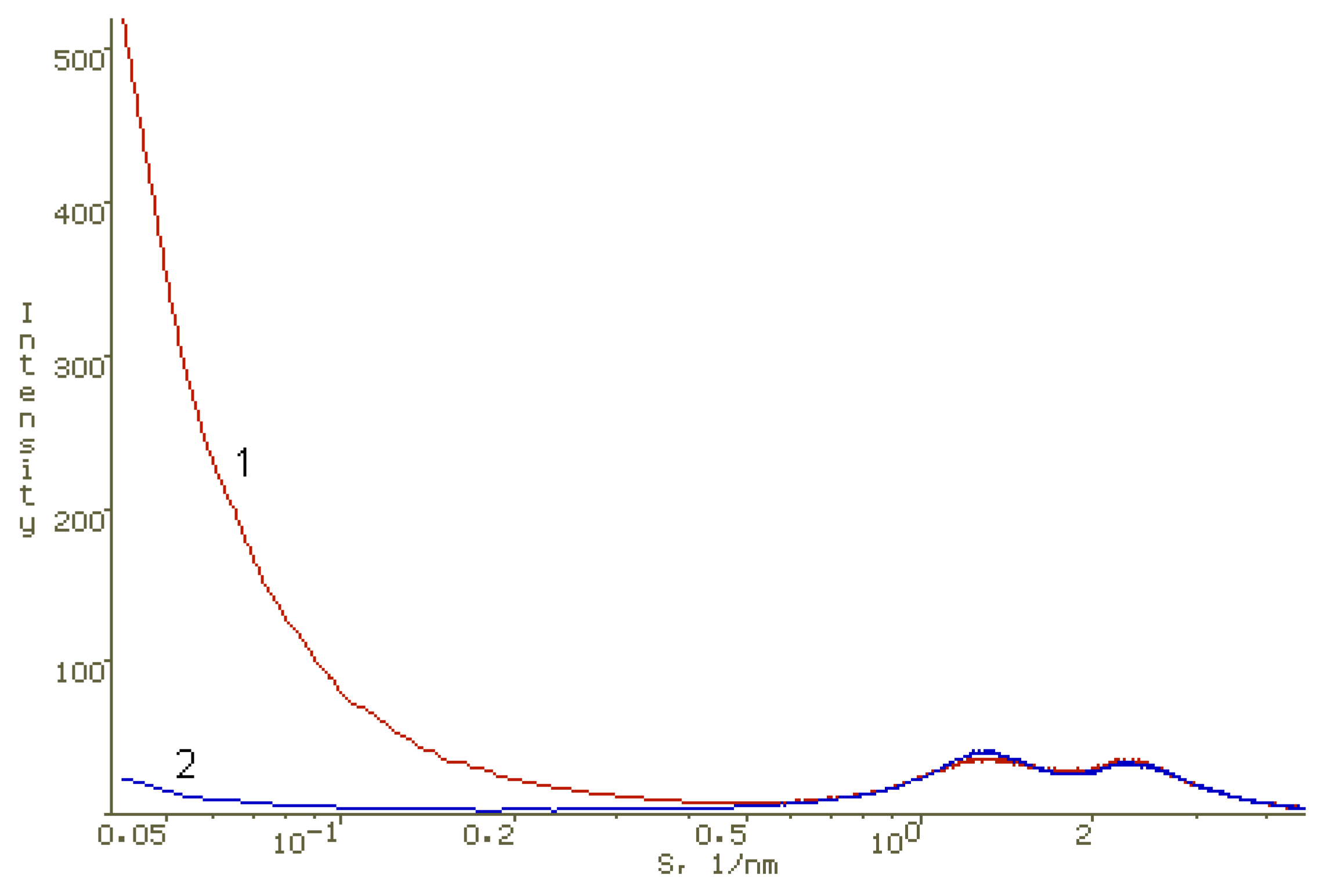
2.4. X-ray Diffraction
2.5. Atomic Force Microscopy
3. Results and Discussion
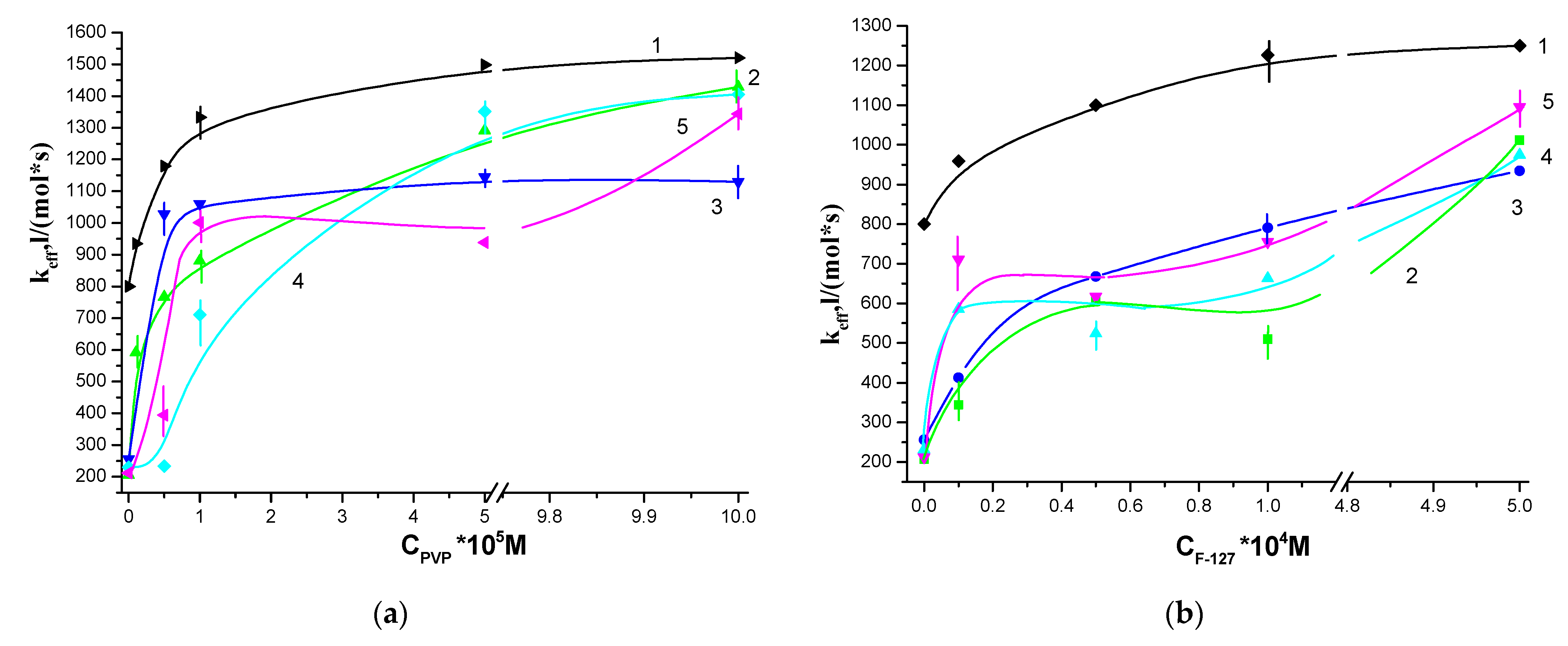
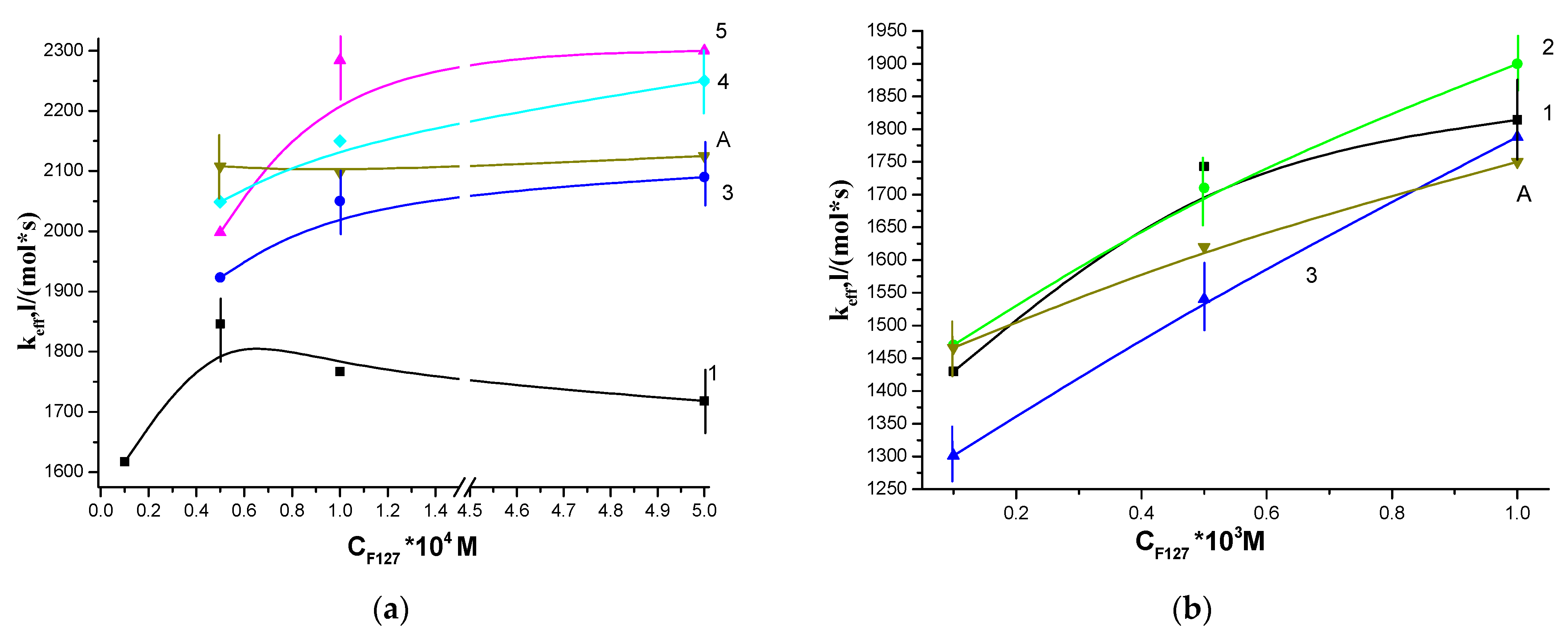
3.1. Photocatalytic Activity of Water-Soluble PS
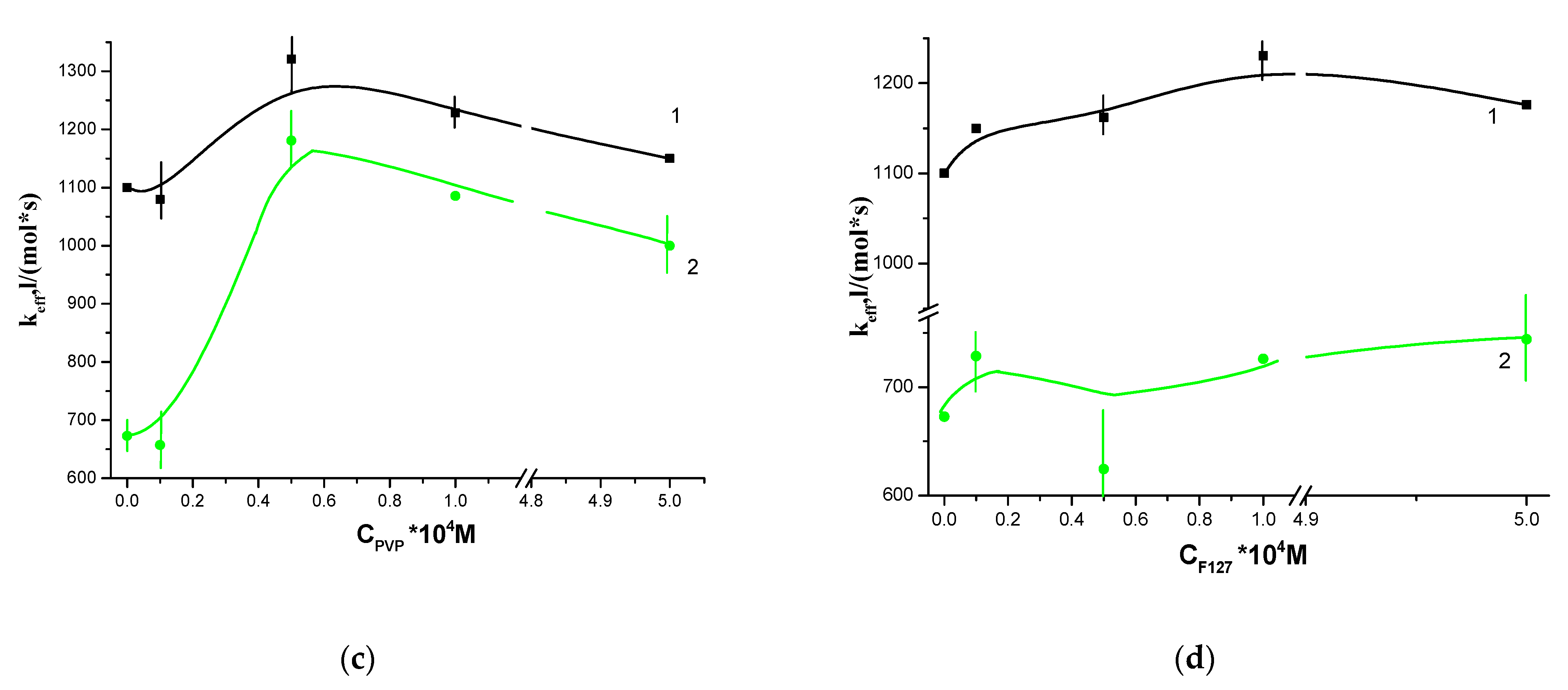
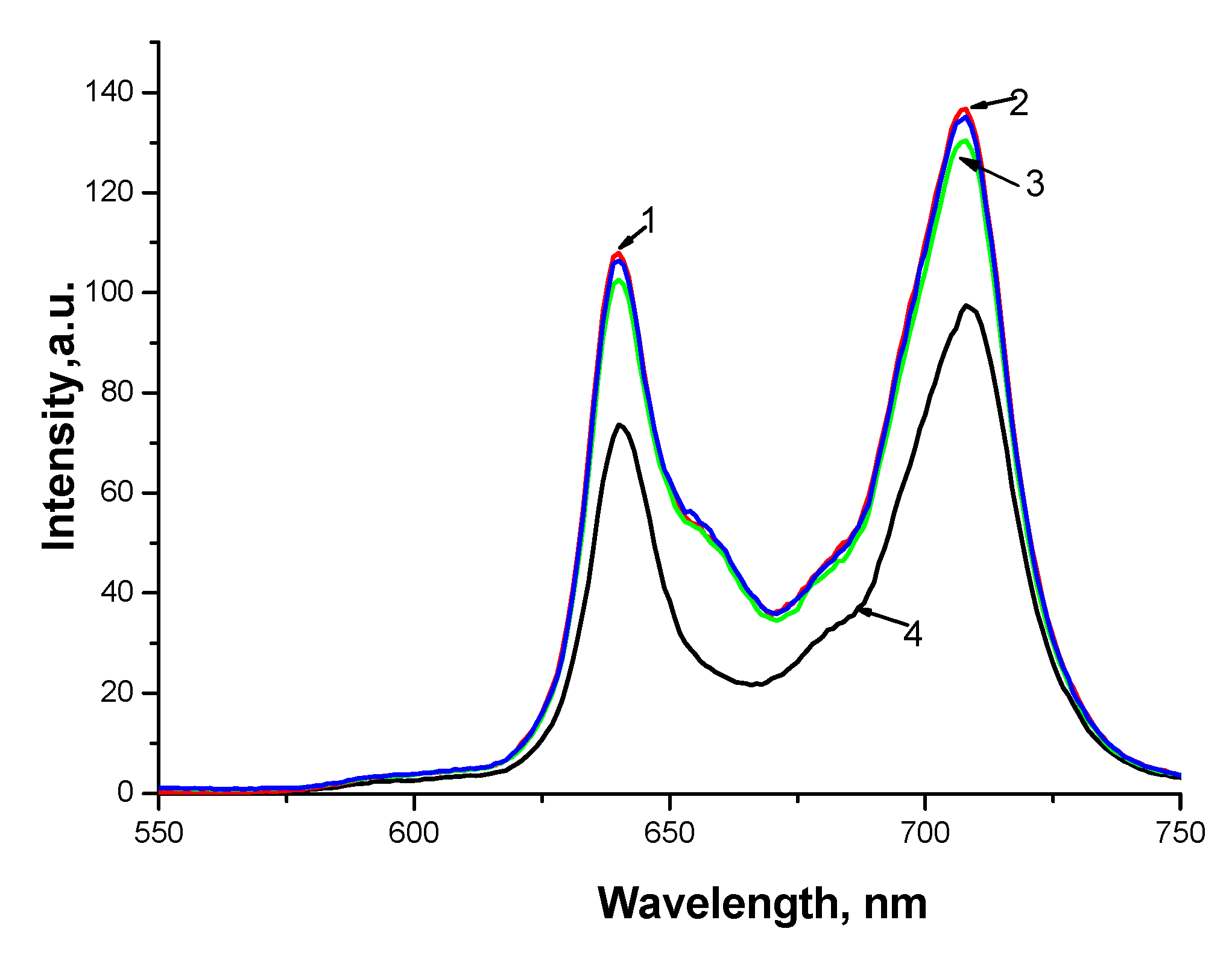
3.2. Photocatalytic Properties of Hydrophobic PS
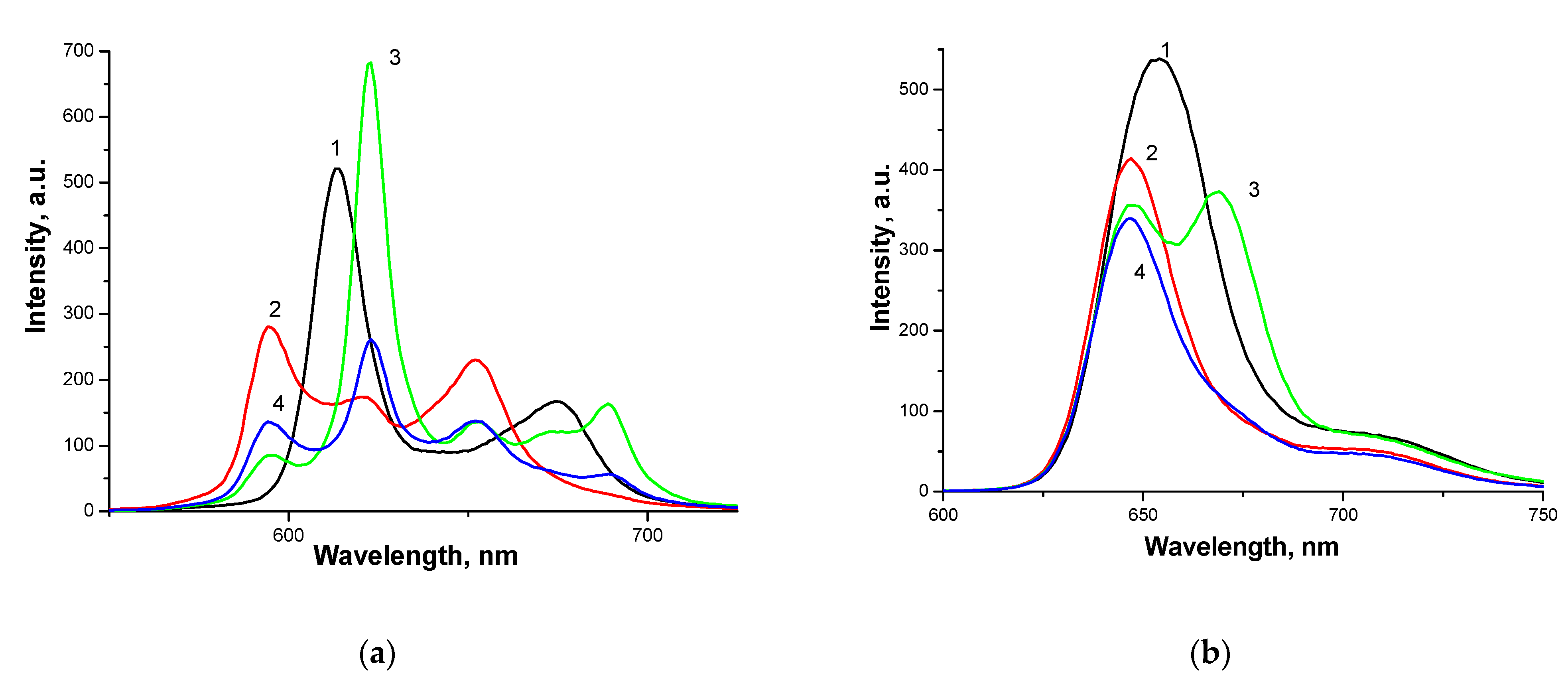
3.3. Methods for Studying Supramolecular Interactions in the PS-AP-CT100 System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akhlyustina, E.V.; Meerovich, G.A.; Tiganova, I.G.; A Makarova, E.; I Philipova, N.; Romanishkin, I.D.; Alekseeva, N.V.; A Lukyanets, E.; Romanova, Y.M.; Loschenov, V.B. New cationic photosensitizers: Photophysical properties and results of preliminary studies of antibacterial efficacy. J. Phys. Conf. Ser. 2019, 1189, 012033. [Google Scholar] [CrossRef]
- Swavey, S.; Tran, M. Porphyrin and Phthalocyanine Photosensitizers as PDT Agents: A New Modality for the Treatment of Melanoma. In Recent Advances in the Biology, Therapy and Management of Melanoma; IntechOpen: Cape Town, South Africa, 2013. [Google Scholar]
- Dai, T.; Huang, Y.-Y.; Hamblin, M.R. Photodynamic therapy for localized infections—State of the art. Photodiagn. Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef] [Green Version]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filonenko, E.V.; Sokolov, V.V. Photodynamic therapy in patients with early central lung cancer. Biomed. Photonics 2013, 2, 3–7. [Google Scholar]
- Istomin, Y.P.; Tzerkovsky, D.A.; Trukhachova, T.V.; Popova, N.A.; Dunets, L.N.; Shlyakhtin, S.V. Photodynamic therapy with local application of the photosensitizer Photolon. Experemental Stud. First Clin. Exp. Patients Skin Basal Cell Carcinoma 2014, 1, 94–107. [Google Scholar]
- Shubina, A.M.; Kaplan, M.A. Potential of photodynamic therapy with the use of photosensitizer photoditazin for the treatment of proriasis. Russ. J. Biother. 2005, 3, 76–79. [Google Scholar]
- Wagner, A.; Denzer, U.W.; Neureiter, D.; Kiesslich, T.; Puespoeck, A.; Rauws, E.A.J.; Emmanuel, K.; Degenhardt, N.; Frick, U.; Beuers, U.; et al. Temoporfin improves efficacy of photodynamic therapy in advanced biliary tract carcinoma: A multicenter prospective phase II study. Hepatology 2015, 62, 1456–1465. [Google Scholar] [CrossRef]
- Nitzan, Y.; Gutterman, M.; Malik, Z.; Ehrenberg, B. Inactivation of gram-negative bacteria by photosensitized porphyrins. Photochem. Photobiol. 1992, 55, 89–96. [Google Scholar] [CrossRef]
- Strakhovskaya, M.G.; Belenikina, N.S.; Nikitina, V.V.; Kovalenko, S.Y.; Kovalenko, I.B.; Averyanov, A.V.; Rubin, A.B.; Galochkina, T.V. Promising Photosensitizer for Antimicrobial Photodynamic Therapy. Clin. Pract. 2013, 4, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiMagno, S.G.; Biffinger, J.C.; Sun, H. Fluorinated Porphyrins and Corroles: Synthesis, Electrochemistry, and Applications. In Fluorine in Heterocyclic Chemistry; Metzler, J.B., Ed.; Springer: Cham, Switzerland, 2014; Volume 1, pp. 589–620. [Google Scholar]
- Logunova, E.V.; Nasedkin, A.N.; Rusanova, E.V. Features and advantages of the antimicrobic photodynamic therappy of chronic tonsillitis. Sci. World 2015, 2, 122–124. Available online: http://elibrary.ru/item.asp?id=25052776 (accessed on 25 February 2021).
- Jakus, J.; Farkas, O. Photosensitizers and antioxidants: A way to new drugs? Photochem. Photobiol. Sci. 2005, 4, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Tamara, F.R.; Lin, C.; Mi, F.-L.; Ho, Y.-C. Antibacterial Effects of Chitosan/Cationic Peptide Nanoparticles. Nanomaterials 2018, 8, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Foster, L.J.R.; Ho, S.; Hook, J.; Basuki, M.; Marçal, H. Chitosan as a Biomaterial: Influence of Degree of Deacetylation on Its Physiochemical, Material and Biological Properties. PLoS ONE 2015, 10, e0135153. [Google Scholar] [CrossRef] [Green Version]
- Zhientaev, T.M.; Melik-Nubarov, N.S.; Litmanovich, E.A.; Aksenova, N.A.; Glagolev, N.N.; Solov’Eva, A.B. The effect of Pluronics on the photocatalytic activity of water-soluble porphyrins. Polym. Sci. Ser. A 2009, 51, 502–511. [Google Scholar] [CrossRef]
- Solovieva, A.; Rudenko, T.; Shekhter, A.; Glagolev, N.; Spokoinyi, A.; Fayzullin, A.; Aksenova, N.; Shpichka, A.; Kardumyan, V.; Timashev, P. Broad-spectrum antibacterial and pro-regenerative effects of photoactivated Photodithazine-Pluronic F127-Chitosan polymer system: In vivo study. J. Photochem. Photobiol. B Biol. 2020, 210, 111954. [Google Scholar] [CrossRef]
- Glagolev, N.N.; Rogovina, S.Z.; Solov’Eva, A.B.; Aksenova, N.A.; Kotova, S.L. Photocatalytic activity of water-soluble tetrapyrrole compounds in the presence of amino-containing polymers. Russ. J. Phys. Chem. A 2006, 80, S72–S76. [Google Scholar] [CrossRef]
- Solovieva, A.B.; Kardumian, V.V.; Aksenova, N.A.; Belovolova, L.V.; Glushkov, M.V.; Bezrukov, E.A.; Timashev, P.S. Optimization of photosensitized tryptophan oxidation in the presence of dimegin-polyvinylpyrrolidone-chitosan systems. Sci. Rep. 2018, 8, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Aksenova, N.A.; Timofeeva, V.A.; Rogovina, S.Z.; Timashev, P.S.; Glagolev, N.N.; Solov’Eva, A.B. Photocatalytic properties and structure of chitosan-based porphyrin-containing systems. Polym. Sci. Ser. B 2010, 52, 67–72. [Google Scholar] [CrossRef]
- Solov’eva, A.B.; Savko, M.A.; Glagolev, N.N.; Aksenova, N.A.; Timashev, P.S.; Bragina, N.A.; Zhdanova, K.A.; Mironov, A.F. Photogeneration of Singlet Oxygen by Tetra(p-Hydroxyphenyl)porphyrins Modified with Oligo- and Polyalkylene Oxides. Russ. J. Phys. Chem. A 2018, 92, 1621–1626. [Google Scholar] [CrossRef]
- Krivandin, A.V.; Solov’Eva, A.B.; Glagolev, N.N.; Shatalova, O.V.; Kotova, S.L. Structure alterations of perfluorinated sulfocationic membranes under the action of ethylene glycol (SAXS and WAXS studies). Polymer 2003, 44, 5789–5796. [Google Scholar] [CrossRef]
- Shatalova, O.V.; Aksenova, N.A.; Solovieva, A.B.; Krivandin, A.V.; Rogovina, S.Z.; Sidohin, F.A. Features of crystallization of chitosan with different molecular mass and its mixtures with pluronic F-127 according to atomic force microscopy and X-ray diffraction data. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2011, 5, 454–459. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Zhang, X.; Liu, B.; Zhao, H.; Chen, D. Directly determining the molecular weight of chitosan with atomic force microscopy. Front. Nanosci. Nanotechnol. 2016, 2, 123–127. [Google Scholar] [CrossRef]
- Schatz, C.; Viton, C.; Delair, T.; Pichot, C.; Domard, A. Typical Physicochemical Behaviors of Chitosan in Aqueous Solution. Biomacromolecules 2003, 4, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Tsaih, M.L.; Chen, R.H. Effect of molecular weight and urea on the conformation of chitosan molecules in dilute solutions. Int. J. Biol. Macromol. 1997, 20, 233–240. [Google Scholar] [CrossRef]
- Chen, R.H.; Tsaih, M.L. Effect of temperature on the intrinsic viscosity and conformation of chitosans in dilute HCl solution. Int. J. Biol. Macromol. 1998, 23, 135–141. [Google Scholar] [CrossRef]
- Parkhats, M.V.; Galievsky, V.A.; Stashevsky, A.S.; Trukhacheva, T.V.; Dzhagarov, B.M. Dynamics and efficiency of the photosensitized singlet oxygen formation by chlorin e 6: The effects of the solution pH and polyvinylpyrrolidone. Opt. Spectrosc. 2009, 107, 974. [Google Scholar] [CrossRef]
- Kardumyan, V.V.; Aksenova, N.A.; Glagolev, N.N.; Timashev, P.S.; Solovieva, A.B. Influence of acetic acid on the photocatalytic activity of photosensitiser–amphiphilic polymer complexes in the oxidation reaction of tryptophan. J. Chem. Phys. 2020, 152, 194901. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Guo, C.; Chen, S.; Ma, J.; Wang, J.; Liang, X.; Zheng, L.; Liu, H. Effect of Acid on the Aggregation of Poly(ethylene oxide)−Poly(propylene oxide)−Poly(ethylene oxide) Block Copolymers. J. Phys. Chem. B 2006, 110, 23068–23074. [Google Scholar] [CrossRef] [PubMed]

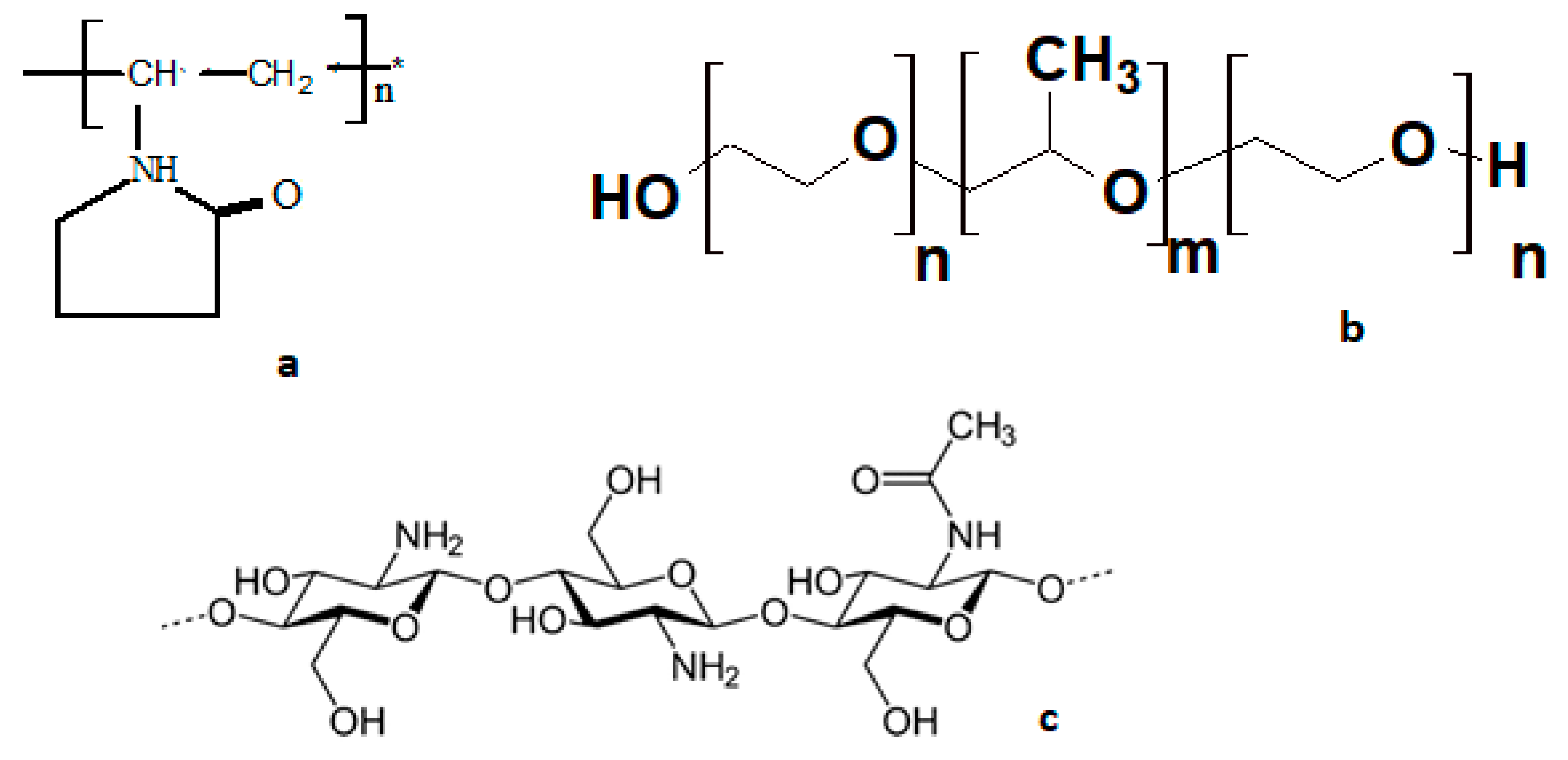

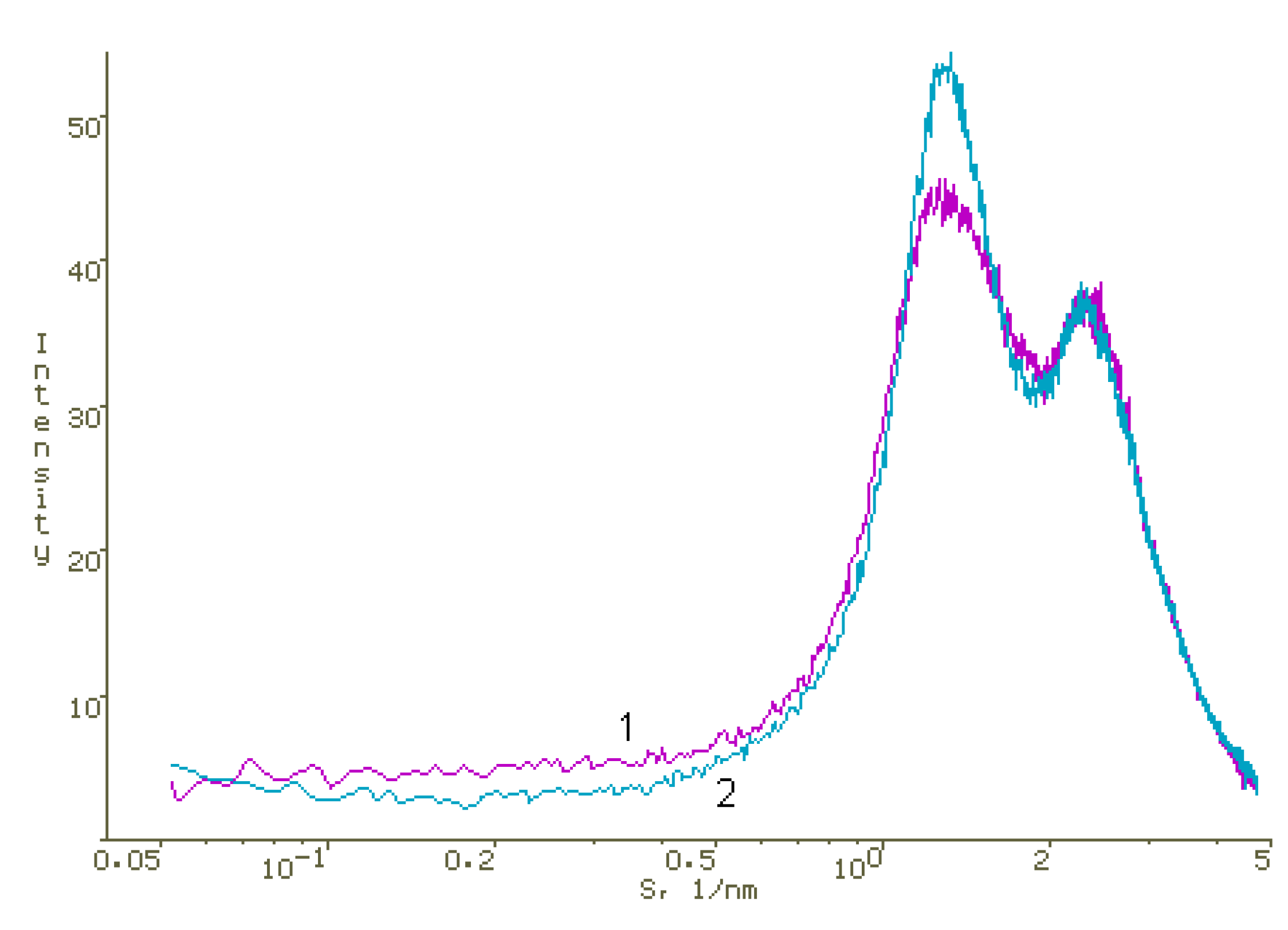
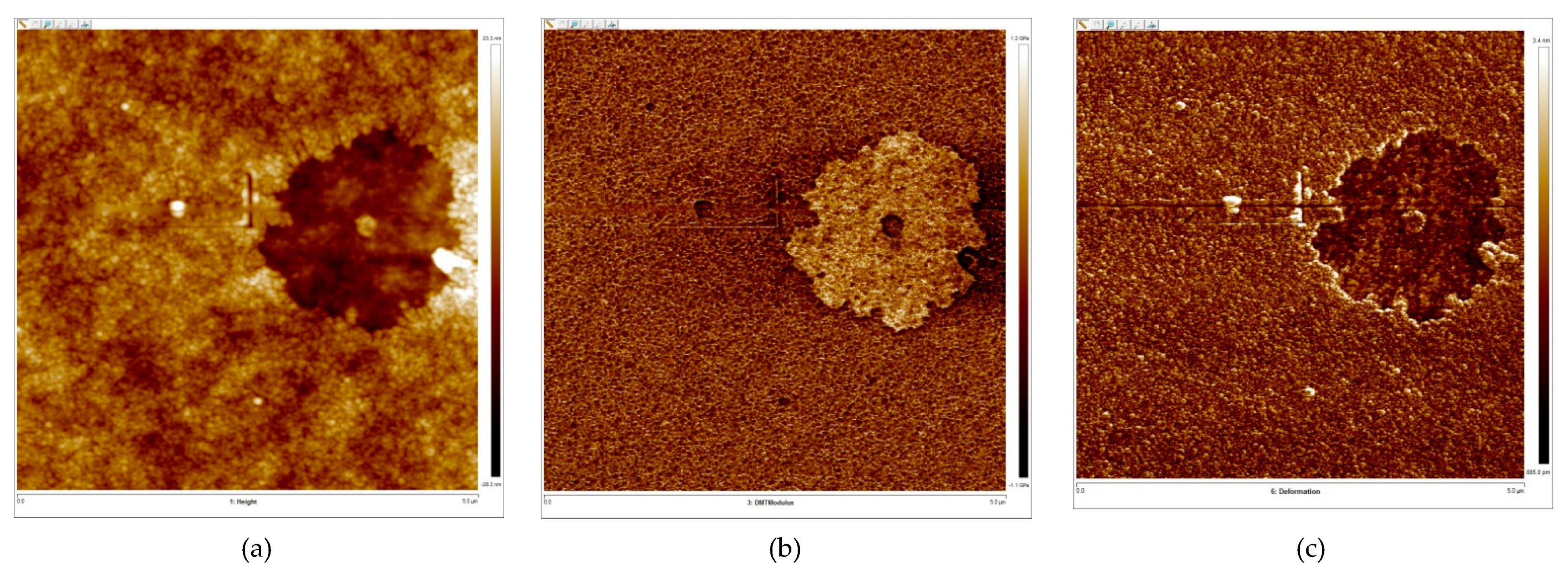
| № | Solution Composition | d, nm | ξ-Potential, mV | ||
|---|---|---|---|---|---|
| Peak1 | Peak2 | Peak3 | |||
| 1 | F127 (2 mass%) | 6.9 | 56 | ||
| 2 | F127 (1 mass%) | 7.2 | 62 | −8 | |
| 3 | F127 (1 mass%) + CH3COOH (1 vol.%) | 7.6 | 53 | 396 | +5 |
| 4 | PVP (1 mass%) | 9 | 165 | ||
| 5 | CT100 (0.05 mass%) | 89 | 960 | +53 | |
| 6 | CT100 (0.2 mass%) | 112 | 790 | ||
| 7 | CT100 (0.2 mass%)-F127 (2 mass%) | 6.8 | 50 | 1250 | |
| 8 | CT100 (0.2 mass%)-PVP (1 mass%) | 10.4 | 154 | 1320 | |
| 9 | TPPF20-F127(1 mass%) | 6.6 | 46 | 490 | +4 |
| 10 | TPPF20-F127(1 mass%)-CT100 (0.05 mass%) | 8 | 50 | 405 | +36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kardumyan, V.V.; Aksenova, N.A.; Timofeeva, V.A.; Krivandin, A.V.; Shatalova, O.V.; Dubovik, A.S.; Plashchina, I.G.; Timashev, P.S.; Solovieva, A.B. Effect of Chitosan on the Activity of Water-Soluble and Hydrophobic Porphyrin Photosensitizers Solubilized by Amphiphilic Polymers. Polymers 2021, 13, 1007. https://doi.org/10.3390/polym13071007
Kardumyan VV, Aksenova NA, Timofeeva VA, Krivandin AV, Shatalova OV, Dubovik AS, Plashchina IG, Timashev PS, Solovieva AB. Effect of Chitosan on the Activity of Water-Soluble and Hydrophobic Porphyrin Photosensitizers Solubilized by Amphiphilic Polymers. Polymers. 2021; 13(7):1007. https://doi.org/10.3390/polym13071007
Chicago/Turabian StyleKardumyan, Valeriya V., Nadejda A. Aksenova, Victoria A. Timofeeva, Alexey V. Krivandin, Olga V. Shatalova, Alexander S. Dubovik, Irina G. Plashchina, Peter S. Timashev, and Anna B. Solovieva. 2021. "Effect of Chitosan on the Activity of Water-Soluble and Hydrophobic Porphyrin Photosensitizers Solubilized by Amphiphilic Polymers" Polymers 13, no. 7: 1007. https://doi.org/10.3390/polym13071007







