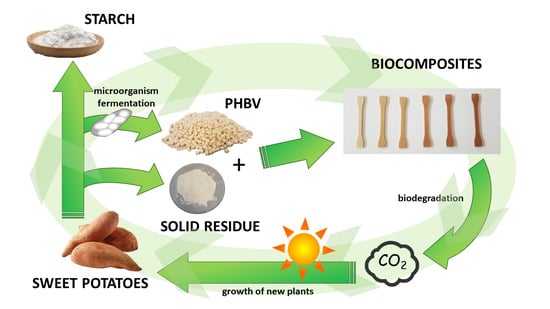
Integrated Efforts for the Valorization of Sweet Potato By-Products within a Circular Economy Concept: Biocomposites for Packaging Applications Close the Loop
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Sweet Potato Residues
2.3. Composition of Sweet Potato Residues
2.4. Composite Preparation
2.5. Filler and Composite Characterizations
3. Results and Discussion
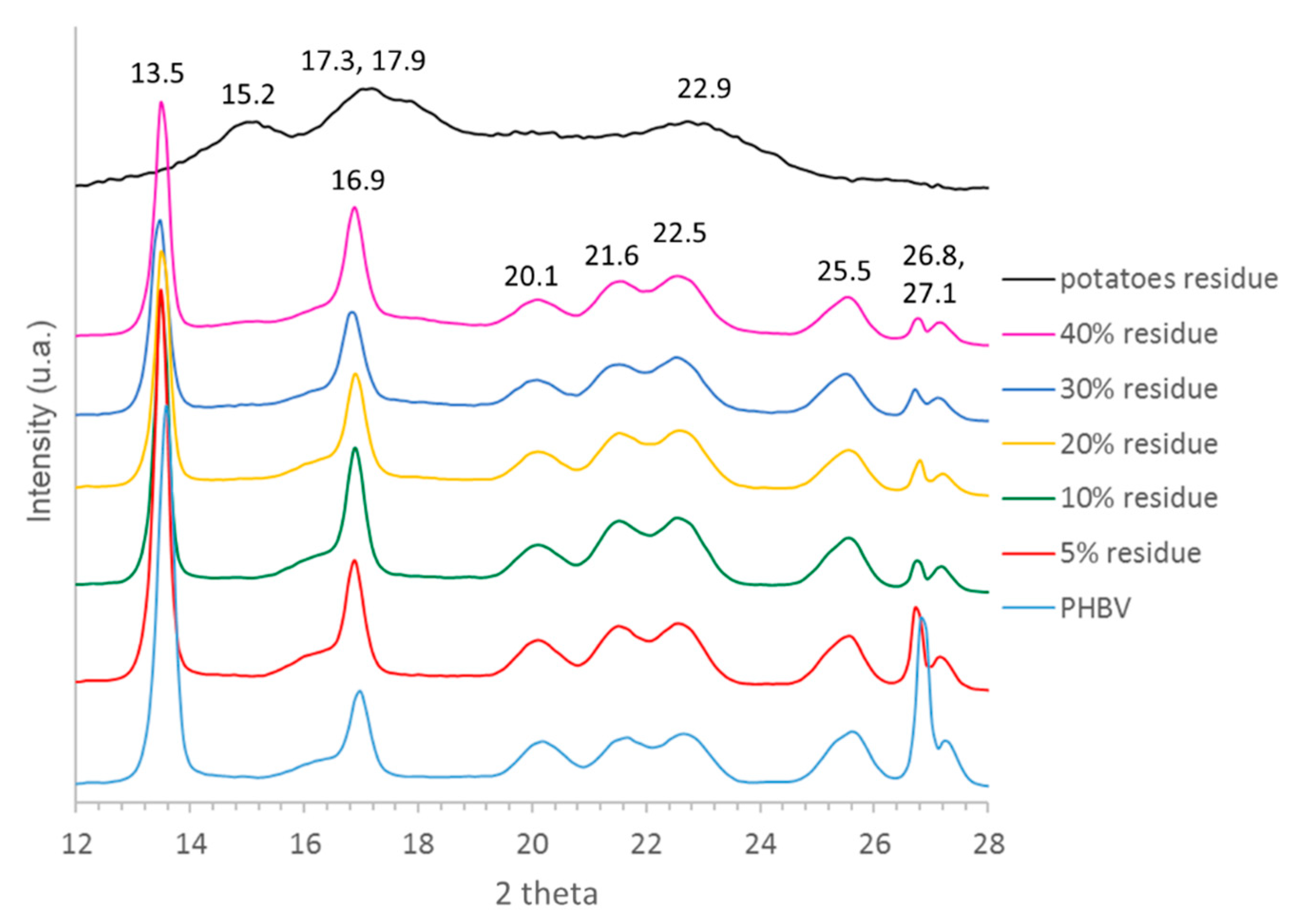
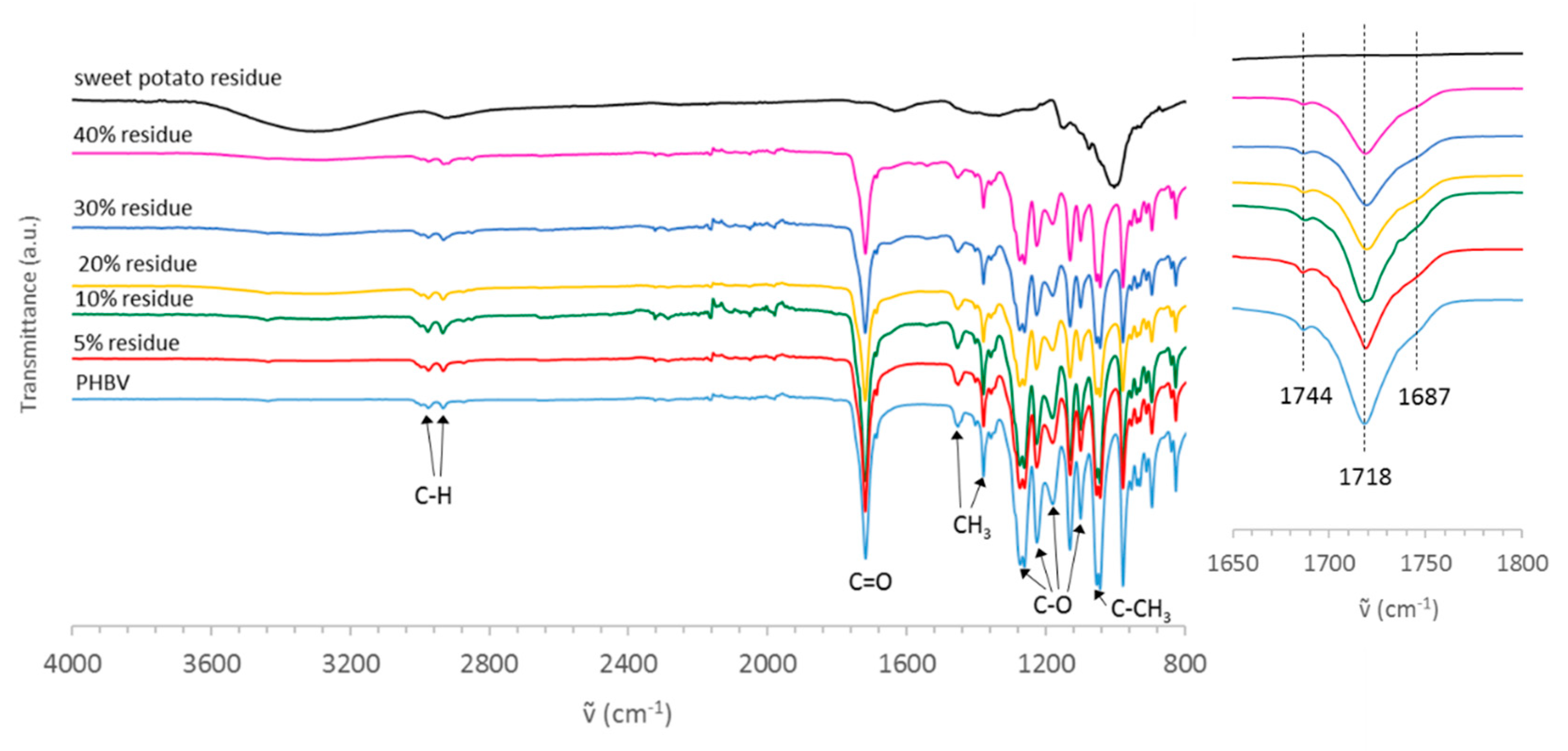
3.1. Characterization of Sweet Potato Processing Residues
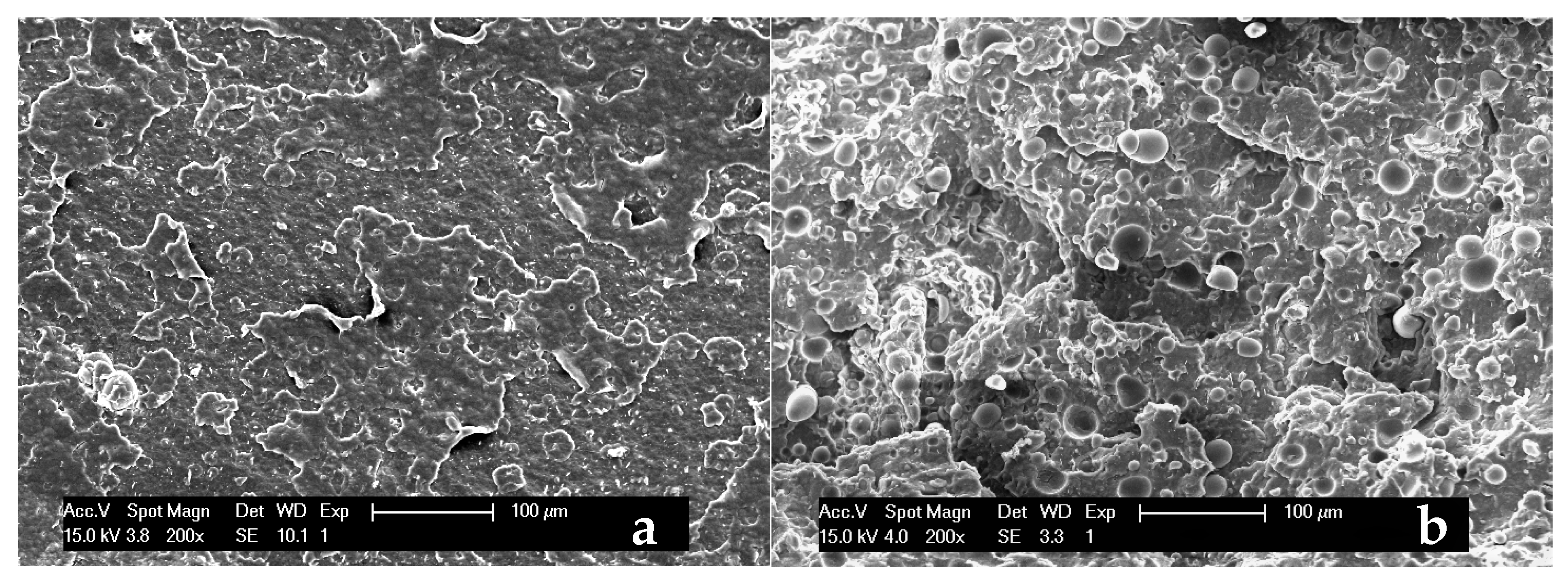
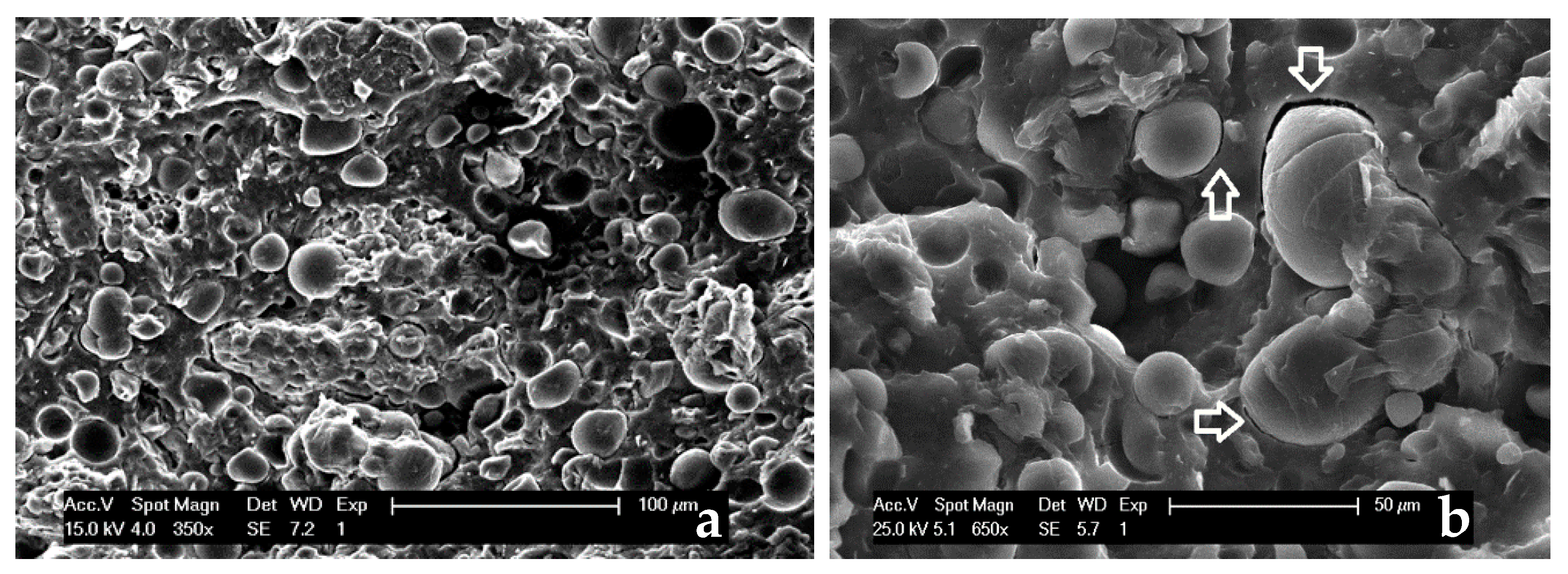
3.2. Characterization of PHBV-Based Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Circular Economy—UK, USA, Europe, Asia & South America—The Ellen MacArthur Foundation. Available online: www.ellenmacarthurfoundation.org (accessed on 26 February 2021).
- United Nations Environment Programme (UNEP)—Converting Waste Agricultural Biomass into a Resource. United Nations Environment Programme Division of Technology, Industry and Economics International Environmental Technology Centre, Osaka/Shiga, Japan. 2009. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/7614/WasteAgriculturalBiomassEST_Compendium.pdf (accessed on 9 March 2021).
- Teigiserova, D.A.; Hamelin, L.; Thomsen, M. Review of high-value food waste and food residues biorefineries with focus on unavoidable wastes from processing. Resour. Conserv. Recy. 2019, 149, 413–426. [Google Scholar] [CrossRef]
- Fritsch, C.; Staebler, A.; Happel, A.; Marquez, M.A.C.; Aguilo-Aguayo, I.; Abadias, M.; Gallur, M.; Cigognini, I.M.; Montanari, A.; Lopez, M.J.; et al. Processing, Valorization and Application of Bio-Waste Derived Compounds from Potato, Tomato, Olive and Cereals: A Review. Sustainability 2017, 9, 1492. [Google Scholar] [CrossRef]
- Torres, M.D.; Dominguez, H. Valorisation of potato wastes. Int. J. Food Sci. Technol. 2020, 55, 2296–2304. [Google Scholar] [CrossRef]
- Tassoni, A.; Tedeschi, T.; Zurlini, C.; Cigognini, I.M.; Petrusan, J.I.; Rodriguez, O.; Neri, S.; Celli, A.; Sisti, L.; Cinelli, P.; et al. State-of-the-Art Production Chains for Peas, Beans and Chickpeas-Valorization of Agro-Industrial Residues and Applications of Derived Extracts. Molecules 2020, 25, 1383. [Google Scholar] [CrossRef]
- Totaro, G.; Sisti, L.; Vannini, M.; Marchese, P.; Tassoni, A.; Lenucci, M.S.; Lamborghini, M.; Kalia, S.; Celli, A. A new route of valorization of rice endosperm by-product: Production of polymeric biocomposites. Compos. B Eng. 2018, 139, 195–202. [Google Scholar] [CrossRef]
- Ricciardi, P.; Cillari, G.; Miino, M.C.; Collivignarelli, M.C. Valorization of agro-industry residues in the building and environmental sector: A review. Waste Manag. Res. 2020, 38, 487–513. [Google Scholar] [CrossRef]
- FAO STAT. Statistical Database. 2019. Available online: www.fao.org (accessed on 26 February 2021).
- Mei, X.; Mu, T.H.; Han, J.J. Composition and Physicochemical Properties of Dietary Fiber Extracted from Residues of 10 Varieties of Sweet Potato by a Sieving Method. J. Agric. Food Chem. 2010, 58, 7305–7310. [Google Scholar] [CrossRef]
- Yokoi, H.; Saitsu, A.; Uchida, H.; Hirose, J.; Hayashi, S.; Takasaki, Y. Microbial hydrogen production from sweet potato starch residue. J. Biosci. Bioeng. 2001, 91, 58–63. [Google Scholar] [CrossRef]
- Wang, Q.J.; Chen, S.W.; Zhang, J.B.; Sun, M.; Liu, Z.D.; Yu, Z.I. Co-producing lipopeptides and poly-gamma-glutamic acid by solid-state fermentation of Bacillus subtilis using soybean and sweet potato residues and its bliocontrol and fertilizer synergistic effects. Bioresour. Technol. 2008, 99, 3318–3323. [Google Scholar] [CrossRef]
- Lu, H.J.; Gui, Y.; Zheng, L.H.; Liu, X. Morphological, crystalline, thermal and physicochemical properties of cellulose nanocrystals obtained from sweet potato residue. Food Res. Int. 2013, 50, 121–128. [Google Scholar] [CrossRef]
- Arachchige, M.P.M.; Mu, T.H.; Ma, M.M. Effect of high hydrostatic pressure-assisted pectinase modification on the Pb2+ adsorption capacity of pectin isolated from sweet potato residue. Chemosphere 2021, 262, 10. [Google Scholar] [CrossRef]
- Ju, D.; Mu, T.; Sun, H. Sweet potato and potato residual flours as potential nutritional and healthy food material. J. Integr. Agric. 2017, 16, 2632–2645. [Google Scholar] [CrossRef]
- Qiao, H.Z.; Shao, H.M.; Zheng, X.J.; Liu, J.W.; Liu, J.Q.; Huang, J.; Zhang, C.Y.; Liu, Z.; Wang, J.R.; Guan, W.T. Modification of sweet potato (Ipomoea batatas Lam.) residues soluble dietary fiber following twin-screw extrusion. Food Chem. 2021, 335, 10. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, H.B.; Li, Y.; Tian, J.Q.; Qin, X.L.; Shabani, K.I.; Liao, C.; Liu, X. Characterization of Pickering emulsions stabilized by OSA-modified sweet potato residue cellulose: Effect of degree of substitute and concentration. Food Hydrocoll. 2020, 108, 13. [Google Scholar] [CrossRef]
- Haas, R.; Jin, B.; Zepf, F.T. Production of poly(3-hydroxybutyrate) from waste potato starch. Biosci. Biotechnol. Biochem. 2008, 72, 253–256. [Google Scholar] [CrossRef]
- Rusendi, D.; Sheppard, J.D. Hydrolysis of potato processing waste for the production of poly-beta-hydroxybutyrate. Bioresour. Technol. 1995, 54, 191–196. [Google Scholar] [CrossRef]
- Kumar, P.; Ray, S.; Kalia, V.C. Production of co-polymers of polyhydroxyalkanoates by regulating the hydrolysis of biowastes. Bioresour. Technol. 2016, 200, 413–419. [Google Scholar] [CrossRef]
- Sanchez-Safont, E.L.; Gonzalez-Ausejo, J.; Gamez-Perez, J.; Lagaron, J.M.; Cabedo, L. Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate)/Purified Cellulose Fiber Composites by Melt Blending: Characterization and Degradation in Composting Conditions. J. Renew. Mater. 2016, 4, 123–132. [Google Scholar] [CrossRef]
- Keskin, G.; Kızıl, G.; Bechelany, M.; Pochat-Bohatier, C.; Öner, M. Potential of polyhydroxyalkanoate (PHA) polymers family as substitutes of petroleum based polymers for packaging applications and solutions brought by their composites to form barrier materials. Pure Appl. Chem. 2017, 89, 1841–1848. [Google Scholar] [CrossRef]
- Hassaini, L.; Kaci, M.; Touati, N.; Pillin, I.; Kervoelen, A.; Bruzaud, S. Valorization of olive husk flour as a filler for biocomposites based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate): Effects of silane treatment. Polym. Test. 2017, 59, 430–440. [Google Scholar] [CrossRef]
- Malmir, S.; Montero, B.; Rico, M.; Barral, L.; Bouza, R. Morphology, thermal and barrier properties of biodegradable films of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) containing cellulose nanocrystals. Compos. Part A 2017, 93, 41–48. [Google Scholar] [CrossRef]
- Kovalcik, A.; Machovsky, M.; Kozakova, Z.; Koller, M. Designing packaging materials with viscoelastic and gas barrier properties by optimized processing of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with lignin. React. Funct. Polym. 2015, 94, 25–34. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in bio-based food packaging applications. Ind. Crops Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef]
- Rivera-Briso, A.L.; Serrano-Aroca, A. Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate): Enhancement Strategies for Advanced Applications. Polymers 2018, 10, 732. [Google Scholar] [CrossRef] [PubMed]
- Bledzki, A.K.; Jaszkiewicz, A. Mechanical performance of biocomposites based on PLA and PHBV reinforced with natural fibres—A comparative study to PP. Compos. Sci. Technol. 2010, 70, 1687–1696. [Google Scholar] [CrossRef]
- Angelini, S.; Cerruti, P.; Immirzi, B.; Santagata, G.; Scarinzi, G.; Malinconico, M. From biowaste to bioresource: Effect of a lignocellulosic filler on the properties of poly(3-hydroxybutyrate). Int. J. Biol. Macromol. 2014, 71, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Berthet, M.A.; Angellier-Coussy, H.; Chea, V.; Guillard, V.; Gastaldi, E.; Gontard, N. Sustainable food packaging: Valorising wheat straw fibres for tuning PHBV-based composites properties. Compos. Part A Appl. Sci. Manuf. 2015, 72, 139–147. [Google Scholar] [CrossRef]
- Kittikorn, T.; Malakul, R.; Stromberg, E.; Ek, M.; Karlsson, S. Enhancement of mechanical, thermal and antibacterial properties of sisal/polyhydroxybutyrate-co-valerate biodegradable composite. J. Met. Mater. Miner. 2018, 28, 52–61. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Hilliou, L.; Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Madalena, D.; Cabedo, L.; Covas, J.A.; Vicente, A.A.; Lagaron, J.M. Melt processability, characterization, and antibacterial activity of compression-molded green composite sheets made of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) reinforced with coconut fibers impregnated with oregano essential oil. Food Packag. Shelf Life 2018, 17, 39–49. [Google Scholar] [CrossRef]
- Singh, S.; Mohanty, A.K.; Sugie, T.; Takai, Y.; Hamada, H. Renewable resource based biocomposites from natural fiber and polyhydroxybutyrate-co-valerate (PHBV) bioplastic. Compos. Part A Appl. Sci. Manuf. 2008, 39, 875–886. [Google Scholar] [CrossRef]
- Righetti, M.C.; Cinelli, P.; Mallegni, N.; Stabler, A.; Lazzeri, A. Thermal and Mechanical Properties of Biocomposites Made of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Potato Pulp Powder. Polymers 2019, 11, 308. [Google Scholar] [CrossRef]
- Wei, L.Q.; Liang, S.B.; McDonald, A.G. Thermophysical properties and biodegradation behavior of green composites made from polyhydroxybutyrate and potato peel waste fermentation residue. Ind. Crops Prod. 2015, 69, 91–103. [Google Scholar] [CrossRef]
- Ferri, M.; Vannini, M.; Ehrnell, M.; Eliasson, L.; Xanthakis, E.; Monari, S.; Sisti, L.; Marchese, P.; Celli, A.; Tassoni, A. From winery waste to bioactive compounds and new polymeric biocomposites: A contribution to the circular economy concept. J. Adv. Res. 2020, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Monari, S.; Ferri, M.; Vannini, M.; Sisti, L.; Marchese, P.; Ehrnell, M.; Xanthakis, E.; Celli, A.; Tassoni, A. Cascade strategies for the full valorisation of Garganega white grape pomace towards bioactive extracts and bio-based materials. PLoS ONE 2020, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Lammi, S.; Le Moigne, N.; Djenane, D.; Gontard, N.; Angellier-Coussy, H. Dry fractionation of olive pomace for the development of food packaging biocomposites. Ind. Crops Prod. 2018, 120, 250–261. [Google Scholar] [CrossRef]
- David, G.; Vannini, M.; Sisti, L.; Marchese, P.; Celli, A.; Gontard, N.; Angellier-Coussy, H. Eco-Conversion of Two Winery Lignocellulosic Wastes into Fillers for Biocomposites: Vine Shoots and Wine Pomaces. Polymers 2020, 12, 1530. [Google Scholar] [CrossRef] [PubMed]
- Totaro, G.; Sisti, L.; Fiorini, M.; Lancellotti, I.; Andreola, F.N.; Saccani, A. Formulation of Green Particulate Composites from PLA and PBS Matrix and Wastes Deriving from the Coffee Production. J. Polym. Environ. 2019, 27, 1488–1496. [Google Scholar] [CrossRef]
- Saccani, A.; Sisti, L.; Manzi, S.; Fiorini, M. PLA composites formulated recycling residuals of the winery industry. Polym. Compos. 2019, 40, 1378–1383. [Google Scholar] [CrossRef]
- No Agro-Waste. Horizon 2020 European Project. Available online: https://noaw2020.eu (accessed on 26 February 2021).
- Gontard, N.; Sonesson, U.; Birkved, M.; Majone, M.; Bolzonella, D.; Celli, A.; Angellier-Coussy, H.; Jang, G.W.; Verniquet, A.; Broeze, J.; et al. A research challenge vision regarding management of agricultural waste in a circular bio-based economy. Crit. Rev. Env. Sci. Tec. 2018, 48, 614–654. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Mu, T.H.; Sun, H.N.; Chen, J.W.; Zhang, M. Comparative study of potato protein concentrates extracted using ammonium sulfate and isoelectric precipitation. Int. J. Food Prop. 2017, 20, 2113–2127. [Google Scholar] [CrossRef]
- Elahi, R.; Mu, T.H. High hydrostatic pressure (HHP)-induced structural modification of patatin and its antioxidant activities. Molecules 2017, 22, 438. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Mu, T.H.; Zhang, M. Optimisation of acid extraction of pectin from sweet potato residues by response surface methodology and its antiproliferation effect on cancer cells. Int. J. Food Sci. Technol. 2013, 48, 778–785. [Google Scholar] [CrossRef]
- Reis, K.C.; Pereira, J.; Smith, A.C.; Carvalho, C.W.P.; Wellner, N.; Yakimets, I. Characterization of polyhydroxybutyrate-hydroxyvalerate (PHB-HV)/maize starch blend films. J. Food Eng. 2008, 89, 361–369. [Google Scholar] [CrossRef]
- Polman, E.M.N.; Gruter, G.J.M.; Parsons, J.R.; Tietema, A. Comparison of the aerobic biodegradation of biopolymers and the corresponding bioplastics: A review. Sci. Total Environ. 2021, 753, 13. [Google Scholar] [CrossRef]
- Florez, J.P.; Fazeli, M.; Simao, R.A. Preparation and characterization of thermoplastic starch composite reinforced by plasma-treated poly (hydroxybutyrate) PHB. Int. J. Biol. Macromol. 2019, 123, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.M.; Mu, T.H.; Zhang, M.; Abegunde, O.K. Composition, structure, and physicochemical properties of sweet potato starches isolated by sour liquid processing and centrifugation. Starke 2013, 65, 162–171. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Claye, S.S.; Idouraine, A.; Weber, C.W. Extraction and fractionation of insoluble fiber from five fiber sources. Food Chem. 1996, 57, 305–310. [Google Scholar] [CrossRef]
- Guo, J.; Liu, L.; Lian, X.; Li, L.; Wu, H. The properties of different cultivars of Jinhai sweet potato starchesin China. Int. J. Biol. Macromol. 2014, 67, 1–6. [Google Scholar] [CrossRef]
- Gamarano, D.D.; Pereira, L.M.; da Silva, M.C.; Mottin, A.C.; Ayres, E. Crystal structure transformations in extruded starch plasticized with glycerol and urea. Polym. Bull. 2020, 77, 4971–4992. [Google Scholar] [CrossRef]
- Gunaratne, A.; Hoover, R. Effect of heat-moisture treatment on the structure and physicochemical properties of tuber and root starches. Carbohydr. Polym. 2002, 49, 425–437. [Google Scholar] [CrossRef]
- Zhou, W.Z.; Yang, J.; Hong, Y.; Liu, G.L.; Zheng, J.L.; Gu, Z.B.; Zhang, P. Impact of amylose content on starch physicochemical properties in transgenic sweet potato. Carbohydr. Polym. 2015, 122, 417–427. [Google Scholar] [CrossRef]
- Gallant, D.J.; Bouchet, B.; Baldwin, P.M. Microscopy of starch: Evidence of a new level of granule organization. Carbohydr. Polym. 1997, 32, 177–191. [Google Scholar] [CrossRef]
- Aggarwal, P.; Dollimore, D.; Heon, K. Comparative thermal analysis study of two biopolymers, starch and cellulose. J. Therm. Anal. Calorim. 1997, 50, 7–17. [Google Scholar] [CrossRef]
- Wang, W.J.; Ma, X.B.; Jiang, P.; Hu, L.L.; Zhi, Z.J.; Chen, J.L.; Ding, T.; Ye, X.Q.; Liu, D.H. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Signori, F.; Pelagaggi, M.; Bronco, S.; Righetti, M.C. Amorphous/crystal and polymer/filler interphases in biocomposites from poly(butylene succinate). Thermochim. Acta 2012, 543, 74–81. [Google Scholar] [CrossRef]
- Gigante, V.; Cinelli, P.; Righetti, M.C.; Sandroni, M.; Polacco, G.; Seggiani, M.; Lazzeri, A. On the Use of Biobased Waxes to Tune Thermal and Mechanical Properties of Polyhydroxyalkanoates-Bran Biocomposites. Polymers 2020, 12, 2615. [Google Scholar] [CrossRef] [PubMed]
- Righetti, M.C.; Tornbari, E.; Di Lorenzo, M.L. The Role of the Crystallization Temperature on the Nanophase Structure Evolution of Poly (R)-3-hydroxybutyrate. J. Phys. Chem. B 2013, 117, 12303–12311. [Google Scholar] [CrossRef]
- Zhang, M.; Thomas, N.L. Preparation and Properties of Polyhydroxybutyrate Blended with Different Types of Starch. J. Appl. Polym. Sci. 2010, 116, 688–694. [Google Scholar] [CrossRef]
- Ahmad, A.N.; Lim, S.A.; Navaranjan, N.; Hsu, Y.I.; Uyama, H. Green sago starch nanoparticles as reinforcing material for green composites. Polymer 2020, 202, 8. [Google Scholar] [CrossRef]
- Fatrozi, S.; Purwanti, L.; Sari, S.K.; Ariesta, M.N.; Kusumaningsih, T.; Marliyana, S.D. Starch-based biofoams reinforced with microcrystalline cellulose from banana stem: Hydrophobicity and Biodegradability. IOP Conf. Ser. Mater. Sci. Eng. 2020, 858, 012037. [Google Scholar] [CrossRef]
- Li, M.; Tian, X.; Jin, R.F.; Li, D. Preparation and characterization of nanocomposite films containing starch and cellulose nanofibers. Ind. Crops Prod. 2018, 123, 654–660. [Google Scholar] [CrossRef]
- Malmir, S.; Montero, B.; Rico, M.; Barral, L.; Bouza, R.; Farrag, Y. Effects of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) microparticles on morphological, mechanical, thermal, and barrier properties in thermoplastic potato starch films. Carbohydr. Polym. 2018, 194, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesan, S.; Govindaraju, I.; Mazumder, N. An Insight into the Ultrastructural and Physiochemical Characterization of Potato Starch: A Review. Am. J. Potato Res. 2020, 97, 464–476. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Reactive compatibilization and performance evaluation of miscanthus biofiber reinforced poly(hydroxybutyrate-co-hydroxyvalerate) biocomposites. J. Appl. Polym. Sci. 2017, 134, 10. [Google Scholar] [CrossRef]
- Vandi, L.J.; Chan, C.M.; Werker, A.; Richardson, D.; Laycock, B.; Pratt, S. Extrusion of wood fibre reinforced poly(hydroxybutyrate-co-hydroxyvalerate) (PHBV) biocomposites: Statistical analysis of the effect of processing conditions on mechanical performance. Polym. Degrad. Stab. 2019, 159, 1–14. [Google Scholar] [CrossRef]

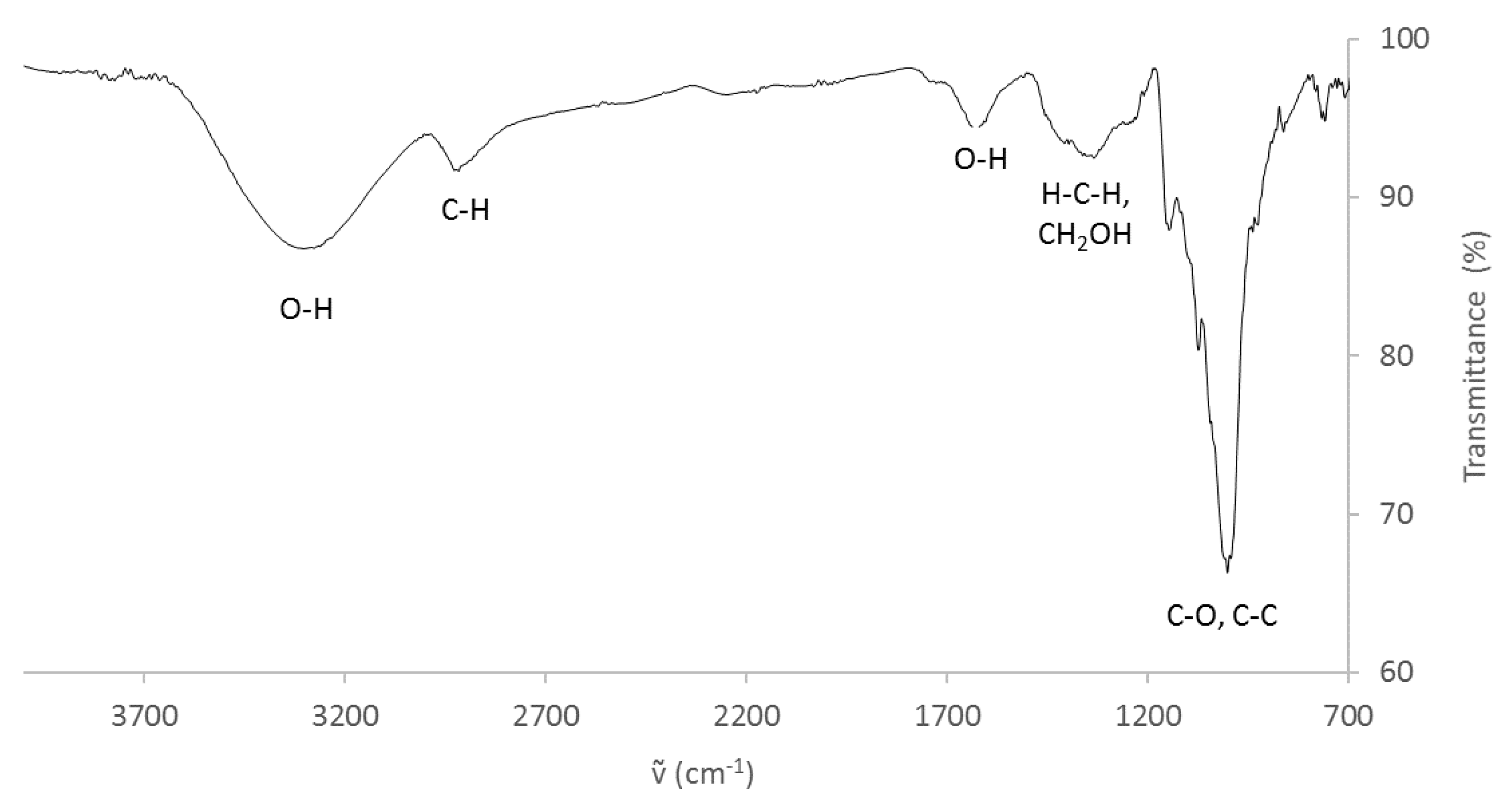
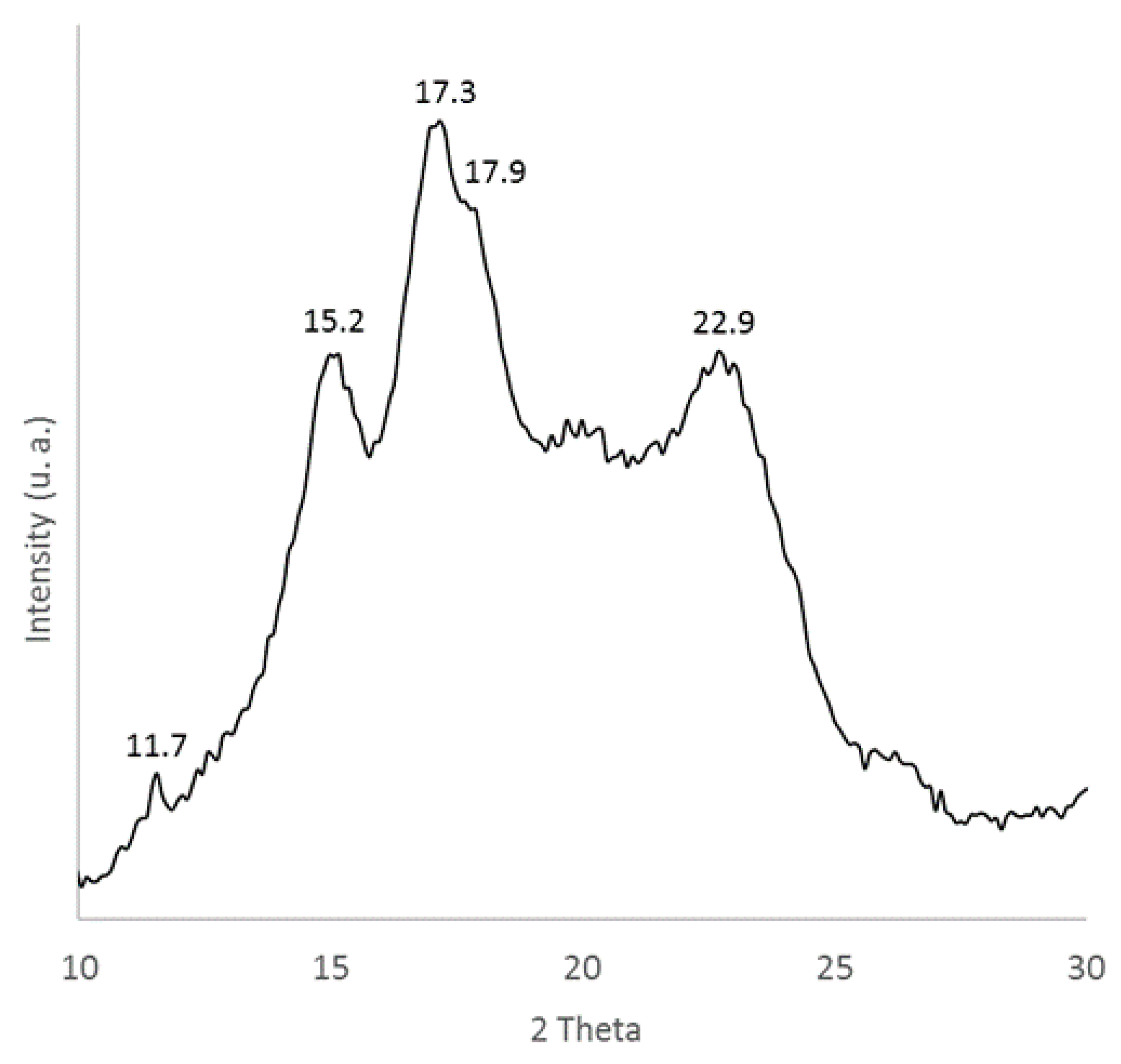
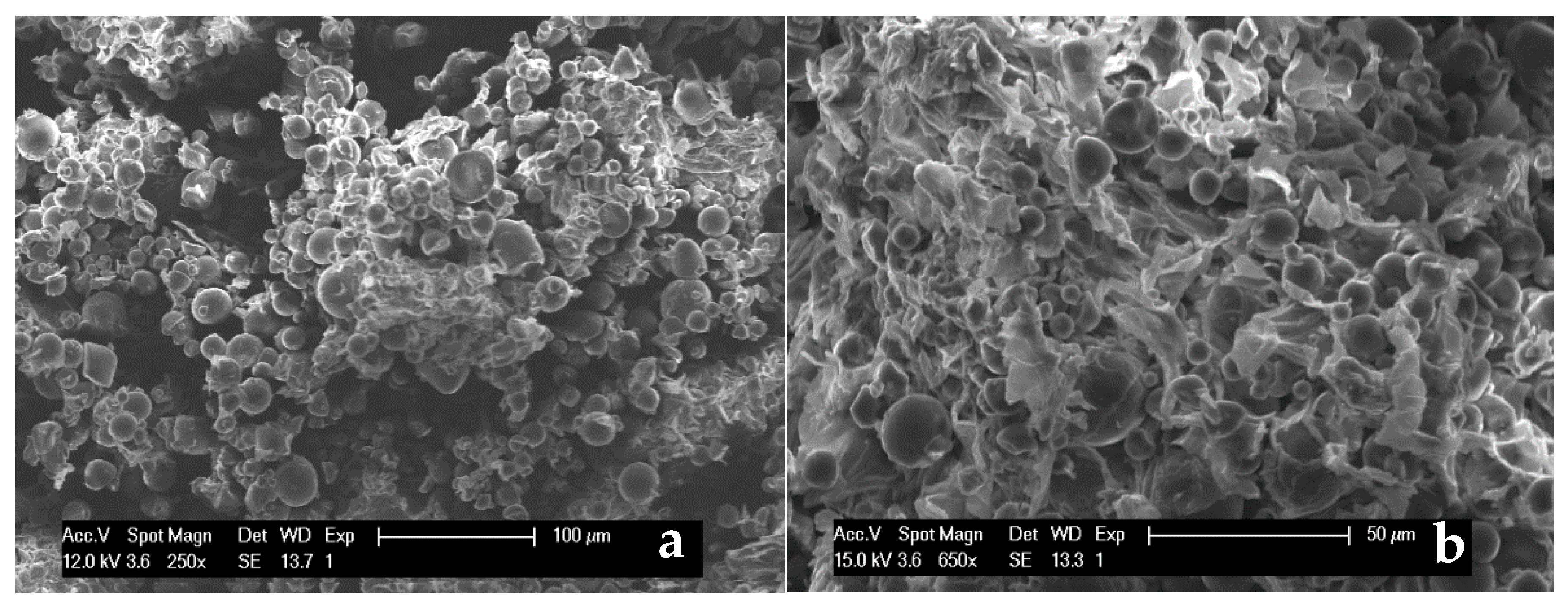
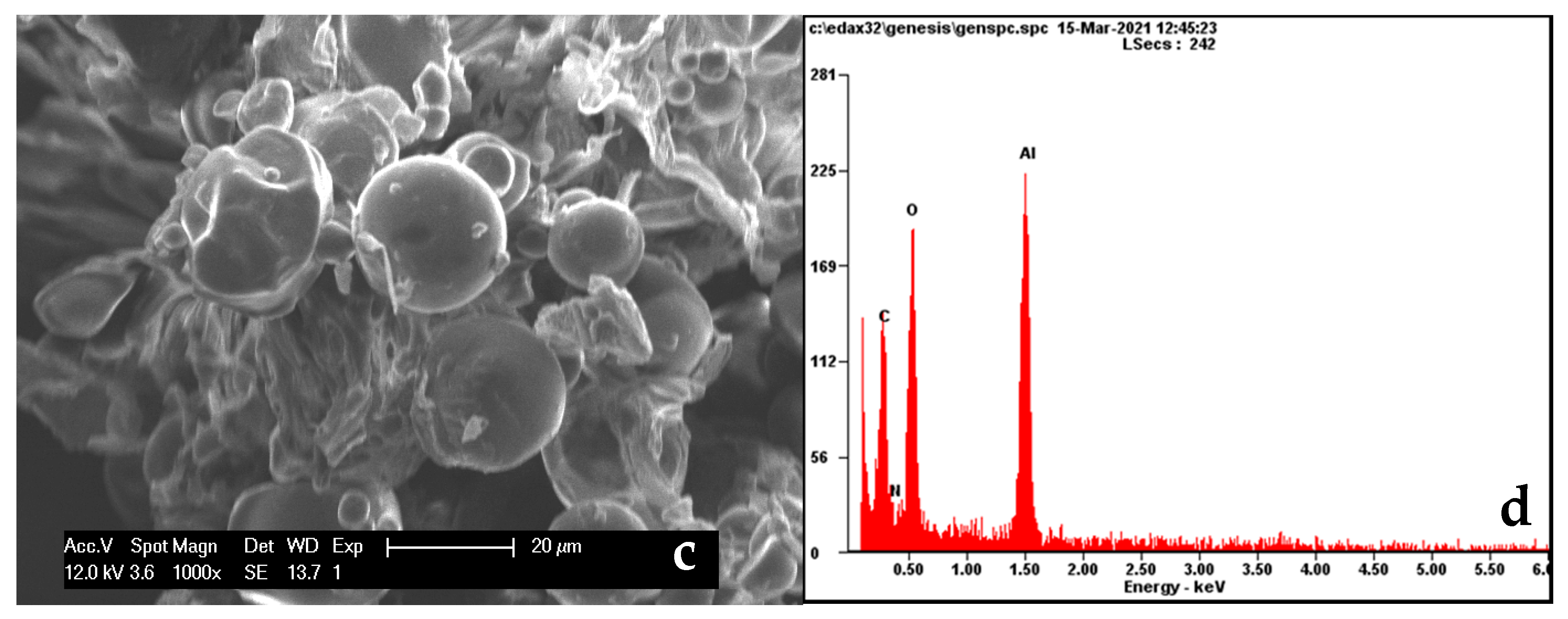
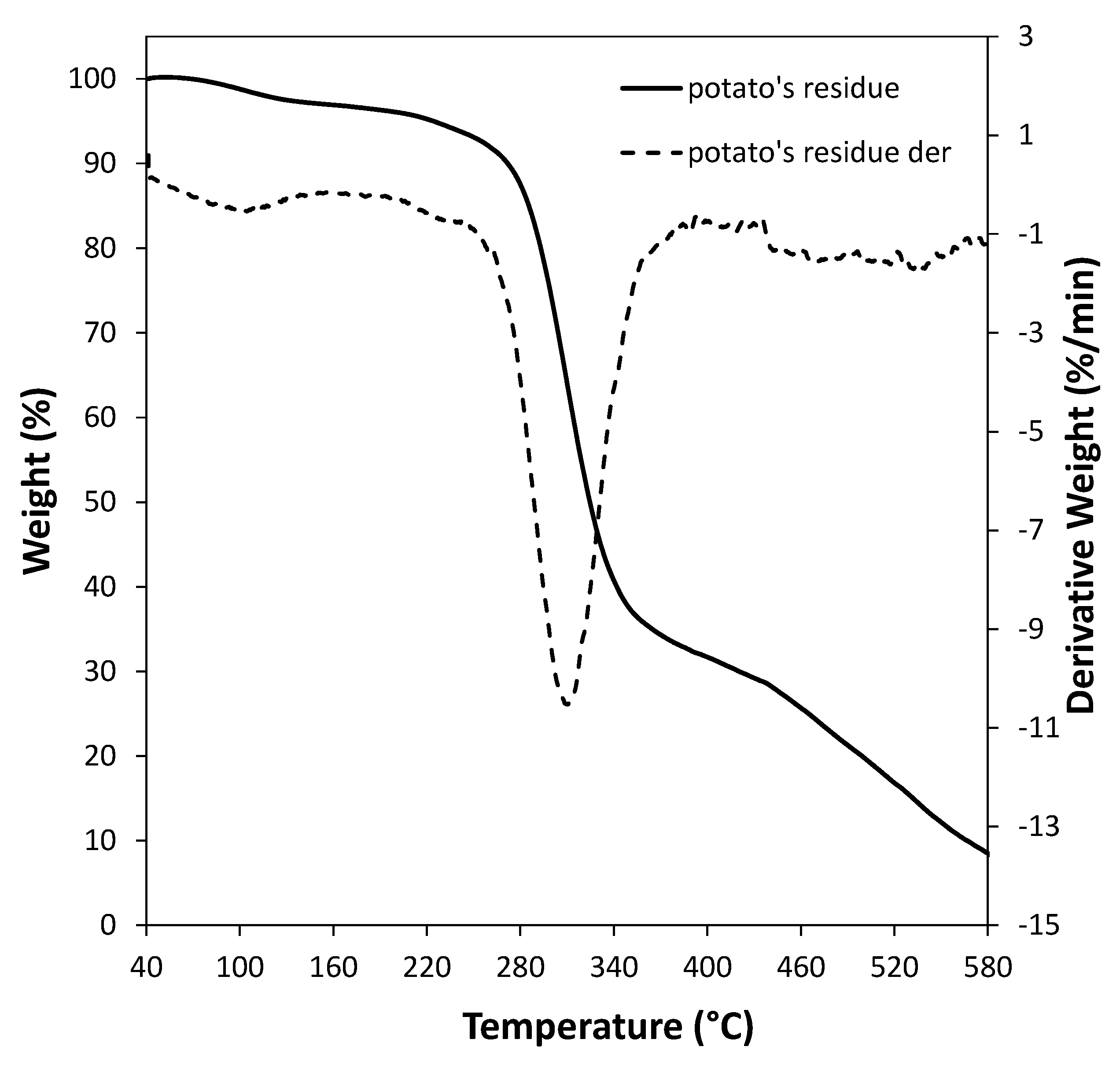

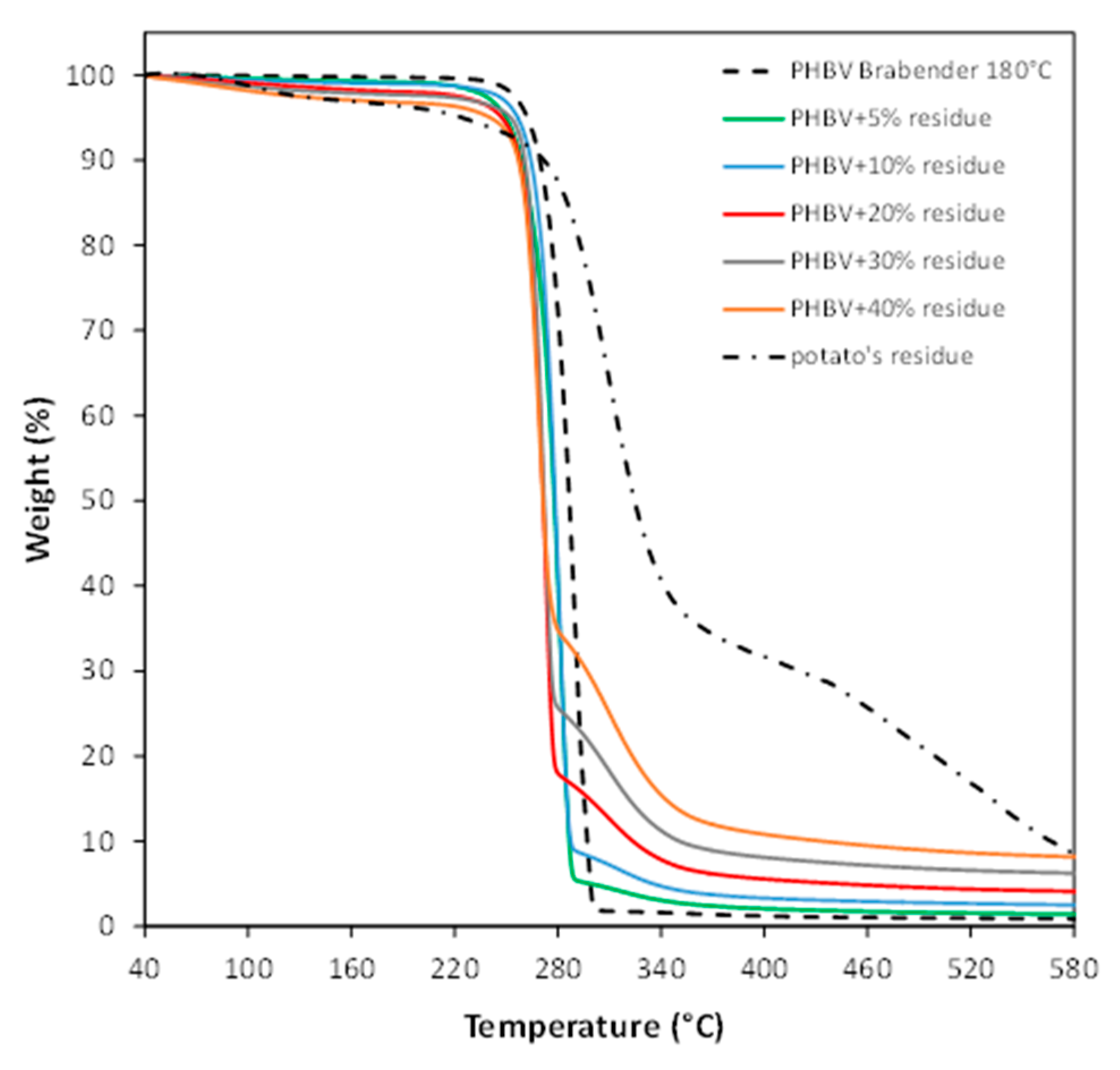
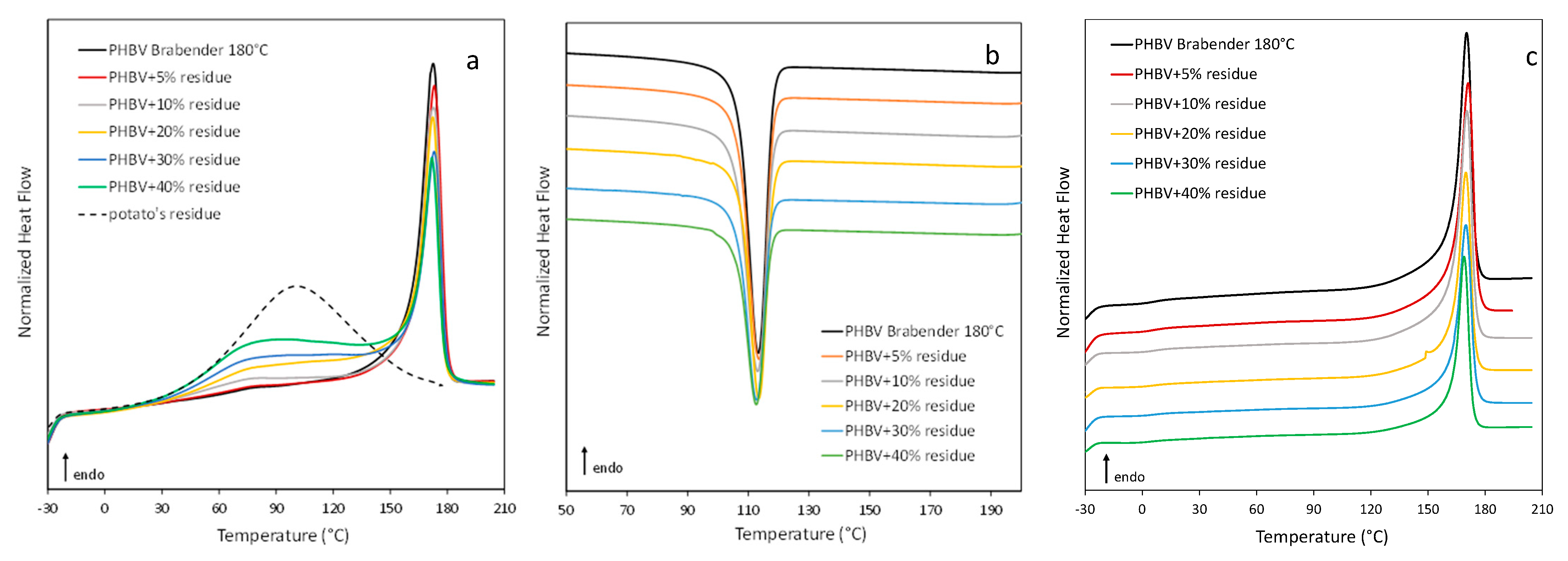


| Component | Amount (wt %) |
|---|---|
| Starch | 61.56 ± 0.72 |
| Protein | 3.81 ± 0.23 |
| Fat | 1.16 ± 0.01 |
| Total dietary fibre | 25.63 ± 0.17 |
| Ash | 1.95 ± 0.13 |
| Pectin | Hemicellulose | Cellulose | Lignin |
|---|---|---|---|
| 24.13 ± 0.79 | 13.66 ± 0.58 | 38.84 ±1.02 | 22.74 ± 0.69 |
| Residue Amount (%) | Mixing Temperature (°C) | Processed by Injection Moulding | Mn · 10−3 | Mw · 10−3 | D |
|---|---|---|---|---|---|
| - | - | no | 126.1 | 306 | 2.4 |
| - | 200 | no | 62.8 | 129 | 2.0 |
| 20 | 200 | no | 63.0 | 133 | 2.1 |
| - | 180 | no | 89.8 | 202 | 2.2 |
| - | 180 | yes | 47.8 | 122 | 2.6 |
| 5 | 180 | no | 90.7 | 217 | 2.4 |
| 5 | 180 | yes | 65.2 | 146 | 2.2 |
| 10 | 180 | no | 87.3 | 210 | 2.4 |
| 10 | 180 | yes | 51.1 | 113 | 2.2 |
| 20 | 180 | no | 88.7 | 217 | 2.4 |
| 20 | 180 | yes | 48.5 | 111 | 2.3 |
| Residue Amount (wt %) | DSC | TGA | GPC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st Scan | Cooling | 2nd Scan | Tonset (°C) | TD (°C) | Mn ·10−3 | Mw ·10−3 | D | |||||||
| Tm (°C) | ΔHm (J/g) | wc | Tc (°C) | ΔHc (J/g) | Tg (°C) | Tm (°C) | ΔHm (J/g) | wc | ||||||
| - | 175 | 79 | 0.55 | 113 | 72 | 7 | 171 | 82 | 0.57 | 276 | 290 | 89.8 | 202 | 2.2 |
| 5 | 174 | 83 | 0.58 | 113 | 74 | 7 | 172 | 82 | 0.57 | 268 | 281 | 90.7 | 217 | 2.4 |
| 10 | 174 | 90 | 0.63 | 113 | 76 | 7 | 171 | 82 | 0.57 | 270 | 282 | 87.3 | 210 | 2.4 |
| 20 | 172 | 80 | 0.56 | 113 | 75 | 7 | 170 | 83 | 0.58 | 264 | 273 | 88.7 | 217 | 2.4 |
| 30 | 174 | 83 | 0.58 | 113 | 74 | 7 | 171 | 86 | 0.60 | 263 | 272 | 86.3 | 209 | 2.4 |
| 40 | 173 | 73 | 0.51 | 113 | 75 | 6 | 170 | 82 | 0.57 | 260 | 269 | 87.2 | 205 | 2.4 |
| Residue Amount (wt %) | Young Modulus (MPa) | Tensile Strength (MPa) | Strain at Break (%) |
|---|---|---|---|
| - | 1853 ± 78 | 36.3 ± 0.9 | 3.6 ± 0.1 |
| 5 | 1817 ± 76 | 34.2 ± 0.9 | 3.7 ± 0.2 |
| 10 | 1804 ± 14 | 30.1 ± 0.2 | 3.2 ± 0.2 |
| 20 | 1827 ± 9 | 25.6 ± 0.5 | 2.2 ± 0.1 |
| 30 | 1950 ± 14 | 22.1 ± 0.4 | 1.9 ± 0.1 |
| 40 | 2006 ± 39 | 19.0 ± 0.3 | 1.7 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vannini, M.; Marchese, P.; Sisti, L.; Saccani, A.; Mu, T.; Sun, H.; Celli, A. Integrated Efforts for the Valorization of Sweet Potato By-Products within a Circular Economy Concept: Biocomposites for Packaging Applications Close the Loop. Polymers 2021, 13, 1048. https://doi.org/10.3390/polym13071048
Vannini M, Marchese P, Sisti L, Saccani A, Mu T, Sun H, Celli A. Integrated Efforts for the Valorization of Sweet Potato By-Products within a Circular Economy Concept: Biocomposites for Packaging Applications Close the Loop. Polymers. 2021; 13(7):1048. https://doi.org/10.3390/polym13071048
Chicago/Turabian StyleVannini, Micaela, Paola Marchese, Laura Sisti, Andrea Saccani, Taihua Mu, Hongnan Sun, and Annamaria Celli. 2021. "Integrated Efforts for the Valorization of Sweet Potato By-Products within a Circular Economy Concept: Biocomposites for Packaging Applications Close the Loop" Polymers 13, no. 7: 1048. https://doi.org/10.3390/polym13071048
APA StyleVannini, M., Marchese, P., Sisti, L., Saccani, A., Mu, T., Sun, H., & Celli, A. (2021). Integrated Efforts for the Valorization of Sweet Potato By-Products within a Circular Economy Concept: Biocomposites for Packaging Applications Close the Loop. Polymers, 13(7), 1048. https://doi.org/10.3390/polym13071048