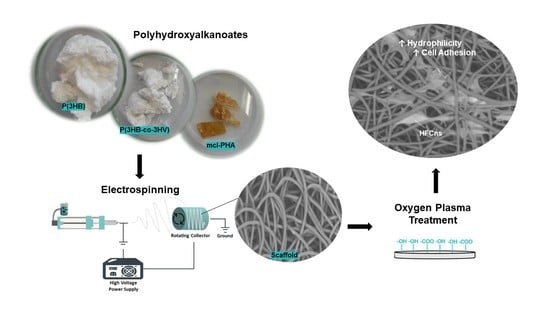
Oxygen Plasma Treated-Electrospun Polyhydroxyalkanoate Scaffolds for Hydrophilicity Improvement and Cell Adhesion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biopolymers
2.2. Preparation of PHA-Based Scaffolds
2.2.1. Electrospinning
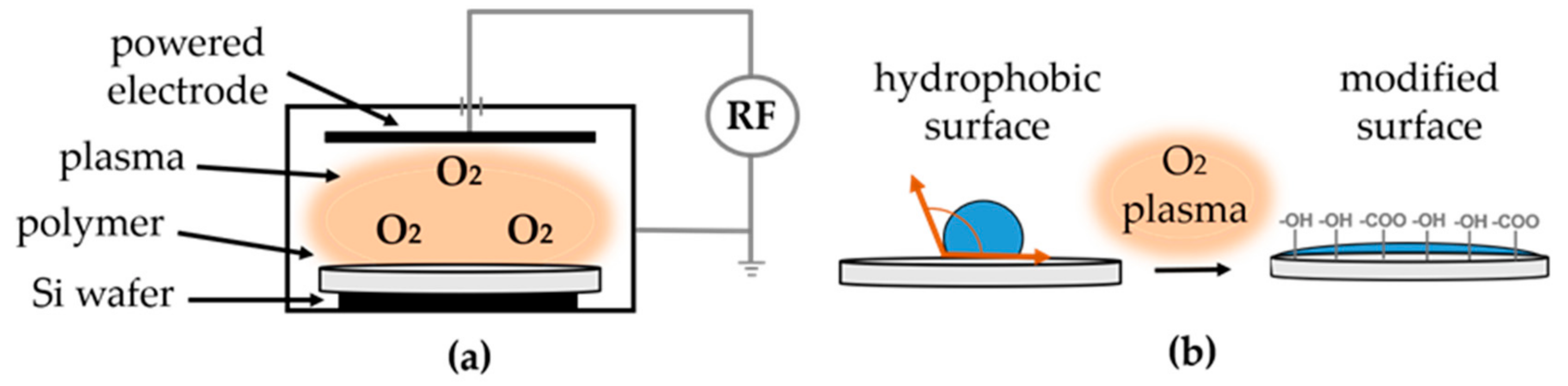
2.2.2. Oxygen Plasma Treatment
2.3. Characterization
2.3.1. Molecular Mass Distribution of the Biopolymers
2.3.2. Thermal Analysis
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Water Contact Angle and Water Uptake Degree
2.4. Biological Assays
2.4.1. Cell Culture
2.4.2. Cell Seeding
2.4.3. Cell Viability (MTT Assay)
2.4.4. Cell Morphology
3. Results and Discussion
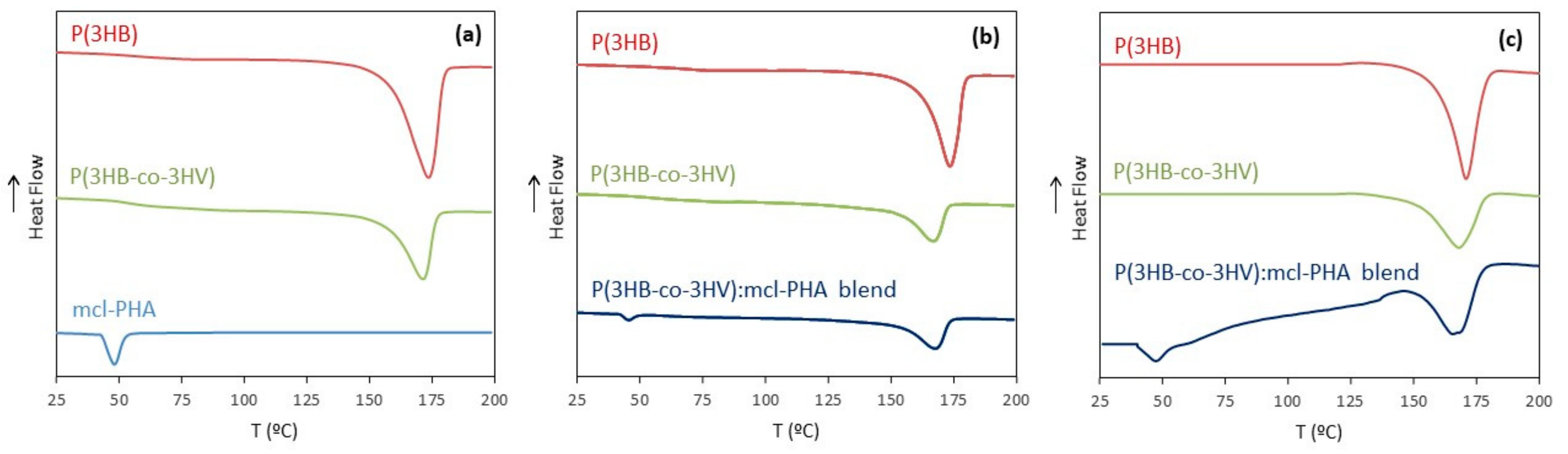
3.1. Biopolymers Characterization
3.2. Scaffolds Preparation
3.2.1. Electrospun Scaffolds Based on P(3HB), P(3HB-co-3HV) and mcl-PHA
3.2.2. Electrospun Scaffolds Based on scl-/mcl-PHA Blends
3.2.3. Oxygen Plasma Treated Scaffolds
3.3. Scaffolds Characterization
3.3.1. Morphology
3.3.2. Molecular Mass Distribution
3.3.3. Thermal Properties
3.3.4. Water Contact Angle and Water Uptake Degree
3.3.5. Fourier Transform Infrared Spectroscopy
3.4. Biological Assays
3.4.1. Cell Viability
3.4.2. Cell Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zubairi, S.I.; Mantalaris, A.; Bismarck, A.; Aizad, S. Polyhydroxyalkanoates (PHAs) for tissue engineering applications: Biotransformation of palm oil mill effluent (POME) to value-added polymers. J. Teknol. 2016, 78, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef] [Green Version]
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È; Chabaud, S.; Bolduc, S. Innovative Human Three-Dimensional Tissue-Engineered Models as an Alternative to Animal Testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell Migration. Compr. Physiol. 2012, 2, 2369–2392. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, C.; Wu, S.; Fan, Y.; Li, X. Influence of the mechanical properties of biomaterials on degradability, cell behaviors and signaling pathways: Current progress and challenges. Biomater. Sci. 2020, 8, 2714–2733. [Google Scholar] [CrossRef]
- Wimmer, R.A.; Leopoldi, A.; Aichinger, M.; Wick, N.; Hantusch, B.; Novatchkova, M.; Taubenschmid, J.; Hämmerle, M.; Esk, C.; Bagley, J.A.; et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nat. Cell Biol. 2019, 565, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.; Singh, G.; Trasatti, J.P.; Bjornsson, C.; Xu, X.; Tran, T.N.; Yoo, S.-S.; Dai, G.; Karande, P. Design and Fabrication of Human Skin by Three-Dimensional Bioprinting. Tissue Eng. Part C Methods 2014, 20, 473–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Chen, H.; Zhang, H.; Guo, C.; Yang, K.; Chen, K.; Cheng, R.; Qian, N.; Sandler, N.; Zhang, Y.S.; et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials 2019, 190–191, 97–110. [Google Scholar] [CrossRef]
- Pusch, J.; Votteler, M.; Göhler, S.; Engl, J.; Hampel, M.; Walles, H.; Schenke-Layland, K. The physiological performance of a three-dimensional model that mimics the microenvironment of the small intestine. Biomaterials 2011, 32, 7469–7478. [Google Scholar] [CrossRef]
- Campuzano, S.; Pelling, A.E. Scaffolds for 3D Cell Culture and Cellular Agriculture Applications Derived From Non-animal Sources. Front. Sustain. Food Syst. 2019, 3, 38. [Google Scholar] [CrossRef] [Green Version]
- Deluzio, T.G.; Seifu, D.G.; Mequanint, K. 3D scaffolds in tissue engineering and regenerative medicine: Beyond structural templates? Pharm. Bioprocess. 2013, 1, 267–281. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayward, A.S.; Sano, N.; Przyborski, S.A.; Cameron, N.R. Acrylic-Acid-Functionalized PolyHIPE Scaffolds for Use in 3D Cell Culture. Macromol. Rapid Commun. 2013, 34, 1844–1849. [Google Scholar] [CrossRef]
- Możejko-Ciesielska, J.; Kiewisz, R. Bacterial polyhydroxyalkanoates: Still fabulous? Microbiol. Res. 2016, 192, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, H.; Deng, B.; Zhao, X. Poly(3-hydroxybutyrate) and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate): Structure, Property, and Fiber. Int. J. Polym. Sci. 2014, 2014, 374368. [Google Scholar] [CrossRef]
- Radu, I.C.; Hudita, A.; Zaharia, C.; Galateanu, B.; Iovu, H.; Vasile, E.; Nitu, S.G.; Ginghina, O.; Negrei, C.; Tsatsakis, A.; et al. Poly(3-hydroxybutyrate-CO-3-hydroxyvalerate) PHBHV biocompatible nanocarriers for 5-FU delivery targeting colorectal cancer. Drug Deliv. 2019, 26, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Basnett, P.; Marcello, E.; Lukasiewicz, B.; Panchal, B.; Nigmatullin, R.; Knowles, J.C.; Roy, I. Biosynthesis and characterization of a novel, biocompatible medium chain length polyhydroxyalkanoate by Pseudomonas mendocina CH50 using coconut oil as the carbon source. J. Mater. Sci. Mater. Med. 2018, 29, 179. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.; Przyborski, S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J. Anat. 2015, 227, 746–756. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Inai, R.; Kotaki, M.; Ramakrishna, S. Aligned biodegradable nanofibrous structure: A potential scaffold for blood vessel engineering. Biomaterials 2004, 25, 877–886. [Google Scholar] [CrossRef]
- Vieira, T.; Silva, J.C.; Botelho do Rego, A.M. Electrospun biodegradable chitosan based-poly(urethane urea)scaffolds for soft tissue engineering. Mater. Sci. Eng. C 2019, 103, 109819. [Google Scholar] [CrossRef]
- Tan, D.; Yin, J.; Chen, G.Q. Production of Polyhydroxyalkanoates. In Current Developments in Biotechnologyand Bioengineering, Production, Isolation and Purification of Industrial Products; Pandey, A., Negi, S., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 655–692. ISBN 978-0-444-6366. [Google Scholar]
- Silva, J.; Pereira, J.; Marreiros, B.; Reis, M.; Freitas, F. Microbial production of medium-chain length polyhydroxyalkanoates. Proc. Biochem. 2021, 102, 393–407. [Google Scholar] [CrossRef]
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174. [Google Scholar] [CrossRef]
- Sangsanoh, P.; Waleetorncheepsawat, S.; Suwantong, O.; Wutticharoenmongkol, P.; Weeranantanapan, O.; Chuenjitbuntaworn, B.; Cheepsunthorn, P.; Pavasant, A.P.; Supaphol, P. In Vitro Biocompatibility of Schwann Cells on Surfaces of Biocompatible Polymeric Electrospun Fibrous and Solution-Cast Film Scaffolds. Biomacromolecules 2007, 8, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Sombatmankhong, K.; Suwantong, O.; Waleetorncheepsawat, S.; Supaphol, P. Electrospun fiber mats of poly(3-hydroxybutyrate), poly(3-hydroxybutyrate-co-3-hydroxyvalerate), and their blends. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2923–2933. [Google Scholar] [CrossRef]
- Volova, T.; Goncharov, D.; Sukovatyi, A.; Shabanov, A.; Nikolaeva, E.; Shishatskaya, E. Electrospinning of polyhydroxyalkanoate fibrous scaffolds: Effects on electrospinning parameters on structure and properties. J. Biomater. Sci. Polym. Ed. 2013, 25, 370–393. [Google Scholar] [CrossRef]
- Cheng, M.-L.; Chen, P.-Y.; Lan, C.-H.; Sun, Y.-M. Structure, mechanical properties and degradation behaviors of the electrospun fibrous blends of phbhhx/pdlla. Polymer 2011, 52, 139–140. [Google Scholar] [CrossRef]
- Canadas, R.F.; Cavalheiro, J.M.; Guerreiro, J.D.; De Almeida, M.C.M.; Pollet, E.; Da Silva, C.L.; Da Fonseca, M.; Ferreira, F.C. Polyhydroxyalkanoates: Waste glycerol upgrade into electrospun fibrous scaffolds for stem cells culture. Int. J. Biol. Macromol. 2014, 71, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Prabhakaran, M.P.; Qin, X.; Ramakrishna, S. Poly-3-hydroxybutyrate-co-3-hydroxyvalerate containing scaffolds and their integration with osteoblasts as a model for bone tissue engineering. J. Biomater. Appl. 2014, 29, 1394–1406. [Google Scholar] [CrossRef]
- Hosseini, F.S.; Soleimanifar, F.; Aidun, A.; Enderami, S.E.; Saburi, E.; Marzouni, H.Z.; Khani, M.; Khojasteh, A.; Ardeshirylajimi, A. Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) improved osteogenic differentiation of the human induced pluripotent stem cells while considered as an artificial extracellular matrix. J. Cell. Physiol. 2018, 234, 11537–11544. [Google Scholar] [CrossRef]
- Li, W.; Cicek, N.; Levin, D.B.; Liu, S. Enabling electrospinning of medium-chain length polyhydroxyalkanoates (PHAs) by blending with short-chain length PHAs. Int. J. Polym. Mater. 2018, 68, 499–509. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Lupescu, I.; Frone, A.N.; Chiulan, I.; Nicolae, C.A.; Tofan, V.; Stefaniu, A.; Somoghi, R.; Trusca, R. Medium Chain-Length Polyhydroxyalkanoate Copolymer Modified by Bacterial Cellulose for Medical Devices. Biomacromolecules 2017, 18, 3222–3232. [Google Scholar] [CrossRef]
- Ndreu, A.; Nikkola, L.; Ylikauppila, H.; Ashammakhi, N.; Hasirci, V. Electrospun biodegradable nanofibrous mats for tissue engineering. Nanomedicine 2008, 3, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Masuoka, T. Degradation properties of PLA and PHBV films treated with CO2-plasma. React. Funct. Polym. 2009, 69, 287–292. [Google Scholar] [CrossRef]
- Mirmohammadi, S.A.; Khorasani, M.T.; Mirzadeh, H.; Irani, S. Investigation of plasma treatment on poly (3-hydroxybutyrate) film surface: Characterization and in vitro assay. Polym. Plast. Technol. Eng. 2012, 51, 1319–1326. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, L.; Zheng, Y.; Chen, X. Improvement in hydrophilicity of PHBV films by plasma treatment. J. Biomed. Mater. Res. Part A 2006, 76, 589–595. [Google Scholar] [CrossRef]
- Denes, F.S.; Manolache, S. Macromolecular plasma-chemistry: An emerging field of polymer science. Prog. Polym. Sci. 2004, 29, 815–885. [Google Scholar] [CrossRef]
- Kim, H.W.; Chung, C.W.; Kim, S.S.; Kim, Y.B.; Rhee, Y.H. Preparation and cell compatibility of acrylamide-grafted poly(3-hydroxyoctanoate). Int. J. Biol. Macromol. 2002, 30, 129–135. [Google Scholar] [CrossRef]
- Ke, Y.; Liu, C.; Zhang, X.; Xiao, M.; Wu, G. Surface modification of polyhydroxyalkanoates toward enhancing cell compatibility and antibacterial activity. Macromol. Mater. Eng. 2017, 302, 1700258. [Google Scholar] [CrossRef]
- Cruz, M.V.; Sarraguça, M.C.; Freitas, F.; Lopes, J.A.; Reis, M.A. Online monitoring of P(3HB) produced from used cooking oil with near-infrared spectroscopy. J. Biotechnol. 2015, 194, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Cai, J.; Liu, Z.; Zheng, Y.; Wang, H.; Li, Q.; He, N. Biosynthesis and Thermal Properties of PHBV Produced from Levulinic Acid by Ralstonia eutropha. PLoS ONE 2013, 8, e60318. [Google Scholar] [CrossRef] [Green Version]
- Meneses, L.; Pereira, J.; Servin, C.; Grandfils, C.; Paiva, A.; Reis, M.A.M.; Freitas, F. Pseudomonas chlororaphis as a multiproduct platform: Conversion of glycerol into high-value biopolymers and phenazines. New Biotechnol. 2020, 55, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.R.; Araújo, D.; Marques, A.C.; Neves, L.A.; Grandfils, C.; Sevrin, C.; Alves, V.D.; Fortunato, E.; Reis, M.A.M.; Freitas, F. Demonstration of the adhesive properties of the medium-chain-length polyhydroxyalkanoate produced by Pseudomonas chlororaphis subsp. aurantiaca from glycerol. Int. J. Biol. Macromol. 2019, 122, 1144–1151. [Google Scholar] [CrossRef]
- Morais, C.; Freitas, F.; Cruz, M.V.; Paiva, A.; Dionísio, M.; Reis, M.A.; Andrade, M.M.D. Conversion of fat-containing waste from the margarine manufacturing process into bacterial polyhydroxyalkanoates. Int. J. Biol. Macromol. 2014, 71, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Rebocho, A.T.; Pereira, J.R.; Freitas, F.; Neves, L.A.; Alves, V.D.; Sevrin, C.; Grandfils, C.; Reis, M.A. Production of medium-chain length polyhydroxyalkanoates by Pseudomonas citronellolis grown in apple pulp waste. Appl. Food Biotechnol. 2019, 6, 71–82. [Google Scholar]
- Singh, M.; Kumar, P.; Ray, S.; Kalia, V.C. Challenges and Opportunities for Customizing Polyhydroxyalkanoates. Indian J. Microbiol. 2015, 55, 235–249. [Google Scholar] [CrossRef] [Green Version]
- Kunasundari, B.; Sudesh, K. Isolation and recovery of microbial polyhydroxyalkanoates. Express Polym. Lett. 2011, 5, 620–634. [Google Scholar] [CrossRef]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; Du Toit, L.C.; Ndesendo, V.M.K. A Review of the Effect of Processing Variables on the Fabrication of Electrospun Nanofibers for Drug Delivery Applications. J. Nanomater. 2013, 2013, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Henriques, C.; Vidinha, R.; Botequim, D.; Borges, J.P.; Silva, J.A.M.C. A Systematic Study of Solution and Processing Parameters on Nanofiber Morphology Using a New Electrospinning Apparatus. J. Nanosci. Nanotechnol. 2009, 9, 3535–3545. [Google Scholar] [CrossRef]
- Azari, P.; Yahya, R.; Wong, C.S.; Gan, S.N. Improved processability of electrospun poly[(R)-3-hydroxybutyricacid] through blending with medium-chain length poly(3-hydroxyalkanoates) produced by Pseudomonas putida from oleic acid. Mater. Res. Innov. 2014, 18, 345–349. [Google Scholar] [CrossRef]
- Puppi, D.; Pecorini, G.; Chiellini, F. Biomedical Processing of Polyhydroxyalkanoates. Bioengineering 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köse, G.; Kenar, H.; Hasırcı, N.; Hasırcı, V.; Hasirci, N.; Hasirci, V. Macroporous poly(3-hydroxybutyrate-co-3-hydroxyvalerate) matrices for bone tissue engineering. Biomaterials 2003, 24, 1949–1958. [Google Scholar] [CrossRef]
- Köse, T.; Ber, S.; Korkusuz, F.; Hasirci, V. Poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) based tissue engineering ma-trices. J. Mat. Sci. Mat. Med. 2003, 14, 121–126. [Google Scholar] [CrossRef]
- Chen, M.; Patra, P.K.; Lovett, M.L.; Kaplan, D.L.; Bhowmick, S. Role of electrospun fibre diameter and corresponding specific surface area (SSA) on cell attachment. J. Tissue Eng. Regen. Med. 2009, 3, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lou, T.; Zhao, W.; Song, G.; Li, C.; Cui, G. The effect of fiber size and pore size on cell proliferation and infiltration in PLLA scaffolds on bone tissue engineering. J. Biomater. Appl. 2016, 30, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Organ, S. Variation in melting point with molecular weight for hydroxybutyrate/hydroxyvalerate copolymers. Polymer 1993, 34, 2175–2179. [Google Scholar] [CrossRef]
- Mottin, A.C.; Ayres, E.; Orefice, R.L.; Câmara, J.J.D. What changes in poly(3-hydroxybutyrate) (PHB) when processed as electrospun nanofibers or thermo-compression molded film? Mater. Res. 2016, 19, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Law, K.-Y. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef]
- Lee, Y.; Sridewei, N.; Ramanathan, S.; Sudesh, K. The influence of electrospinning parameters and drug loading on polyhydrox-yalkanoate (pha) nanofibers for drug delivery. Int. J. Biotech. Well. Ind. 2017, 4, 103–113. [Google Scholar]
- Correia, D.; Ribeiro, C.; Ferreira, J.C.; Botelho, G.; Ribelles, J.L.G.; Lanceros-Méndez, S.; Sencadas, V. Influence of electrospinning parameters on poly(hydroxybutyrate) electrospun membranes fiber size and distribution. Polym. Eng. Sci. 2013, 54, 1608–1617. [Google Scholar] [CrossRef]
- Ouyang, S.P.; Luo, R.C.; Chen, S.S.; Liu, Q.; Chung, A.; Wu, Q.; Chen, G.Q. Production of polyhydroxyalkanoates with high 3-hydroxydodecanoate monomer content by fadB and fadA knockout mutant of Pseudomonas putida KT2442. Biomacromolecules 2007, 8, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
- Gumel, A.; Annuar, M.; Heidelberg, T. Growth kinetics, effect of carbon substrate in biosynthesis of mcl-PHA by Pseudomonas putida Bet001. Braz. J. Microbiol. 2014, 45, 427–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoyle, H.W.; Smith, L.A.; Williams, R.J.; Przyborski, S.A. Applications of novel bioreactor technology to enhance the viability and function of cultured cells and tissues. Interface Focus 2020, 10, 20190090. [Google Scholar] [CrossRef]
- Brancato, V.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L.; Kundu, S.C. Could 3D models of cancer enhance drug screening? Biomaterialis 2020, 232, 119744. [Google Scholar] [CrossRef]
- Dikici, B.A.; Sherborne, C.; Reilly, G.C.; Claeyssens, F. Emulsion templated scaffolds manufactured from photocurable polycaprolactone. Polymer 2019, 175, 243–254. [Google Scholar] [CrossRef]
- Giancotti, F.G.; Ruoslahti, E. Integrin signaling. Science 1999, 285, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Fomby, P.; Cherlin, A.J.; Hadjizadeh, A.; Doillon, C.J.; Sueblinvong, V.; Weiss, D.J.; Bates, J.H.T.; Gilbert, T.; Liles, W.C.; Lutzko, C.; et al. Stem cells and cell therapies in lung biology and diseases: Conference report. Ann. Am. Thorac. Soc. 2010, 12, 181–204. [Google Scholar]
- Karahaliloğlu, Z. Cell-compatible PHB/silk fibroin composite nanofiber mat for tissue engineering applications. Turkish J. Biol. 2017, 41, 503–513. [Google Scholar] [CrossRef]

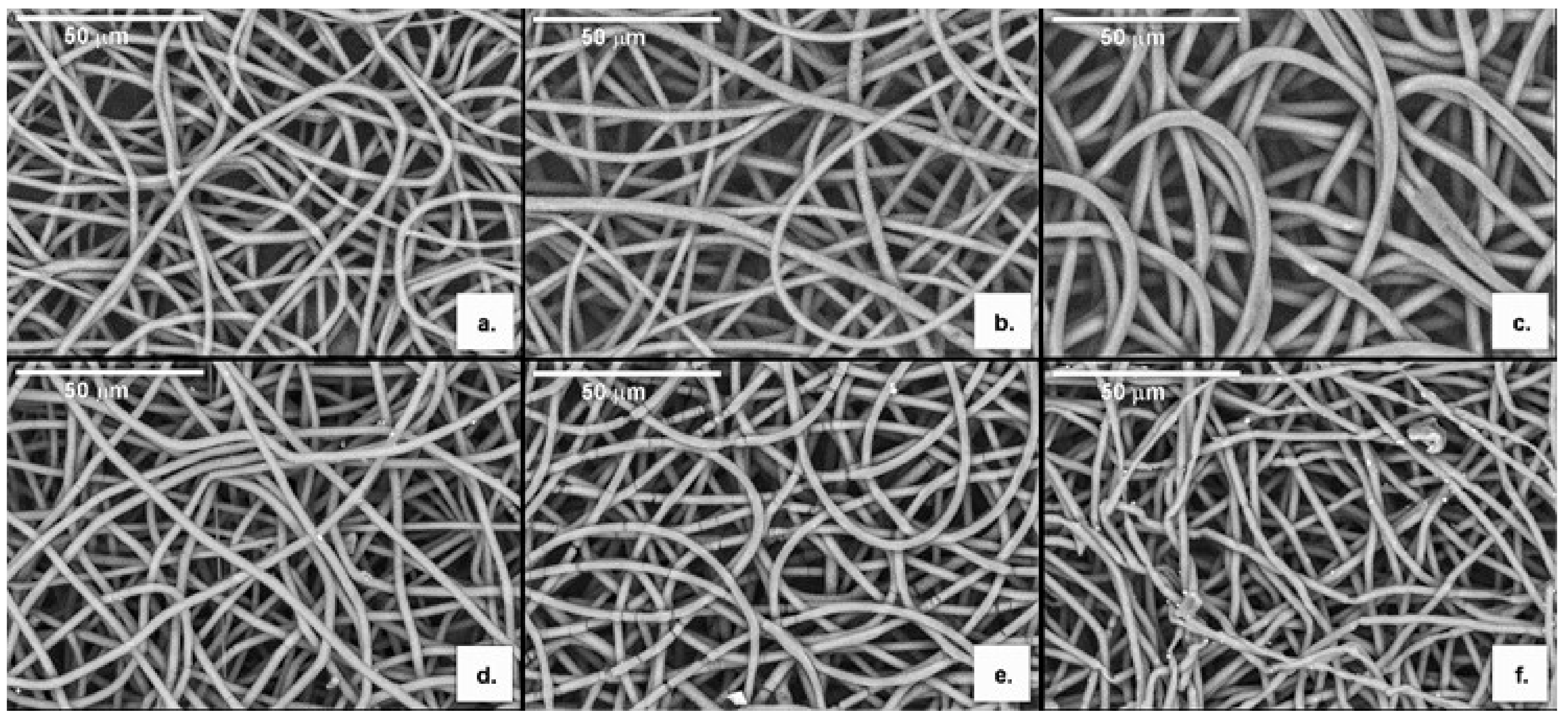


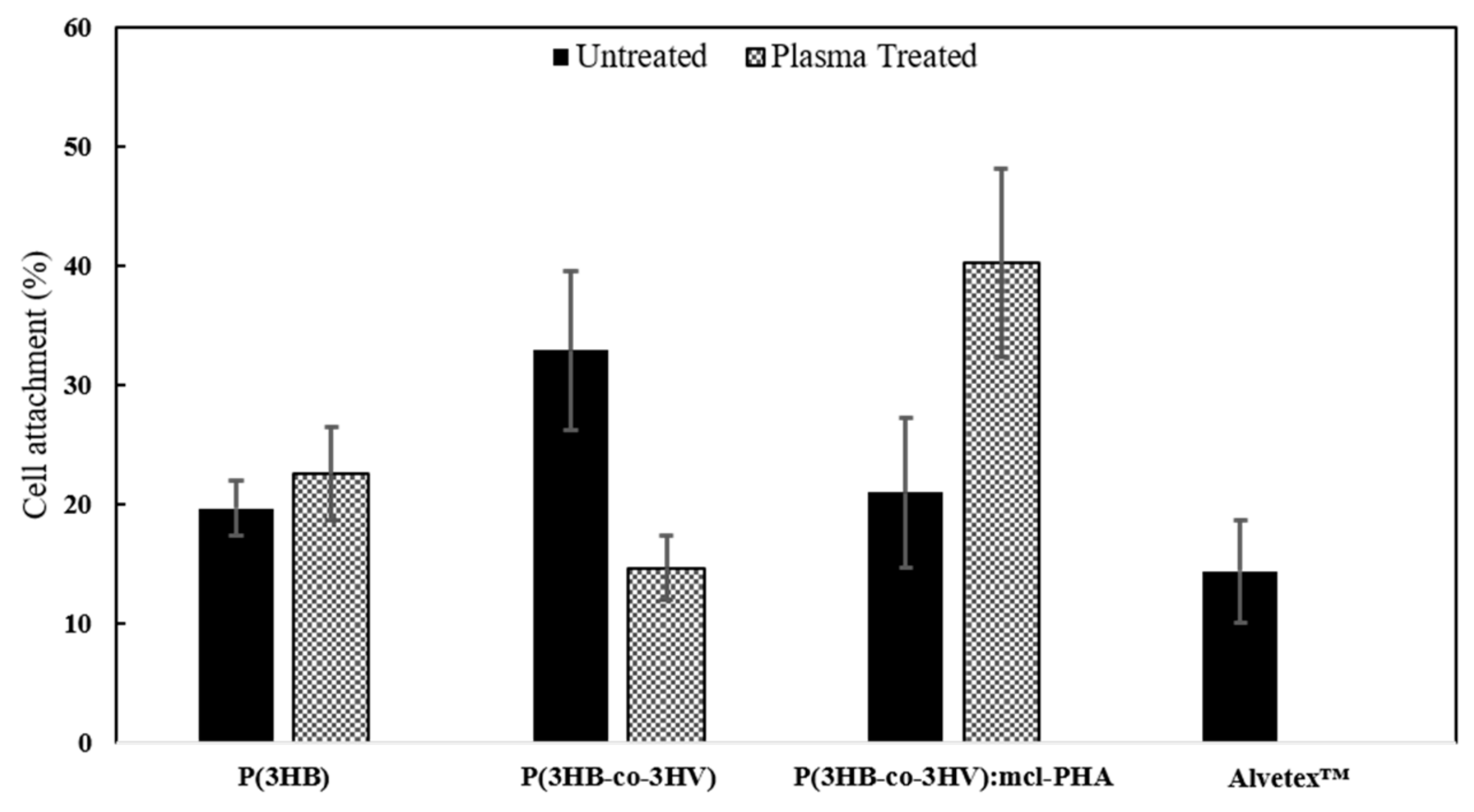
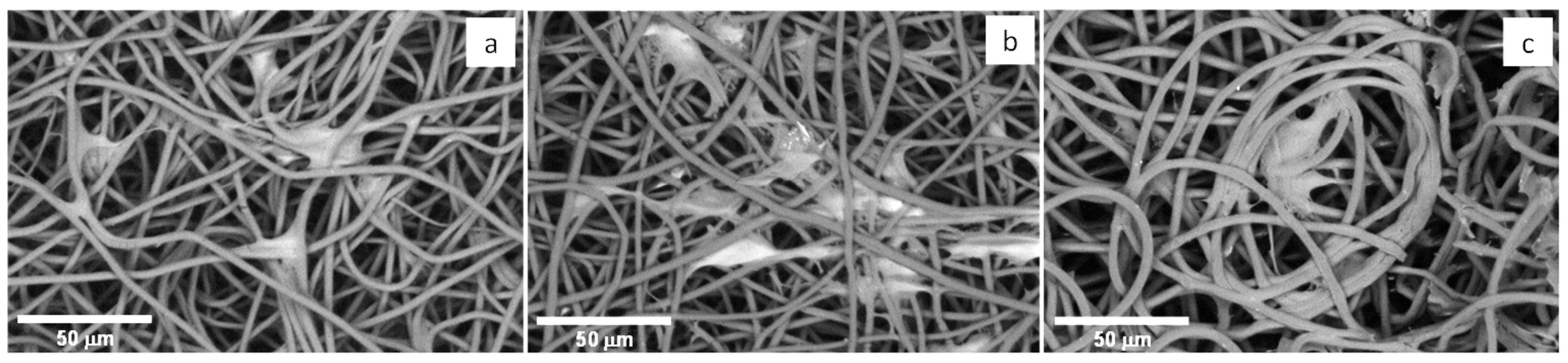
| Processing/Material | Mw (Da) | PDI | Tm (°C) | Tdeg (°C) | ∆Hm (J g−1) | Xc (%) |
|---|---|---|---|---|---|---|
| None | ||||||
| P(3HB) | 5.20 × 105 | 1.80 | 176 | 293 | 76.5 | 52.4 |
| P(3HB-co-3HV) | 5.60 × 105 | 1.60 | 171 | 292 | 34.5 | 23.6 |
| mcl-PHA | 0.69 × 105 | 1.50 | 48 | 292 | 8.2 | 5.6 |
| Electrospinning | ||||||
| P(3HB) scaffold | 4.20 × 105 | 1.63 | 173 | 289 | 77.5 | 53.1 |
| P(3HB-co-3HV) scaffold | 4.10 × 105 | 1.68 | 167 | 288 | 36.1 | 24.7 |
| P(3HB-co-3HV): mcl-PHA blend scaffold | 3.50×105 | 2.79 | 168/46 | 290 | 30.5 | 20.9 |
| Electrospinning + O2 Plasma Treatment | ||||||
| P(3HB) scaffold | 3.50 × 105 | 1.90 | 171 | 291 | 56.6 | 38.8 |
| P(3HB-co-3HV) scaffold | 3.50 × 105 | 2.00 | 168 | 293 | 26.3 | 18.0 |
| P(3HB-co-3HV): mcl-PHA blend scaffold | 3.50 × 105 | 2.90 | 168/46 | 293 | 14.9 | 10.2 |
| Solution | d (cm) | ϕ (mL h−1) | V (kV) |
|---|---|---|---|
| P(3HB) | 20 | 0.5 | 12 |
| P(3HB-co-3HV) | 20 | 0.5 | 12 |
| P(3HB-co-3HV): mcl-PHA blend | 25 | 0.5 | 15 |
| Processing/Scaffold | Pore Size (µm) | Water Contact Angle (ϴ) | Water Uptake Degree (%) |
|---|---|---|---|
| Electrospinning | |||
| P(3HB) | 9.4 ± 3.5 | 84.3 ± 1.8 | 0 |
| P(3HB-co-3HV) | 10.2 ± 2.7 | 96.8 ± 1.3 | 77 |
| P(3HB-co-3HV): mcl-PHA blend | 10.2 ± 3.3 | 113.5 ± 0.7 | 0 |
| Electrospinning + O2 Plasma Treatment | |||
| P(3HB) | 10.0 ± 2.9 | 0 | 294 |
| P(3HB-co-3HV) | 9.5 ± 2.5 | 0 | 205 |
| P(3HB-co-3HV): mcl-PHA blend | 10.4 ± 3.4 | 0 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esmail, A.; Pereira, J.R.; Zoio, P.; Silvestre, S.; Menda, U.D.; Sevrin, C.; Grandfils, C.; Fortunato, E.; Reis, M.A.M.; Henriques, C.; et al. Oxygen Plasma Treated-Electrospun Polyhydroxyalkanoate Scaffolds for Hydrophilicity Improvement and Cell Adhesion. Polymers 2021, 13, 1056. https://doi.org/10.3390/polym13071056
Esmail A, Pereira JR, Zoio P, Silvestre S, Menda UD, Sevrin C, Grandfils C, Fortunato E, Reis MAM, Henriques C, et al. Oxygen Plasma Treated-Electrospun Polyhydroxyalkanoate Scaffolds for Hydrophilicity Improvement and Cell Adhesion. Polymers. 2021; 13(7):1056. https://doi.org/10.3390/polym13071056
Chicago/Turabian StyleEsmail, Asiyah, João R. Pereira, Patrícia Zoio, Sara Silvestre, Ugur Deneb Menda, Chantal Sevrin, Christian Grandfils, Elvira Fortunato, Maria A. M. Reis, Célia Henriques, and et al. 2021. "Oxygen Plasma Treated-Electrospun Polyhydroxyalkanoate Scaffolds for Hydrophilicity Improvement and Cell Adhesion" Polymers 13, no. 7: 1056. https://doi.org/10.3390/polym13071056
APA StyleEsmail, A., Pereira, J. R., Zoio, P., Silvestre, S., Menda, U. D., Sevrin, C., Grandfils, C., Fortunato, E., Reis, M. A. M., Henriques, C., Oliva, A., & Freitas, F. (2021). Oxygen Plasma Treated-Electrospun Polyhydroxyalkanoate Scaffolds for Hydrophilicity Improvement and Cell Adhesion. Polymers, 13(7), 1056. https://doi.org/10.3390/polym13071056