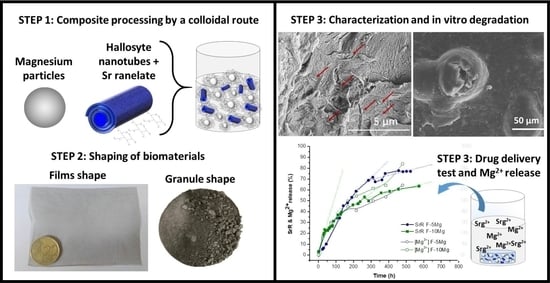
Controlled SrR Delivery by the Incorporation of Mg Particles on Biodegradable PLA-Based Composites
Abstract
1. Introduction
2. Materials and Methods
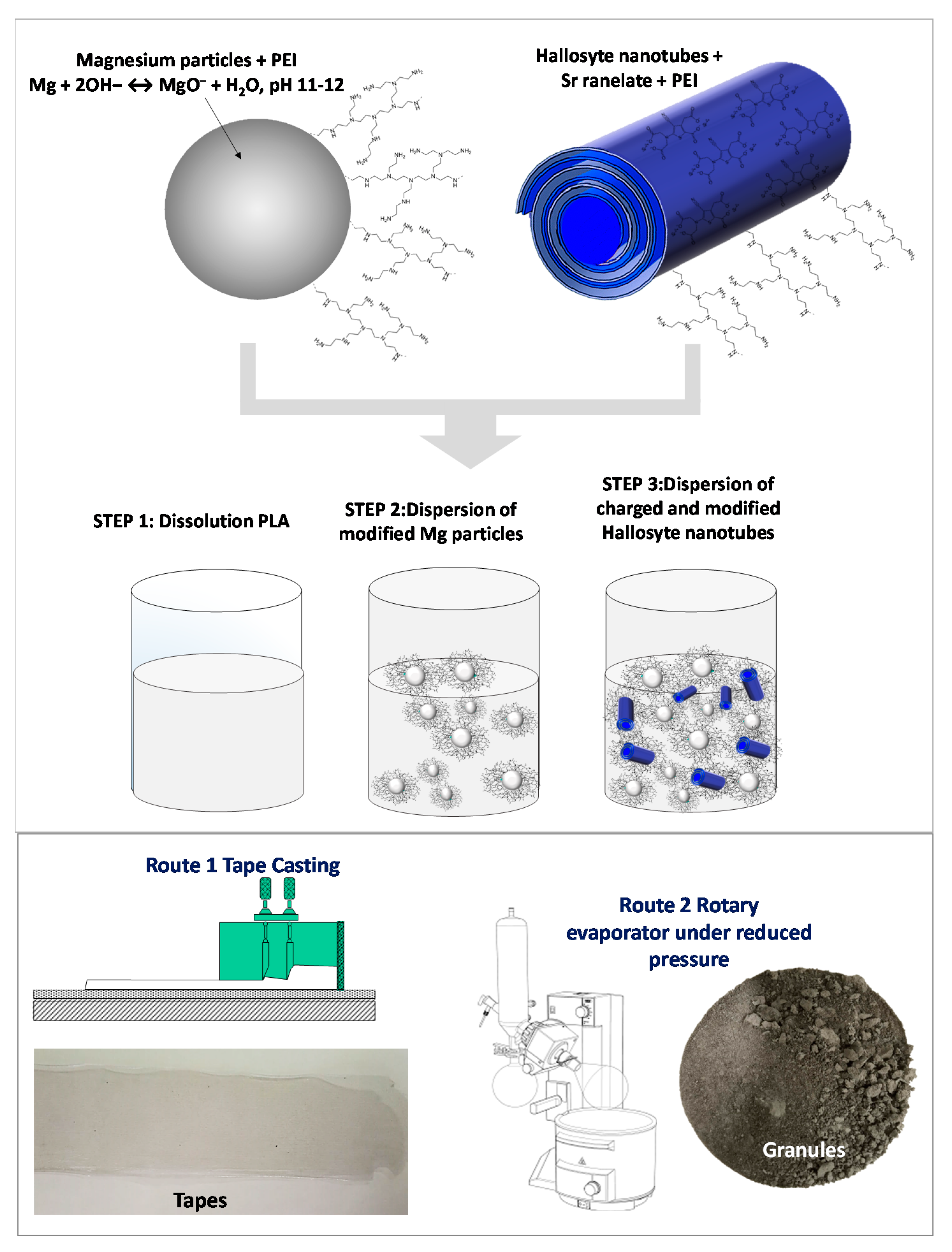
2.1. Preparation of Colloidal Suspensions
2.2. Granules and Films Preparation
2.3. Granules and Films Characterization
3. Results and discussion
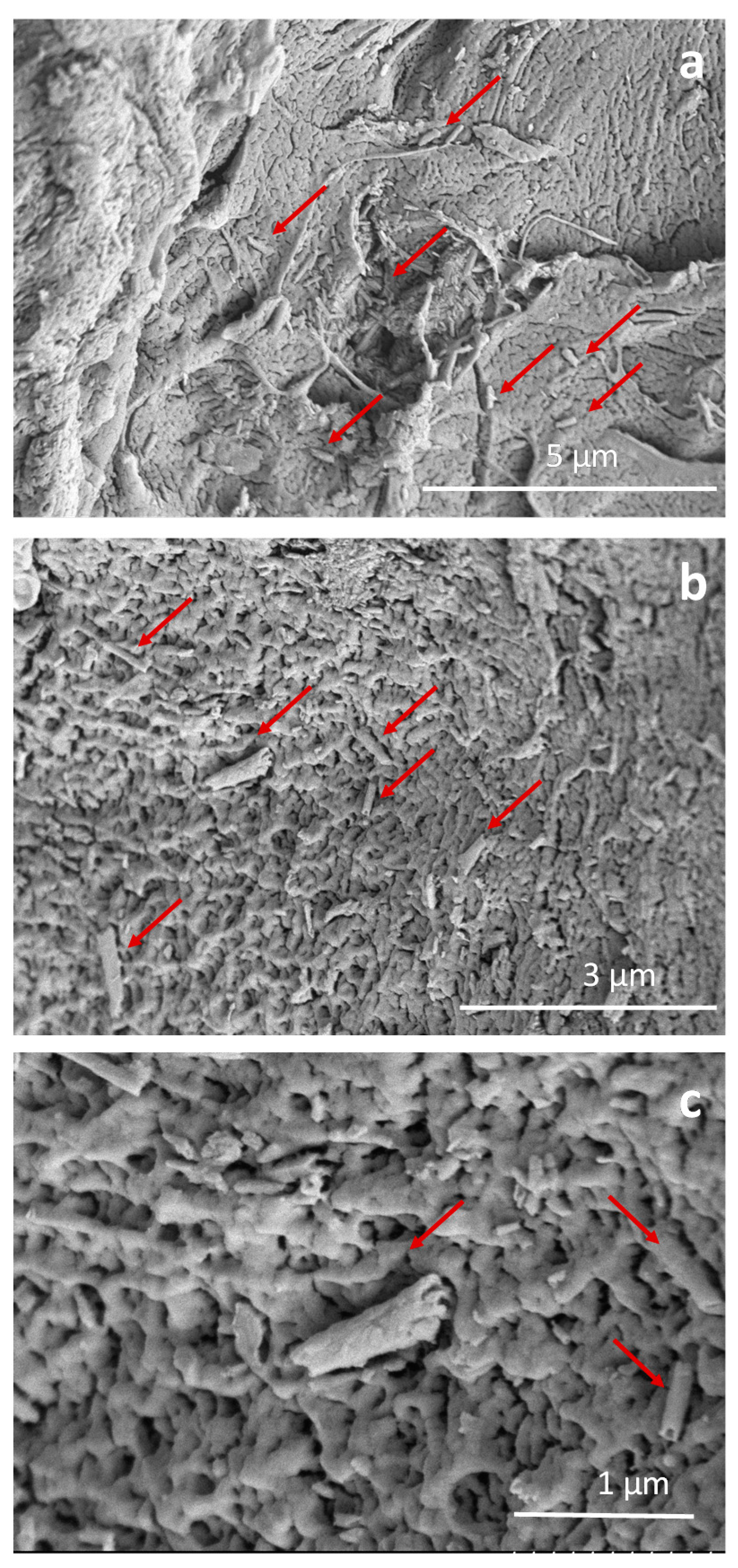
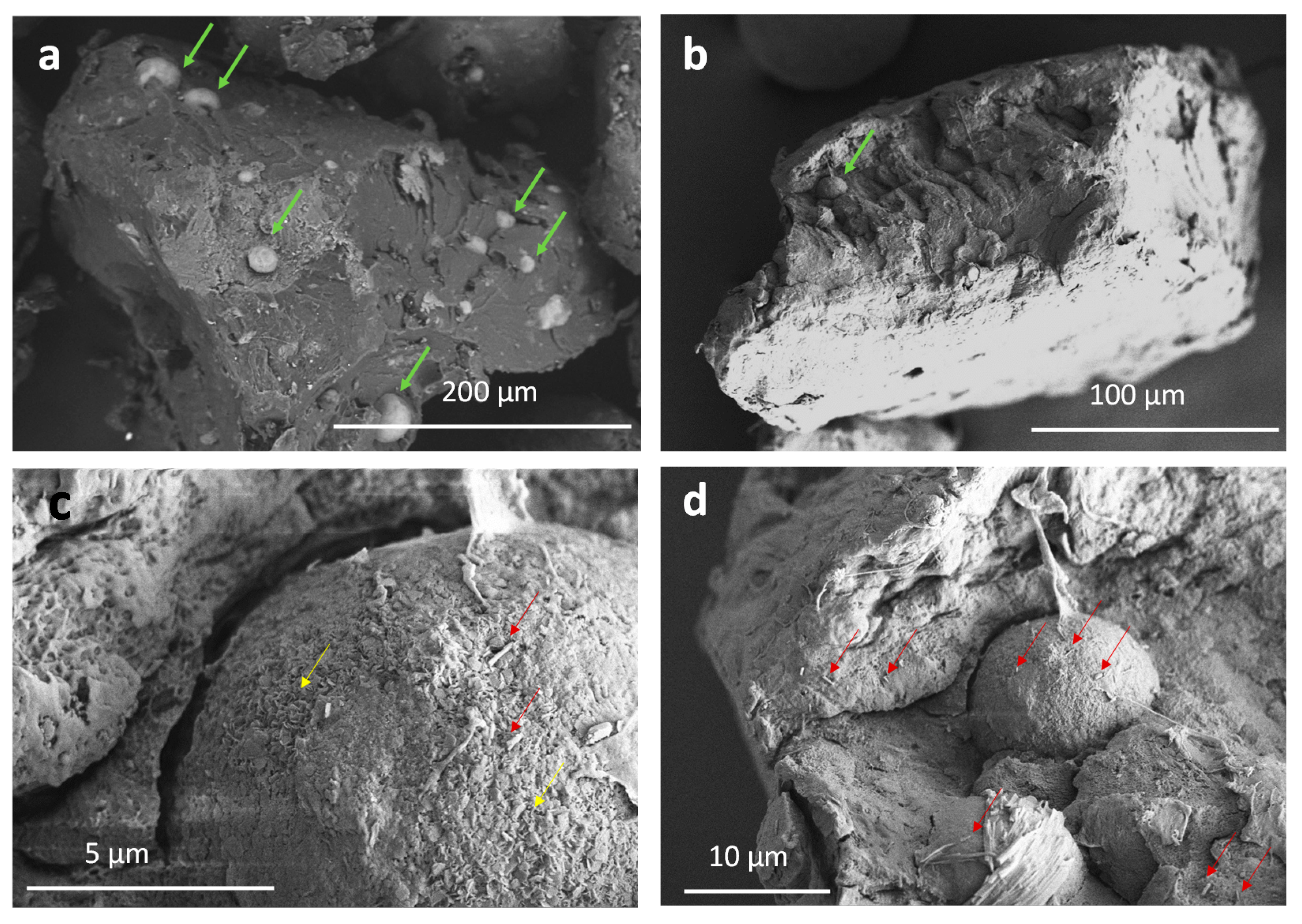
3.1. Drug Delivery in Composite Granules
3.2. Drug Delivery in Composites Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aaseth, J.; Boivin, G.; Andersen, O. Osteoporosis and trace elements—An overview. J. Trace Elem. Med. Biol. 2012, 26, 149–152. [Google Scholar] [CrossRef]
- Marx, D.; Rahimnejad Yazdi, A.; Papini, M.; Towler, M. A review of the latest insights into the mechanism of action of strontium in bone. Bone Rep. 2020, 12, 100273. [Google Scholar] [CrossRef]
- Pilmane, M.; Salma-Ancane, K.; Loca, D.; Locs, J.; Berzina-Cimdina, L. Strontium and strontium ranelate: Historical review of some of their functions. Mater. Sci. Eng. C 2017, 78, 1222–1230. [Google Scholar] [CrossRef]
- Kyllönen, L.; D’Este, M.; Alini, M.; Eglin, D. Local drug delivery for enhancing fracture healing in osteoporotic bone. Acta Biomater. 2015, 11, 412–434. [Google Scholar] [CrossRef]
- Deepthi, S.; Abdul Gafoor, A.A.; Sivashanmugam, A.; Nair, S.V.; Jayakumar, R. Nanostrontium ranelate incorporated injectable hydrogel enhanced matrix production supporting chondrogenesis in vitro. J. Mater. Chem. B 2016, 4, 4092–4103. [Google Scholar] [CrossRef]
- Loca, D.; Smirnova, A.; Locs, J.; Dubnika, A.; Vecstaudza, J.; Stipniece, L.; Makarova, E.; Dambrova, M. Development of local strontium ranelate delivery systems and long term in vitro drug release studies in osteogenic medium. Sci. Rep. 2018, 8, 16754. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, W.; Wu, S.; Kuss, M.; Jiang, X.; Untrauer, J.B.; Reid, S.P.; Duan, B. 3D printed composite scaffolds with dual small molecule delivery for mandibular bone regeneration. Biofabrication 2020, 12, 035020. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.P.; Sindhu, M.; Nair, P.D. Polycaprolactone-laponite composite scaffold releasing strontium ranelate for bone tissue engineering applications. Colloids Surf. B. Biointerfaces 2016, 143, 423–430. [Google Scholar] [CrossRef]
- Abdollahi Boraei, S.B.; Nourmohammadi, J.; Bakhshandeh, B.; Dehghan, M.M.; Gholami, H.; Calle Hernández, D.; Gonzalez, Z.; Ferrari, B. Enhanced osteogenesis of gelatin-halloysite nanocomposite scaffold mediated by loading strontium ranelate. Int. J. Polym. Mater. Polym. Biomater. 2020, 1–11. [Google Scholar] [CrossRef]
- Abdollahi Boraei, S.B.; Nourmohammadi, J.; Sadat Mahdavi, F.; Yus, J.; Ferrandez-Montero, A.; Sanchez-Herencia, A.J.; Gonzalez, Z.; Ferrari, B. Effect of SrR delivery in the biomarkers of bone regeneration during the in vitro degradation of HNT/GN coatings prepared by EPD. Colloids Surf. B Biointerfaces 2020, 190, 110944. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloy. 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Ferrandez-Montero, A.; Lieblich, M.; Benavente, R.; González-Carrasco, J.L.; Ferrari, B. New approach to improve polymer-Mg interface in biodegradable PLA/Mg composites through particle surface modification. Surf. Coat. Technol. 2020, 383, 125285. [Google Scholar] [CrossRef]
- Ferrández-Montero, A.; Lieblich, M.; Benavente, R.; González-Carrasco, J.L.; Ferrari, B. Study of the matrix-filler interface in PLA/Mg composites manufactured by Material Extrusion using a colloidal feedstock. Addit. Manuf. 2020, 33, 101142. [Google Scholar] [CrossRef]
- Cifuentes, S.C.C.; Gavilán, R.; Lieblich, M.; Benavente, R.; González-Carrasco, J.L.L. In vitro degradation of biodegradable polylactic acid/magnesium composites: Relevance of Mg particle shape. Acta Biomater. 2016, 32, 348–357. [Google Scholar] [CrossRef]
- Xu, L.; Yamamoto, A. In vitro degradation of biodegradable polymer-coated magnesium under cell culture condition. Appl. Surf. Sci. 2012, 258, 6353–6358. [Google Scholar] [CrossRef]
- Cifuentes, S.C.; Bensiamar, F.; Gallardo-Moreno, A.M.; Osswald, T.A.; González-Carrasco, J.L.; Benavente, R.; González-Martín, M.L.; García-Rey, E.; Vilaboa, N.; Saldaña, L. Incorporation of Mg particles into PDLLA regulates mesenchymal stem cell and macrophage responses. J. Biomed. Mater. Res. Part A 2016, 104, 866–878. [Google Scholar] [CrossRef]
- Brown, A.; Zaky, S.; Ray Jr, H.; Sfeir, C. Porous magnesium/PLGA composite scaffolds for enhanced bone regeneration following tooth extraction. Acta Biomater. 2015, 11, 543–553. [Google Scholar] [CrossRef]
- Ferrández-Montero, A.; Lieblich, M.; González-Carrasco, J.L.; Benavente, R.; Lorenzo, V.; Detsch, R.; Boccaccini, A.R.; Ferrari, B. Development of biocompatible and fully bioabsorbable PLA/Mg films for tissue regeneration applications. Acta Biomater. 2019. [Google Scholar] [CrossRef]
- Fernández-Calderón, M.C.; Romero-Guzmán, D.; Ferrández-Montero, A.; Pérez-Giraldo, C.; González-Carrasco, J.L.; Lieblich, M.; Benavente, R.; Ferrari, B.; González-Martín, M.L.; Gallardo-Moreno, A.M. Impact of PLA/Mg films degradation on surface physical properties and biofilm survival. Colloids Surf. B Biointerfaces 2020, 185, 110617. [Google Scholar] [CrossRef]
- Cifuentes, S.C.; Frutos, E.; Benavente, R.; Lorenzo, V.; González-Carrasco, J.L. Assessment of mechanical behavior of PLA composites reinforced with Mg micro-particles through depth-sensing indentations analysis. J. Mech. Behav. Biomed. Mater. 2017, 65, 781–790. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, H.; Ni, J.; Zhang, S.; Zhang, X. Development of PLA/Mg composite for orthopedic implant: Tunable degradation and enhanced mineralization. Compos. Sci. Technol. 2017, 147, 8–15. [Google Scholar] [CrossRef]
- Li, X.; Chu, C.; Wei, Y.; Qi, C.; Bai, J.; Guo, C.; Xue, F.; Lin, P.; Chu, P.K. In vitro degradation kinetics of pure PLA and Mg/PLA composite: Effects of immersion temperature and compression stress. Acta Biomater. 2017, 48, 468–478. [Google Scholar] [CrossRef]
- Shuai, C.; Li, Y.; Feng, P.; Guo, W.; Yang, W.; Peng, S. Positive feedback effects of Mg on the hydrolysis of poly-l-lactic acid (PLLA): Promoted degradation of PLLA scaffolds. Polym. Test. 2018, 68, 27–33. [Google Scholar] [CrossRef]
- Fernández, J.M.; Molinuevo, M.S.; McCarthy, A.D.; Cortizo, A.M. Strontium ranelate stimulates the activity of bone-specific alkaline phosphatase: Interaction with Zn2+ and Mg2+. BioMetals 2014, 27, 601–607. [Google Scholar] [CrossRef]
- Hu, D.; Li, K.; Xie, Y.; Pan, H.; Zhao, J.; Huang, L.; Zheng, X. Different response of osteoblastic cells to Mg2+, Zn2+ and Sr2+ doped calcium silicate coatings. J. Mater. Sci. Mater. Med. 2016, 27, 56. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Pascall, A.J.; Dudukovic, N.; Worsley, M.A.; Kuntz, J.D.; Duoss, E.B.; Spadaccini, C.M. Colloidal Materials for 3D Printing. Annu. Rev. Chem. Biomol. Eng. 2019, 10, 17–42. [Google Scholar] [CrossRef]
- Sangiorgi, A.; Gonzalez, Z.; Ferrandez-Montero, A.; Yus, J.; Sanchez-Herencia, A.J.; Galassi, C.; Sanson, A.; Ferrari, B. 3D printing of photocatalytic filters using a biopolymer to immobilize TiO2 nanoparticles. J. Electrochem. Soc. 2019, 166. [Google Scholar] [CrossRef]
- Gonzalez, Z.; Yus, J.; Sanchez-Herencia, A.J.; Dewalque, J.; Manceriu, L.; Henrist, C.; Ferrari, B. A colloidal approach to prepare binder and crack-free TiO2 multilayer coatings from particulate suspensions: Application in DSSCs. J. Eur. Ceram. Soc. 2019, 39, 366–375. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Heim, H.P.; Feldmann, M. Effects of surface modification on dispersion, mechanical, thermal and dynamic mechanical properties of injection molded PLA-hydroxyapatite composites. Compos. Part A 2017, 103, 96–105. [Google Scholar] [CrossRef]
- Guo, M.; Muhammad, F.; Wang, A.; Qi, W.; Wang, N.; Guo, Y.; Wei, Y.; Zhu, G. Magnesium hydroxide nanoplates: A pH-responsive platform for hydrophobic anticancer drug delivery. J. Mater. Chem. B 2013, 1, 5273–5278. [Google Scholar] [CrossRef] [PubMed]
- Muthu, R.N.; Rajashabala, S.; Kannan, R. Synthesis, characterization of hexagonal boron nitride nanoparticles decorated halloysite nanoclay composite and its application as hydrogen storage medium. Renew. Energy 2016, 90, 554–564. [Google Scholar] [CrossRef]
- Gouda, R.; Baishya, H.; Qing, Z. Application of Mathematical Models in Drug Release Kinetics of Carbidopa and Levodopa ER Tablets. J. Dev. Drugs 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G.; Turrini, F.; Domenicotti, C. Dendrimer Nanodevices and Gallic Acid as Novel Strategies to Fight Chemoresistance in Neuroblastoma Cells. Nanomaterials 2020, 10, 1243. [Google Scholar] [CrossRef]
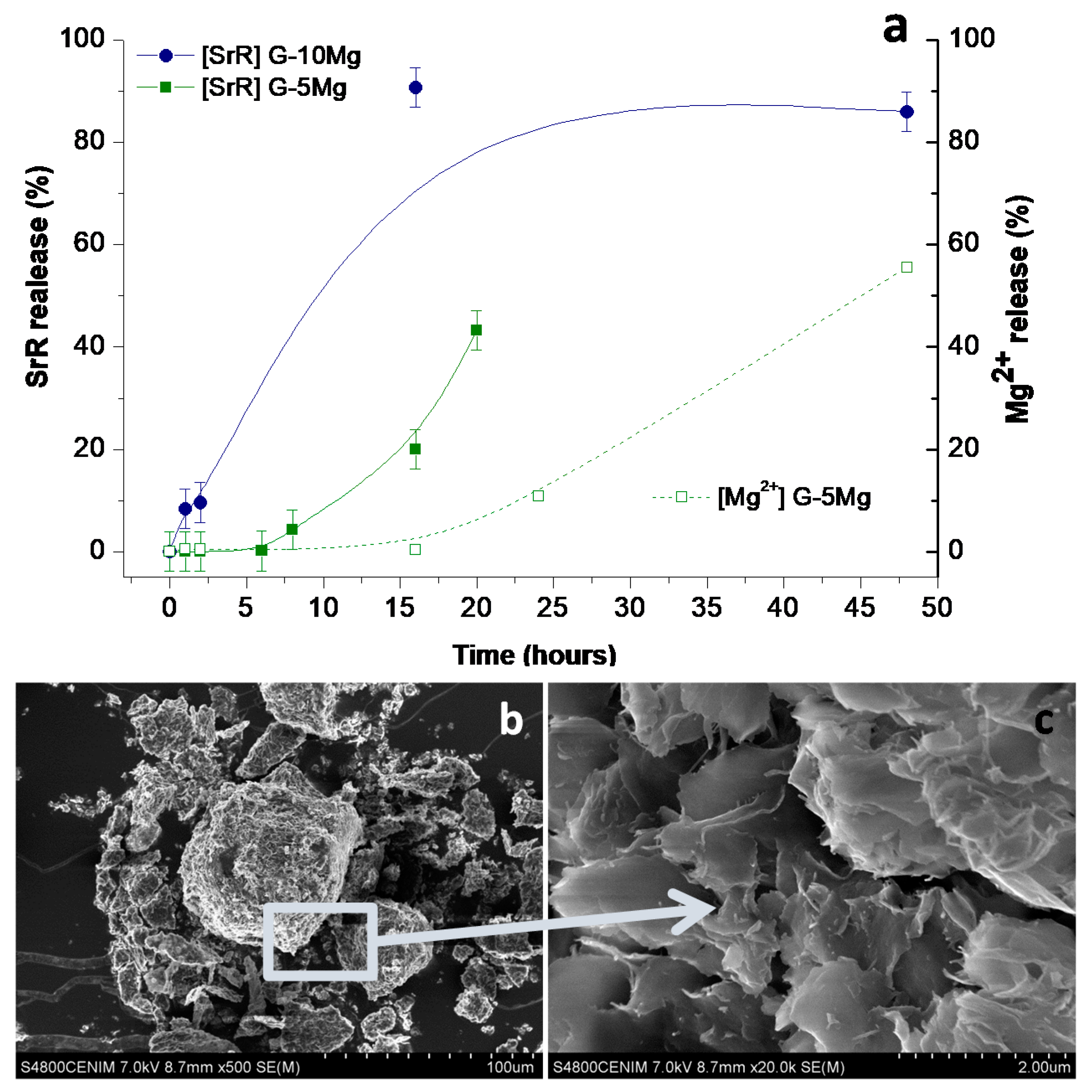
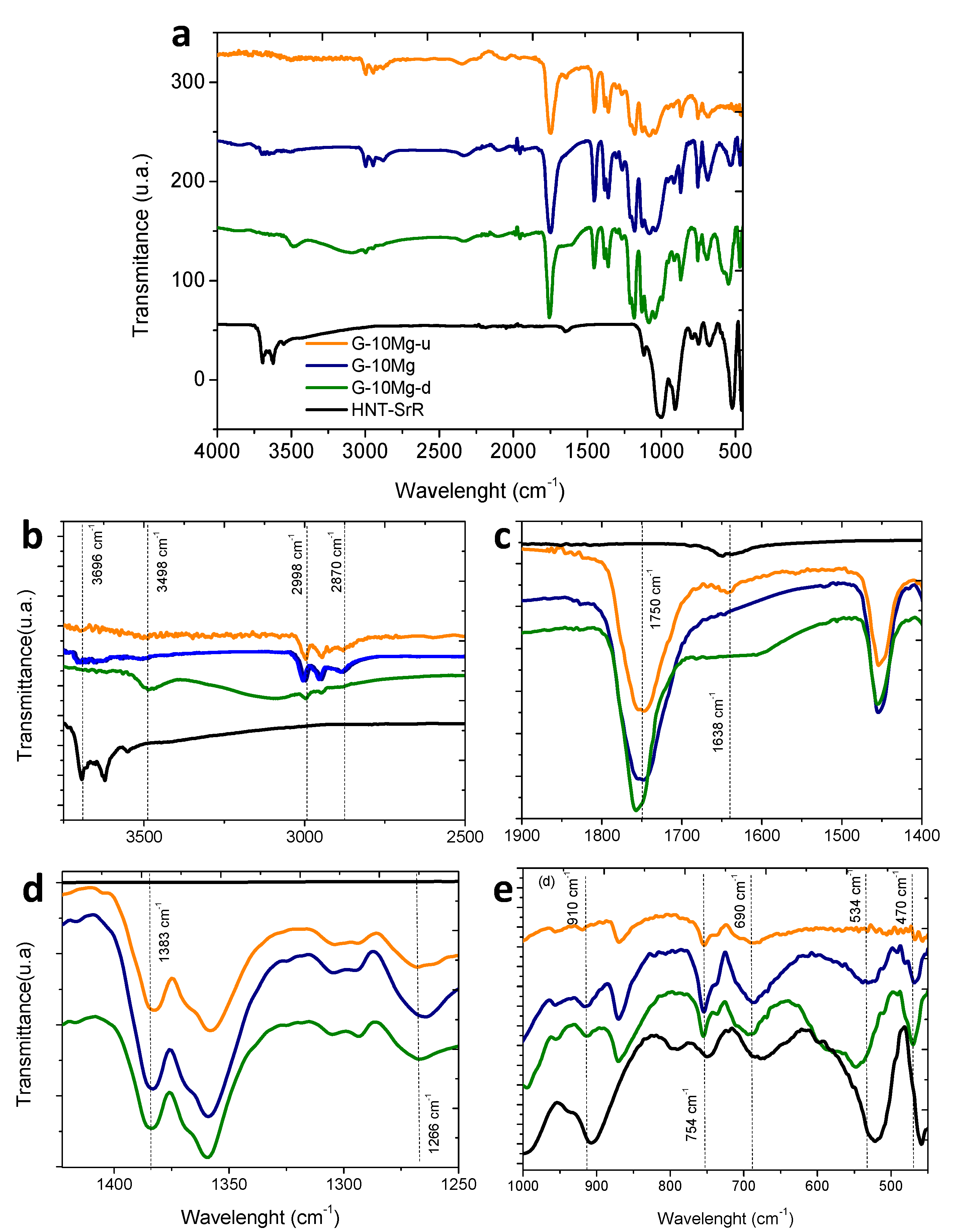


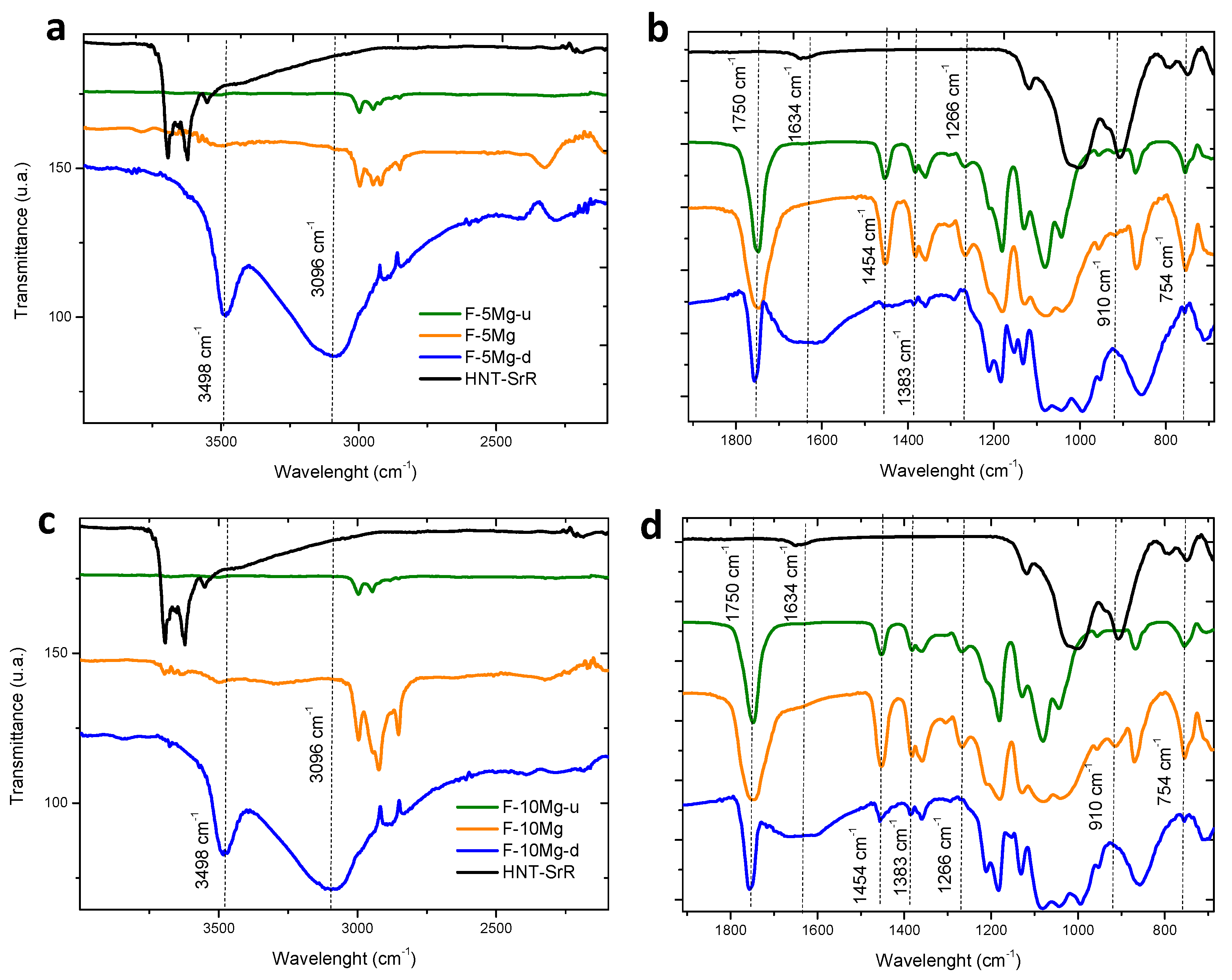
| Nomenclature | Composite Formulation and Feature (Weight Basis) |
|---|---|
| G-HNT | Granules of 96% PLA/4% HNT |
| G-5Mg | Granules of 91% PLA/5%Mg/4% HNT–SrR (3.88% HNT + 0.12% SrR) |
| G-10Mg | Granules of 86% PLA/10%Mg/4% HNT–SrR (3.88% HNT + 0.12% SrR) |
| F-5Mg | Films of 91% PLA/5%Mg/4% HNT–SrR (3.88% HNT + 0.12% SrR) |
| F-10Mg | Films of 86% PLA/10%Mg/4% HNT–SrR (3.88% HNT + 0.12% SrR) |
| F-10Mg | F-5Mg | ||||
|---|---|---|---|---|---|
| SrR Release (%) | Time (h) | Kinetics (%/h) | SrR Release (%) | Time (h) | Kinetics (%/h) |
| up to 23 | 38 | 0.53 | up to 45 | 142 | 0.31 |
| up to 52 | 270 | 0.11 | up to 72 | 310 | 0.16 |
| up to 64 | 575 | 0.04 | up to 77 | 525 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrández-Montero, A.; Eguiluz, A.; Vazquez, E.; Guerrero, J.D.; Gonzalez, Z.; Sanchez-Herencia, A.J.; Ferrari, B. Controlled SrR Delivery by the Incorporation of Mg Particles on Biodegradable PLA-Based Composites. Polymers 2021, 13, 1061. https://doi.org/10.3390/polym13071061
Ferrández-Montero A, Eguiluz A, Vazquez E, Guerrero JD, Gonzalez Z, Sanchez-Herencia AJ, Ferrari B. Controlled SrR Delivery by the Incorporation of Mg Particles on Biodegradable PLA-Based Composites. Polymers. 2021; 13(7):1061. https://doi.org/10.3390/polym13071061
Chicago/Turabian StyleFerrández-Montero, Ana, Alvaro Eguiluz, Elena Vazquez, Joab David Guerrero, Zoilo Gonzalez, Antonio Javier Sanchez-Herencia, and Begoña Ferrari. 2021. "Controlled SrR Delivery by the Incorporation of Mg Particles on Biodegradable PLA-Based Composites" Polymers 13, no. 7: 1061. https://doi.org/10.3390/polym13071061
APA StyleFerrández-Montero, A., Eguiluz, A., Vazquez, E., Guerrero, J. D., Gonzalez, Z., Sanchez-Herencia, A. J., & Ferrari, B. (2021). Controlled SrR Delivery by the Incorporation of Mg Particles on Biodegradable PLA-Based Composites. Polymers, 13(7), 1061. https://doi.org/10.3390/polym13071061