Abstract
Polymeric membranes are employed in guided bone regeneration (GBR) as physical barriers to facilitate bone in-growth. A bioactive and biomimetic membrane with the ability to participate in the healing and regeneration of the bone is necessary. The aim of the present study was to analyze how novel silicon dioxide composite membranes functionalized with zinc or doxycycline can modulate the osteoblasts’ proliferation, differentiation, and expression of selected antigenic markers related to immunomodulation. Nanostructured acrylate-based membranes were developed, blended with silica, and functionalized with zinc or doxycycline. They were subjected to MG63 osteoblast-like cells culturing. Proliferation was assessed by MTT-assay, differentiation by evaluating the alkaline phosphatase activity by a spectrophotometric method and antigenic phenotype was assessed by flow cytometry for selected markers. Mean comparisons were conducted by one-way ANOVA and Tukey tests (p < 0.05). The blending of silica nanoparticles in the tested non-resorbable polymeric scaffold improved the proliferation and differentiation of osteoblasts, but doxycycline doped scaffolds attained the best results. Osteoblasts cultured on doxycycline functionalized membranes presented higher expression of CD54, CD80, CD86, and HLA-DR, indicating a beneficial immunomodulation activity. Doxycycline doped membranes may be a potential candidate for use in GBR procedures in several challenging pathologies, including periodontal disease.
1. Introduction
Guided bone regeneration (GBR) is a frequently used surgical technique. GBR techniques are intended to obtain enough alveolar bone volume to accomplish a successful therapy with osseointegrated dental implants. In order to achieve this goal, the use of a membrane, that should not only act as a physical barrier but also as a modulatory barrier, protecting the growth of osteoblastic cells is required [1]. GBR is a tissue engineering therapy that greatly relies on the appropriate selection of the biomaterial. Commercially available membranes can be resorbable or non-resorbable [2]. The shortcoming for resorbable membranes is mainly a lack of space maintenance due to short resorption times. On the other hand, for non-resorbable membranes, higher rates of wound dehiscences and the need for a second surgery in order to retrieve the membrane are the main drawbacks, as these kinds of membranes do not integrate with the host bone tissue [3].
The search for new osteogenic, bioactive, and biomimetic non-resorbable membranes that integrate with the bone tissue structure is crucial. The electrospinning technique can be used for obtaining scaffolds with attractive features, such as the similarity to the extracellular matrix [4], and may also be successfully used for drug delivery and tissue regeneration [5,6]. Recently, a new membrane barrier based on the electrospun of a polymer mixture of (MMA)1-co-(HEMA)1 and (MA)3-co-(HEA)2 has been developed. These nanofibrous scaffolds closely mimic the bone collagen matrix (fibers with diameters ranging from 50 to 500 nm) [7]. The composite membranes were formed with silica nanoparticles and functionalized with zinc or doxycycline. These new composite membranes combine the mechanical properties of polymeric materials, the bioactivity of SiO2-NPs [7], osteogenicity provided by the doped zinc [8], and antibacterial properties conferred by the doxycycline [9]. Moreover, zinc has been tested in vivo and in vitro studies, concluding that it plays a crucial role in inducing the viability and proliferation of bone cells as well as enhancing new bone formation and mineralization of extracellular matrix [8,10,11]. Similarly, doxycycline appears to enhance maturation and cells’ osteogenic capacity [7,12,13].
These novel membranes should also be tested on the cell’s environment. It would be desirable that the membranes favored osteoblast proliferation and differentiation. It may be positive that the membranes could contribute to a positive immunomodulation, hindering the establishment of a pro-inflammatory atmosphere and hampering the penetration of pro-inflammatory cells and cytokines, thus improving tissue regeneration [14]. A growing body of evidence has been found about the immunoregulatory potential of biomaterials. It has been already shown that interactions of osteoblasts with biomaterials can regulate the extent of the response initiated by macrophages, mainly by osteoblastic release of interleukin-6, prostaglandin-E2, or granulocyte macrophage colony-stimulating factor [15,16]. The bone and immune systems are closely related through cellular and molecular interactions [17]. Several regulatory molecules are shared by the immune and skeletal systems, which include cytokines, receptors, signaling molecules, and transcription factors [17,18].
The cluster of differentiation (CD) is a protocol used for the identification of cells’ surface antigens, providing targets for immunophenotyping of cells. In terms of physiology, CD molecules can act in numerous ways, often acting as important receptors or ligands to the cell. The behavior of the cell is usually modified by CD proteins playing a role in cell signaling or other functions, such as cell adhesion. Assessing CD osteoblasts’ antigenic phenotype is possible by means of flow cytometry. It is an extremely robust technique that has been successfully implemented to probe phenotypic cellular activity in living cells. Namely, the in vitro immunomodulatory properties of bone cells may be ascertained by determining the deficiency or overexpression of the related CD antigenic markers of interest at a specific time point of cells’ differentiation stages. It may provide information about cells’ potential role in immunomodulation [19].
The aim of the present study was to analyze the proliferation, differentiation potential, and immunomodulation ability of osteoblastic cells cultured on silicon dioxide composite membranes functionalized with zinc or doxycycline. The null hypothesis is that zinc or doxycycline addition on membranes does not affect the proliferation, differentiation, and antigenic phenotype expression of the cultured osteoblasts.
2. Materials and Methods
2.1. Preparation of Nanostructured Polymeric Membranes
Nanostructured membranes were produced by electrospinning using a novel polymeric blend: (MMA)1-co-(HEMA)1/(MA)3-co-(HEA)2 50/50 wt.%, doped with 5 wt.% of SiO2-NPs (NanomyP®, Granada, Spain). The membranes were incubated for 2 h in a sodium carbonate buffer solution (333 mM; pH = 12.5), so as to activate them with carboxyl groups (HOOC-Si-M). The partial hydrolysis of ester bonds and the disposal of carboxyl groups on the surface of the artificial tissue made this functionalization possible [20]. The membranes were then rinsed with distilled water and dried using a vacuum oven [21]. Secondly, the membranes were functionalized with zinc or doxycycline (Dox). Zinc was incorporated using the ability of carboxyl groups to complex divalent cations. On the other hand, Dox was immobilized on the membranes by acid–base reactions between amino groups of Dox and carboxyl groups present in the membranes. HOOC-Si-M were immersed under continuous shaking at room temperature and in aqueous solutions (pH = 7) of both 330 mgL−1 of ZnCl2 or 800 mgL−1 of Dox [7]. Four different membranes were tested: (1) non-functionalized and SiO2-NPs undoped membrane (HOOC-M), (2) SiO2-NPs doped membrane (HOOC-Si-M), (3) SiO2-NPs doped membrane functionalized with Zn (Zn-HOOC-Si-M), and (4) SiO2-NPs doped membrane functionalized with Dox (Dox-HOOC-Si-M). The membranes were placed at the bottom of a 24-well plate (Falcon, Becton Dickinson Labware, Franklin Lakes, NJ, USA) and sterilized using an ultraviolet radiation sterilization desk (J.P. SELECTA, Barcelona, Spain).
2.2. Cell Culture
The human MG63 osteosarcoma cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). This cell line has been widely used as an osteoblast model since it shares most of the same characteristics with primary human osteoblasts. MG63 osteoblast-like cells have no interspecies differences with primary human osteoblasts, they have a shorter isolation time and there is unlimited accessibility [1]. The MG63 cell line was maintained in Dulbecco’s modified Eagle medium (DMEM; Invitrogen Gibco Cell Culture Products, Carlsbad, CA, USA) with penicillin 100 IU/mL (Lab Roger SA, Barcelona, Spain), gentamicin 50 mg/mL (Braum Medical SA, Jaen, Spain), amphotericin B 2.5 mg/mL (Sigma, St. Louis, MO, USA), 1% glutamine (Sigma), and 2% HEPES (Sigma) supplemented with 10% of fetal bovine serum (FBS; Gibco, Paisley, UK), as described by Díaz Rodríguez et al. [22]. Cultures were kept in a humidified atmosphere at 37 °C of 95% air and 5% CO2. Cells were detached from the flask using 0.05% trypsin (Sigma) and 0.02% ethylenediaminetetraacetic acid (EDTA; Sigma) solution. After this process, they were rinsed and resuspended in complete culture medium with 10% FBS as described by Manzano-Moreno et al. [19].
2.3. Cell Proliferation Assay
Osteoblasts were seeded at 1 × 104 cells/mL per well onto the membranes placed inside the 24-well plate. The cells were then cultured in a humid atmosphere of 95% air and 5% CO2 at 37 C for 48 h. After this time, cell proliferation was assessed by means of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay. For this purpose, the media was replaced by phenol red-free DNEM with MTT 0.5 mg/mL (Sigma) and incubated for 4 h. MTT cellular reduction resulted in the formation of insoluble crystal deposits of formazan that were dissolved by adding dimethyl sulfoxide (Merck Biosciences, Darmstadt, Germany). Absorbance was then measured with a spectrophotometer (Sunrise, Tecan, Männedorf, Switzerland) at 570 nm [23]. The results were expressed as mean absorbance ± standard deviation (SD). At least three experiments were conducted for each type of membrane.
2.4. Field Emission Scanning Electron Microscopy (FESEM)
Osteoblasts were also seeded at 1 × 104 cells/mL per well onto the membranes placed inside the 24-well plate. The cells were then cultured in a humid atmosphere of 95% air and 5% CO2 at 37 °C for 48 h. After this time, two membranes of each experimental group were submitted to FESEM (GEMINI, Carl Zeiss SMT, Oberkochen, Germany) observation. Samples were first critical point dried and covered with carbon.
2.5. Alkaline Phosphatase Activity
Early osteoblasts differentiation was assessed by evaluating the alkaline phosphatase (ALP) activity, a colorimetric assay (Diagnostic kit 104-LL, Sigma). This method, measures the conversion of the colorless substrate p-nitrophenyl phosphate to the yellow p-nitrophenol, accomplished by the ALP enzyme. The rate of color shift corresponded with the amount of enzyme present in the culture. Standards of p nitrophenol (0–250 μM) were prepared from dilutions of a 1000 μM stock solution and assayed in parallel. The ALP assay was performed as previously described by Manzano-Moreno et al. [23]. In brief, cell cultures, for 72 h on the membranes placed inside the 24-well plate, were trypsinized (0.05% trypsin, 1 mM EDTA, (Invitrogen Gibco)) and lysed in 100 μL 1 M Tris pH 8.00 by ultrasonification for 4 min. Then, the suspension was mixed with a 7.6 mM p-nitrophenyl phosphate solution at a proportion of 1:10 and incubated for 15 min at 37 °C. Substrate solution was prepared by merging an aqueous solution of 4 mg/mL of 4-nitrophenyl phosphate disodium salt (Sigma) with an equal volume of 1.5 M alkaline buffer (Sigma). The reaction was stopped by adding 1 mL 0.05 N NaOH, and the final absorbance was measured with a spectrophotometer (Sunrise, Tecan, Männedorf, Switzerland) at 405 nm. The total protein content was estimated by the Bradford method using a protein assay kit from Bio-Rad Laboratories (Bio-Rad Laboratories, Nazareth-Eke, Belgium). All samples were conducted in triplicate.
2.6. Antigenic Phenotype
Antigenic phenotype was assessed by flow cytometry at 48 h of culture using 0.05% trypsin (Sigma) and 0.02% EDTA (Sigma) solution 0.4% EDTA solution. They were then washed and suspended in PBS at a concentration of 2 × 104 cells/mL. Osteoblasts were labeled by direct staining with anti-CD54, CD80, CD86, and HLA-DR monoclonal antibodies (MAbs); CD54/IOL1b, CD80, CD86, and OKDR, respectively (Invitrogen Corp). 100 μL of cell suspension were incubated with 10 μL of each MAb for 30 min at 4 °C in the absence of light. Cells were rinsed and re-suspended in PBS 1 mL, and analyzed at a wavelength of 488 nm in a flow cytometer with a diode laser (FACSCanton II, Becton-Dickinson, Palo Alto, CA, USA) to determine the percentage of fluorescent cells [24].
2.7. Phagocytic Activity
Phagocytic activity was also studied by flow cytometry following the method described by Díaz-Rodriguez et al. [25]. In brief, cultured human MG-63 osteosarcoma cells were cultured for 48 h on the tested membranes. After this time, they were detached from the membranes, washed, and then suspended in complete culture medium with 10% FBS at a density of 2 × 104 cells/mL. Cells were treated by direct staining with labeled latex beads; a 100-µL cell suspension was incubated with 5 μL carboxylated FICT-labeled latex beads 2 μL in diameter (Sigma) for 90 min in darkness at 37 °C. Cells were washed, suspended in 1 mL of PBS, and analyzed in a flow cytometer (Facs Vantage; Becton–Dickinson, Palo Alto, CA, USA). Results were expressed as a percentage of cells positive for phagocytosis and mean channel fluorescence, which correlates with the number of particles phagocytosed by the cells. Tests were performed in triplicate and control assays were carried out at 4 °C, to eliminate the background fluorescence. Results were obtained as the percentage of cells positive for phagocytosis.
2.8. Statistical Analysis
Mean comparisons were conducted by one-way ANOVA and Student–Newman–Keuls multiple comparisons. Significance was set at p < 0.05. Data were expressed as means ± standard deviation (SD) in all cases. Before ANOVA, normal distribution was probed by Kolmogorv–Smirnov tests (p > 0.05).
3. Results
3.1. Cell Proliferation Assay
Mean and standard deviations of osteoblasts cell proliferation determined by the MTT assay are presented in Figure 1. Significant differences were found between all tested groups and attained means follow the trend: COOH-M < HOOC-Si-M < Zn-HOOC-Si-M < Dox-HOOC-Si-M.
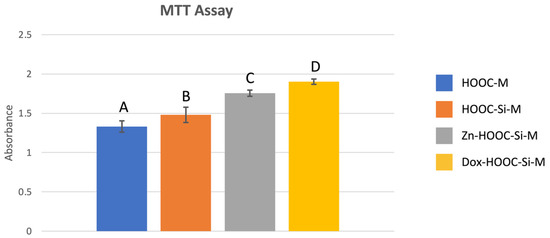
Figure 1.
Absorbance mean values and standard deviations obtained after the MTT assay for the different membranes. Distinct letter indicates significant difference between membranes after ANOVA and Student–Newman–Keuls multiple comparisons (p < 0.05).
3.2. Field Emission Scanning Electron Microscopy (FESEM)
Selected images from the FESEM analysis are displayed in Figure 2 and Figure 3. Osteoblasts cultured on COOH-Si-M displayed an elongated spindle-shaped morphology and a similar situation was observed for those grown on Zn-COOH-Si-M. By contrast, osteoblasts on COOH-M are round shaped. For osteoblasts on the Dox-COOH-Si-M the elongated morphology was accompanied by larger and more cytoplasmic membrane protrusions compared with those grown on the other membranes. There also exists an alignment of the elongated cells on the tested surfaces, which provided large bio-adhesive areas for the cells. Cell spreading and cell layer thickness is lower at COOH-M than in the other membranes.
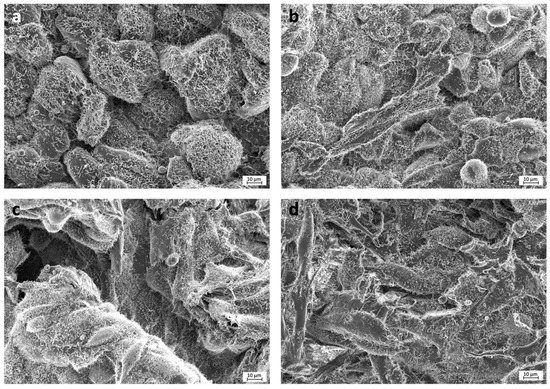
Figure 2.
FESEM images of the experimental membranes (a) COOH-M, (b) COOH-Si-M, (c) Zn-COOH-Si, and (d) Dox-COOH-Si-M. Cells seeded on COOH-M are round shaped and filapodia are not observed. In the rest of the membranes, elongated cells are observed, some filapodia may also be detected emerging from osteoblasts cytoplasm. Osteoblasts are covered by fibrilar substance and rounded mineral deposits.
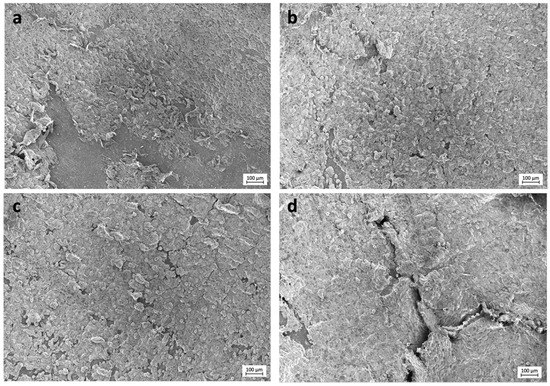
Figure 3.
FESEM images of the experimental membranes (a) COOH-M, (b) COOH-Si-M, (c) Zn-COOH-Si-M, and (d) Dox-COOH-Si-M. Several aligned osteoblasts connected to each other may be seen. Thicker layers of osteoblasts, constituting a tri-dimensional structure, are evidenced on Zn-COOH-Si-M and Dox-COOH-Si-M.
3.3. Alkaline Phosphatase (AP) Activity
Mean and standard deviations of alkaline phosphatase expressed as international units (IU) of AP per mg of total proteins are presented in Figure 4. Significant differences were found between all tested groups and attained means follow the trend: COOH-M < HOOC-Si-M < Zn-HOOC-Si-M < Dox-HOOC-Si-M.
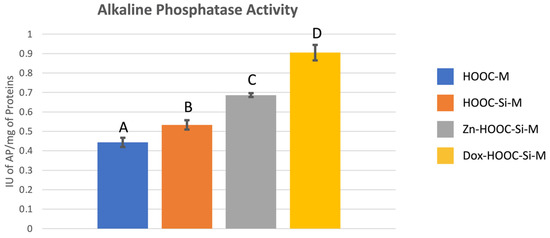
Figure 4.
Mean and standard deviation of international units of AP per mg of proteins values obtained with the different membranes. Distinct letter indicates significant difference between membranes after ANOVA and Student–Newman–Keuls multiple comparisons (p < 0.05).
3.4. Antigenic Phenotype
The characterization of cultured osteoblasts on the distinct membranes, for the presence of selected surface markers is presented in Figure 5. Osteoblasts cultured on doxycycline functionalized membranes presented higher expression of CD54, CD80, CD86, and HLA-DR (p < 0.05), than the rest of the groups. Differences were about (38%, 36%, 53%, and 64%, respectively). Zinc functionalization reduced CD54 expression on osteoblasts about 64% (p < 0.001).
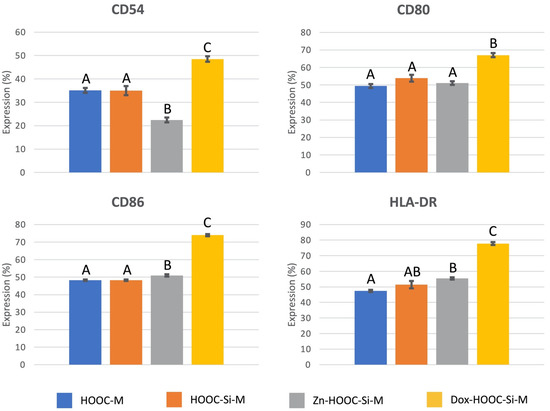
Figure 5.
CD-marker antigenic expression on osteoblasts, cultured on the different experimental membranes. Values are presented as percentage of cells expressing the antigen phenotype (CD54, CD80, CD86, and HLA-DR). ANOVA and Student-Newman-Keuls multiple comparisons were performed (p < 0.05). Distinct letter indicates significant difference between membranes and always compares each CD-marker independently.
3.5. Phagocytic Activity
Osteoblasts cultured on tested membranes were negative for phagocytosis, except for those osteoblasts grown on the COOH-M where 24.33% of cells were positive, and the standard deviation was 1.52%.
4. Discussion
This research manuscript implies an approach to the study of how osteoblasts’ proliferation and differentiation are affected by the different studied nanostructured polymeric membranes. MG-63 osteoblast-like cells were used for this in vitro study. They are one of the cell lines most widely used in literature since there are no interspecies differences with primary human osteoblasts, they have a shorter isolation time and there is unlimited accessibly [1,26]. However, the results need to be extrapolated with caution, taking into account that a tumor line may have an alternative pattern of differentiation from primary human osteoblasts [27,28]. The MTT assay and FESEM were used in order to quantitively measure osteoblasts’ proliferation while their differentiation potential was evaluated by means of alkaline phosphatase activity, antigenic phenotype expression, phagocytic activity, and FESEM.
The previous surface characterization of the present membranes demonstrated that the mean fiber diameter was of around 765 nm [7]. Taking into account that mineralized collagen fibrils are about 800 nm in human trabecular bone, these membranes imitate the osseous structure. This collagen bone mimicking has been shown to increase cell attachment on membranes by about 1.7 fold [29]. The best strategy when designing an ideal scaffold is trying to replicate the native tissue and the fibrillar structure, enhancing cellular attachment, proliferation, and differentiation [1,7].
For topical drug application, the in vitro drug release assay does not correlate with the expected drug activity. In these cases, product performance tests in similar conditions to the clinical scenario have been employed. The present methodology involves the determination of the drug activity on osteoblastic cells, considering the liberated drug and the amount of drug permeated into the cells. The effect on cells of the quantity of drug which is not liberated but remained doing its action on the functionalized membrane is also considered. Moreover, it should also be taken into account that membranes are non-resorbable and do not swell or dissolve. Therefore, doxycycline and zinc release is controlled by diffusion of the drug/ions through the liquid permeating the membrane, but as membranes are bioactive [7], an apatite layer can be formed on the surface, hindering the diffusion of the drug/ions to the surrounding tissue [30].
Osteoblasts’ proliferation increased on silica-doped membranes, but the best results were obtained on doxycycline functionalized membranes (Figure 1). It has been previously reported that tetracyclines promote the proliferation of osteoblasts [4]; but, the underlying mechanism is not completely understood. Two different events may explain this fact: (i) osteoblasts’ proliferation is usually enhanced in the presence of antioxidants, as reactive oxidant species diminish osteoblasts’ reproduction [31], and doxycycline presents a potent antioxidant effect [32,33]. (ii) Doxycycline possesses calcium chelating ability that may facilitate calcium deposit on membranes [7]. Increases in extracellular calcium concentration have been shown to stimulate osteoblastic lineage cells, leading to bone formation and osteoconductivity [34].
Osteoblasts with an elongated morphology were observed on the doxycycline doped membranes, with filapodia extensions from the cells to the substrate, producing an interlocked cell structure (Figure 2). This suggests that interplay between the cell and the membrane surface allows for enhanced dynamic propagation and an overall increase in osteoblast activation, as indicated by the filopodia. Correlations between cell morphology and both proliferation and metabolic activity have been previously stated [1,35,36]. Hence, osteoblast spreading apparently favored the metabolic activity and cell elongation favored proliferation, while cellular roundness decreased mitotic activity [35,36]. The same correlation was found in the present study, obtaining the highest osteoblasts proliferation for the Dox-COOH-Si-M. Rounded and less spread cell shape was found on the COOH-M (Figure 2), coinciding with a lower cell proliferation. The described correlation of cell spreading and proliferation has already been reported, not only for osteoblasts but for other cell types [35]. The cellular morphology seems to be also affected by the presence of silica, inducing a three-dimensional growth (Figure 3). This three-dimensional cellular network is the basis for the known in vitro earlier differentiation of osteoblasts and better osseointegration in vivo [37].
Regarding osteoblasts differentiation, it may be ascertained from phosphatase activity assays that the silica blending and doxycycline doping of the membranes extremely induces osteoblasts’ differentiation since it increased the phosphatase activity in about 100% respect COOM-M (p < 0.001). The AP activity was also enhanced by 50% when osteoblasts were seeded on Zn-COOH-Si-M when compared with COOH-M (p < 0.001). It could be also ascertained that regarding AP activity, Dox and Zn could be said to favor osteoblasts’ differentiation, as doping COOH-Si-M with Dox and Zn, raises the AP activity by 70 and 30%, respectively (p < 0.001 in both cases). In previous investigations, the modulation of genes related to the osteogenic functional capacity of osteoblastic cells exerted by the studied membranes was evaluated. One of these genes was the one encoding the alkaline phosphatase, and the same tendency that the observed in the present study was registered [28].
It was found that osteoblasts expressed major histocompatibility complex molecules as HLA-DR, and adhesion molecules as CD54, as well as some signaling or co-stimulatory molecules as CD80 and CD86 [38]. The expression of these molecules is up-regulated by the tested doxycycline-doped membranes (Figure 5). Expression of HLA-DR, which has also been described on osteoblasts obtained after mandibular surgery [39], is related to osteoblasts’ osteocalcin production when stimulated with 1,25-di-hydroxyvitamin D3 [40]. The co-stimulatory molecules CD80 and CD86 are withal expressed by antigen presenting cells and play pivotal roles in inducing T-cell immunity or tolerance [41,42]. CD80 may primarily inhibit the immune response, working on T-lymphocytes [43].
The co-stimulatory molecule CD86 is a marker expressed in different cell populations such as dendritic cells. To understand the meaning of its over-expression it should be taken into account that the differentiation of osteoclasts is tightly regulated by bone-forming osteoblasts [44]. Osteoblasts express cytokines essential for osteoclastic differentiation, like the receptor activator of NF-kB ligand (RANKL) [45,46], in response to osteotropic hormones and factors [47]. Doxycycline and minocycline have been previously shown to induce the production of dendritic cell markers, like CD86, in the presence of RANKL [48]. Osteoclast’s precursors express RANK (RANKL receptors) and differentiate into osteoclasts in response to RANKL emitted by osteoblasts [46,47]. But tetracyclines convert the differentiation pathway, resulting in dendritic-like cells rather than osteoclasts, in the presence of RANKL in vitro and in vivo [48]. As a result, tetracyclines prevent bone loss [48], but the mechanism involved is only partially known. Further research is required in this field. Doxycycline and minocycline inhibited RANKL, which induced osteoclastogenesis, however, no other clear effect on cell growth and phagocytic activity of osteoclasts has been described.
Zinc functionalization reduced CD54 expression on osteoblasts and doxycycline produced its over-expression (Figure 5). CD54 is an adhesion molecule and its presence on osteoblasts is well documented [39,49]. CD54 upregulation on osteoblastic cells has been shown to facilitate osteoclastogenesis [50,51]. CD54 is also known as intercellular adhesion molecule 1, a highly glycosylated immunoglobulin superfamily member that binds the leukocyte integrins. CD54 is constitutively expressed on leukocytes, epithelial, endothelial, and other cells, and it is up-regulated in response to inflammatory mediators [38,52]. CD54 is the mediating protein involved in the contact between mesenchymal stromal cells and macrophages. It has an essential role in regulating immunosuppressive properties of mesenchymal stromal cells [53]. When CD54 is over-expressed by cells, they become more immunosuppressive in inflammatory environments [54]. There is an unconventional but functional CD54-mediated interaction between pro-inflammatory macrophages (M1) and mesenquimal cells. This crosstalk modulates the immunosuppressive functions of mesenquimal cells and opens important perspectives in therapies for autoimmune and inflammatory diseases [53].
Doxycycline has previously been described as a potent anti-inflammatory and antioxidant substance [33]. These properties are related to beneficial doxycycline actions stated on alveolar tissue inflammatory infiltrates [13]. Oral diseases producing bone loss, such as periodontitis or periimplantitis, are characterized by inflammation and produce the activation of immunological cells leading to the release of pro-inflammatory cytokines and the recruitment of phagocytes and lymphocytes [55].
Phagocytosis is a biological cellular activity through which cells can protect themselves from infectious and non-infectious environmental particles. The process of phagocytosis has been previously identified in osteoblasts [25,56] and requires the recognition of ligands by specific receptors expressed by the phagocyte. Receptor engagement triggers intracellular signaling pathways that initiate appropriate innate immune and pro-inflammatory responses [57]. Osteoblasts grown on tested functionalized membranes possess a diminished phagocytic activity, as an additional sign of anti-inflammatory response to tested doped biomaterials. Just the COOH-M presented about 25% of osteoblasts positive to phagocytosis activity.
Biomaterial-mediated inflammatory response is crucial for bone regeneration. Excessive inflammation may lead to the formation of fibrous tissue, preventing bone cells from integrating with the membranes, resulting in the failure of bone regeneration. However, moderate inflammatory responses may enhance the recruitment and differentiation of osteoblasts, improving osteogenesis [58]. Further research with macrophages in co-culture is required determining immunomodulation and osteogenic differentiation.
Despite recent advancements, the therapeutic capability of biomaterials to regulate the osteoblastic cells-host immune system crosstalk, and the mechanism underlying this immunoregulation is poorly understood [14]. The most important limitation of the present study is the lack of mechanistic assays. When the application of detailed mechanistic assays is being intended [31], it may help to gain insight into a particular mechanism of action, but it also hinders the multi-regulatory processes that are necessary in the biological complexity. However, the efficient use of methods, enabling the identification of cells antigenic phenotype, has an enhanced probability of success in translation of new biomaterials through to the clinic. It presents a different perspective on signaling focused on integrated or holistic responses rather than the resolution of individual events. However, it is recognized that further screening efforts are necessary in order to discover an array that really allows the study and probing of detailed mechanistic studies, for example how these receptors are activated/inactivated and then to ascertain the dissection of cellular pathways. Rather than simplifying, the increasing number of tools should contribute to refine models and to cover further levels of biological complexity. Analogous to a puzzle built from multiple individual pieces, the full representation of cellular activity may transcend following the assembly of different functional outputs.
Since membrane exposure to the oral cavity and contamination of the surgical site are common problems, the linking of antimicrobial [9], anti-inflammatory, and bone regeneration property of doxycycline functionalized membrane makes it a favorable alternative therapeutic tool for GBR procedures prior to implant placement.
5. Conclusions
In the present study, it has been demonstrated that silica loading may offer beneficial effects to experimental membranes in terms of osteogenicity (osteoblasts proliferation and differentiation). Adding zinc to the membranes’ formulation showed an enhancement in the proliferation capacity of osteoblast. Furthermore, even better results were obtained when the scaffolds were functionalized with doxycycline. An up-regulation of several antigenic markers with immune-modulatory potential has also been demonstrated for these Dox-COOH-Si-Ms, which may be potential candidates for use in GBR procedures in several challenging pathologies, including periodontal disease.
Author Contributions
Conceptualization, M.T.-O., F.J.M.-M., M.T., C.R., and R.O.; methodology, M.T.-O., F.J.M.-M., M.T., C.R., and R.O.; validation, M.T.-O., M.T., and R.O.; formal analysis, M.T.-O., F.J.M.-M., M.T., and R.O.; investigation, M.T.-O., F.J.M.-M., A.L.M.-C., and V.J.C.-R.; resources, M.T., C.R. and R.O.; data curation, M.T.-O., and F.J.M.-M.; writing—original draft preparation, M.T.-O., F.J.M.-M., and R.O.; writing—review and editing, M.T.-O., F.J.M.-M., M.T., C.R., and R.O.; supervision, M.T., C.R., and R.O.; project administration, M.T., C.R., and R.O.; funding acquisition, M.T., C.R., and R.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by (1) the Ministry of Economy and Competitiveness and European Regional Development Fund [Project MAT2017-85999-P MINECO/AEI/FEDER/UE], (2) University of Granada/Regional Government of Andalusia Research Fund from Spain and European Regional Development Fund (A-BIO-157-UGR-18/FEDER). This research is part of Manuel Toledano-Osorio’s PhD research study.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Toledano-Osorio, M.; Manzano-Moreno, F.J.; Ruiz, C.; Toledano, M.; Osorio, R. Testing Active Membranes for Bone Regeneration: A Review. J. Dent. 2021, 103580. [Google Scholar] [CrossRef] [PubMed]
- de Azambuja Carvalho, P.H.; Dos Santos Trento, G.; Moura, L.B.; Cunha, G.; Gabrielli, M.A.C.; Pereira-Filho, V.A. Horizontal Ridge Augmentation Using Xenogenous Bone Graft-Systematic Review. Oral Maxillofac. Surg. 2019, 23, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Bienz, S.P.; Figuero, E.; Jung, R.E.; Sanz-Martín, I. Efficacy of Lateral Bone Augmentation Performed Simultaneously with Dental Implant Placement: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 257–276. [Google Scholar] [CrossRef]
- Ma, Y.; Song, J.; Almassri, H.N.S.; Zhang, D.; Zhang, T.; Cheng, Y.; Wu, X. Minocycline-Loaded PLGA Electrospun Membrane Prevents Alveolar Bone Loss in Experimental Peridontitis. Drug Deliv. 2020, 27, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Bonino, C.A.; Efimenko, K.; Jeong, S.I.; Krebs, M.D.; Alsberg, E.; Khan, S.A. Three-Dimensional Electrospun Alginate Nanofiber Mats via Tailored Charge Repulsions. Small 2012, 8, 1928–1936. [Google Scholar] [CrossRef]
- Zhao, C.; Tan, A.; Pastorin, G.; Ho, H.K. Nanomaterial Scaffolds for Stem Cell Proliferation and Differentiation in Tissue Engineering. Biotechnol. Adv. 2013, 31, 654–668. [Google Scholar] [CrossRef]
- Osorio, R.; Carrasco-Carmona, Á.; Toledano, M.; Osorio, E.; Medina-Castillo, A.L.; Iskandar, L.; Marques, A.; Deb, S.; Toledano-Osorio, M. Ex Vivo Investigations on Bioinspired Electrospun Membranes as Potential Biomaterials for Bone Regeneration. J. Dent. 2020, 98, 103359. [Google Scholar] [CrossRef]
- Toledano, M.; Toledano-Osorio, M.; Osorio, R.; Carrasco-Carmona, Á.; Gutiérrez-Pérez, J.-L.; Gutiérrez-Corrales, A.; Serrera-Figallo, M.-A.; Lynch, C.D.; Torres-Lagares, D. Doxycycline and Zinc Loaded Silica-Nanofibrous Polymers as Biomaterials for Bone Regeneration. Polymers 2020, 12, 1201. [Google Scholar] [CrossRef]
- Bueno, J.; del Sánchez, M.C.; Toledano-Osorio, M.; Figuero, E.; Toledano, M.; Medina-Castillo, A.L.; Osorio, R.; Herrera, D.; Sanz, M. Antimicrobial Effect of Nanostructured Membranes for Guided Tissue Regeneration: An in Vitro Study. Dent. Mater. 2020, 36, 1566–1577. [Google Scholar] [CrossRef]
- Guo, H.; Xia, D.; Zheng, Y.; Zhu, Y.; Liu, Y.; Zhou, Y. A Pure Zinc Membrane with Degradability and Osteogenesis Promotion for Guided Bone Regeneration: In Vitro and in Vivo Studies. Acta Biomater. 2020, 106, 396–409. [Google Scholar] [CrossRef]
- O’Connor, J.P.; Kanjilal, D.; Teitelbaum, M.; Lin, S.S.; Cottrell, J.A. Zinc as a Therapeutic Agent in Bone Regeneration. Materials 2020, 13, 2211. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.S.; Resende, M.; Fernandes, M.H. Doxycycline Restores the Impaired Osteogenic Commitment of Diabetic-Derived Bone Marrow Mesenchymal Stromal Cells by Increasing the Canonical WNT Signaling. Mol. Cell. Endocrinol. 2020, 518, 110975. [Google Scholar] [CrossRef]
- Do Nascimento Gomes, K.; Negreiros Nunes Alves, A.P.; Goes Pinheiro Dutra, P.; Socorro de Barros Viana, G. Doxycycline Induces Bone Repair and Changes in Wnt Signalling. Int. J. Oral Sci. 2017, 9, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Pouraghaei Sevari, S.; Ansari, S.; Chen, C.; Moshaverinia, A. Harnessing Dental Stem Cell Immunoregulation Using Cell-Laden Biomaterials. J. Dent. Res. 2021, 22034520985820. [Google Scholar] [CrossRef]
- Vallés, G.; Gil-Garay, E.; Munuera, L.; Vilaboa, N. Modulation of the Cross-Talk between Macrophages and Osteoblasts by Titanium-Based Particles. Biomaterials 2008, 29, 2326–2335. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Roger, P.-M. From Crosstalk between Immune and Bone Cells to Bone Erosion in Infection. Int. J. Mol. Sci. 2019, 20, 5154. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Osteoimmunology: Shared Mechanisms and Crosstalk between the Immune and Bone Systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef]
- Lorenzo, J.; Horowitz, M.; Choi, Y. Osteoimmunology: Interactions of the Bone and Immune System. Endocr. Rev. 2008, 29, 403–440. [Google Scholar] [CrossRef]
- Manzano-Moreno, F.J.; Ramos-Torrecillas, J.; De Luna-Bertos, E.; Reyes-Botella, C.; García-Martínez, O.; Ruiz, C. Effect of Clodronate on Antigenic Profile, Growth, and Differentiation of Osteoblast-Like Cells. J. Oral Maxillofac. Surg. 2016, 74, 1765–1770. [Google Scholar] [CrossRef]
- Punet, X.; Mauchauffé, R.; Rodríguez-Cabello, J.C.; Alonso, M.; Engel, E.; Mateos-Timoneda, M.A. Biomolecular Functionalization for Enhanced Cell-Material Interactions of Poly(Methyl Methacrylate) Surfaces. Regen. Biomater. 2015, 2, 167–175. [Google Scholar] [CrossRef]
- Osorio, R.; Alfonso-Rodríguez, C.A.; Osorio, E.; Medina-Castillo, A.L.; Alaminos, M.; Toledano-Osorio, M.; Toledano, M. Novel Potential Scaffold for Periodontal Tissue Engineering. Clin. Oral Investig. 2017, 21, 2695–2707. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rodríguez, L.; García-Martínez, O.; Arroyo-Morales, M.; Reyes-Botella, C.; Ruiz, C. Antigenic Phenotype and Phagocytic Capacity of MG-63 Osteosarcoma Line. Ann. N. Y. Acad. Sci. 2009, 1173 (Suppl. S1), E46–E54. [Google Scholar] [CrossRef]
- Manzano-Moreno, F.J.; Rodríguez-Martínez, J.B.; Ramos-Torrecillas, J.; Vallecillo-Capilla, M.F.; Ruiz, C.; García-Martínez, O.; Reyes-Botella, C. Proliferation and Osteogenic Differentiation of Osteoblast-like Cells Obtained from Two Techniques for Harvesting Intraoral Bone Grafts. Clin. Oral Investig. 2013, 17, 1349–1356. [Google Scholar] [CrossRef]
- Manzano-Moreno, F.J.; Ramos-Torrecillas, J.; De Luna-Bertos, E.; Reyes-Botella, C.; Ruiz, C.; García-Martínez, O. Nitrogen-Containing Bisphosphonates Modulate the Antigenic Profile and Inhibit the Maturation and Biomineralization Potential of Osteoblast-like Cells. Clin. Oral Investig. 2015, 19, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rodríguez, L.; García-Martínez, O.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Ruiz, C. Effect of Ibuprofen on Proliferation, Differentiation, Antigenic Expression, and Phagocytic Capacity of Osteoblasts. J. Bone Min. Metab. 2012, 30, 554–560. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In Search of an Osteoblast Cell Model for in Vitro Research. Eur. Cell. Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.; Pabst, A.; Ziebart, T.; Klein, M.; Al-Nawas, B. Bisphosphonates Affect Migration Ability and Cell Viability of HUVEC, Fibroblasts and Osteoblasts in Vitro. Oral Dis. 2011, 17, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Osorio, M.; Manzano-Moreno, F.J.; Toledano, M.; Osorio, R.; Medina-Castillo, A.L.; Costela-Ruiz, V.J.; Ruiz, C. Doxycycline-Doped Membranes Induced Osteogenic Gene Expression on Osteoblastic Cells. J. Dent. 2021, in press. [Google Scholar]
- Woo, K.M.; Chen, V.J.; Ma, P.X. Nano-Fibrous Scaffolding Architecture Selectively Enhances Protein Adsorption Contributing to Cell Attachment. J. Biomed. Mater. Res. Part A 2003, 67, 531–537. [Google Scholar] [CrossRef]
- Ginebra, M.-P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium Phosphate Cements as Drug Delivery Materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef]
- Luo, M.-L.; Jiao, Y.; Gong, W.-P.; Li, Y.; Niu, L.-N.; Tay, F.R.; Chen, J.-H. Macrophages Enhance Mesenchymal Stem Cell Osteogenesis via Down-Regulation of Reactive Oxygen Species. J. Dent. 2020, 94, 103297. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Toledano-Osorio, M.; Navarro-Hortal, M.D.; Varela-López, A.; Osorio, R.; Quiles, J.L. Novel Polymeric Nanocarriers Reduced Zinc and Doxycycline Toxicity in the Nematode Caenorhabditis Elegans. Antioxidants 2019, 8, 550. [Google Scholar] [CrossRef]
- Leite, L.M.; Carvalho, A.G.G.; Ferreira, P.L.F.T.; Pessoa, I.X.; Gonçalves, D.O.; Lopes, A.d.A.; Góes, J.G.d.S.; Alves, V.C.d.C.; Leal, L.K.A.M.; Brito, G.A.; et al. Anti-Inflammatory Properties of Doxycycline and Minocycline in Experimental Models: An in Vivo and in Vitro Comparative Study. Inflammopharmacology 2011, 19, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.C.; Carlier, A.; Bolander, J.; Roberts, S.J.; Geris, L.; Schrooten, J.; Van Oosterwyck, H.; Luyten, F.P. Current Views on Calcium Phosphate Osteogenicity and the Translation into Effective Bone Regeneration Strategies. Acta Biomater. 2012, 8, 3876–3887. [Google Scholar] [CrossRef] [PubMed]
- Rabel, K.; Kohal, R.-J.; Steinberg, T.; Tomakidi, P.; Rolauffs, B.; Adolfsson, E.; Palmero, P.; Fürderer, T.; Altmann, B. Controlling Osteoblast Morphology and Proliferation via Surface Micro-Topographies of Implant Biomaterials. Sci. Rep. 2020, 10, 12810. [Google Scholar] [CrossRef]
- Kouhi, M.; Jayarama Reddy, V.; Fathi, M.; Shamanian, M.; Valipouri, A.; Ramakrishna, S. Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Fibrinogen/Bredigite Nanofibrous Membranes and Their Integration with Osteoblasts for Guided Bone Regeneration. J. Biomed. Mater. Res. A 2019, 107, 1154–1165. [Google Scholar] [CrossRef]
- Schmidt, C.; Kaspar, D.; Sarkar, M.R.; Claes, L.E.; Ignatius, A.A. A Scanning Electron Microscopy Study of Human Osteoblast Morphology on Five Orthopedic Metals. J. Biomed. Mater. Res. 2002, 63, 252–261. [Google Scholar] [CrossRef]
- Montjovent, M.-O.; Bocelli-Tyndall, C.; Scaletta, C.; Scherberich, A.; Mark, S.; Martin, I.; Applegate, L.A.; Pioletti, D.P. In Vitro Characterization of Immune-Related Properties of Human Fetal Bone Cells for Potential Tissue Engineering Applications. Tissue Eng. Part A 2009, 15, 1523–1532. [Google Scholar] [CrossRef]
- Reyes-Botella, C.; Montes, M.J.; Vallecillo-Capilla, M.F.; Olivares, E.G.; Ruiz, C. Expression of Molecules Involved in Antigen Presentation and T Cell Activation (HLA-DR, CD80, CD86, CD44 and CD54) by Cultured Human Osteoblasts. J. Periodontol. 2000, 71, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Skjødt, H.; Hughes, D.E.; Dobson, P.R.; Russell, R.G. Constitutive and Inducible Expression of HLA Class II Determinants by Human Osteoblast-like Cells in Vitro. J. Clin. Investig. 1990, 85, 1421–1426. [Google Scholar] [CrossRef]
- Gutcher, I.; Becher, B. APC-Derived Cytokines and T Cell Polarization in Autoimmune Inflammation. J. Clin. Investig. 2007, 117, 1119–1127. [Google Scholar] [CrossRef]
- Hodge, J.W.; Greiner, J.W.; Tsang, K.-Y.; Sabzevari, H.; Kudo-Saito, C.; Grosenbach, D.W.; Gulley, J.L.; Arlen, P.M.; Marshall, J.L.; Panicali, D.; et al. Costimulatory Molecules as Adjuvants for Immunotherapy. Front. Biosci. 2006, 11, 788–803. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hong, S.; Qian, J.; Zheng, Y.; Yang, J.; Yi, Q. Cross Talk between the Bone and Immune Systems: Osteoclasts Function as Antigen-Presenting Cells and Activate CD4+ and CD8+ T Cells. Blood 2010, 116, 210–217. [Google Scholar] [CrossRef]
- Roodman, G.D. Regulation of Osteoclast Differentiation. Ann. N. Y. Acad. Sci. 2006, 1068, 100–109. [Google Scholar] [CrossRef]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Mochizuki, S.; Tomoyasu, A.; Yano, K.; Goto, M.; Murakami, A.; et al. Osteoclast Differentiation Factor Is a Ligand for Osteoprotegerin/Osteoclastogenesis-Inhibitory Factor and Is Identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 1998, 95, 3597–3602. [Google Scholar] [CrossRef]
- Kong, Y.Y.; Yoshida, H.; Sarosi, I.; Tan, H.L.; Timms, E.; Capparelli, C.; Morony, S.; Oliveira-dos-Santos, A.J.; Van, G.; Itie, A.; et al. OPGL Is a Key Regulator of Osteoclastogenesis, Lymphocyte Development and Lymph-Node Organogenesis. Nature 1999, 397, 315–323. [Google Scholar] [CrossRef]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of Osteoclast Differentiation and Function by the New Members of the Tumor Necrosis Factor Receptor and Ligand Families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Kinugawa, S.; Koide, M.; Kobayashi, Y.; Mizoguchi, T.; Ninomiya, T.; Muto, A.; Kawahara, I.; Nakamura, M.; Yasuda, H.; Takahashi, N.; et al. Tetracyclines Convert the Osteoclastic-Differentiation Pathway of Progenitor Cells To Produce Dendritic Cell-like Cells. J. Immunol. 2012, 188, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Morimoto, I.; Nakano, Y.; Okada, Y.; Hirota, S.; Nomura, S.; Nakamura, T.; Eto, S. Osteoblasts Are Regulated by the Cellular Adhesion through ICAM-1 and VCAM-1. J. Bone Min. Res. 1995, 10, 1462–1469. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakayamada, S.; Okada, Y. Osteoblasts and Osteoclasts in Bone Remodeling and Inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Fujii, K.; Nakano, K.; Tanaka, Y. Crosslinking of CD44 on Human Osteoblastic Cells Upregulates ICAM-1 and VCAM-1. FEBS Lett. 2003, 539, 45–50. [Google Scholar] [CrossRef]
- Roebuck, K.A.; Finnegan, A. Regulation of Intercellular Adhesion Molecule-1 (CD54) Gene Expression. J. Leukoc. Biol. 1999, 66, 876–888. [Google Scholar] [CrossRef]
- Espagnolle, N.; Balguerie, A.; Arnaud, E.; Sensebé, L.; Varin, A. CD54-Mediated Interaction with Pro-Inflammatory Macrophages Increases the Immunosuppressive Function of Human Mesenchymal Stromal Cells. Stem Cell Rep. 2017, 8, 961–976. [Google Scholar] [CrossRef]
- Ren, G.; Zhao, X.; Zhang, L.; Zhang, J.; L’Huillier, A.; Ling, W.; Roberts, A.I.; Le, A.D.; Shi, S.; Shao, C.; et al. Inflammatory Cytokine-Induced Intercellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule-1 in Mesenchymal Stem Cells Are Critical for Immunosuppression. J. Immunol. 2010, 184, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, A.; Gigante, I.; Colucci, S.; Grano, M. Periodontal Disease: Linking the Primary Inflammation to Bone Loss. Clin. Dev. Immunol. 2013, 2013, 503754. [Google Scholar] [CrossRef]
- Hamza, T.; Li, B. Differential Responses of Osteoblasts and Macrophages upon Staphylococcus Aureus Infection. BMC Microbiol. 2014, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Platt, N.; Fineran, P. Chapter 14—Measuring the phagocytic activity of cells. In Methods in Cell Biology; Platt, F., Platt, N., Eds.; Lysosomes and Lysosomal Diseases; Academic Press: Cambridge, MA, USA, 2015; Volume 126, pp. 287–304. [Google Scholar]
- Chen, Z.; Bachhuka, A.; Han, S.; Wei, F.; Lu, S.; Visalakshan, R.M.; Vasilev, K.; Xiao, Y. Tuning Chemistry and Topography of Nanoengineered Surfaces to Manipulate Immune Response for Bone Regeneration Applications. ACS Nano 2017, 11, 4494–4506. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).