A Comprehensive Review on Plant-Derived Mucilage: Characterization, Functional Properties, Applications, and Its Utilization for Nanocarrier Fabrication
Abstract
:1. Introduction
2. Origin of Mucilage in Different Plant Parts
3. Extraction of Plant-Derived Mucilage
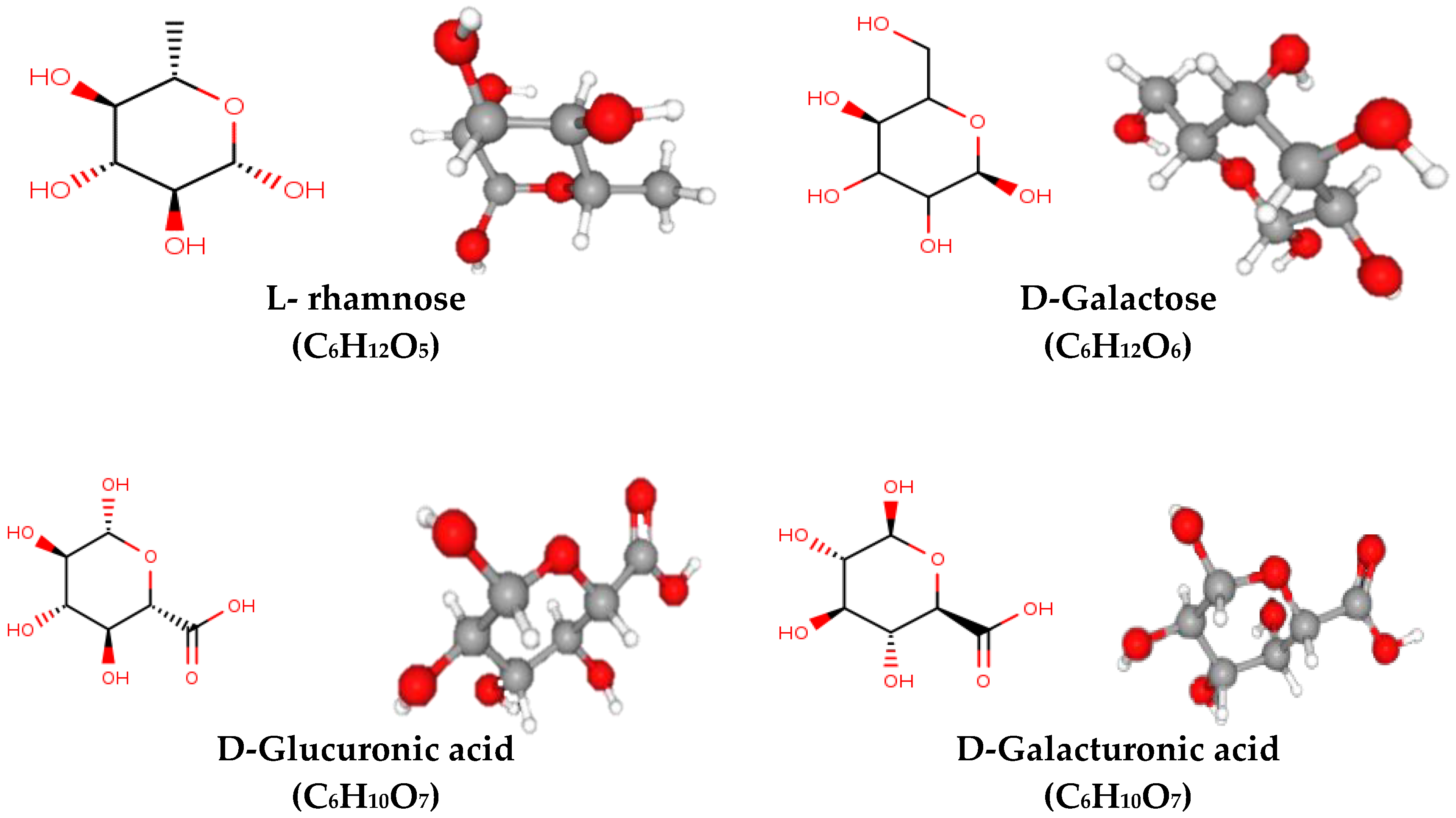
4. Structural Chemistry of Mucilage
5. Characterization of the Mucilage
6. Functional Properties of Mucilage
6.1. Water Holding Capacity of Mucilage
6.2. Oil Holding Capacity of Mucilage
6.3. Emulsifying Property of Mucilage
6.4. Foaming Property of Mucilage
6.5. Antioxidant Property of Mucilage
6.6. Antimicrobial Activity of Mucilage
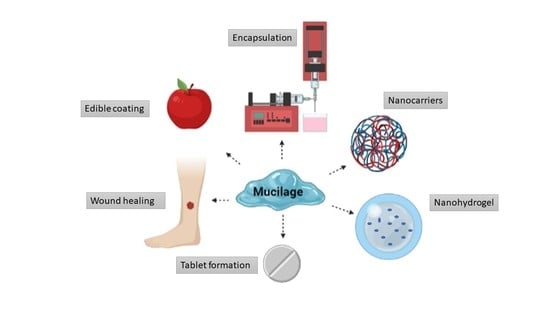
7. Application of Mucilage
7.1. Mucilage as a Coating Material
7.2. Application of Mucilage as Encapsulation Agents
7.3. Application of Mucilage in Wound Healing
7.4. Application of Mucilage as an Emulsifying or Suspending Agent
7.5. Application of Mucilage in Tablet Formations
7.6. Application of Mucilage for Removal of Contaminants from Water
8. Therapeutic Importance of Mucilage
9. Mucilage Based Nanocarriers and Their Application
Synthesis of Nanoparticles with Mucilage
10. Market Outlook of Mucilage
11. Conclusions, Future Research Perspectives, and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, F.; Wang, R.; Li, X.; Kang, W.; Bell, A.E.; Zhao, D.; Liu, X.; Chen, W. Physical properties of mucilage polysaccharides from dioscorea Opposita Thunb. Food Chem. 2020, 311. [Google Scholar] [CrossRef]
- Singh, R.; Barreca, D.N. Analysis of gums and mucilages. In Recent Advances in Natural Products Analysis; Silva, A.S., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 663–676. [Google Scholar] [CrossRef]
- Freitas, T.K.F.S.; Oliveira, V.M.; de Souza, M.T.F.; Geraldino, H.C.L.; Almeida, V.C.; Fávaro, S.L.; Garcia, J.C. Optimization of coagulation-flocculation process for treatment of industrial textile wastewater using okra (A. esculentus) mucilage as natural coagulant. Ind. Crop. Prod. 2015, 76, 538–544. [Google Scholar] [CrossRef]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11. [Google Scholar] [CrossRef] [Green Version]
- Chawla, P.; Kumar, N.; Bains, A.; Dhull, S.B.; Kumar, M.; Kaushik, R.; Punia, S. Gum arabic capped copper nanoparticles: Synthesis, characterization, and applications. Int. J. Biol. Macromol. 2020, 146, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Archana, G.; Sabina, K.; Babuskin, S.; Radhakrishnan, K.; Fayidh, M.A.; Azhagu Saravana Babu, P.; Sivarajan, M.; Sukumar, M. Preparation and characterization of mucilage polysaccharide for biomedical applications. Carbohydr. Polym. 2013, 98, 89–94. [Google Scholar] [CrossRef]
- Ameri, A.; Heydarirad, G.; Jafari, J.M.; Ghobadi, A.; Rezaeizadeh, H.; Choopani, R. Medicinal plants contain mucilage used in traditional Persian medicine (TPM). Pharm. Biol. 2015, 53, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, S.S.; de las Mercedes Salas-Mellado, M. Addition of chia seed mucilage for reduction of fat content in bread and cakes. Food Chem. 2017, 227, 237–244. [Google Scholar] [CrossRef]
- Beikzadeh, S.; Khezerlou, A.; Jafari, S.M.; Pilevar, Z.; Mortazavian, A.M. Seed Mucilages as the functional ingredients for biodegradable films and edible coatings in the food industry. Adv. Colloid Interface Sci. 2020, 280, 102164. [Google Scholar] [CrossRef]
- Gebresamuel, N.; Gebre-Mariam, T. Comparative physico-chemical characterization of the mucilages of two cactus pears (Opuntia Spp.) obtained from Mekelle, Northern Ethiopia. J. Biomater. Nanobiotechnol. 2012, 3, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P. Pharmaceutical applications of various natural gums, mucilages and their modified forms. Carbohydr. Polym. 2013, 92, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Petera, B.; Delattre, C.; Pierre, G.; Wadouachi, A.; Elboutachfaiti, R.; Engel, E.; Poughon, L.; Michaud, P.; Fenoradosoa, T.A. Characterization of arabinogalactan-rich mucilage from cereus triangularis cladodes. Carbohydr. Polym. 2015, 127, 372–380. [Google Scholar] [CrossRef]
- Alpizar-Reyes, E.; Carrillo-Navas, H.; Gallardo-Rivera, R.; Varela-Guerrero, V.; Alvarez-Ramirez, J.; Pérez-Alonso, C. Functional properties and physicochemical characteristics of tamarind (Tamarindus indica L.) seed mucilage powder as a novel hydrocolloid. J. Food Eng. 2017, 209, 68–75. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Hemmati, K.; Ghaemy, M. Synthesis of nanohydrogels based on tragacanth gum biopolymer and investigation of swelling and drug delivery. Int. J. Biol. Macromol. 2016, 82, 806–815. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Devi, K.; Sharma, S.; Naushad, M.; Ghfar, A.A.; Ahamad, T.; Stadler, F.J. Guar Gum-Crosslinked-Soya lecithin nanohydrogel sheets as effective adsorbent for the removal of thiophanate methyl fungicide. Int. J. Biol. Macromol. 2018, 114, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Lee, D.I.; Park, J.M. Biopolymer-based microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2009, 34, 1261–1282. [Google Scholar] [CrossRef]
- Suner, S.S.; Sahiner, M.; Sengel, S.B.; Rees, D.J.; Reed, W.F.; Sahiner, N. Responsive biopolymer-based microgels/nanogels for drug delivery applications. Stimuli Responsive Polym. Nanocarriers Drug Deliv. Appl. 2018, 1, 453–500. [Google Scholar] [CrossRef]
- Mukherjee, T.; Lerma-Reyes, R.; Thompson, K.A.; Schrick, K. Making glue from seeds and gums: Working with plant-based polymers to introduce students to plant biochemistry. Biochem. Mol. Biol. Educ. 2019, 47, 468–475. [Google Scholar] [CrossRef]
- Viudes, S.; Burlat, V.; Dunand, C. Seed mucilage evolution: Diverse molecular mechanisms generate versatile ecological functions for particular environments. Plant Cell Environ. 2020, 43, 2857–2870. [Google Scholar] [CrossRef]
- Ferreira, B.; Montesinos, D.; Sales, F. Mucilage in Portuguese Lamiaceae. Bot. Lett. 2020, 167, 430–438. [Google Scholar] [CrossRef]
- Cuomo, F.; Iacovino, S.; Cinelli, G.; Messia, M.C.; Marconi, E.; Lopez, F. Effect of additives on chia mucilage suspensions: A rheological approach. Food Hydrocoll. 2020, 109, 106118. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Ji, Y.; Walck, J.L.; Tao, J. Seed Biology of Lepidium apetalum (Brassicaceae), with particular reference to dormancy and mucilage development. Plants 2020, 9, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Baskin, J.M.; Baskin, C.C.; Huang, Z. More than just a coating: Ecological importance, taxonomic occurrence and phylogenetic relationships of seed coat mucilage. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 434–442. [Google Scholar] [CrossRef]
- Cai, M.; Wang, N.; Xing, C.; Wang, F.; Wu, K.; Du, X. Immobilization of aluminum with mucilage secreted by root cap and root border cells is related to aluminum resistance in Glycine max L. Environ. Sci. Pollut. Res. 2013, 20, 8924–8933. [Google Scholar] [CrossRef] [PubMed]
- Hawes, M.C.; Curlango-Rivera, G.; Xiong, Z.; Kessler, J.O. Roles of root border cells in plant defense and regulation of rhizosphere microbial populations by extracellular DNA ‘trappin’. Plant Soil 2012, 355, 1–16. [Google Scholar] [CrossRef]
- Rashid, F.; Ahmed, Z.; Hussain, S.; Huang, J.Y.; Ahmad, A. Linum usitatissimum L. seeds: Flax gum extraction, physicochemical and functional characterization. Carbohydr. Polym. 2019, 215, 29–38. [Google Scholar] [CrossRef]
- Coorey, R.; Tjoe, A.; Jayasena, V. Gelling properties of chia seed and flour. J. Food Sci. 2014, 79, E859–E866. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, D.; Pradhan, J.; Hasnain, M.S. Fenugreek seed mucilage-alginate mucoadhesive beads of metformin HCl: Design, optimization and evaluation. Int. J. Biol. Macromol. 2013, 54, 144–154. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, D.; Pany, D.R.; Mohanty, B. Evaluation of Spinacia oleracea L. leaves mucilage as an innovative suspending agent. J. Adv. Pharm. Technol. Res. 2010, 1, 338–341. [Google Scholar] [CrossRef]
- Tavares, L.S.; Junqueira, L.A.; de Oliveira Guimarães, Í.C.; de Resende, J.V. Cold extraction method of chia seed mucilage (Salvia hispanica L.): Effect on yield and rheological behavior. J. Food Sci. Technol. 2018, 55, 457–466. [Google Scholar] [CrossRef]
- Jindal, M.; Kumar, V.; Rana, V.; Tiwary, A.K. Exploring potential new gum source aegle marmelos for food and pharmaceuticals: Physical, chemical and functional performance. Ind. Crop. Prod. 2013, 45, 312–318. [Google Scholar] [CrossRef]
- Hung, P.Y.; Lai, L.S. Structural characterization and rheological properties of the water extracted mucilage of basella alba and the starch/aqueous mucilage blends. Food Hydrocoll. 2019, 93, 413–421. [Google Scholar] [CrossRef]
- Souza, G.; Siqueira dos Santos, S.; Bergamasco, R.; Antigo, J.; Madrona, G.S. Antioxidant activity, extraction and application of psyllium mucilage in chocolate drink. Nutr. Food Sci. 2020, 50, 1175–1185. [Google Scholar] [CrossRef]
- Andrade, L.A.; de Oliveira Silva, D.A.; Nunes, C.A.; Pereira, J. Experimental techniques for the extraction of taro mucilage with enhanced emulsifier properties using chemical characterization. Food Chem. 2020, 327, 127095. [Google Scholar] [CrossRef] [PubMed]
- Kamel, R.; Afifi, S.M.; Kassem, I.A.A.; Elkasabgy, N.A.; Farag, M.A. Arabinoxylan and rhamnogalacturonan mucilage: Outgoing and potential trends of pharmaceutical, environmental, and medicinal merits. Int. J. Biol. Macromol. 2020, 165, 2550–2564. [Google Scholar] [CrossRef]
- Andrade, L.A.; Nunes, C.A.; Pereira, J. Relationship between the chemical components of taro rhizome mucilage and its emulsifying property. Food Chem. 2015, 178, 331–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elboutachfaiti, R.; Delattre, C.; Quéro, A.; Roulard, R.; Duchêne, J.; Mesnard, F.; Petit, E. Fractionation and structural characterization of six purified rhamnogalacturonans type i from flaxseed mucilage. Food Hydrocoll. 2017, 62, 273–279. [Google Scholar] [CrossRef]
- Luo, M.; Cao, Y.; Wang, W.; Chen, X.; Cai, J.; Wang, L.; Xiao, J. Sustained-release antimicrobial gelatin film: Effect of chia mucilage on physicochemical and antimicrobial properties. Food Hydrocoll. 2019, 87, 783–791. [Google Scholar] [CrossRef]
- Behbahani, B.A.; Noshad, M.; Jooyandeh, H. Improving oxidative and microbial stability of beef using Shahri Balangu seed mucilage loaded with Cumin essential oil as a bioactive edible coating. Biocatal. Agric. Biotechnol. 2020, 24, 101563. [Google Scholar] [CrossRef]
- Pereira, G.A.; Silva, E.K.; Peixoto Araujo, N.M.; Arruda, H.S.; Meireles, M.A.A.; Pastore, G.M. Mutamba seed mucilage as a novel emulsifier: Stabilization mechanisms, kinetic stability and volatile compounds retention. Food Hydrocoll. 2019, 97, 105190. [Google Scholar] [CrossRef]
- Soukoulis, C.; Gaiani, C.; Hoffmann, L. Plant seed mucilage as emerging biopolymer in food industry applications. Curr. Opin. Food Sci. 2018, 22, 28–42. [Google Scholar] [CrossRef]
- Vardhanabhuti, B.; Ikeda, S. Isolation and characterization of hydrocolloids from monoi (Cissampelos pareira) leaves. Food Hydrocoll. 2006, 20, 885–891. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Razavi, S.M.A. Functional properties and applications of basil seed gum: An overview. Food Hydrocoll. 2017, 73, 313–325. [Google Scholar] [CrossRef]
- Hesarinejad, M.A.; Sami Jokandan, M.; Mohammadifar, M.A.; Koocheki, A.; Razavi, S.M.A.; Ale, M.T.; Attar, F.R. The effects of concentration and heating-cooling rate on rheological properties of plantago lanceolata seed mucilage. Int. J. Biol. Macromol. 2018, 115, 1260–1266. [Google Scholar] [CrossRef] [Green Version]
- Hosseini-Parvar, S.H.; Matia-Merino, L.; Goh, K.K.T.; Razavi, S.M.A.; Mortazavi, S.A. Steady shear flow behavior of gum extracted from Ocimum basilicum L. seed: Effect of concentration and temperature. J. Food Eng. 2010, 101, 236–243. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Barrow, C.J.; Adhikari, B. Physicochemical and functional properties of protein isolate produced from Australian chia seeds. Food Chem. 2016, 212, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, H.; Amid, B.T. A Review study on chemical composition and molecular structure of newly plant gum exudates and seed gums. Food Res. Int. 2012, 46, 387–398. [Google Scholar] [CrossRef]
- Deore, U.V.; Mahajan, H.S. Isolation and characterization of natural polysaccharide from cassia obtustifolia seed mucilage as film forming material for drug delivery. Int. J. Biol. Macromol. 2018, 115, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.; Noshad, M.; Rastegarzadeh, S.; Hojjati, M.; Fazlara, A. Electrospun chia seed mucilage/pva encapsulated with green cardamonmum essential oils: Antioxidant and antibacterial property. Int. J. Biol. Macromol. 2020, 161, 1–9. [Google Scholar] [CrossRef]
- Devi, R.; Bhatia, M. Thiol Functionalization of Flaxseed Mucilage: Preparation, characterization and evaluation as mucoadhesive polymer. Int. J. Biol. Macromol. 2019, 126, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Thessrimuang, N.; Prachayawarakorn, J. Development, modification and characterization of new biodegradable film from basil seed (Ocimum basilicum L.) mucilage. J. Sci. Food Agric. 2019, 99, 5508–5515. [Google Scholar] [CrossRef]
- Fahami, A.; Fathi, M. Development of cress seed mucilage/PVA nanofibers as a novel carrier for vitamin a delivery. Food Hydrocoll. 2018, 81, 31–38. [Google Scholar] [CrossRef]
- Kurd, F.; Fathi, M.; Shekarchizadeh, H. Basil seed mucilage as a new source for electrospinning: Production and physicochemical characterization. Int. J. Biol. Macromol. 2017, 95, 689–695. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Razavi, S.M.A.; Mohebbi, M.; Malaekeh-Nikouei, B. New studies on basil (Ocimum bacilicum L.) seed gum: Part i—Fractionation, physicochemical and surface activity characterization. Food Hydrocoll. 2016, 52, 350–358. [Google Scholar] [CrossRef]
- Pratik, P.; Shadique, A. Antimicrobial examination of mucilage obtained from fruits of tilkor [Mamoradica monadelpha]: A potential medicinal plant. J. Biol. Chem. Chron. 2020, 6, 5–9. [Google Scholar] [CrossRef]
- Punia, S.; Dhull, S.B. Chia seed (Salvia hispanica L.) mucilage (a heteropolysaccharide): Functional, thermal, rheological behaviour and its utilization. Int. J. Biol. Macromol. 2019, 140, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Capitani, M.I.; Ixtaina, V.Y.; Nolasco, S.M.; Tomás, M.C. Microstructure, chemical composition and mucilage exudation of chia (Salvia hispanica L.) nutlets from Argentina. J. Sci. Food Agric. 2013, 93, 3856–3862. [Google Scholar] [CrossRef]
- Abbastabar, B.; Azizi, M.H.; Adnani, A.; Abbasi, S. Determining and modeling rheological characteristics of quince seed gum. Food Hydrocoll. 2015, 43, 259–264. [Google Scholar] [CrossRef]
- Keshani-Dokht, S.; Emam-Djomeh, Z.; Yarmand, M.S.; Fathi, M. Extraction, chemical composition, rheological behavior, antioxidant activity and functional properties of Cordia myxa mucilage. Int. J. Biol. Macromol. 2018, 118, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Hadad, S.; Goli, S.A.H. Fabrication and characterization of electrospun nanofibers using flaxseed (Linum usitatissimum) Mucilage. Int. J. Biol. Macromol. 2018, 114, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bothara, S.B. Manilkara zapota (Linn.) seeds: A potential source of natural gum. ISRN Pharm. 2014, 2014, 647174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Alvarenga Pinto Cotrim, M.; Mottin, A.C.; Ayres, E. Preparation and characterization of Okra Mucilage (Abelmoschus esculentus) edible films. Macromol. Symp. 2016, 367, 90–100. [Google Scholar] [CrossRef]
- Pawar, H.A.; Lalitha, K.G. Isolation, purification and characterization of galactomannans as an excipient from Senna tora seeds. Int. J. Biol. Macromol. 2014, 65, 167–175. [Google Scholar] [CrossRef]
- Viebke, C.; Al-Assaf, S.; Phillips, G.O. Food hydrocolloids and health claims. Bioact. Carbohydr. Diet. Fibre 2014, 4, 101–114. [Google Scholar] [CrossRef]
- Kang, S.; Wang, H.; Xia, L.; Chen, M.; Li, L.; Cheng, J.; Li, X.; Jiang, S. Colorimetric film based on polyvinyl alcohol/okra mucilage polysaccharide incorporated with rose anthocyanins for shrimp freshness monitoring. Carbohydr. Polym. 2020, 229, 115402. [Google Scholar] [CrossRef]
- Gemede, H.F.; Haki, G.D.; Beyene, F.; Rakshit, S.K.; Woldegiorgis, A.Z. Indigenous Ethiopian Okra (Abelmoschus Esculentus) Mucilage: A Novel Ingredient with Functional and Antioxidant Properties. Food Sci. Nutr. 2018, 6, 563–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ubeyitogullari, A.; Ciftci, O.N. Fabrication of bioaerogels from camelina seed mucilage for food applications. Food Hydrocoll. 2020, 102, 105597. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, R.; Punia, S. Characterization of mucilages extracted from different flaxseed (Linum usitatissiumum L.) cultivars: A heteropolysaccharide with desirable functional and rheological properties. Int. J. Biol. Macromol. 2018, 117, 919–927. [Google Scholar] [CrossRef]
- Lan, Y.; Ohm, J.B.; Chen, B.; Rao, J. Physicochemical properties and aroma profiles of flaxseed proteins extracted from whole flaxseed and flaxseed meal. Food Hydrocoll. 2020, 104, 105731. [Google Scholar] [CrossRef]
- Hassan, L.K.; Haggag, H.F.; ElKalyoubi, M.H.; Abd EL-Aziz, M.; El-Sayed, M.M.; Sayed, A.F. Physico-chemical properties of yoghurt containing cress seed mucilage or guar gum. Ann. Agric. Sci. 2015, 60, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Dhull, S.B.; Sandhu, K.S.; Punia, S.; Kaur, M.; Chawla, P.; Malik, A. Functional, thermal and rheological behavior of fenugreek (Trigonella foenum–graecum L.) gums from different cultivars: A comparative study. Int. J. Biol. Macromol. 2020, 159, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Galla, N.R.; Dubasi, G.R. Chemical and functional characterization of Gum Karaya (Sterculia urens L.) seed meal. Food Hydrocoll. 2010, 24, 479–485. [Google Scholar] [CrossRef]
- Felisberto, M.H.F.; Wahanik, A.L.; Gomes-Ruffi, C.R.; Clerici, M.T.P.S.; Chang, Y.K.; Steel, C.J. Use of chia (Salvia hispanica L.) mucilage gel to reduce fat in pound cakes. LWT Food Sci. Technol. 2015, 63, 1049–1055. [Google Scholar] [CrossRef] [Green Version]
- Monrroy, M.; García, E.; Ríos, K.; García, J.R. Extraction and physicochemical characterization of mucilage from Opuntia cochenillifera (L.) Miller. J. Chem. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Kalegowda, P.; Chauhan, A.S.; Nanjaraj Urs, S.M. Opuntia dillenii (Ker-Gawl) haw cladode mucilage: Physico-chemical, rheological and functional behavior. Carbohydr. Polym. 2017, 157, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Hayati, I.N.; Ching, C.W.; Rozaini, M.Z.H. Flow properties of o/w emulsions as affected by xanthan gum, guar gum and carboxymethyl cellulose interactions studied by a mixture regression modelling. Food Hydrocoll. 2016, 53, 199–208. [Google Scholar] [CrossRef]
- Quinzio, C.; Ayunta, C.; López de Mishima, B.; Iturriaga, L. Stability and rheology properties of oil-in-water emulsions prepared with mucilage extracted from opuntia Ficus-indica (L). Miller. Food Hydrocoll. 2018, 84, 154–165. [Google Scholar] [CrossRef]
- Sangeethapriya, M.; Siddhuraju, P. Health related functional characteristics and antioxidant potential of mucilage (dietary fiber) from Zizyphus mauritiana fruits. Food Sci. Hum. Wellness 2014, 3, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Behrouzian, F.; Razavi, S.M.A.; Phillips, G.O. Cress seed (Lepidium sativum) mucilage, an overview. Bioact. Carbohydr. Diet. Fibre 2014, 3, 17–28. [Google Scholar] [CrossRef]
- Jouki, M.; Mortazavi, S.A.; Yazdi, F.T.; Koocheki, A. Characterization of antioxidant-antibacterial quince seed mucilage films containing thyme essential oil. Carbohydr. Polym. 2014, 99, 537–546. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Sessa, M.; Chouaibi, M.; Ferrari, G.; Donsì, F.; Hamdi, S. assessment of emulsifying ability of almond gum in comparison with gum arabic using response surface methodology. Food Hydrocoll. 2014, 37, 49–59. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Tabatabaei Yazdi, F.; Shahidi, F.; Hesarinejad, M.A.; Mortazavi, S.A.; Mohebbi, M. Plantago major seed mucilage: Optimization of extraction and some physicochemical and rheological aspects. Carbohydr. Polym. 2017, 155, 68–77. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Ebrahimabadi, A.H.; Djafari-Bidgoli, Z.; Batooli, H. GC/MS analysis and in vitro antioxidant activity of essential oil and methanol extracts of Thymus caramanicus Jalas and its main constituent carvacrol. Food Chem. 2009, 115, 1524–1528. [Google Scholar] [CrossRef]
- Roche, A.; Ross, E.; Walsh, N.; O’Donnell, K.; Williams, A.; Klapp, M.; Fullard, N.; Edelstein, S. Representative literature on the phytonutrients category: Phenolic acids. Crit. Rev. Food Sci. Nutr. 2017, 57, 1089–1096. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxidative Med. Cell. Longev. 2016, 64, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Dong, B.; Chen, J.; Zhao, B.; Wang, X.; Wang, L.; Zha, S.; Wang, Y.; Zhang, J.; Wang, Y. Effect of drying methods on physicochemical properties and antioxidant activities of wolfberry (Lycium barbarum) polysaccharide. Carbohydr. Polym. 2015, 127, 176–181. [Google Scholar] [CrossRef]
- Liguori, G.; Gentile, C.; Gaglio, R.; Perrone, A.; Guarcello, R.; Francesca, N.; Fretto, S.; Inglese, P.; Settanni, L. Effect of addition of Opuntia ficus-indica mucilage on the biological leavening, physical, nutritional, antioxidant and sensory aspects of bread. J. Biosci. Bioeng. 2020, 129, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Silveira Coelho, M.; de las Mercedes Salas-Mellado, M. Chemical characterization of chia (Salvia Hispanica L.) for use in food products. J. Food Nutr. Res. 2014, 2, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Tantiwatcharothai, S.; Prachayawarakorn, J. Property improvement of antibacterial wound dressing from basil seed (O. basilicum L.) mucilage-ZnO nanocomposite by borax crosslinking. Carbohydr. Polym. 2020, 227, 115360–115367. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Akyuz, L.; Koc, B.; Kaya, M.; Ilk, S.; Cansaran-Duman, D.; Martinez, A.S.; Cakmak, Y.S.; Labidi, J.; Boufi, S. Novel, multifunctional mucilage composite films incorporated with cellulose nanofibers. Food Hydrocoll. 2019, 89, 20–28. [Google Scholar] [CrossRef]
- Mohammadi, H.; Kamkar, A.; Misaghi, A. Nanocomposite films based on cmc, okra mucilage and ZnO nanoparticles: Physico mechanical and antibacterial properties. Carbohydr. Polym. 2018, 181, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Heydari, S.; Jooyandeh, H.; Alizadeh Behbahani, B.; Noshad, M. The Impact of Qodume Shirazi seed mucilage-based edible coating containing lavender essential oil on the quality enhancement and shelf life improvement of fresh ostrich meat: An experimental and modeling study. Food Sci. Nutr. 2020, 8, 6497–6512. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, H.; Alizadeh Behbahani, B.; Mehrnia, M.A. Quality retention and shelf life extension of fresh beef using Lepidium sativum seed mucilage-based edible coating containing Heracleum lasiopetalum essential oil: An experimental and modeling study. Food Sci. Biotechnol. 2020, 29, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Gheribi, R.; Puchot, L.; Verge, P.; Jaoued-Grayaa, N.; Mezni, M.; Habibi, Y.; Khwaldia, K. Development of plasticized edible films from Opuntia ficus-indica mucilage: A comparative study of various polyol plasticizers. Carbohydr. Polym. 2018, 190, 204–211. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.N.; Wani, A.A.; Shah, M.A. Effect of plant extracts on the techno-functional properties of biodegradable packaging films. Trends Food Sci. Technol. 2018, 80, 141–154. [Google Scholar] [CrossRef]
- Espino-Díaz, M.; De Jesús Ornelas-Paz, J.; Martínez-Téllez, M.A.; Santillán, C.; Barbosa-Cánovas, G.V.; Zamudio-Flores, P.B.; Olivas, G.I. Development and characterization of edible films based on mucilage of Opuntia ficus-indica (L.). J. Food Sci. 2010, 75, E347–E352. [Google Scholar] [CrossRef] [PubMed]
- Kozlu, A.; Elmacı, Y. Quince seed mucilage as edible coating for mandarin fruit; determination of the quality characteristics during storage. J. Food Process. Preserv. 2020, 44, e14854. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Nikou, A.; Tzortzakis, N. Effectiveness of Aloe vera gel coating for maintaining tomato fruit quality. N. Z. J. Crop Hortic. Sci. 2016, 44, 203–217. [Google Scholar] [CrossRef]
- Nourozi, F.; Sayyari, M. Enrichment of Aloe vera gel with basil seed mucilage preserve bioactive compounds and postharvest quality of apricot fruits. Sci. Hortic. 2020, 262, 109041. [Google Scholar] [CrossRef]
- Allegra, A.; Inglese, P.; Sortino, G.; Settanni, L.; Todaro, A.; Liguori, G. The influence of Opuntia ficus-indica mucilage edible coating on the quality of ‘Hayward’ kiwifruit slices. Postharvest Biol. Technol. 2016, 120, 45–51. [Google Scholar] [CrossRef]
- Vignesh, R.M.; Nair, B.R. Improvement of shelf life quality of tomatoes using a novel edible coating formulation. Plant Sci. Today 2019, 2, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Soleimani-Rambod, A.; Zomorodi, S.; Naghizadeh Raeisi, S.; Khosrowshahi Asl, A.; Shahidi, S.-A. The Effect of Xanthan Gum and Flaxseed Mucilage as Edible Coatings in Cheddar Cheese during Ripening. Coatings 2018, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Song, H.Y.; Jo, W.S.; Song, N.B.; Min, S.C.; Song, K.B. Quality change of apple slices coated with Aloe vera gel during storage. J. Food Sci. 2013, 78, 817–822. [Google Scholar] [CrossRef]
- Tamri, P.; Hemmati, A.; Boroujerdnia, M.G. wound healing properties of quince seed mucilage: In vivo evaluation in rabbit full-thickness wound model. Int. J. Surg. 2014, 12, 843–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, B.; Suchithra, T.V. Plant-derived bioadhesives for wound dressing and drug delivery system. Fitoterapia 2019, 137, 104241. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, M.; Manzoor, K.; Purwar, R.; Ikram, S. A review on latest innovations in natural gums based hydrogels: Preparations & applications. Int. J. Biol. Macromol. 2019, 136, 870–890. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, E.; Mekhloufi, G.; Rosilio, V.; Grossiord, J.L.; Agnely, F. Proteins, polysaccharides, and their complexes used as stabilizers for emulsions: Alternatives to synthetic surfactants in the pharmaceutical field? Int. J. Pharm. 2012, 436, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Campos, B.E.; Dias Ruivo, T.; da Silva Scapim, M.R.; Madrona, G.S.; Bergamasco, R.D. Optimization of the mucilage extraction process from chia seeds and application in ice cream as a stabilizer and emulsifier. LWT Food Sci. Technol. 2016, 65, 874–883. [Google Scholar] [CrossRef]
- Ahuja, M.; Kumar, A.; Yadav, P.; Singh, K. Mimosa pudica seed mucilage: Isolation; characterization and evaluation as tablet disintegrant and binder. Int. J. Biol. Macromol. 2013, 57, 105–110. [Google Scholar] [CrossRef]
- Kaleemullah, M.; Jiyauddin, K.; Thiban, E.; Rasha, S.; Al-Dhalli, S.; Budiasih, S.; Gamal, O.E.; Fadli, A.; Eddy, Y. Development and evaluation of Ketoprofen sustained release matrix tablet using Hibiscus rosa-sinensis leaves mucilage. Saudi Pharm. J. 2017, 25, 770–779. [Google Scholar] [CrossRef]
- Chaudhary, A.; Kulkarnı, G.T.; Awasthı, R.; Kumar, P. Investigation on binding properties of Grewi asiatica mucilage in tablet formulations. Marmara Pharm. J. 2016, 20, 353–367. [Google Scholar] [CrossRef] [Green Version]
- Raj, V.; Shim, J.J.; Lee, J. Grafting modification of okra mucilage: Recent findings, applications, and future directions. Carbohydr. Polym. 2020, 116653. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Bouafif, H.; Neculita, C.M.; Koubaa, A. Activated biochar as an effective sorbent for organic and inorganic contaminants in water. Water Air Soil Pollut. 2018, 229–230. [Google Scholar] [CrossRef]
- Hanjra, M.A.; Blackwell, J.; Carr, G.; Zhang, F.; Jackson, T.M. wastewater irrigation and environmental health: Implications for water governance and public policy. Int. J. Hyg. Environ. Health 2012, 215, 255–269. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.O.; John, O.O.; Luke, C.; Ochieng, A.; Bassey, B.J. Application of mucilage from dicerocaryum eriocarpum plant as biosorption medium in the removal of selected heavy metal ions. J. Environ. Manag. 2016, 177, 365–372. [Google Scholar] [CrossRef]
- Fox, D.I.; Pichler, T.; Yeh, D.H.; Alcantar, N.A. Removing heavy metals in water: The interaction of cactus mucilage and arsenate (As (V)). Environ. Sci. Technol. 2012, 46, 4553–4559. [Google Scholar] [CrossRef]
- Anastasakis, K.; Kalderis, D.; Diamadopoulos, E. Flocculation behavior of mallow and okra mucilage in treating wastewater. Desalination 2009, 249, 786–791. [Google Scholar] [CrossRef]
- Bouatay, F.; Eljebsi, N.; Dridi-Dhaouadi, S.; Mhenni, F. Valorization of the vicia faba mucilage on textile wastewater treatment as a bio-flocculant: Process development and optimization using response surface methodology (RSM). Water Sci. Technol. 2017, 75, 629–642. [Google Scholar] [CrossRef]
- Sindhu, G.; Ratheesh, M.; Shyni, G.L.; Nambisan, B.; Helen, A. Anti-inflammatory and antioxidative effects of mucilage of Trigonella Foenum Graecum (fenugreek) on adjuvant induced arthritic rats. Int. Immunopharmacol. 2012, 12, 205–211. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.; Naushad, M.; Kumar, A.; Rana, S.; Sharma, S.; Bhatnagar, A.; Stadler, F.J.; Ghfar, A.A.; Khan, M.R. Efficient removal of coomassie brilliant blue r-250 dye using starch/poly(alginic acid-cl-acrylamide) nanohydrogel. Process Saf. Environ. Prot. 2017, 109, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Lodhi, B.A.; Hussain, M.A.; Sher, M.; Haseeb, M.T.; Ashraf, M.U.; Hussain, S.Z.; Hussain, I.; Bukhari, S.N.A. Polysaccharide-based superporous, superabsorbent, and stimuli responsive hydrogel from sweet basil: A novel material for sustained drug release. Adv. Polym. Technol. 2019, 2119, 9583516. [Google Scholar] [CrossRef] [Green Version]
- Setia, A.; Ahuja, P. Nanohydrogels. In Organic Materials as Smart Nanocarriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 293–368. [Google Scholar] [CrossRef]
- Okutan, N.; Terzi, P.; Altay, F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocoll. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Fathi, M.; Martín, Á.; McClements, D.J. Nanoencapsulation of food ingredients using carbohydrate based delivery systems. Trends Food Sci. Technol. 2014, 39, 18–39. [Google Scholar] [CrossRef]
- Prusty, K.; Swain, S.K. Release of ciprofloxacin drugs by nano gold embedded cellulose grafted polyacrylamide hybrid nanocomposite hydrogels. Int. J. Biol. Macromol. 2019, 126, 765–775. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Kumar, A.; Ranjbar-Mohammadi, M.; Ashrafizadeh, M.; Han, S.S.; Khang, G.; Roveimiab, Z. Recent advances in natural gum-based biomaterials for tissue engineering and regenerative medicine: A review. Polymers 2020, 12, 176. [Google Scholar] [CrossRef] [Green Version]
- Thakur, V.K.; Thakur, M.K. Recent Trends in Hydrogels Based on Psyllium Polysaccharide: A Review. J. Clean. Prod. 2014, 82, 1–15. [Google Scholar] [CrossRef]
- Rayegan, A.; Allafchian, A.; Abdolhosseini Sarsari, I.; Kameli, P. Synthesis and characterization of basil seed mucilage coated Fe3O4 magnetic nanoparticles as a drug carrier for the controlled delivery of cephalexin. Int. J. Biol. Macromol. 2018, 113, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Seyyed, M.; Tabrizi, H.M.; Behrouz, E.; Vahid, J. Biosynthesis of pure zinc oxide nanoparticles using Quince seed mucilage for photocatalytic dye degradation. J. Alloy. Compd. 2020, 821, 153519. [Google Scholar] [CrossRef]
- Prasad, A.R.; Garvasis, J.; Oruvil, S.K.; Joseph, A. Improving oxidative and microbial stability of beef using Shahri Balangu seed mucilage loaded with Cumin essential oil as a bioactive edible coating. J. Phys. Chem. Solids 2019, 127, 265–274. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Javanbakht, V. Fabrication of Zn-based magnetic zeolitic imidazolate framework bionanocomposite using basil seed mucilage for removal of azo cationic and anionic dyes from aqueous solution. Int. J. Biol. Macromol. 2021, 167, 1076–1090. [Google Scholar] [CrossRef] [PubMed]
- Pathak, D.; Kumar, P.; Kuppusamy, G.; Gupta, A.; Kamble, B.; Wadhwani, A. Physicochemical characterization and toxicological evaluation of plant-based anionic polymers and their nanoparticulated system for ocular delivery. Nanotoxicology 2014, 8, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Akbari, I.; Ghoreishi, S.M.; Habibi, N. Generation and precipitation of paclitaxel nanoparticles in basil seed mucilage via combination of supercritical gas antisolvent and phase inversion techniques. J. Supercrit. Fluids 2014, 94, 182–188. [Google Scholar] [CrossRef]
- Haseeb, M.T.; Khaliq, N.U.; Yuk, S.H.; Hussain, M.A.; Bashir, S. Linseed polysaccharides based nanoparticles for controlled delivery of docetaxel: Design, in vitro drug release and cellular uptake. J. Drug Deliv. Sci. Technol. 2019, 49, 143–151. [Google Scholar] [CrossRef]
- Chawla, P.; Kumar, N.; Kaushik, R.; Dhull, S.B. Synthesis, characterization and cellular mineral absorption of nanoemulsions of Rhododendron arboreum flower extracts stabilized with gum Arabic. J. Food Sci. Technol. 2019, 56, 5194–5203. [Google Scholar] [CrossRef]
- Carmona, J.C.; Robert, P.; Vergara, C.; Sáenz, C. Microparticles of yellow-orange cactus pear pulp (Opuntia ficus-indica) with cladode mucilage and maltodextrin as a food coloring in yogurt. LWT 2021, 138, 110672. [Google Scholar] [CrossRef]
- Miart, F.; Fournet, F.; Dubrulle, N.; Petit, E.; Demailly, H.; Dupont, L.; Zabijak, L.; Marcelo, P.; Boudaoud, A.; Pineau, C.; et al. Cytological approaches combined with chemical analysis reveals the layered nature of flax mucilage. Front. Plant Sci. 2019, 10, 684. [Google Scholar] [CrossRef]
- Waghmare, R.; Moses, J.A.; Anandharamakrishnan, C. Mucilages: Sources, extraction methods, and characteristics for their use as encapsulation agents. Crit. Rev. Food Sci. Nutr. 2021, 1–22. [Google Scholar] [CrossRef]
- Soto-Cerda, B.J.; Cloutier, S.; Quian, R.; Gajardo, H.A.; Olivos, M.; You, F.M. Genome-wide association analysis of mucilage and hull content in flax (Linum usitatissimum L.) seeds. Int. J. Mol. Sci. 2018, 19, 2870. [Google Scholar] [CrossRef] [Green Version]
- Orrego, D.; Zapata-Zapata, A.D.; Kim, D. Optimization and scale-up of coffee mucilage fermentation for ethanol production. Energies 2018, 11, 786. [Google Scholar] [CrossRef] [Green Version]
- Añibarro-Ortega, M.; Pinela, J.; Barros, L.; Ćirić, A.; Silva, S.P.; Coelho, E.; Mocan, A.; Calhelha, R.C.; Soković, M.; Coimbra, M.A.; et al. Compositional features and bioactive properties of aloe vera leaf (Fillet, mucilage, and rind) and flower. Antioxidants 2019, 8, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allegra, A.; Sortino, G.; Inglese, P.; Settanni, L.; Todaro, A.; Gallotta, A. The effectiveness of Opuntia ficus-indica mucilage edible coating on post-harvest maintenance of ‘Dottato’fig (Ficus carica L.) fruit. Food Packag. Shelf Life 2017, 12, 135–141. [Google Scholar] [CrossRef]




| Source of Mucilage | Part | Yield of Mucilage | Extraction Method | References |
|---|---|---|---|---|
| Linum usitatissimum L. | Seed | 7.3% | Extracted by centrifugation | [28] |
| Spinacia oleracea L. | Leaves | 12.62% | Extracted by acetone | [29] |
| Salvia hispanica | Seed | 8.3% 6.4% | Non-thermal extraction Thermal extraction | [30] |
| Plantago major | Seed | 15.18% | Thermal extraction | [31] |
| Basella alba | Leaves Stem | 4.83% 6.20% | Hot water extraction | [32] |
| Psyllium seed | Husk | 37–52% | Ultrasonic bath extraction | [33] |
| Mucilage Source | Functional Property | References |
|---|---|---|
| Chia seed mucilage | Stabilizing agent, thickening agent, fat replacer, and emulsifying agent. | [21,56] |
| Okra seed mucilage | Oil absorption, water absorption, emulsion stability, foaming capacity, and emulsifying capacity. | [65,66] |
| Tamarind seed mucilage | Water holding capacity, oil holding capacity, and solubility. | [13,67] |
| Flaxseed mucilage | Water holding capacity, thickening agent, gelling agent, emulsifying agent, and foaming agent. | [40,68,69] |
| Cress seed mucilage | water absorption, Film-forming agent, and gelling agent. | [70] |
| Mucilage | Incorporated Agent | Gram-Positive Bacteria | Gram-Negative Bacteria | References |
|---|---|---|---|---|
| Basil seed mucilage | Zinc oxide nanocomposites crosslinking with borax | Staphylococcus aureus | Escherichia coli | [89] |
| Quince seed mucilage | Thyme essential oil | Bacillus cereus, Listeria monocytogenes | Salmonella typhimurium, Escherichia coli, Vibrio cholera, Pseudomonas aeruginosa, Yersinia enterocolitica | [80] |
| Chia seed mucilage | Oregano essential oils, cellulose nanofibers | Staphylococcus aureus, Staphylococcus mutans, Bacillus thuringiensis | Escherichia coli, Pseudomonas aeruginosa, Salmonella typhmurium | [37,90] |
| Okra mucilage | Zinc oxide nanoparticles and carboxymethylcellulose (CMC) | Staphylococcus aureus | Escherichia coli | [91] |
| Qodume shirazi seed mucilage | Lavender essential oil | Staphylococcus aureus | Escherichia coli | [92] |
| Lepidium sativum seed mucilage | Heracleum lasiopetalum essential oil | Staphylococcus aureus | Escherichia coli | [93] |
| Source of Mucilage | Coating on Food | Reference |
|---|---|---|
| Aloe vera | Application on tomatoes | [98] |
| Aloe vera and Basil mucilage | Application on Apricots | [99] |
| Barbery fig mucilage | Application coating on kiwi slices | [100] |
| Cress mucilage | Application on fresh beef | [93] |
| Shahri Balangu seed mucilage with cumin essential oil | Application on beef slices | [39] |
| Hibiscus mucilage | Application on tomato | [101] |
| Flaxseed mucilage and xanthan gum | Application on cheddar cheese | [102] |
| Aloe vera gel | Application on apple slices | [103] |
| Seed Mucilage | Nanocarrier | Applications | References |
|---|---|---|---|
| Basil seed mucilage | Magnetic nanoparticles (Fe3O4) | Application for the controlled delivery of antibiotic (Cephalexin) | [130] |
| Cress seed mucilage | Nanofibers | Application for the delivery of vitamin A | [18] |
| Quince seed mucilage | Zinc oxide nanoparticles | Application for photocatalytic dye degradation | [131] |
| Quince seed mucilage | Magnetic nanocomposites | Application for removal of cationic dyes from the aqueous solutions | [132] |
| Basil seed mucilage | Zinc based magnetic bio nanocomposites | Application for removal of azo anionic and cationic dyes from the aqueous solutions | [133] |
| Okra seed mucilage | Zinc oxide nanoparticles | Application for nanocomposites-based films | [91] |
| Basil seed mucilage | ZnO nanocomposites | Application for wound healing | [68] |
| Chia seed mucilage | Nanoencapsulation | Application as wall material | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tosif, M.M.; Najda, A.; Bains, A.; Kaushik, R.; Dhull, S.B.; Chawla, P.; Walasek-Janusz, M. A Comprehensive Review on Plant-Derived Mucilage: Characterization, Functional Properties, Applications, and Its Utilization for Nanocarrier Fabrication. Polymers 2021, 13, 1066. https://doi.org/10.3390/polym13071066
Tosif MM, Najda A, Bains A, Kaushik R, Dhull SB, Chawla P, Walasek-Janusz M. A Comprehensive Review on Plant-Derived Mucilage: Characterization, Functional Properties, Applications, and Its Utilization for Nanocarrier Fabrication. Polymers. 2021; 13(7):1066. https://doi.org/10.3390/polym13071066
Chicago/Turabian StyleTosif, Mansuri M., Agnieszka Najda, Aarti Bains, Ravinder Kaushik, Sanju Bala Dhull, Prince Chawla, and Magdalena Walasek-Janusz. 2021. "A Comprehensive Review on Plant-Derived Mucilage: Characterization, Functional Properties, Applications, and Its Utilization for Nanocarrier Fabrication" Polymers 13, no. 7: 1066. https://doi.org/10.3390/polym13071066
APA StyleTosif, M. M., Najda, A., Bains, A., Kaushik, R., Dhull, S. B., Chawla, P., & Walasek-Janusz, M. (2021). A Comprehensive Review on Plant-Derived Mucilage: Characterization, Functional Properties, Applications, and Its Utilization for Nanocarrier Fabrication. Polymers, 13(7), 1066. https://doi.org/10.3390/polym13071066