3D Bioprinted Bacteriostatic Hyperelastic Bone Scaffold for Damage-Specific Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. 3D Bioprinting of Bone Constructs
2.2. Microscopy, Spectroscopy, and Thermogravimetric Analyses of the 3D Bone Constructs
2.3. Mechanical Testing
2.4. Computed Tomography (CT) Analysis
2.5. Cell Culture—Cell Seeding of Printed Constructs
2.5.1. Osteogenic Cultures
2.5.2. Macrophage Cultures
2.6. Bacteria and Macrophage Culture—Antibacterial Activity Assays
2.7. Animals
2.8. In Vivo Application of Bioprinted HB Scaffolds
2.9. Histological Analysis
2.10. Statistical Approaches
3. Results and Discussion
3.1. 3D Bioprinting of Damage-Specific SPION-Loaded Bone Constructs—Material Characterization of Bioprinted Scaffolds
3.2. Mechanical Characterization of Bioprinted Scaffolds
3.3. In Vitro Cell Viability and Growth in Bioprinted HB Scaffolds
3.4. In Vitro Computed Tomography (CT) Imaging of Bioprinted SPION-Loaded Constructs
3.5. Bacteriostatic Activity of Bioprinted HB Scaffolds
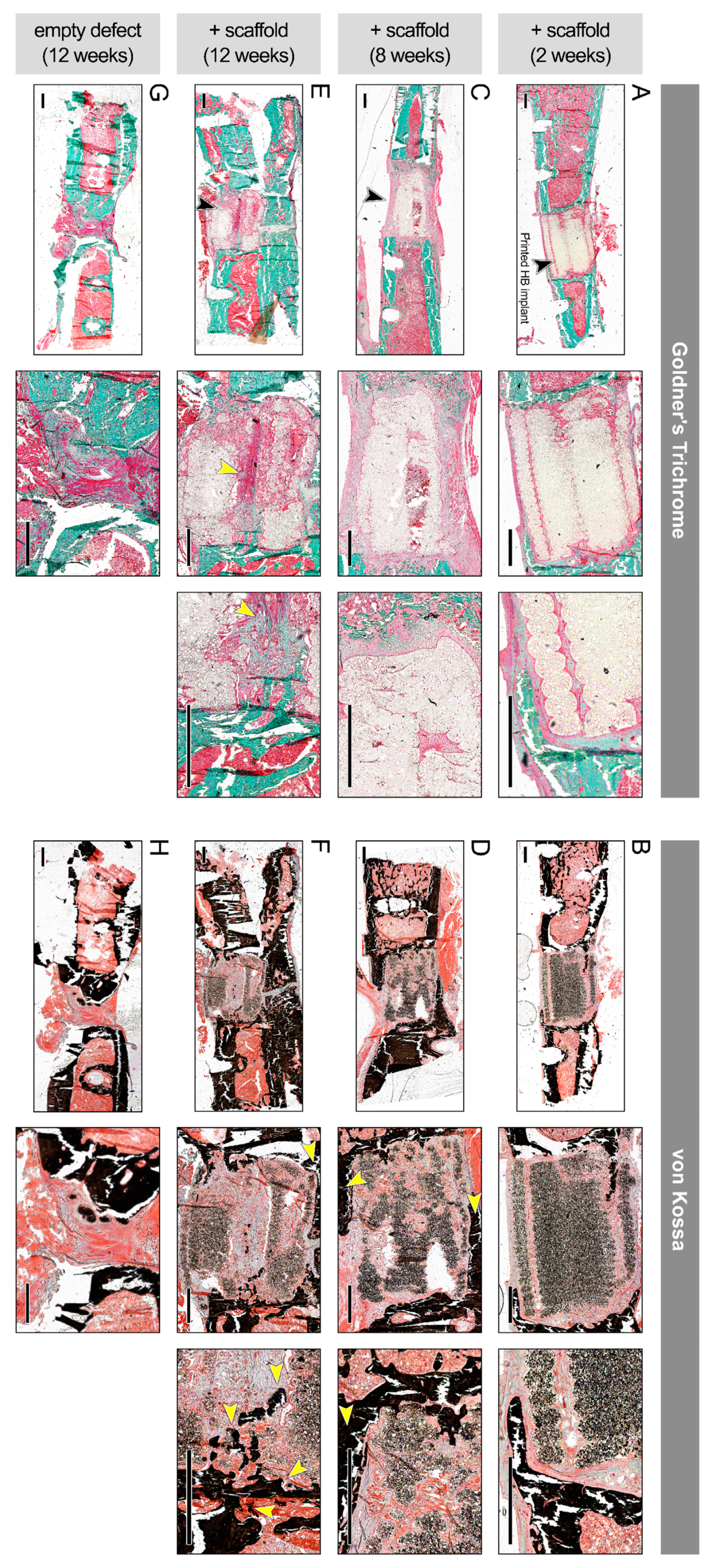
3.6. In Vivo Regenerative Capacity of Bioprinted Damage-Specific HB Constructs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroll, A.; Schiaparelli, F.F.; de Simoni, C.; Slevin, O.; Hirschmann, M.T. Bone void fillers in osteotomies: If, when, and which type? Orthopade 2017, 46, 596–600. [Google Scholar]
- Kirkpatrick, J.S.; Cornell, C.N.; Hoang, B.H.; Hsu, W.; Watson, J.T.; Watters, W.C., 3rd; Turkelson, C.M.; Wies, J.L.; Anderson, S. Bone void fillers. J. Am. Acad. Orthop. Surg. 2010, 18, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.H.; Liu, S.W.; Xiong, L.; Qiu, P.; Ding, L.H.; Xiong, S.L.; Li, J.T.; Liao, X.G.; Tang, Z.M. Scaffolds for the repair of bone defects in clinical studies: A systematic review. J. Orthop. Surg. Res. 2018, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Crowley, C.; Wong, J.M.; Fisher, D.M.; Khan, W.S. A systematic review on preclinical and clinical studies on the use of scaffolds for bone repair in skeletal defects. Curr. Stem Cell Res. 2013, 8, 243–252. [Google Scholar] [CrossRef]
- Winkler, T.; Sass, F.A.; Duda, G.N.; Schmidt-Bleek, K. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: The unsolved challenge. Bone Jt. Res. 2018, 7, 232–243. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Mazzoni, E.; Manfrini, M.; D’Agostino, A.; Trevisiol, L.; Nocini, R.; Trombelli, L.; Barbanti-Brodano, G.; Martini, F.; Tognon, M. Innovative Biomaterials for Bone Regrowth. Int J. Mol. Sci 2019, 20, 618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerado, E.; Caso, E. Challenges of bone tissue engineering in orthopaedic patients. World J. Orthop. 2017, 8, 87–98. [Google Scholar] [CrossRef]
- Mellonig, J.T.; Bowers, G.M.; Cotton, W.R. Comparison of bone graft materials. Part II. New bone formation with autografts and allografts: A histological evaluation. J. Periodontol. 1981, 52, 297–302. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Annabi, N.; Nikkhah, M.; Bae, H.; Binan, L.; Park, S.; Kang, Y.; Yang, Y.; Khademhosseini, A. Vascularized Bone Tissue Engineering: Approaches for Potential Improvement. Tissue Eng. Part B Rev. 2012, 18, 363–382. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, C.; Lindner, M.; Zhang, W.; Koczur, K.; Kirsten, A.; Telle, R.; Fischer, H. 3D printing of bone substitute implants using calcium phosphate and bioactive glasses. J. Eur. Ceram. Soc. 2010, 30, 2563–2567. [Google Scholar] [CrossRef]
- Mercado-Pagán, Á.E.; Stahl, A.M.; Shanjani, Y.; Yang, Y. Vascularization in Bone Tissue Engineering Constructs. Ann. Biomed. Eng. 2015, 43, 718–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.J.; Ebied, M.; Xu, J.; Zreiqat, H. Current Approaches to Bone Tissue Engineering: The Interface between Biology and Engineering. Adv. Healthc. Mater. 2018, 7, e1701061. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Petretta, M.; Cavallo, C.; Roseti, L.; Durante, S.; Albisinni, U.; Grigolo, B. Patient-specific meniscus prototype based on 3D bioprinting of human cell-laden scaffold. Bone Jt. Res. 2019, 8, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Serpooshan, V.; Guvendiren, M. Editorial for the Special Issue on 3D Printing for Tissue Engineering and Regenerative Medicine. Micromachines (Basel) 2020, 11, 366. [Google Scholar] [CrossRef] [Green Version]
- Trombetta, R.; Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D Printing of Calcium Phosphate Ceramics for Bone Tissue Engineering and Drug Delivery. Ann. Biomed. Eng. 2017, 45, 23–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomov, M.L.; Cetnar, A.; Do, K.; Bauser-Heaton, H.; Serpooshan, V. Patient-Specific 3-Dimensional-Bioprinted Model for In Vitro Analysis and Treatment Planning of Pulmonary Artery Atresia in Tetralogy of Fallot and Major Aortopulmonary Collateral Arteries. J. Am. Heart Assoc. 2019, 8, e014490. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, S.H.; Lewis, G.S.; Bushman, Z.J.; Adair, J.H.; Donahue, H.J. 3D Printing of Personalized Artificial Bone Scaffolds. 3d Print Addit. Manuf. 2015, 2, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31 (Suppl. 5), S20–S22. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef]
- Moreno Madrid, A.P.; Vrech, S.M.; Sanchez, M.A.; Rodriguez, A.P. Advances in additive manufacturing for bone tissue engineering scaffolds. Mater. Sci. Eng. C 2019, 100, 631–644. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chimene, D.; Miller, L.; Cross, L.M.; Jaiswal, M.K.; Singh, I.; Gaharwar, A.K. Nanoengineered Osteoinductive Bioink for 3D Bioprinting Bone Tissue. ACS Appl. Mater. Interfaces 2020, 12, 15976–15988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, S.; Zhou, C.; Cheng, L.; Gao, X.; Xie, X.; Sun, J.; Wang, H.; Weir, M.D.; Reynolds, M.A.; et al. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhang, N.; An, Y.H.; Wen, X. Biomaterial strategies to reduce implant-associated infections. Int. J. Artif. Organs. 2007, 30, 828–841. [Google Scholar] [CrossRef]
- Jakus, A.E.; Rutz, A.L.; Jordan, S.W.; Kannan, A.; Mitchell, S.M.; Yun, C.; Koube, K.D.; Yoo, S.C.; Whiteley, H.E.; Richter, C.P.; et al. Hyperelastic “bone”: A highly versatile, growth factor-free, osteoregenerative, scalable, and surgically friendly biomaterial. Sci. Transl. Med. 2016, 8, 358ra127. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.H.; Jakus, A.E.; Jordan, S.W.; Dumanian, Z.; Parker, K.; Zhao, L.; Patel, P.K.; Shah, R.N. Three-Dimensionally Printed Hyperelastic Bone Scaffolds Accelerate Bone Regeneration in Critical-Size Calvarial Bone Defects. Plast. Reconstr. Surg. 2019, 143, 1397–1407. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.S.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef] [Green Version]
- Vakili-Ghartavol, R.; Momtazi-Borojeni, A.A.; Vakili-Ghartavol, Z.; Aiyelabegan, H.T.; Jaafari, M.R.; Rezayat, S.M.; Arbabi Bidgoli, S. Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues. Artif. CellsNanomed. Biotechnol. 2020, 48, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [Green Version]
- Roohani-Esfahani, S.I.; Newman, P.; Zreiqat, H. Design and Fabrication of 3D printed Scaffolds with a Mechanical Strength Comparable to Cortical Bone to Repair Large Bone Defects. Sci. Rep. 2016, 6, 19468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivares-Navarrete, R.; Lee, E.M.; Smith, K.; Hyzy, S.L.; Doroudi, M.; Williams, J.K.; Gall, K.; Boyan, B.D.; Schwartz, Z. Substrate Stiffness Controls Osteoblastic and Chondrocytic Differentiation of Mesenchymal Stem Cells without Exogenous Stimuli. PLoS ONE 2017, 12, e0170312. [Google Scholar] [CrossRef]
- Corona-Gomez, J.; Chen, X.; Yang, Q. Effect of Nanoparticle Incorporation and Surface Coating on Mechanical Properties of Bone Scaffolds: A Brief Review. J. Funct. Biomater. 2016, 7, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.-L.; Teow, S.-Y.; Pushpamalar, J. Application of Metal Nanoparticle–Hydrogel Composites in Tissue Regeneration. Bioengineering 2019, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Maharaj, D.; Bhushan, B. Scale effects of nanomechanical properties and deformation behavior of Au nanoparticle and thin film using depth sensing nanoindentation. Beilstein J. Nanotechnol. 2014, 5, 822–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ker, D.F.; Sharma, R.; Wang, E.T.; Yang, Y.P. Development of mRuby2-Transfected C3H10T1/2 Fibroblasts for Musculoskeletal Tissue Engineering. PLoS ONE 2015, 10, e0139054. [Google Scholar] [CrossRef]
- McMurtrey, R.J. Analytic Models of Oxygen and Nutrient Diffusion, Metabolism Dynamics, and Architecture Optimization in Three-Dimensional Tissue Constructs with Applications and Insights in Cerebral Organoids. Tissue Eng. Part C Methods 2016, 22, 221–249. [Google Scholar] [CrossRef] [Green Version]
- Rouwkema, J.; Koopman, B.; Blitterswijk, C.; Dhert, W.; Malda, J. Supply of nutrients to cells in engineered tissues. Biotechnol. Genet. Eng. Rev. 2010, 26, 163–178. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Holsbeeks, I.; Impens, S.; Sonnaert, M.; Bloemen, V.; Luyten, F.; Schrooten, J. Noninvasive real-time monitoring by alamarBlue((R)) during in vitro culture of three-dimensional tissue-engineered bone constructs. Tissue Eng. Part C Methods 2013, 19, 720–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbiahdoss, G.; Sharifi, S.; Grijpma, D.W.; Laurent, S.; van der Mei, H.C.; Mahmoudi, M.; Busscher, H.J. Magnetic targeting of surface-modified superparamagnetic iron oxide nanoparticles yields antibacterial efficacy against biofilms of gentamicin-resistant staphylococci. Acta Biomater. 2012, 8, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Serpooshan, V. Silver-coated engineered magnetic nanoparticles are promising for the success in the fight against antibacterial resistance threat. Acs Nano 2012, 6, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Borgwardt, L.; Hoiby, N.; Wu, H.; Sorensen, T.S.; Borgwardt, A. Prosthesis infections after orthopedic joint replacement: The possible role of bacterial biofilms. Orthop. Rev. (Pavia) 2013, 5, 65–71. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shokouhimehr, M.; Theus, A.S.; Kamalakar, A.; Ning, L.; Cao, C.; Tomov, M.L.; Kaiser, J.M.; Goudy, S.; Willett, N.J.; Jang, H.W.; et al. 3D Bioprinted Bacteriostatic Hyperelastic Bone Scaffold for Damage-Specific Bone Regeneration. Polymers 2021, 13, 1099. https://doi.org/10.3390/polym13071099
Shokouhimehr M, Theus AS, Kamalakar A, Ning L, Cao C, Tomov ML, Kaiser JM, Goudy S, Willett NJ, Jang HW, et al. 3D Bioprinted Bacteriostatic Hyperelastic Bone Scaffold for Damage-Specific Bone Regeneration. Polymers. 2021; 13(7):1099. https://doi.org/10.3390/polym13071099
Chicago/Turabian StyleShokouhimehr, Mohammadreza, Andrea S. Theus, Archana Kamalakar, Liqun Ning, Cong Cao, Martin L. Tomov, Jarred M. Kaiser, Steven Goudy, Nick J. Willett, Ho Won Jang, and et al. 2021. "3D Bioprinted Bacteriostatic Hyperelastic Bone Scaffold for Damage-Specific Bone Regeneration" Polymers 13, no. 7: 1099. https://doi.org/10.3390/polym13071099
APA StyleShokouhimehr, M., Theus, A. S., Kamalakar, A., Ning, L., Cao, C., Tomov, M. L., Kaiser, J. M., Goudy, S., Willett, N. J., Jang, H. W., LaRock, C. N., Hanna, P., Lechtig, A., Yousef, M., Martins, J. D. S., Nazarian, A., Harris, M. B., Mahmoudi, M., & Serpooshan, V. (2021). 3D Bioprinted Bacteriostatic Hyperelastic Bone Scaffold for Damage-Specific Bone Regeneration. Polymers, 13(7), 1099. https://doi.org/10.3390/polym13071099










