Improving Rheological and Mechanical Properties of Various Virgin and Recycled Polypropylenes by Blending with Long-Chain Branched Polypropylene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
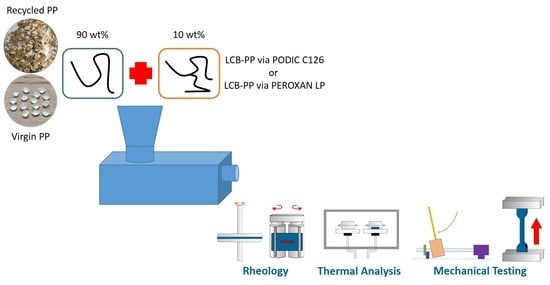
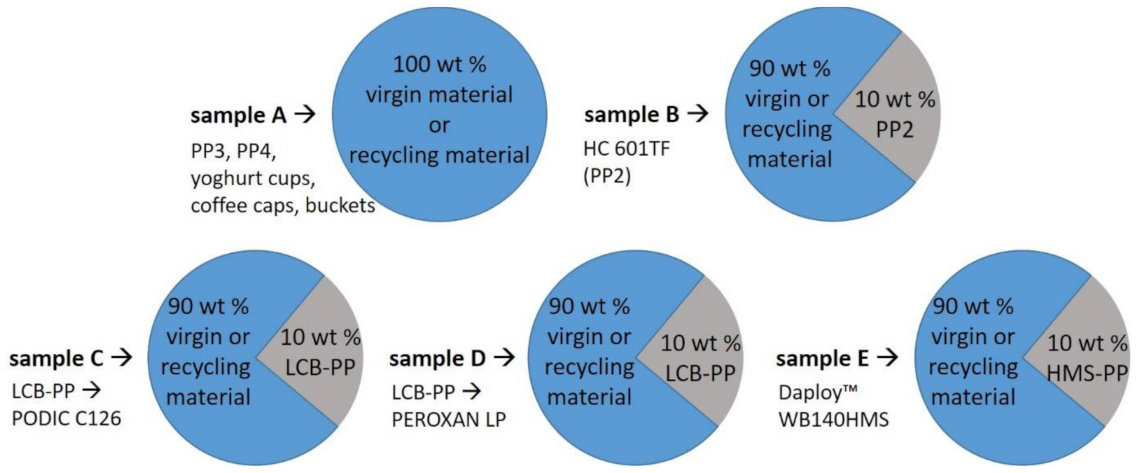
2.2. Sample Preparation
2.3. Melt Flow Rate (MFR)
2.4. Differential Scanning Caloremtry (DSC)
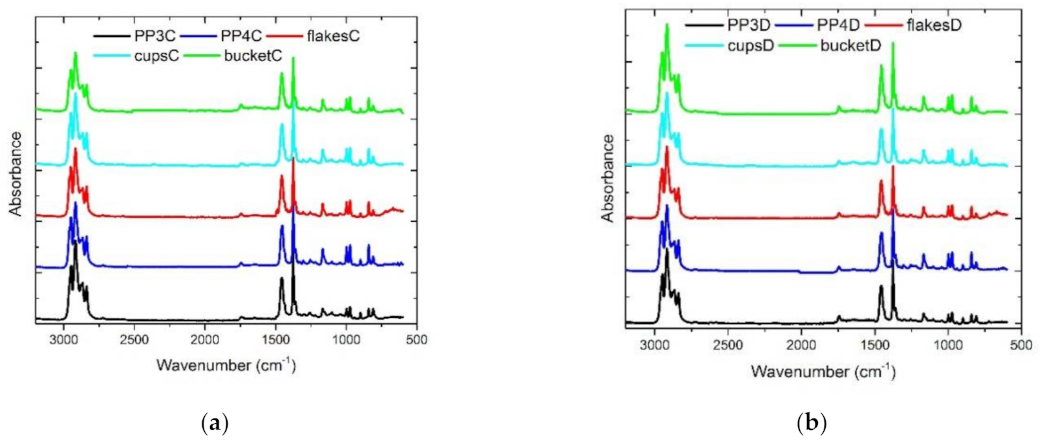
2.5. Fourier Transform Infared (FTIR) Measurements
2.6. Rheology Properties
2.7. Mechanical Properties
2.8. Scanning Electron Microscopy (SEM)
3. Results
3.1. Melt Flow Rate
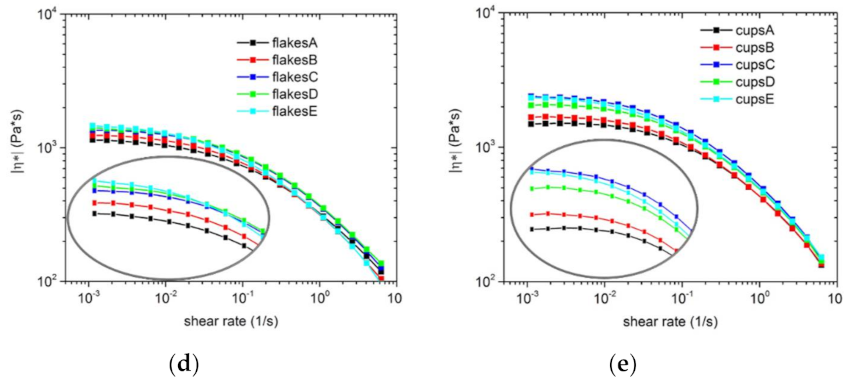
3.2. Dynamic Rheology Properties
3.3. Extensional Rheology
3.4. Thermal Characterization
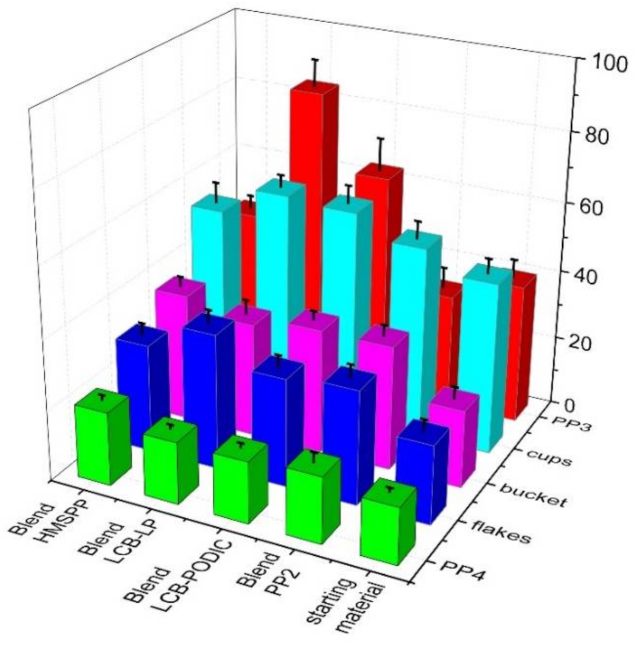
3.5. Mechanical Properties
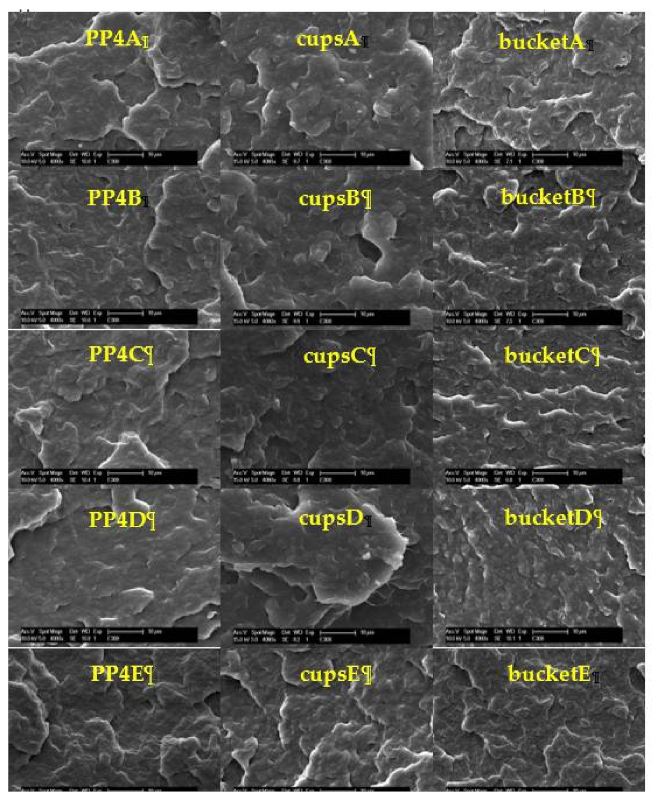
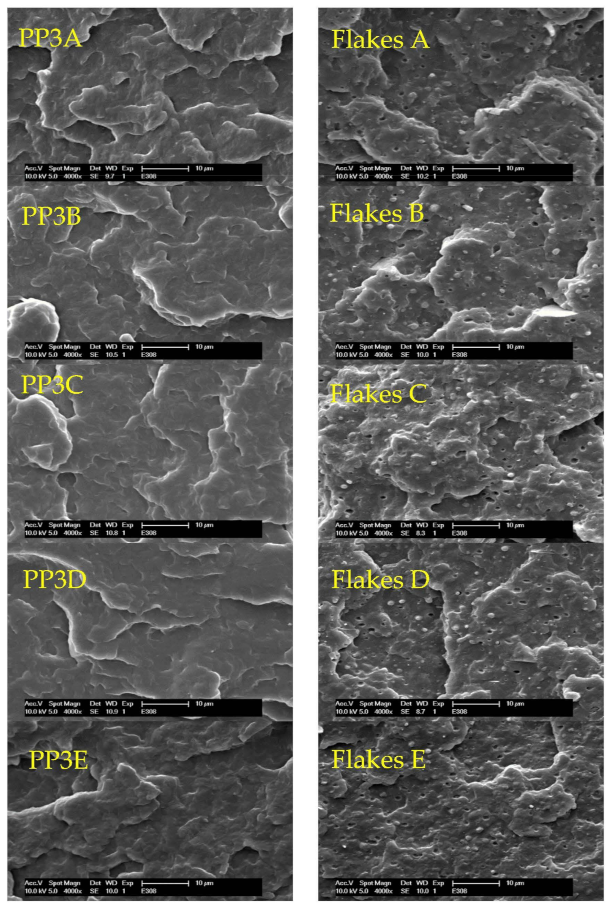
3.6. Scanning Electron Microscopy (SEM)
3.7. Characterization of Modified Polymers Long-Chain Branched Polypropylenes (LCB-PP)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Plastic Europe. Plastics—The Facts 2019; Plastic Europe: Brussels, Belgium, 2020. [Google Scholar]
- Pasquini, N.; Addeo, A. (Eds.) Polypropylene Handbook; Hanser Publications: Munich, Germany, 2005. [Google Scholar]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, D.; Aoustin, E.; Buclet, N.; Brandt, N. Plastic waste management in the context of a European recycling society: Comparing results and uncertainties in a life cycle perspective. Resour. Conserv. Recycl. 2010, 55, 246–259. [Google Scholar] [CrossRef]
- Gu, F.; Hall, P.; Miles, N.J. Performance evaluation for composites based on recycled polypropylene using principal component analysis and cluster analysis. J. Clean. Prod. 2016, 115, 343–353. [Google Scholar] [CrossRef]
- Gu, F.; Hall, P.; Miles, N.J. Development of composites based on recycled polypropylene for injection moulding automobile parts using hierarchical clustering analysis and principal component estimate. J. Clean. Prod. 2016, 137, 632–643. [Google Scholar] [CrossRef]
- Wäger, P.A.; Hischier, R. Life cycle assessment of post-consumer plastics production from waste electrical and electronic equipment (WEEE) treatment residues in a Central European plastics recycling plant. Sci. Total Environ. 2015, 529, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Al-Maaded, M.; Madi, N.K.; Kahraman, R.; Hodzic, A.; Ozerkan, N.G. An Overview of Solid Waste Management and Plastic Recycling in Qatar. J. Polym. Environ. 2012, 20, 186–194. [Google Scholar] [CrossRef]
- Strapasson, R.; Amico, S.C.; Pereira, M.F.R.; Sydenstricker, T.H.D. Tensile and impact behavior of polypropylene/low density polyethylene blends. Polym. Test. 2005, 24, 468–473. [Google Scholar] [CrossRef]
- Meran, C.; Ozturk, O.; Yuksel, M. Examination of the possibility of recycling and utilizing recycled polyethylene and polypropylene. Mater. Des. 2008, 29, 701–705. [Google Scholar] [CrossRef]
- Brachet, P.; Høydal, L.T.; Hinrichsen, E.L.; Melum, F. Modification of mechanical properties of recycled polypropylene from post-consumer containers. Waste Manag. 2008, 28, 2456–2464. [Google Scholar] [CrossRef]
- Kowalska, E.; Wielgosz, Z.; Pelka, J. Use of Post-Life Waste and Production Waste in Thermoplastic Polymer Compositions. Polym. Polym. Compos. 2002, 10, 83–92. [Google Scholar] [CrossRef]
- Tri Phuong, N.; Gilbert, V.; Chuong, B. Preparation of Recycled Polypropylene/Organophilic Modified Layered Silicates Nanocomposites Part I: The Recycling Process of Polypropylene and the Mechanical Properties of Recycled Polypropylene/Organoclay Nanocomposites. J. Reinf. Plast. Compos. 2008, 27, 1983–2000. [Google Scholar] [CrossRef]
- Bahlouli, N.; Pessey, D.; Raveyre, C.; Guillet, J.; Ahzi, S.; Dahoun, A.; Hiver, J.M. Recycling effects on the rheological and thermomechanical properties of polypropylene-based composites. Mater. Des. 2012, 33, 451–458. [Google Scholar] [CrossRef]
- Bourmaud, A.; Baley, C. Rigidity analysis of polypropylene/vegetal fibre composites after recycling. Polym. Degrad. Stab. 2009, 94, 297–305. [Google Scholar] [CrossRef]
- Dehghani, A.; Madadi Ardekani, S.; Al-Maadeed, M.A.; Hassan, A.; Wahit, M.U. Mechanical and thermal properties of date palm leaf fiber reinforced recycled poly (ethylene terephthalate) composites. Mater. Des. 2013, 52, 841–848. [Google Scholar] [CrossRef]
- Al-Maadeed, M.A.; Shabana, Y.M.; Khanam, P.N. Processing, characterization and modeling of recycled polypropylene/glass fibre/wood flour composites. Mater. Des. 2014, 58, 374–380. [Google Scholar] [CrossRef]
- Sommerhuber, P.F.; Welling, J.; Krause, A. Substitution potentials of recycled HDPE and wood particles from post-consumer packaging waste in Wood–Plastic Composites. Waste Manag. 2015, 46, 76–85. [Google Scholar] [CrossRef]
- Sommerhuber, P.F.; Wang, T.; Krause, A. Wood–plastic composites as potential applications of recycled plastics of electronic waste and recycled particleboard. J. Clean. Prod. 2016, 121, 176–185. [Google Scholar] [CrossRef]
- Vazquez, Y.V.; Barbosa, S.E. Recycling of mixed plastic waste from electrical and electronic equipment. Added value by compatibilization. Waste Manag. 2016, 53, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Kamleitner, F.; Duscher, B.; Koch, T.; Knaus, S.; Archodoulaki, V.M. Upcycling of polypropylene—The influence of polyethylene impurities. Polym. Eng. Sci. 2017, 57, 1374–1381. [Google Scholar] [CrossRef]
- Jahani, Y.; Ghetmiri, M.; Vaseghi, M.R. The effects of long chain branching of polypropylene and chain extension of poly(ethylene terephthalate) on the thermal behavior, rheology and morphology of their blends. RSC Adv. 2015, 5, 21620–21628. [Google Scholar] [CrossRef]
- Nam, G.J.; Yoo, J.H.; Lee, J.W. Effect of long-chain branches of polypropylene on rheological properties and foam-extrusion performances. J. Appl. Polym. Sci. 2005, 96, 1793–1800. [Google Scholar] [CrossRef]
- Gotsis, A.D.; Zeevenhoven, B.L.F.; Hogt, A.H. The effect of long chain branching on the processability of polypropylene in thermoforming. Polym. Eng. Sci. 2004, 44, 973–982. [Google Scholar] [CrossRef]
- Tian, J.; Yu, W.; Zhou, C.J.P. The preparation and rheology characterization of long chain branching polypropylene. Polymer 2006, 47, 7962–7969. [Google Scholar] [CrossRef]
- Stange, J.; Münstedt, H. Rheological properties and foaming behavior of polypropylenes with different molecular structures. J. Rheol. 2006, 50, 907–923. [Google Scholar] [CrossRef]
- Passaglia, E.; Coiai, S.; Cicogna, F.; Ciardelli, F. Some recent advances in polyolefin functionalization. Polym. Int. 2014, 63, 12–21. [Google Scholar] [CrossRef]
- Parent, J.S.; Bodsworth, A.; Sengupta, S.S.; Kontopoulou, M.; Chaudhary, B.I.; Poche, D.; Cousteaux, S. Structure–rheology relationships of long-chain branched polypropylene: Comparative analysis of acrylic and allylic coagent chemistry. Polymer 2009, 50, 85–94. [Google Scholar] [CrossRef]
- El Mabrouk, K.; Parent, J.S.; Chaudhary, B.I.; Cong, R. Chemical modification of PP architecture: Strategies for introducing long-chain branching. Polymer 2009, 50, 5390–5397. [Google Scholar] [CrossRef]
- Kim, D.Y.; Cha, J.H.; Seo, K.H. Effects of chain extender on properties and foaming behavior of polypropylene foam. RSC Adv. 2019, 9, 25496–25507. [Google Scholar] [CrossRef] [Green Version]
- Weng, W.; Hu, W.; Dekmezian, A.H.; Ruff, C.J. Long chain branched isotactic polypropylene. Macromolecules 2002, 35, 3838–3843. [Google Scholar] [CrossRef]
- Langston, J.A.; Colby, R.H.; Chung, T.C.M.; Shimizu, F.; Suzuki, T.; Aoki, M. Synthesis and Characterization of Long Chain Branched Isotactic Polypropylene via Metallocene Catalyst and T-Reagent. Macromolecules 2007, 40, 2712–2720. [Google Scholar] [CrossRef]
- Krause, B.; Stephan, M.; Volkland, S.; Voigt, D.; Häußler, L.; Dorschner, H. Long-chain branching of polypropylene by electron-beam irradiation in the molten state. J. Appl. Polym. Sci. 2006, 99, 260–265. [Google Scholar] [CrossRef]
- Lugão, A.B.; Otaguro, H.; Parra, D.F.; Yoshiga, A.; Lima, L.F.C.P.; Artel, B.W.H.; Liberman, S. Review on the production process and uses of controlled rheology polypropylene—Gamma radiation versus electron beam processing. Radiat. Phys. Chem. 2007, 76, 1688–1690. [Google Scholar] [CrossRef]
- Ali, Z.I.; Youssef, H.A.; Said, H.M.; Saleh, H.H. Influence of electron beam irradiation and polyfunctional monomer loading on the physico-chemical properties of polyethylene/polypropylene blends. Adv. Polym. Technol. J. Polym. Process. Inst. 2006, 25, 208–217. [Google Scholar] [CrossRef]
- Graebling, D. Synthesis of Branched Polypropylene by a Reactive Extrusion Process. Macromolecules 2002, 35, 4602–4610. [Google Scholar] [CrossRef]
- Su, F.H.; Huang, H.X. Rheology and thermal behavior of long branching polypropylene prepared by reactive extrusion. J. Appl. Polym. Sci. 2009, 113, 2126–2135. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Wagner, M.H. Impact of processing history on rheological properties for branched polypropylene. Polymer 2006, 47, 3629–3635. [Google Scholar] [CrossRef]
- Kamleitner, F.; Duscher, B.; Koch, T.; Knaus, S.; Schmid, K.; Archodoulaki, V.-M. Influence of the Molar Mass on Long-Chain Branching of Polypropylene. Polymers 2017, 9, 442. [Google Scholar] [CrossRef] [Green Version]
- Stanic, S.; Gottlieb, G.; Koch, T.; Göpperl, L.; Schmid, K.; Knaus, S.; Archodoulaki, V.-M. Influence of Different Types of Peroxides on the Long-Chain Branching of PP via Reactive Extrusion. Polymers 2020, 12, 886. [Google Scholar] [CrossRef] [Green Version]
- Maris, J.; Bourdon, S.; Brossard, J.-M.; Cauret, L.; Fontaine, L.; Montembault, V. Mechanical recycling: Compatibilization of mixed thermoplastic wastes. Polym. Degrad. Stab. 2018, 147, 245–266. [Google Scholar] [CrossRef]
- Moad, G. The synthesis of polyolefin graft copolymers by reactive extrusion. Prog. Polym. Sci. 1999, 24, 81–142. [Google Scholar] [CrossRef]
- Moore Junior, E. Polypropylene Handbook: Polymerization, Characterization, Properties, Processing, Applications; Hanser Publishers: New York, NY, USA, 1996. [Google Scholar]
- Zweifel, H.; Maier, R.D.; Schiller, M.; Amos, S.E. Plastics Additives Handbook; Hanser Pub Inc.: Munich, Germany, 2001. [Google Scholar]
- Azizi, H.; Ghasemi, I. Reactive extrusion of polypropylene: Production of controlled-rheology polypropylene (CRPP) by peroxide-promoted degradation. Polym. Test. 2004, 23, 137–143. [Google Scholar] [CrossRef]
- Azizi, H.; Ghasemi, I.; Karrabi, M. Controlled-peroxide degradation of polypropylene: Rheological properties and prediction of MWD from rheological data. Polym. Test. 2008, 27, 548–554. [Google Scholar] [CrossRef]
- Stange, J.; Uhl, C.; Münstedt, H. Rheological behavior of blends from a linear and a long-chain branched polypropylene. J. Rheol. 2005, 49, 1059–1079. [Google Scholar] [CrossRef]
- Fang, Y.; Sadeghi, F.; Fleuret, G.; Carreau, P.J. Properties of blends of linear and branched polypropylenes in film blowing. Can. J. Chem. Eng. 2008, 86, 6–14. [Google Scholar] [CrossRef]
- McCallum, T.J.; Kontopoulou, M.; Park, C.B.; Muliawan, E.B.; Hatzikiriakos, S.G. The rheological and physical properties of linear and branched polypropylene blends. Polym. Eng. Sci. 2007, 47, 1133–1140. [Google Scholar] [CrossRef]
- Stanic, S.; Koch, T.; Schmid, K.; Knaus, S.; Archodoulaki, V.-M. Upcycling of polypropylene with various concentrations of peroxydicarbonate and dilauroyl peroxide and two processing steps. J. Appl. Polym. Sci. 2021, 50659. [Google Scholar] [CrossRef]
- ISO 1133. Plastics—Determination of the Melt Mass-Flow Rate (MFR) and Melt Volume-Flow Rate (MVR) of Thermoplastics—Part 1: Standard Method; ISO: Geneva, Switzerland, 2011. [Google Scholar]
- ISO 527. Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- ISO 8256. Plastics—Determination of Tensile-Impact Strength; ISO: Geneva, Switzerland, 2004. [Google Scholar]
- Lagendijk, R.P.; Hogt, A.H.; Buijtenhuijs, A.; Gotsis, A.D. Peroxydicarbonate modification of polypropylene and extensional flow properties. Polymer 2001, 42, 10035–10043. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, J.; Li, L.; Zhao, S.; Shi, Y.; Xin, Z. Relationship between Peroxide Initiators and Properties of Styrene Grafted Polypropylene via Reactive Extrusion. J. Macromol. Sci. Part B 2018, 57, 377–394. [Google Scholar] [CrossRef]
- An, Y.; Zhang, Z.; Bi, W.; Wang, Y.; Tang, T. Characterization of high melt strength polypropylene synthesized via silane grafting initiated by in situ heat induction reaction. J. Appl. Polym. Sci. 2008, 110, 3727–3732. [Google Scholar] [CrossRef]
- Drabek, J.; Zatloukal, M.; Martyn, M. Effect of molecular weight, branching and temperature on dynamics of polypropylene melts at very high shear rates. Polymer 2018, 144, 179–183. [Google Scholar] [CrossRef]
- Su, F.-H.; Huang, H.-X. Influence of polyfunctional monomer on melt strength and rheology of long-chain branched polypropylene by reactive extrusion. J. Appl. Polym. Sci. 2010, 116, 2557–2565. [Google Scholar] [CrossRef]
- Su, F.-H.; Huang, H.-X. Rheology and melt strength of long chain branching polypropylene prepared by reactive extrusion with various peroxides. Polym. Eng. Sci. 2010, 50, 342–351. [Google Scholar] [CrossRef]
- Sugimoto, M.; Masubuchi, Y.; Takimoto, J.; Koyama, K. Melt rheology of polypropylene containing small amounts of high molecular weight chain. I. Shear flow. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 2692–2704. [Google Scholar] [CrossRef]
- Wong, B.; Baker, W.J.P. Melt rheology of graft modified polypropylene. Polymer 1997, 38, 2781–2789. [Google Scholar] [CrossRef]
- Malmberg, A.; Gabriel, C.; Steffl, T.; Münstedt, H.; Löfgren, B. Long-Chain Branching in Metallocene-Catalyzed Polyethylenes Investigated by Low Oscillatory Shear and Uniaxial Extensional Rheometry. Macromolecules 2002, 35, 1038–1048. [Google Scholar] [CrossRef]
- Larson, R.G. The Structure and Rheology of Complex Fluids; Oxford University Press: New York, NY, USA, 1999; Volume 150. [Google Scholar]
- Wang, L.; Wan, D.; Zhang, Z.; Liu, F.; Xing, H.; Wang, Y.; Tang, T. Synthesis and Structure–Property Relationships of Polypropylene-g-poly(ethylene-co-1-butene) Graft Copolymers with Well-Defined Long Chain Branched Molecular Structures. Macromolecules 2011, 44, 4167–4179. [Google Scholar] [CrossRef]
- Ajji, A.; Sammut, P.; Huneault, M.A. Elongational rheology of LLDPE/LDPE blends. J. Appl. Polym. Sci. 2003, 88, 3070–3077. [Google Scholar] [CrossRef]
- Bernreitner, K.; Neiβl, W.; Gahleitner, M. Correlation between molecular structure and rheological behaviour of polypropylene. Polym. Test. 1992, 11, 89–100. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook: For Users of Rotational and Oscillatory Rheometers; Vincentz Network GmbH & Co KG: Hannover, Germany, 2006. [Google Scholar]
- Tzoganakis, C. A rheological evaluation of linear and branched controlled-rheology polypropylenes. Can. J. Chem. Eng. 1994, 72, 749–754. [Google Scholar] [CrossRef]
- Morshedian, J.; Karbalaei-Bagher, M.; Bayazian, H.; Jamshidi, A.; Razavi-Nouri, M. Unraveling the Bimodality of Polypropylene Film Grades Using Rheological Shear and Elongational Measurements: Inconsistent Results of Gel Permeation Chromatography. Polym. Sci. Ser. A 2018, 60, 523–529. [Google Scholar] [CrossRef]
- Fleissner, M. Characterization of polymer molecular mass distribution from rheological measurements. In Makromolekulare Chemie. Macromolecular Symposia; Hüthig & Wepf Verlag: Basel, Switzerland, 1992; Volume 61, pp. 324–341. [Google Scholar] [CrossRef]
- Borg, T.; Pääkkönen, E.J. Linear viscoelastic models: Part II. Recovery of the molecular weight distribution using viscosity data. J. Non-Newton. Fluid Mech. 2009, 156, 129–138. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Teishev, A.E. Flow curve–molecular weight distribution: Is the solution of the inverse problem possible? Polym. Eng. Sci. 1991, 31, 1590–1596. [Google Scholar] [CrossRef]
- Friedrich, C.; Loy, R.J.; Anderssen, R.S. Relaxation time spectrum molecular weight distribution relationships. Rheol. Acta 2008, 48, 151. [Google Scholar] [CrossRef]
- Gotsis, A.D.; Zeevenhoven, B.L.F.; Tsenoglou, C. Effect of long branches on the rheology of polypropylene. J. Rheol. 2004, 48, 895–914. [Google Scholar] [CrossRef]
- Kamleitner, F.; Duscher, B.; Koch, T.; Knaus, S.; Archodoulaki, V.-M. Long chain branching as an innovative up-cycling process of polypropylene post-consumer waste—Possibilities and limitations. Waste Manag. 2017, 68, 32–37. [Google Scholar] [CrossRef]
- Münstedt, H.; Auhl, D. Rheological measuring techniques and their relevance for the molecular characterization of polymers. J. Non-Newton. Fluid Mech. 2005, 128, 62–69. [Google Scholar] [CrossRef]
- Münstedt, H.; Laun, H.M. Elongational properties and molecular structure of polyethylene melts. Rheol. Acta 1981, 20, 211–221. [Google Scholar] [CrossRef]
- Spitael, P.; Macosko, C.W. Strain hardening in polypropylenes and its role in extrusion foaming. Polym. Eng. Sci. 2004, 44, 2090–2100. [Google Scholar] [CrossRef]
- Dealy, J.M.; Wissbrun, K.F. Melt Rheology and Its Role in Plastics Processing: Theory and Applications; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Stadler, F.J.; Nishioka, A.; Stange, J.; Koyama, K.; Münstedt, H. Comparison of the elongational behavior of various polyolefins in uniaxial and equibiaxial flows. Rheol. Acta 2007, 46, 1003–1012. [Google Scholar] [CrossRef]
- Duscher, B.; Schausberger, A. Influence of processing on the flow properties of long-chain branched polypropylene. Polym. Eng. Sci. 2018, 58, 1596–1603. [Google Scholar] [CrossRef]
- Seier, M.; Stanic, S.; Koch, T.; Archodoulaki, V.-M. Effect of Different Compatibilization Systems on the Rheological, Mechanical and Morphological Properties of Polypropylene/Polystyrene Blends. Polymers 2020, 12, 2335. [Google Scholar] [CrossRef]
- Münstedt, H. Extensional Rheology and Processing of Polymeric Materials. Int. Polym. Process. 2018, 33, 594–618. [Google Scholar] [CrossRef]
- Vlachopoulos, J.; Polychronopoulos, N.; Kontopoulou, M. Basic Concepts in Polymer Melt Rheology and Their Importance in Processing. Appl. Polym. Rheol. 2011, 1–27. [Google Scholar] [CrossRef]
- Trouton, F.T. On the coefficient of viscous traction and its relation to that of viscosity. Proc. R. Soc. Lond. Ser. A Contain. Pap. Math. Phys. Character 1906, 77, 426–440. [Google Scholar] [CrossRef]
- Tabatabaei, S.H.; Carreau, P.J.; Ajji, A. Rheological and thermal properties of blends of a long-chain branched polypropylene and different linear polypropylenes. Chem. Eng. Sci. 2009, 64, 4719–4731. [Google Scholar] [CrossRef]
- Lampman, S. Characterization and Failure Analysis of Plastics; ASM International: Almere, The Netherlands, 2003. [Google Scholar]
- Grellmann, W.; Seidler, S. Kunststoffprüfung; Carl Hanser Verlag GmbH Co KG: Munich, Germany, 2015. [Google Scholar]
- Mark, J.E. Physical Properties of Polymers Handbook; Springer: Berlin/Heidelberg, Germany, 2007; Volume 1076. [Google Scholar]
- Cao, J.; Wen, N.; Zheng, Y.-Y. Effect of long chain branching on the rheological behavior, crystallization and mechanical properties of polypropylene random copolymer. Chin. J. Polym. Sci. 2016, 34, 1158–1171. [Google Scholar] [CrossRef]
- Li, Y.; Jia, S.; Du, S.; Wang, Y.; Lv, L.; Zhang, J. Improved properties of recycled polypropylene by introducing the long chain branched structure through reactive extrusion. Waste Manag. 2018, 76, 172–179. [Google Scholar] [CrossRef]
- Wang, X.; Tzoganakis, C.; Rempel, G.L. Chemical modification of polypropylene with peroxide/pentaerythritol triacrylate by reactive extrusion. J. Appl. Polym. Sci. 1996, 61, 1395–1404. [Google Scholar] [CrossRef]
- Flores, M.; Fernández-Francos, X.; Ramis, X.; Serra, A. Novel epoxy-anhydride thermosets modified with a hyperbranched polyester as toughness enhancer. I. Kinetics study. Thermochim. Acta 2012, 544, 17–26. [Google Scholar] [CrossRef]



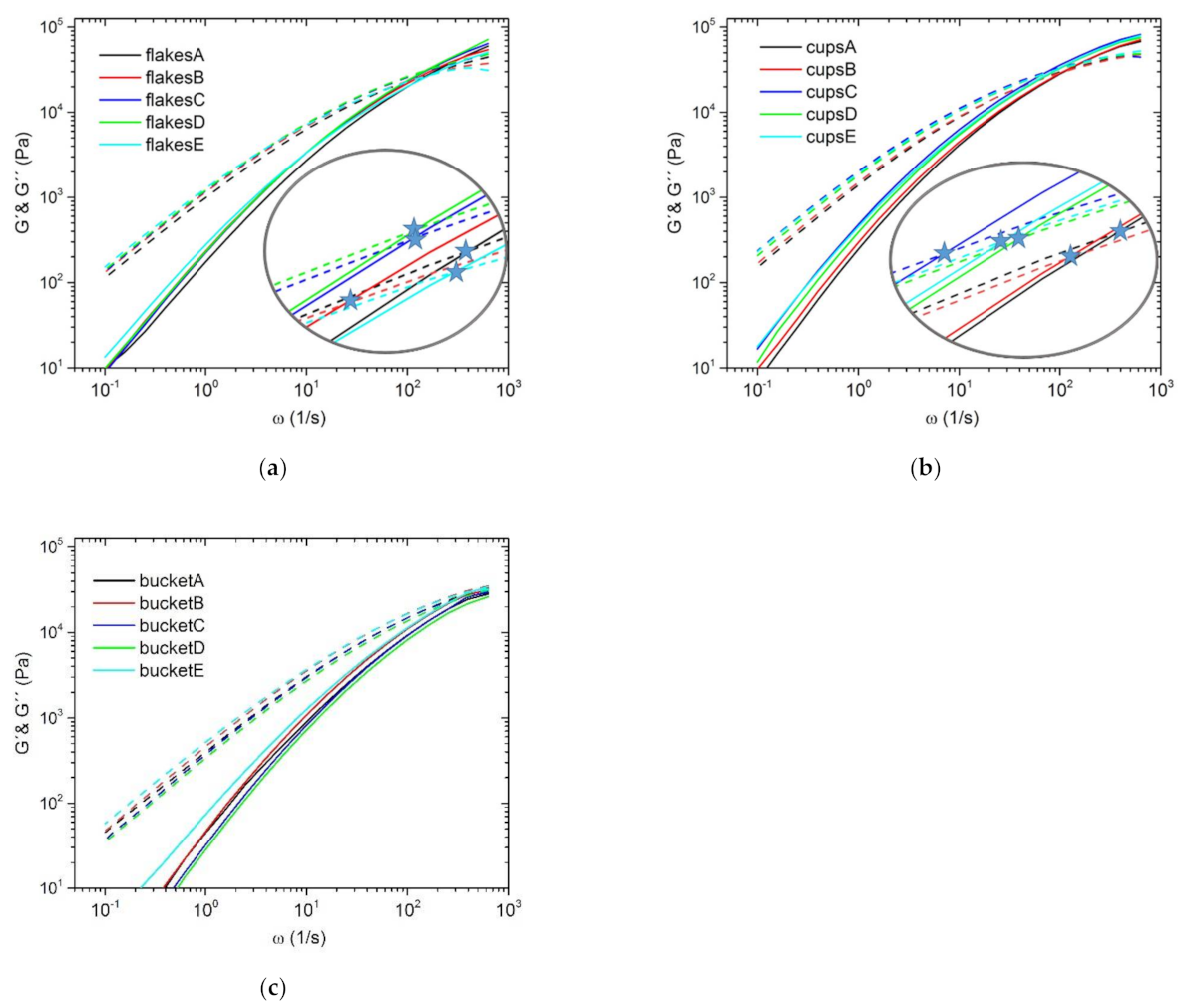

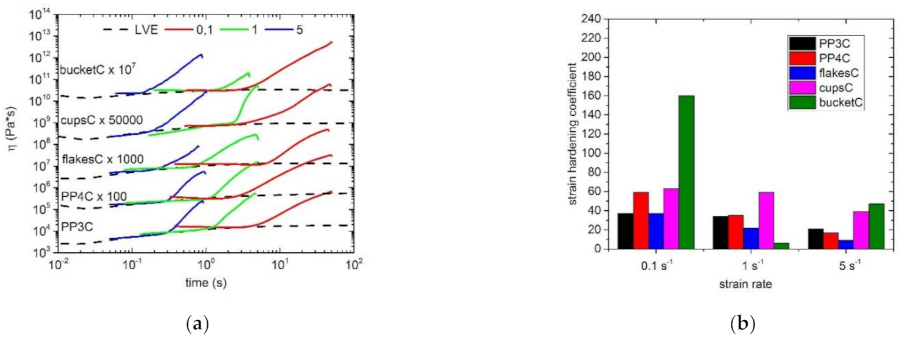
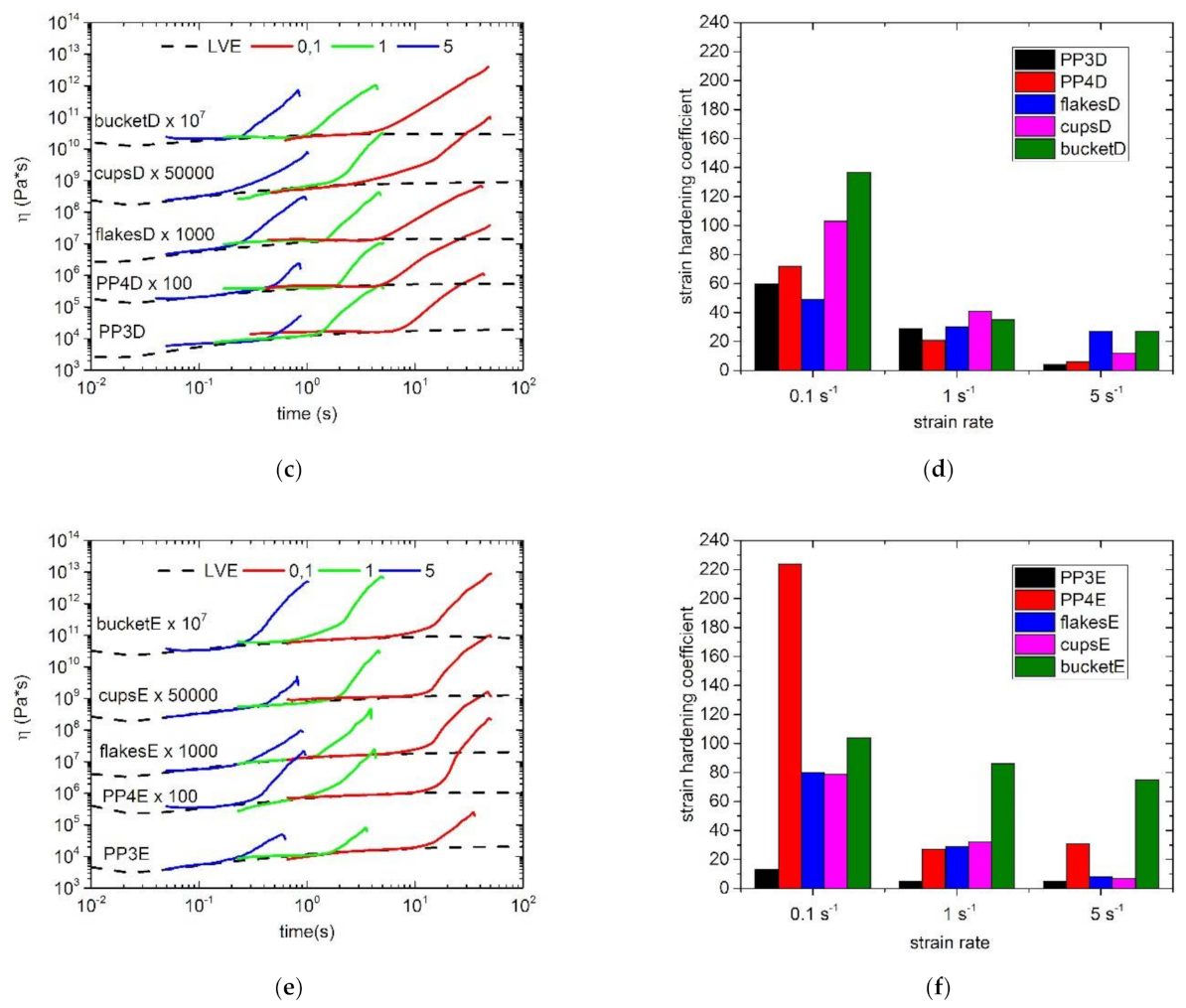
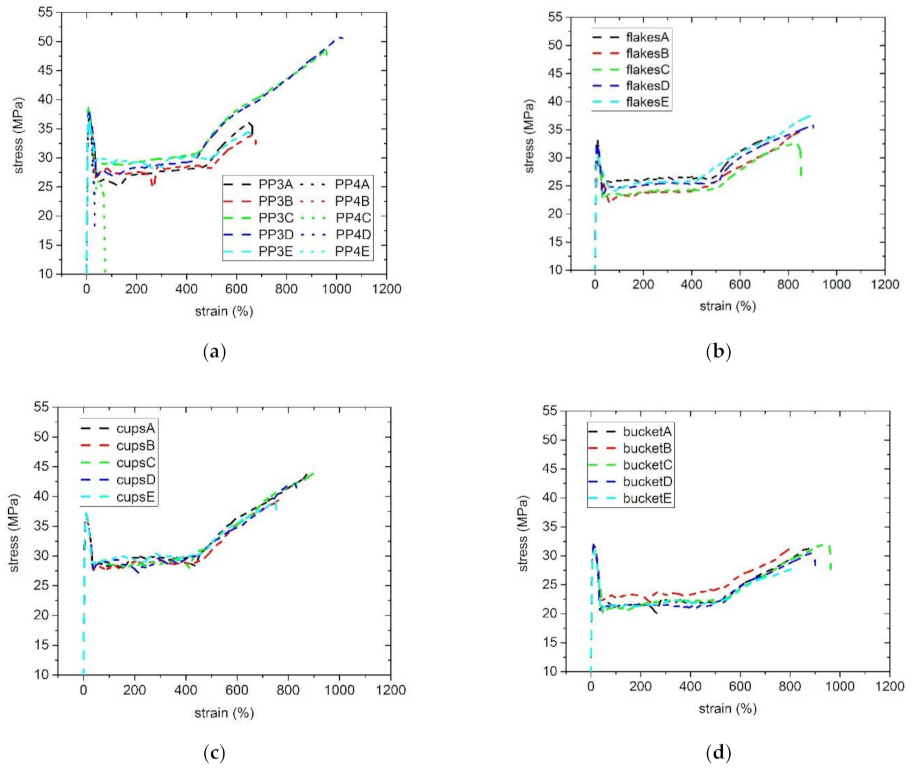

| Sample | ωC (rad/s) | GC (kPa) | Mw/MMD |
|---|---|---|---|
| PP3A | 102 | 24.52 | |
| PP3B | 78 | 20.26 | Mw ↑, MMD ↑ |
| PP3C | 80 | 23.51 | Mw ↑, MMD ↑ |
| PP3D | 81 | 22.96 | Mw ↑, MMD ↑ |
| PP3E | 100 | 24.09 | Mw ↑, MMD ↑ |
| PP4A | 255 | 21.18 | |
| PP4B | 170 | 15.29 | Mw ↑, MMD ↑ |
| PP4C | 238 | 19.37 | Mw ↑, MMD ↑ |
| PP4D | 232 | 18.87 | Mw ↑, MMD ↑ |
| PP4E | 201 | 16.96 | Mw ↑, MMD ↑ |
| FlakesA | 177 | 32.47 | |
| FlakesB | 124 | 25.02 | Mw ↑, MMD ↑ |
| FlakesC | 150 | 30.16 | Mw ↑, MMD ↑ |
| FlakesD | 152 | 31.01 | Mw ↑, MMD ↑ |
| FlakesE | 170 | 27.47 | Mw ↑, MMD ↑ |
| cupsA | 123 | 31.86 | |
| cupsB | 106 | 29.61 | Mw ↑, MMD ↑ |
| cupsC | 76 | 30.00 | Mw ↑, MMD ↑ |
| cupsD | 93 | 31.22 | Mw ↑, MMD ↑ |
| cupsE | 90 | 31.14 | Mw ↑, MMD ↑ |
| Sample | Tc (°C) | Tm (°C) | XC (%) | Et (MPa) | εB (%) | σm (MPa) |
|---|---|---|---|---|---|---|
| PP3A | 115 | 167 | 50 | 2039 ± 139 | 689 ± 65 | 38.7 ± 0.5 |
| PP3B | 119 | 169 | 49 | 1848 ± 115 | 650 ± 105 | 36.5 ± 0.7 |
| PP3C | 121 | 167 | 50 | 2181 ± 140 | 921 ± 77 | 38.9 ± 0.3 |
| PP3D | 115 | 165 | 50 | 1904 ± 108 | 1008 ± 34 | 38.1 ± 0.5 |
| PP3E | 129 | 168 | 53 | 2037 ± 81 | 681 ± 57 | 36.9 ± 0.3 |
| PP4A | 118 | 164 | 50 | 1960 ± 18 | 23 ± 4 | 37.1 ± 0.5 |
| PP4B | 118 | 164 | 48 | 1898 ± 151 | 35 ± 4 | 37.0 ± 0.5 |
| PP4C | 120 | 165 | 50 | 1980 ± 164 | 77 ± 25 | 36.9 ± 0.3 |
| PP4D | 117 | 165 | 50 | 1950 ± 125 | 26 ± 6 | 36.7 ± 0.7 |
| PP4E | 128 | 167 | 50 | 1966 ± 53 | 43 ± 5 | 36.8 ± 0.4 |
| FlakesA | 121 | 167 | 42 | 1864 ± 55 | 748 ± 85 | 32.8 ± 0.6 |
| FlakesB | 121 | 165 | 43 | 1436 ± 35 | 863 ± 48 | 29.8 ± 0.4 |
| FlakesC | 122 | 165 | 44 | 1717 ± 51 | 917 ± 38 | 31.7 ± 0.2 |
| FlakesD | 121 | 165 | 44 | 1733 ± 66 | 916 ± 49 | 32.2 ± 0.3 |
| FlakesE | 125 | 166 | 45 | 1502 ± 123 | 912 ± 21 | 30.5 ± 0.4 |
| cupsA | 129 | 167 | 51 | 1794 ± 143 | 859 ± 39 | 37.1 ± 0.3 |
| cupsB | 129 | 167 | 50 | 1913 ± 188 | 758 ± 35 | 37.5 ± 0.4 |
| cupsC | 129 | 167 | 50 | 1708 ± 123 | 913 ± 32 | 36.9 ± 0.4 |
| cupsD | 129 | 167 | 51 | 1818 ± 114 | 826 ± 29 | 37.1 ± 0.2 |
| cupsE | 130 | 167 | 52 | 1976 ± 188 | 757 ± 48 | 37.9 ± 0.3 |
| bucketA | 114 | 151 | 42 | 1570 ± 94 | 892 ± 73 | 30.9 ± 1.3 |
| bucketB | 114 | 155 | 44 | 1537 ± 75 | 813 ± 129 | 32.8 ± 0.5 |
| bucketC | 114 | 154 | 54 | 1488 ± 81 | 880 ± 134 | 30.9 ± 1.1 |
| bucketD | 114 | 153 | 54 | 1560 ± 70 | 864 ± 67 | 30.4 ± 1.3 |
| bucketE | 124 | 157 | 45 | 1476 ± 50 | 817 ± 69 | 30.6 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanic, S.; Koch, T.; Schmid, K.; Knaus, S.; Archodoulaki, V.-M. Improving Rheological and Mechanical Properties of Various Virgin and Recycled Polypropylenes by Blending with Long-Chain Branched Polypropylene. Polymers 2021, 13, 1137. https://doi.org/10.3390/polym13071137
Stanic S, Koch T, Schmid K, Knaus S, Archodoulaki V-M. Improving Rheological and Mechanical Properties of Various Virgin and Recycled Polypropylenes by Blending with Long-Chain Branched Polypropylene. Polymers. 2021; 13(7):1137. https://doi.org/10.3390/polym13071137
Chicago/Turabian StyleStanic, Sascha, Thomas Koch, Klaus Schmid, Simone Knaus, and Vasiliki-Maria Archodoulaki. 2021. "Improving Rheological and Mechanical Properties of Various Virgin and Recycled Polypropylenes by Blending with Long-Chain Branched Polypropylene" Polymers 13, no. 7: 1137. https://doi.org/10.3390/polym13071137