Characteristics of Metakaolin-Based Geopolymer with Cathode Ray Tube Glass
Abstract
1. Introduction
1.1. CRT Glass and Its Recycling Methods
1.2. Application of CRT Glass in the Concrete
1.3. Geopolymer: An Environmental Friendly Material
1.4. Application of CRT Glass in the Geopolymer
2. Materials and Methods
2.1. Materials
2.2. Determination of the Optimal Mixture Composition
2.3. Influence of Curing Temperature on the Mechanical Behavior
2.4. Measurement of the Temperature and Mass Changes
2.5. Development of Mechanical Behavior with Time
2.6. Atomic Absorption Apectrometry
2.7. Determination of Phytotoxicity
3. Results
3.1. Determination of the Optimal Mixture Composition
3.2. Influence of Curing Temperature on the Mechanical Behavior
3.3. Measurement of the Temperature and Mass Changes
3.4. Development of Mechanical Behavior with Time
3.5. Atomic Absorption Apectrometry
3.6. Phytotoxicity Test
4. Discussion
4.1. Determination of the Optimal Mixture Composition
4.2. Influence of Curing Temperature on the Mechanical Behavior
4.3. Measurement of the Temperature and Mass Changes
4.4. Development of Mechanical Behavior with Time
4.5. Atomic Absorption Apectrometry and Phytotoxicity Test
5. Conclusions
- No strict dependence between CRT glass content and geopolymer strength was observed. The obtained flexural strength ranged from 3.6 to 6.2 MPa (samples containing metakaolin-to-CRT-glass ratios of 2:1 and 1:1, respectively), while the compressive strength ranged from 41.4 to 51.0 MPa (metakaolin-to-CRT-glass ratios of 3:1 and 1:1, respectively).
- Mixtures containing metakaolin-to-CRT-glass-mass ratios equal to 1:1 and 1:3 were considered optimal taking into account environmental, economic, and mechanical issues. However, the flexural and compressive strengths of the geopolymer with a precursor-to-aggregate ratio of 1:3 showed alarming tendency to decrease with time (compressive strength decreased by over 16% within the 7th and 28th days of curing). The strength of the geopolymer containing a metakaolin-to-CRT-glass-mass ratio equal to 1:1 showed promising tendency to increase with time.
- The curing of samples for the first 24 h at 40 °C was decided as optimal due to the high strength and possibility of quick demolding. Samples containing a metakaolin-to-CRT-glass ratio of 1:3 achieved after 7 days a flexural strength of 4.3 MPa and a compressive strength of 51.4 MPa, while samples with an ingredients ratio of 1:1 obtained strengths equal to 5.7 MPa and 51.3 MPa, respectively. After just one day, the compressive strength of the geopolymer made out of both mixtures exceeded 45 MPa.
- The highest temperature (47.06 °C) was registered in the samples containing more metakaolin (50% by mass) after about 6 h of curing. The loss in mass was also higher in samples containing a metakaolin-to-CRT glass ratio of 1:1.
- The amount of chosen toxic elements in the aqueous extract from CRT glass is much higher than that in an extract from a geopolymer containing CRT glass. The following elements were determined: Fe, Mn, Cu, Ni, Cr, Co, Zn, Pb, and Cd. The most significant difference was observed in the case of Pb, for which the concentration in the CRT extract was almost 14 times higher than in the geopolymer. The amount of all investigated elements (including toxic ones) in the leachate from the geopolymer fulfilled the regulatory limits. Therefore, it may be concluded that the CRT glass was successfully immobilized within the geopolymer matrix.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qi, Y.; Xiao, X.; Lu, Y.; Shu, J.; Wang, J.; Chen, M. Cathode ray tubes glass recycling: A review. Sci. Total Environ. 2019, 650, 2842–2849. [Google Scholar] [CrossRef]
- Poon, C. Management of CRT glass from discarded computer monitors and TV sets. Waste Manag. 2008, 28, 1499. [Google Scholar] [CrossRef] [PubMed]
- Directive 2002/96/EC of the European Parlament and of the Council of 27 January 2003 on Waste Electrical and Electronic Equipment (WEEE). Official Journal EU. 2003, Volume 007, pp. 359–374. Available online: https://eur-lex.europa.eu/eli/dir/2002/96/oj (accessed on 31 March 2021).
- König, J.; Petersen, R.R.; Yue, Y. Fabrication of highly insulating foam glass made from CRT panel glass. Ceram. Int. 2015, 41, 9793–9800. [Google Scholar] [CrossRef]
- Andreola, F.; Barbieri, L.; Corradi, A.; Lancellotti, I.; Falcone, R.; Hreglich, S. Glass-ceramics obtained by the recycling of end of life cathode ray tubes glasses. Waste Manag. 2005, 25, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Andreola, F.; Barbieri, L.; Corradi, A.; Ferrari, A.M.; Lancellotti, I.; Neri, P. Recycling of EOL CRT glass into ceramic glaze formulations and its environmental impact by LCA approach. Int. J. Life Cycle Assess. 2006, 12, 448–454. [Google Scholar] [CrossRef]
- Singh, N.; Li, J.; Zeng, X. Solutions and challenges in recycling waste cathode-ray tubes. J. Clean. Prod. 2016, 133, 188–200. [Google Scholar] [CrossRef]
- Meng, W.; Wang, X.; Yuan, W.; Wang, J.; Song, G. The Recycling of Leaded Glass in Cathode Ray Tube (CRT). Procedia Environ. Sci. 2016, 31, 954–960. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, F.-S.; Zhu, J. Detoxification of cathode ray tube glass by self-propagating process. J. Hazard. Mater. 2009, 165, 980–986. [Google Scholar] [CrossRef]
- Miyoshi, H.; Chen, D.; Akai, T. A Novel Process Utilizing Subcritical Water to Remove Lead from Wasted Lead Silicate Glass. Chem. Lett. 2004, 33, 956–957. [Google Scholar] [CrossRef]
- Yao, Z.; Ling, T.-C.; Sarker, P.; Su, W.; Liu, J.; Wu, W.; Tang, J. Recycling difficult-to-treat e-waste cathode-ray-tube glass as construction and building materials: A critical review. Renew. Sustain. Energy Rev. 2018, 81, 595–604. [Google Scholar] [CrossRef]
- Ling, T.-C.; Poon, C.-S. Feasible use of recycled CRT funnel glass as heavyweight fine aggregate in barite concrete. J. Clean. Prod. 2012, 33, 42–49. [Google Scholar] [CrossRef]
- Ling, T.-C.; Poon, C.-S.; Lam, W.-S.; Chan, T.-P.; Fung, K.K.-L. Utilization of recycled cathode ray tubes glass in cement mortar for X-ray radiation-shielding applications. J. Hazard. Mater. 2012, 199–200, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Song, W.; Zou, D.; Li, L. Dynamic mechanical analysis of cement mortar prepared with recycled cathode ray tube (CRT) glass as fine aggregate. J. Clean. Prod. 2018, 174, 1436–1443. [Google Scholar] [CrossRef]
- Song, W.; Zou, D.; Liu, T.; Teng, J.; Li, L. Effects of recycled CRT glass fine aggregate size and content on mechanical and damping properties of concrete. Constr. Build. Mater. 2019, 202, 332–340. [Google Scholar] [CrossRef]
- Pauzi, N.; Jamil, M.; Hamid, R.; Abdin, A.; Zain, M. Influence of spherical and crushed waste Cathode-Ray Tube (CRT) glass on lead (Pb) leaching and mechanical properties of concrete. J. Build. Eng. 2019, 21, 421–428. [Google Scholar] [CrossRef]
- Walczak, P.; Małolepszy, J.; Reben, M.; Rzepa, K. Mechanical Properties of Concrete Mortar Based on Mixture of CRT Glass Cullet and Fluidized Fly Ash. Procedia Eng. 2015, 108, 453–458. [Google Scholar] [CrossRef]
- Romero, D.; James, J.; Mora, R.; Hays, C.D. Study on the mechanical and environmental properties of concrete containing cathode ray tube glass aggregate. Waste Manag. 2013, 33, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Quinlan, M.; Yen, T.F. Encapsulation of lead from hazardous CRT glass wastes using biopolymer cross-linked concrete systems. Waste Manag. 2009, 29, 321–328. [Google Scholar] [CrossRef]
- Rashad, A.M. Recycled cathode ray tube and liquid crystal display glass as fine aggregate replacement in cementitious materials. Constr. Build. Mater. 2015, 93, 1236–1248. [Google Scholar] [CrossRef]
- Liu, T.; Qin, S.; Zou, D.; Song, W. Experimental investigation on the durability performances of concrete using cathode ray tube glass as fine aggregate under chloride ion penetration or sulfate attack. Constr. Build. Mater. 2018, 163, 634–642. [Google Scholar] [CrossRef]
- Davidovits, J.; Comrie, D.C.; Paterson, J.H.; Ritcey, D.J. Geopolymeric concretes for environmental protection. Concr. Int. 1990, 12, 30–40. [Google Scholar]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Brümmer, M.; Sáez-Pérez, M.P.; Suárez, J.D.; Fangueiro, R.; Rana, S. Hemp-Clay Concretes for Environmental Building—Features that Attribute to Drying, Stabilization with Lime, Water Uptake and Mechanical Strength. In Advances in Natural Fibre Composites; Metzler, J.B., Ed.; Springer: Berlin, Germany, 2017; pp. 249–265. [Google Scholar]
- Feduik, R. Reducing permeability of fiber concrete using composite binders. Spéc. Top. Rev. Porous Media Int. J. 2018, 9, 79–89. [Google Scholar] [CrossRef]
- Sair, S.; Mandili, B.; Taqi, M.; El Bouari, A. Development of a new eco-friendly composite material based on gypsum reinforced with a mixture of cork fibre and cardboard waste for building thermal insulation. Compos. Commun. 2019, 16, 20–24. [Google Scholar] [CrossRef]
- Liu, L.; Zou, S.; Li, H.; Deng, L.; Bai, C.; Zhang, X.; Wang, S.; Li, N. Experimental physical properties of an eco-friendly bio-insulation material based on wheat straw for buildings. Energy Build. 2019, 201, 19–36. [Google Scholar] [CrossRef]
- Sandanayake, M.; Gunasekara, C.; Law, D.; Zhang, G.; Setunge, S. Greenhouse gas emissions of different fly ash based geopolymer concretes in building construction. J. Clean. Prod. 2018, 204, 399–408. [Google Scholar] [CrossRef]
- Sedira, N.; Castro-Gomes, J. Effect of activators on hybrid alkaline binder based on tungsten mining waste and ground granulated blast furnace slag. Constr. Build. Mater. 2020, 232, 117176. [Google Scholar] [CrossRef]
- Emdadi, Z.; Asim, N.; Amin, M.H.; Yarmo, M.A.; Maleki, A.; Azizi, M.; Sopian, K. Development of Green Geopolymer Using Agricultural and Industrial Waste Materials with High Water Absorbency. Appl. Sci. 2017, 7, 514. [Google Scholar] [CrossRef]
- Toniolo, N.; Rincón, A.; Avadhut, Y.; Hartmann, M.; Bernardo, E.; Boccaccini, A. Novel geopolymers incorporating red mud and waste glass cullet. Mater. Lett. 2018, 219, 152–154. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mohapatra, S.; Gaur, A.; Dwivedi, G.; Soni, A. Study of various properties of geopolymer concrete—A review. In Proceedings of the Materials Today: Proceedings; Elsevier BV: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Ayzenshtadt, A.; Lesovik, V.; Frolova, M.; Tutygin, A.; Danilov, V. Nanostructured Wood Mineral Composite. Procedia Eng. 2015, 117, 45–51. [Google Scholar] [CrossRef]
- Long, W.-J.; Li, H.-D.; Ma, H.; Fang, Y.; Xing, F. Green alkali-activated mortar: Sustainable use of discarded cathode-ray tube glass powder as precursor. J. Clean. Prod. 2019, 229, 1082–1092. [Google Scholar] [CrossRef]
- Moncea, A.-M.; Georgescu, M.; Badanoiu, A.; Matei, E.; Stoleriu, S. Alkali activated binders as matrices for the immobilization of glass waste with Pb content. In Proceedings of the 18th International Conference on Building Materials (IBAUSIL 2012), Weimar, Germany, 12–15 September 2012; Volume 1, p. 9. [Google Scholar]
- Mary, B.O.; Ikpeni, S.E. Metakaolin clay-derived geopolymer for recycling of waste cathode ray tube glass. Afr. J. Pure Appl. Chem. 2018, 12, 42–49. [Google Scholar] [CrossRef]
- Guzmán-Carrillo, H.; Gasca-Tirado, J.; López-Romero, J.; Luis, M.A.-C.; Eric, M.R.-M.; Pineda-Piñón, J.; Pérez-Bueno, J.; Feregrino-Montes, C.; López-Naranjo, E.; Manzano-Ramírez, A. Encapsulation of toxic heavy metals from waste CRT using calcined kaolin base-geopolymer. Mater. Chem. Phys. 2021, 257, 123745. [Google Scholar] [CrossRef]
- de Gutiérrez, R.M.; Villaquirán-Caicedo, M.A.; Guzmán-Aponte, L.A. Alkali-activated metakaolin mortars using glass waste as fine aggregate: Mechanical and photocatalytic properties. Constr. Build. Mater. 2020, 235, 117510. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Kashani, A. Sustainable one-part geopolymer foams with glass fines versus sand as aggregates. Constr. Build. Mater. 2018, 171, 223–231. [Google Scholar] [CrossRef]
- Khan, N.N.; Sarker, P.K. Effect of waste glass fine aggregate on the strength, durability and high temperature resistance of alkali-activated fly ash and GGBFS blended mortar. Constr. Build. Mater. 2020, 263, 120177. [Google Scholar] [CrossRef]
- Zhang, B.; He, P.; Poon, C.S. Improving the high temperature mechanical properties of alkali activated cement (AAC) mortars using recycled glass as aggregates. Cem. Concr. Compos. 2020, 112, 103654. [Google Scholar] [CrossRef]
- Barroca, N.; Borges, L.M.; Velez, F.J.; Monteiro, F.; Górski, M.; Castro-Gomes, J. Wireless sensor networks for temperature and humidity monitoring within concrete structures. Constr. Build. Mater. 2013, 40, 1156–1166. [Google Scholar] [CrossRef]
- CSN E. 15309: Characterization of Waste and Soil-Determination of Elemental Composition by X-Ray Fluorescence; European Standard Organizations, Polish Committee for Standardization: Warsaw, Poland, 2013. [Google Scholar]
- Wielgus, N.; Górski, M.; Kubica, J. Discarded Cathode Ray Tube Glass as an Alternative for Aggregate in a Metakaolin-Based Geopolymer. Sustainability 2021, 13, 479. [Google Scholar] [CrossRef]
- Part 1: Determination of strength. In EN 196-1 Method of Testing Cement; European Committee for Standardization: Brussels, Belgium, 2016.
- Principles of selection and evaluation of biotests for ecotoxicological characteristics of soil and soil materials. In Standard: PN-ISO 17616: 2010, Soil Quality; Polish Committee for Standardization: Warsaw, Poland, 2010.
- Determination of the influence of pollutants on the soil flora. Part I: Method for measuring root growth inhibition. In Standard: PN-EN ISO 11269: 2013-06. Soil Quality; Polish Committee for Standardization: Warsaw, Poland, 2013.
- Rozporządzenie Ministra Gospodarki Morskiej i Żeglugi Śródlądowej z Dnia 12 Lipca 2019 r. w Sprawie Substancji Szczególnie Szkodliwych dla Środowiska Wodnego oraz Warunków, Jakie Należy Spełnić Przy Wprowadzaniu do wód lub do ziemi Ścieków, a Także przy Odprowadzaniu wód Opadowych lub Roztopowych do wód lub do Urządzeń Wodnych. In Regulation of the Ministry of Marine Economy and Inland Navigation of 12 July 2019 on Substances Particularly Harmful to the Aquatic Environment and the Conditions to be Met When Discharging Sewage into Waters or Soil, as well as Discharging Rainwater or Meltwater into Waters or into Water Facilities; Min. Gospodarki Morskiej i Żeglugi Śródlądowej (Min. Maritime And Inland Navigation): Warszawa, Poland, 2019.
- Moncea, A.M.; Badanoiu, A.; Georgescu, M.; Stoleriu, S. Cementitious composites with glass waste from recycling of cathode ray tubes. Mater. Struct. 2013, 46, 2135–2144. [Google Scholar] [CrossRef]
- Badanoiu, A.; Iordache, E.; Ionescu, R.; Voicu, G.; Matei, E. Effect of composition and curing regime on some properties of geopolymers based on cathode ray tubes glass waste and fly ash. Rom. J. Mater. 2015, 45, 3–13. [Google Scholar]
- Opletal, T. Možnosti Zpracování Odpadního Obrazovkového Skla. Master´s Thesis, Univerzita Palackého v Olomouci, Olomouc, Czech Republic, 2013. [Google Scholar]
- Alonso, S.; Palomo, A. Alkaline activation of metakaolin and calcium hydroxide mixtures: Influence of temperature, activator concentration and solids ratio. Mater. Lett. 2001, 47, 55–62. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A. One year geopolymerisation of sodium silicate activated fly ash and metakaolin geopolymers. Cem. Concr. Compos. 2019, 95, 98–110. [Google Scholar] [CrossRef]
- Mo, B.-H.; Zhu, H.; Cui, X.-M.; He, Y.; Gong, S.-Y. Effect of curing temperature on geopolymerization of metakaolin-based geopolymers. Appl. Clay Sci. 2014, 99, 144–148. [Google Scholar] [CrossRef]
- Ekaputri, J.J.; Junaedi, S. Wijaya Effect of Curing Temperature and Fiber on Metakaolin-based Geopolymer. Procedia Eng. 2017, 171, 572–583. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Davidovits, R.; Plelegris, C.; Davidovits, J. Standardized Method in Testing Commercial Metakaolins for Geopolymer Formulations; Geopolymer Institute Library: Saint-Quentin, France, 2019; p. 9. Available online: http://www.geopolymer.org (accessed on 30 March 2021).
- Yao, X.; Zhang, Z.; Zhu, H.; Chen, Y. Geopolymerization process of alkali–metakaolinite characterized by isothermal calorimetry. Thermochim. Acta 2009, 493, 49–54. [Google Scholar] [CrossRef]
- Malolepszy, J.; Deja, J. Immobilization of heavy metal ions by the alkali activated slag cementitious materials. Stud. Environ. Sci. 1994, 60, 519–524. [Google Scholar]
- Małolepszy, J.; Deja, J. Effect of Heavy Metals Immobilization on the Properties of Alkali Activated Slag Mortars. Spec. Publ. 1995, 153, 1087–1102. [Google Scholar]
- Ji, Z.; Su, L.; Pei, Y. Synthesis and toxic metals (Cd, Pb, and Zn) immobilization properties of drinking water treatment residuals and metakaolin-based geopolymers. Mater. Chem. Phys. 2020, 242, 122535. [Google Scholar] [CrossRef]
- Phair, J.; Van Deventer, J.; Smith, J. Effect of Al source and alkali activation on Pb and Cu immobilisation in fly-ash based “geopolymers”. Appl. Geochem. 2004, 19, 423–434. [Google Scholar] [CrossRef]
- Palomo, A.; Palacios, M. Alkali-activated cementitious materials: Alternative matrices for the immobilisation of hazardous wastes. Cem. Concr. Res. 2003, 33, 289–295. [Google Scholar] [CrossRef]
- Catauro, M.; Lancellotti, I.; Leonelli, C. Addition of WEEE Glass to Metakaolin-Based Geopolymeric Binder: A Cytotoxicity Study. Environments 2017, 4, 89. [Google Scholar] [CrossRef]



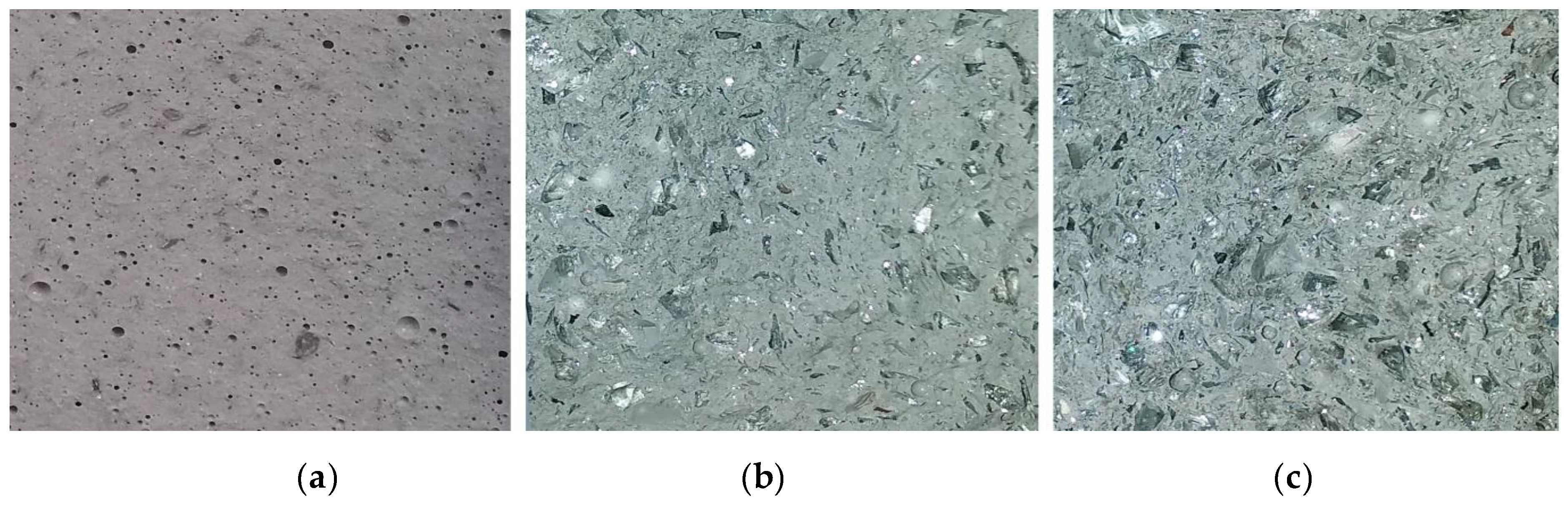
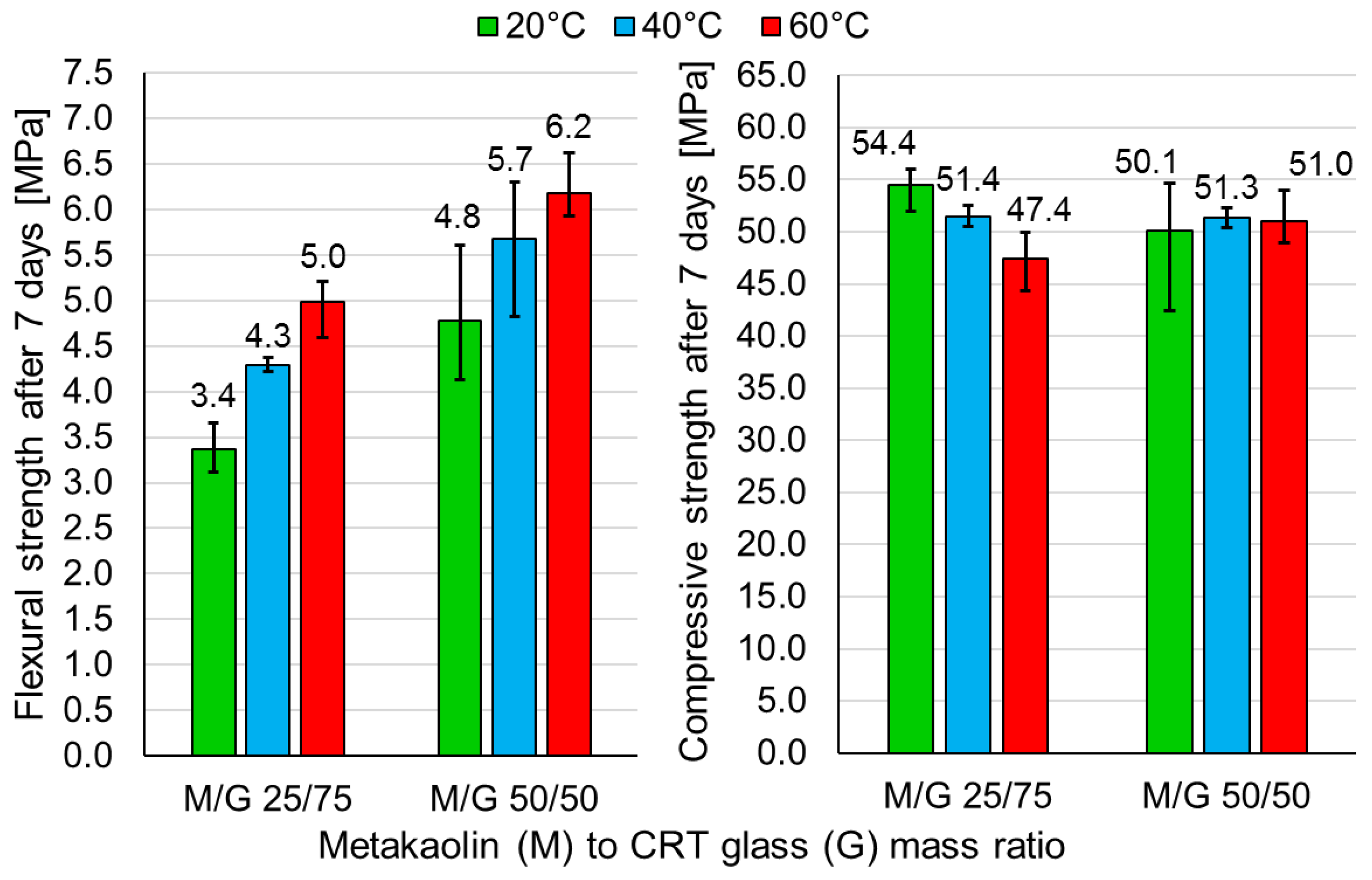
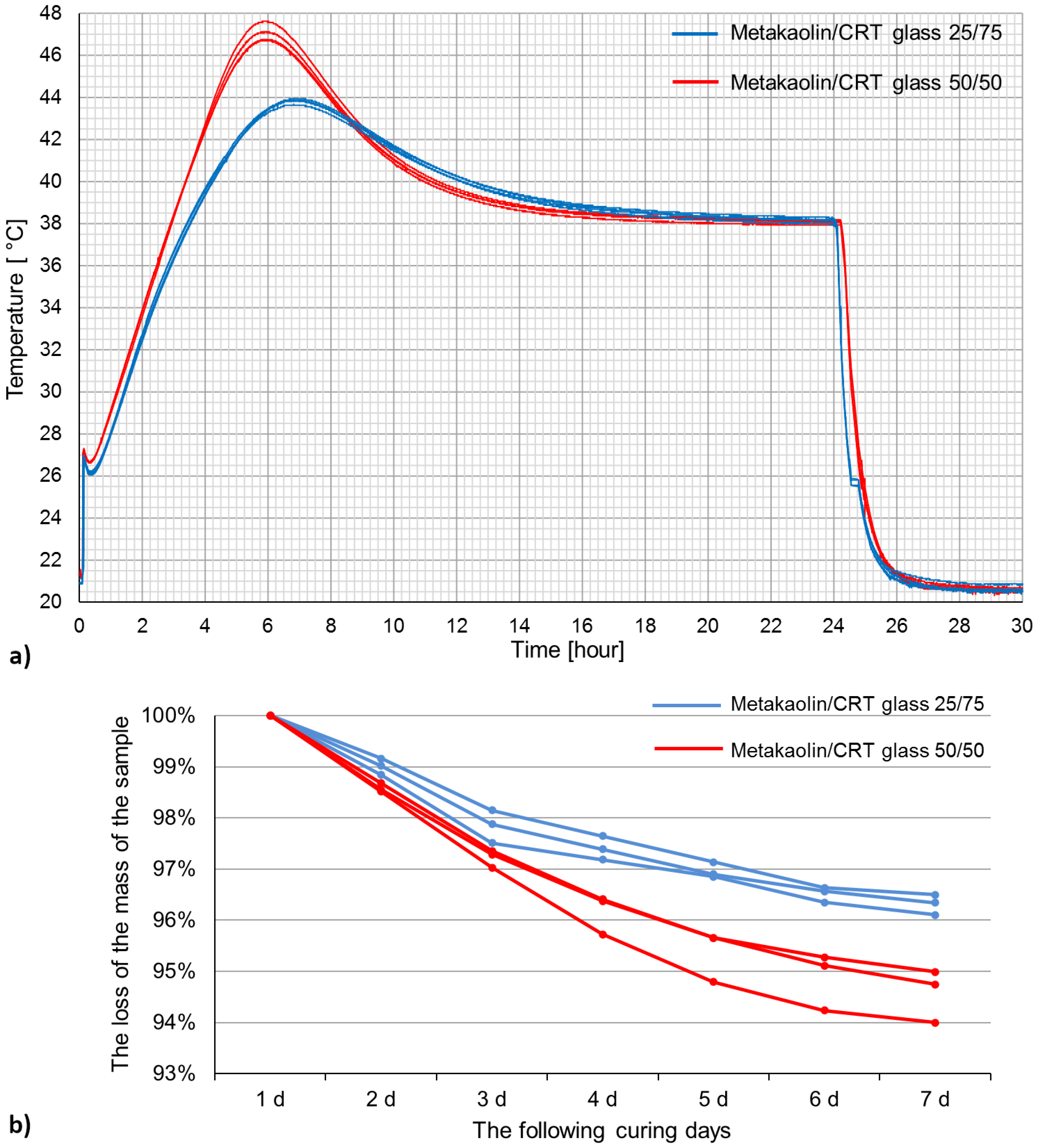
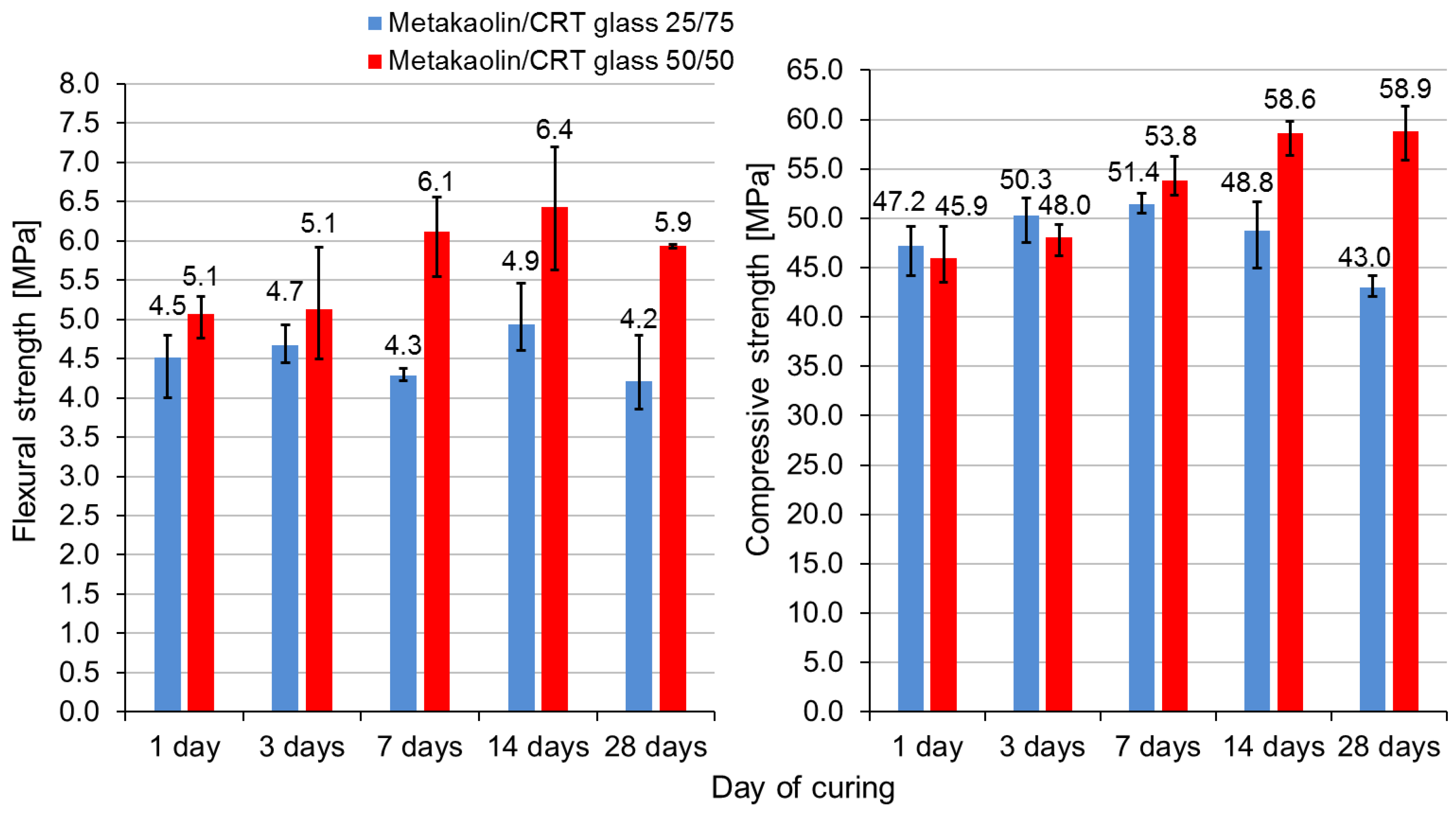
| Oxide Composition (wt %) | CRT Glass | Metakaolin |
|---|---|---|
| SiO2 | 76.10 | 53.12 |
| Na2O | 6.25 | 0.09 |
| CaO | 5.24 | 0.44 |
| BaO | 2.62 | - |
| K2O | 2.36 | 0.73 |
| MgO | 1.64 | 0.26 |
| PbO | 1.61 | - |
| SrO | 1.42 | - |
| Al2O3 | 1.37 | 42.14 |
| SO3 | 0.55 | - |
| Fe2O3 | 0.38 | 0.45 |
| ZrO2 | 0.28 | - |
| TiO2 | 0.12 | 0.64 |
| ZnO | 0.05 | - |
| As2O3 | 0.01 | - |
| H2O- | - | 0.22 |
| P2O5 | - | 0.03 |
| Cl | - | 0.02 |
| S | - | 0.01 |
| MnO | - | 0.01 |
| Mixture | Metakaolin (kg/m3) | CRT (kg/m3) | Sodium Silicate (kg/m3) | NaOH (kg/m3) | Si/Al 1 | Na/Al 1 |
|---|---|---|---|---|---|---|
| M/G 25/75 | 524 | 1572 | 314 | 157 | 1.40 | 0.55 |
| M/G 33/67 | 657 | 1335 | 365 | 182 | 1.37 | 0.51 |
| M/G 40/60 | 755 | 1133 | 417 | 208 | 1.37 | 0.50 |
| M/G 50/50 | 898 | 898 | 449 | 225 | 1.34 | 0.46 |
| M/G 60/40 | 995 | 663 | 521 | 260 | 1.36 | 0.48 |
| M/G 67/33 | 1037 | 510 | 553 | 276 | 1.36 | 0.49 |
| M/G 75/25 | 1078 | 359 | 586 | 293 | 1.37 | 0.48 |
| M/G 100/0 | 1083 | 0 | 675 | 337 | 1.41 | 0.57 |
| Samples Made of a Mixture | M/G 25/75 | M/G 33/67 | M/G 40/60 | M/G 50/50 | M/G 60/40 | M/G 67/33 | M/G 75/25 | M/G 100/0 |
|---|---|---|---|---|---|---|---|---|
| Density (kg/m3) | 2090 | 1960 | 1940 | 1920 | 1770 | 1720 | 1690 | 1500 |
| 20 °C | 40 °C | 60 °C | |
|---|---|---|---|
| M/G 25/75 | 2240 | 2110 | 2090 |
| M/G 50/50 | 2040 | 1890 | 1920 |
| 1 Day | 3 Days | 7 Days | 14 Days | 28 Days | |
|---|---|---|---|---|---|
| M/G 25/75 | 2200 | 2130 | 2110 | 2090 | 2070 |
| M/G 50/50 | 1970 | 1930 | 1920 | 1900 | 1870 |
| Designation | Unit | Aqueous Extract from Geopolymer | Aqueous Extract from CRT Glass | Limit Values [48] |
|---|---|---|---|---|
| pH | - | 11.0 | 6.3 | 6.5–9 |
| Total hardness | mval/dm3 | 0.08 | 0.82 | - |
| Chloride | gCl/dm3 | 0.0138 | 0.0138 | 1 |
| Total acidity | mval/dm3 | 0.0 | 0.8 | - |
| Mineral acidity | mval/dm3 | 0.0 | 0.0 | - |
| Total alkalinity | mval/dm3 | 6.0 | 0.0 | - |
| Mineral alkalinity | mval/dm3 | 10.2 | 0.6 | - |
| Fe | ppm | 0.12 | 0.42 | 10 |
| Mn | ppm | <0.015 | <0.015 | - |
| Cu | ppm | 0.01 | 0.02 | 0.1 |
| Ni | ppm | <0.02 | <0.02 | 0.1 |
| Cr | ppm | <0.03 | <0.03 | 0.05 |
| Co | ppm | <0.025 | <0.025 | 0.1 |
| Zn | ppm | 0.03 | 0.31 | 2 |
| Pb | ppm | 0.12 | 1.66 | 0.5 |
| Cd | ppm | <0.006 | <0.006 | 0.07 |
| Unit | Control Sample | CRT Glass Water Extraction | Water Extract of a Polymer Containing CRT Glass | ||||
|---|---|---|---|---|---|---|---|
| Stalk | Root | Stalk | Root | Stalk | Root | ||
| Average value | mm | 8.9 | 15.5 | 11.9 | 18.2 | 6.6 | 12.9 |
| Standard deviation | mm | 3.3 | 4.8 | 3.6 | 5.2 | 2.4 | 11.8 |
| Germination index | % | 100 | 100 | 134.5 | 117.2 | 74.5 | 82.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górski, M.; Wielgus, N.; Loska, K.; Kozioł, M.; Landrat, M.; Ścierski, W.; Pikoń, K. Characteristics of Metakaolin-Based Geopolymer with Cathode Ray Tube Glass. Polymers 2021, 13, 1149. https://doi.org/10.3390/polym13071149
Górski M, Wielgus N, Loska K, Kozioł M, Landrat M, Ścierski W, Pikoń K. Characteristics of Metakaolin-Based Geopolymer with Cathode Ray Tube Glass. Polymers. 2021; 13(7):1149. https://doi.org/10.3390/polym13071149
Chicago/Turabian StyleGórski, Marcin, Natalia Wielgus, Krzysztof Loska, Michał Kozioł, Marcin Landrat, Waldemar Ścierski, and Krzysztof Pikoń. 2021. "Characteristics of Metakaolin-Based Geopolymer with Cathode Ray Tube Glass" Polymers 13, no. 7: 1149. https://doi.org/10.3390/polym13071149
APA StyleGórski, M., Wielgus, N., Loska, K., Kozioł, M., Landrat, M., Ścierski, W., & Pikoń, K. (2021). Characteristics of Metakaolin-Based Geopolymer with Cathode Ray Tube Glass. Polymers, 13(7), 1149. https://doi.org/10.3390/polym13071149








