UV-Light Curing of 3D Printing Inks from Vegetable Oils for Stereolithography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Fourier-Transform Infrared Spectroscopy
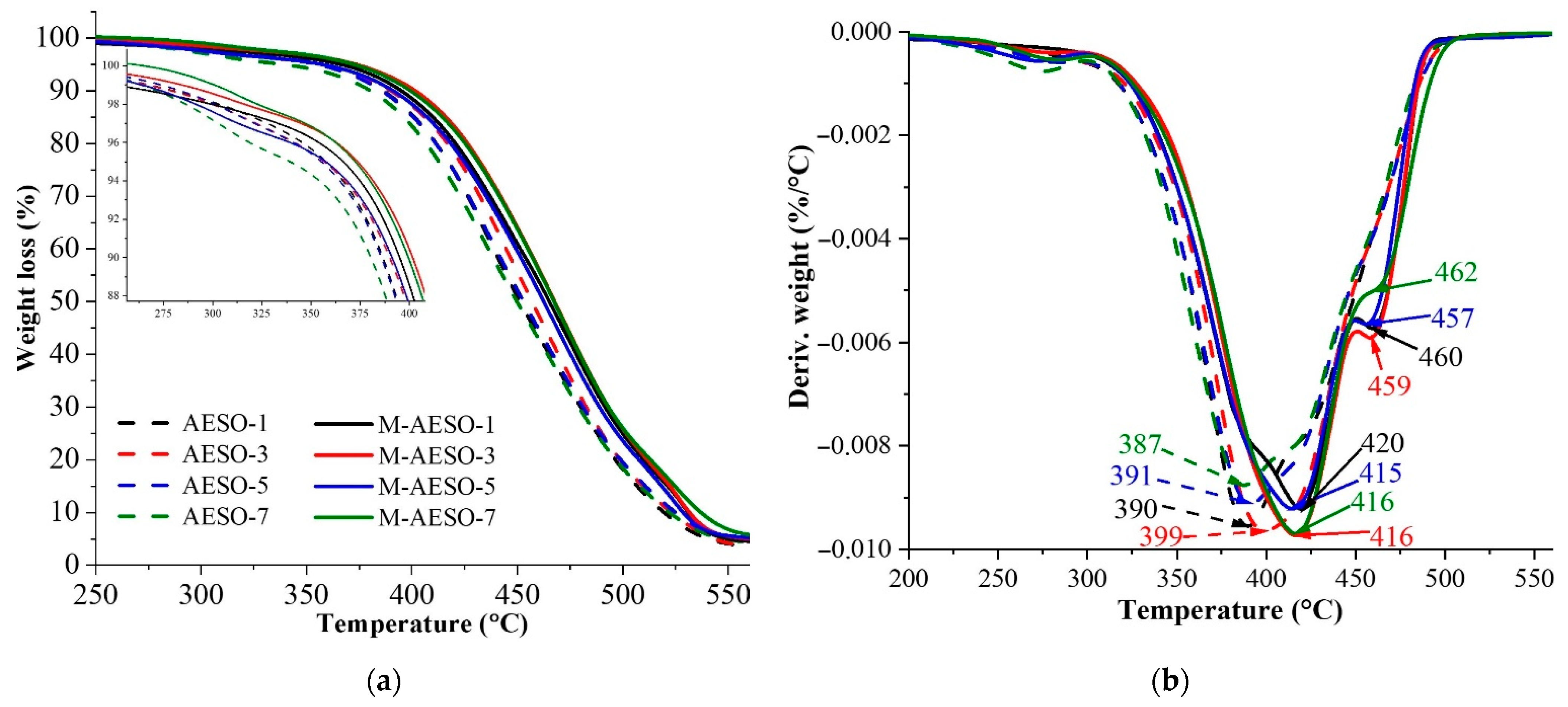
2.2.2. Thermogravimetric Analysis
2.2.3. Photorheology
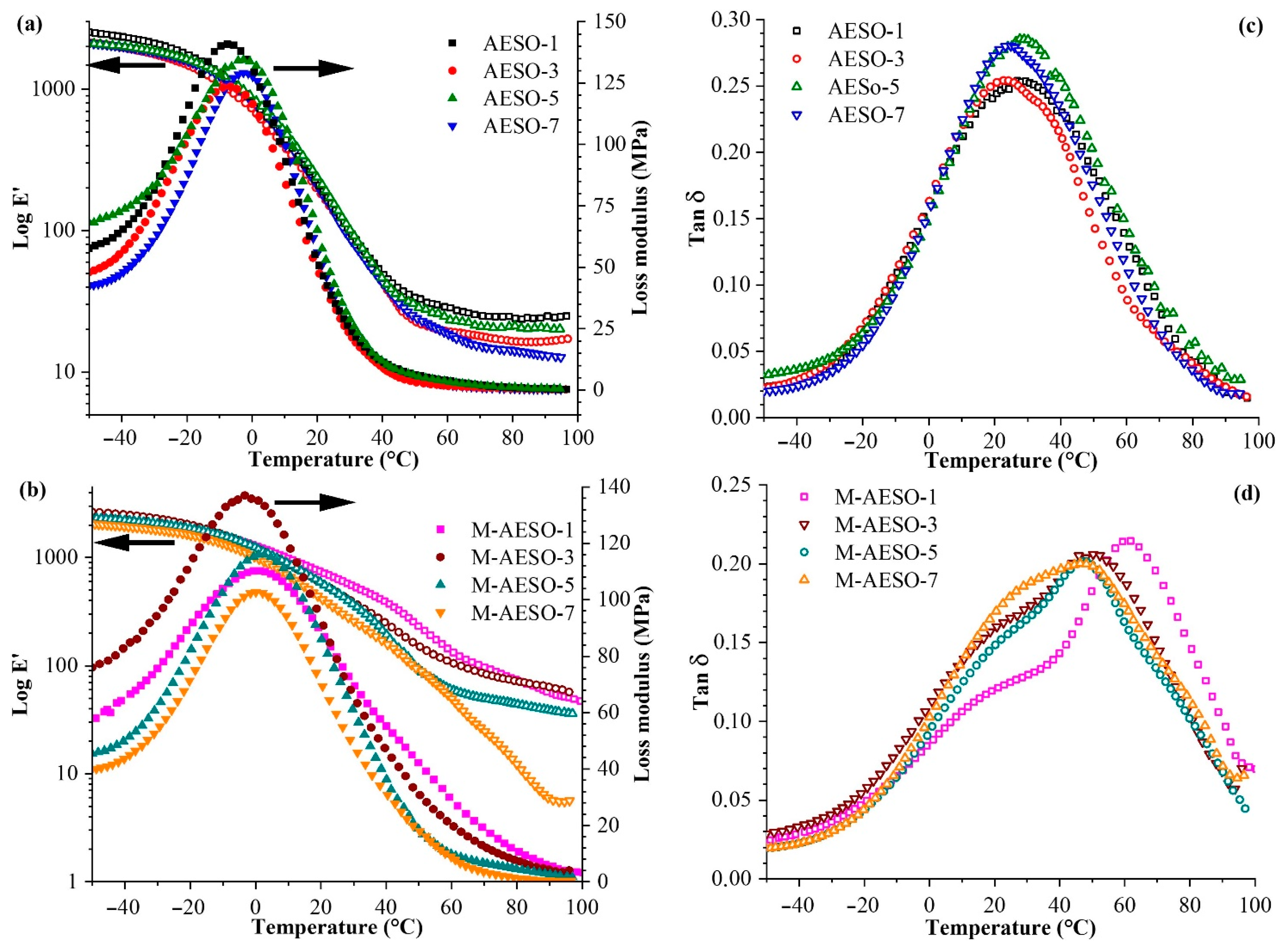
2.2.4. Dynamic Mechanical Analysis
2.3. Resins Formulation and Curing
2.4. 3D Printing of Resins
3. Results and Discussion
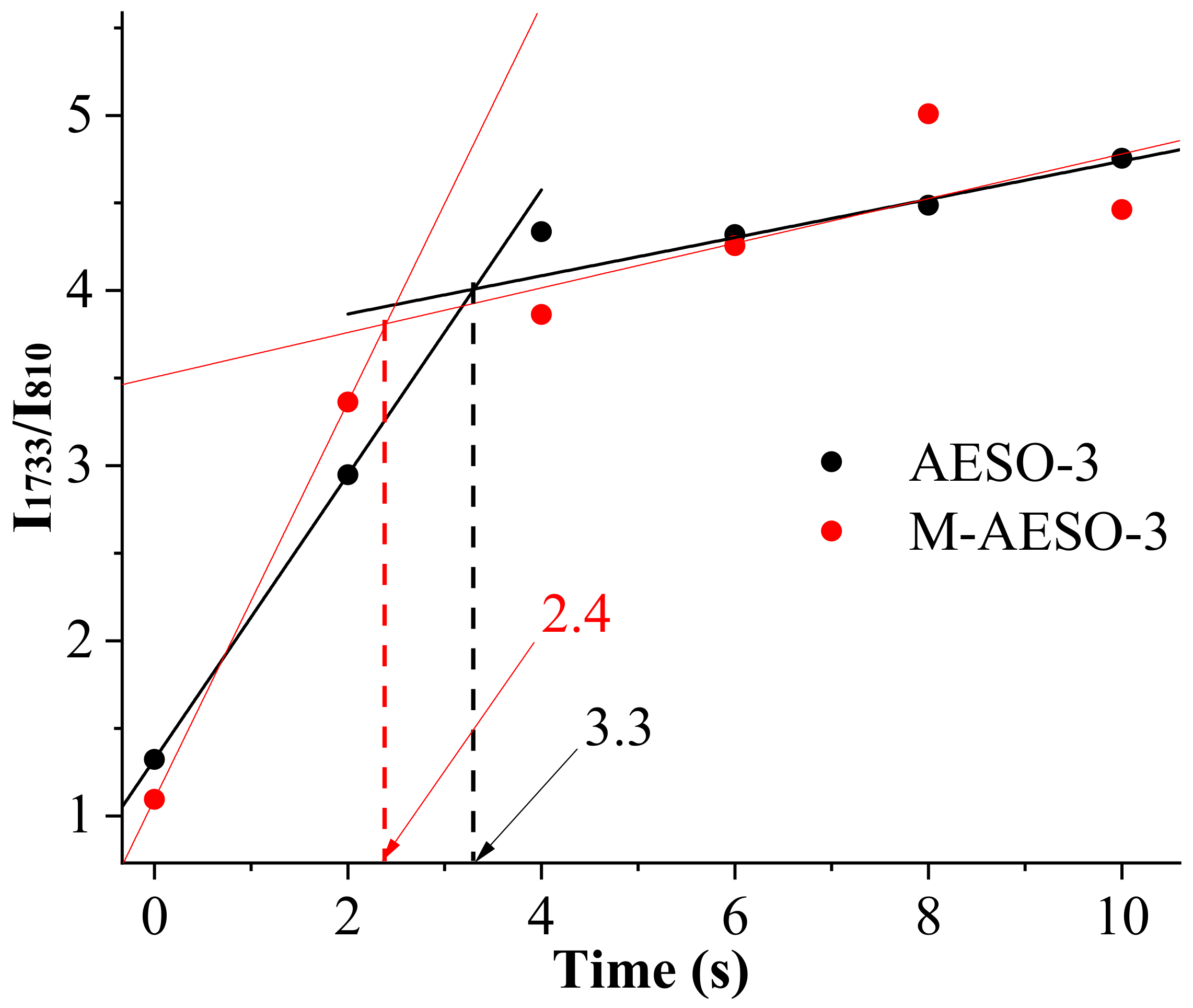
3.1. Investigation of UV-Crosslinking Process
3.2. Thermomechnical Investigation of UV-Cured Materials
3.3. Printability of the Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3d printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Jiménez, M.; Romero, L.; Domínguez, I.A.; Espinosa, M.d.M.; Domínguez, M. Additive manufacturing technologies: An overview about 3d printing methods and future prospects. Complexity 2019, 2019, 9656938. [Google Scholar] [CrossRef] [Green Version]
- Niaki, M.K.; Torabi, S.A.; Nonino, F. Why manufacturers adopt additive manufacturing technologies: The role of sustainability. J. Clean. Prod. 2019, 222, 381–392. [Google Scholar] [CrossRef]
- Calignano, F.; Manfredi, D.; Ambrosio, E.P.; Biamino, S.; Lombardi, M.; Atzeni, E.; Salmi, A.; Minetola, P.; Iuliano, L.; Fino, P. Overview on additive manufacturing technologies. Proc. IEEE 2017, 105, 593–612. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3d printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Qin, Q.; Wang, J. A review of stereolithography: Processes and systems. Processes 2020, 8, 1138. [Google Scholar] [CrossRef]
- Ji, A.; Zhang, S.; Bhagia, S.; Yoo, C.G.; Ragauskas, A.J. 3d printing of biomass-derived composites: Application and characterization approaches. RSC Adv. 2020, 10, 21698–21723. [Google Scholar] [CrossRef]
- Liu, J.; Sun, L.; Xu, W.; Wang, Q.; Yu, S.; Sun, J. Current advances and future perspectives of 3d printing natural-derived biopolymers. Carbohydr. Polym. 2019, 207, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.T.; Rajan, K.; Harper, D.P.; Chmely, S.C. Lignin-containing photoactive resins for 3d printing by stereolithography. ACS Appl. Mater. Interfaces 2018, 10, 36456–36463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, M.; Hao, N.; Ragauskas, A.J. Stereolithography 3d printing of lignin-reinforced composites with enhanced mechanical properties. ACS Omega 2019, 4, 20197–20204. [Google Scholar] [CrossRef] [Green Version]
- Ding, R.; Du, Y.; Goncalves, R.B.; Francis, L.F.; Reineke, T.M. Sustainable near uv-curable acrylates based on natural phenolics for stereolithography 3d printing. Polym. Chem. 2019, 10, 1067–1077. [Google Scholar] [CrossRef]
- Kim, S.-S.; Ha, H.; Ellison, C.J. Soybean oil-based thermoset films and fibers with high biobased carbon content via thiol–ene photopolymerization. ACS Sustain. Chem. Eng. 2018, 6, 8364–8373. [Google Scholar] [CrossRef]
- Kousaalya, A.B.; Ayalew, B.; Pilla, S. Photopolymerization of acrylated epoxidized soybean oil: A photocalorimetry-based kinetic study. ACS Omega 2019, 4, 21799–21808. [Google Scholar] [CrossRef] [Green Version]
- Goliszek, M.; Podkościelna, B.; Klepka, T.; Sevastyanova, O. Preparation, thermal, and mechanical characterization of uv-cured polymer biocomposites with lignin. Polymers 2020, 12, 1159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Galià, M.; de Espinosa, L.M.; Ronda, J.C.; Lligadas, G.; Cádiz, V. Vegetable oil-based thermosetting polymers. Eur. J. Lipid Sci. Technol. 2010, 112, 87–96. [Google Scholar] [CrossRef]
- Mosiewicki, M.A.; Aranguren, M.I. A short review on novel biocomposites based on plant oil precursors. Eur. Polym. J. 2013, 49, 1243–1256. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, Y.; Wang, L.; Gao, Z.; Kessler, M.R. Synthesis and characterization of methacrylated eugenol as a sustainable reactive diluent for a maleinated acrylated epoxidized soybean oil resin. ACS Sustain. Chem. Eng. 2017, 5, 8876–8883. [Google Scholar] [CrossRef]
- Alagi, P.; Ghorpade, R.; Jang, J.H.; Patil, C.; Jirimali, H.; Gite, V.; Hong, S.C. Functional soybean oil-based polyols as sustainable feedstocks for polyurethane coatings. Ind. Crop. Prod. 2018, 113, 249–258. [Google Scholar] [CrossRef]
- Tan, S.G.; Chow, W.S. Biobased epoxidized vegetable oils and its greener epoxy blends: A review. Polym. Plast. Technol. Eng. 2010, 49, 1581–1590. [Google Scholar] [CrossRef]
- Black, M.; Rawlins, J.W. Thiol-ene uv-curable coatings using vegetable oil macromonomers. Eur. Polym. J. 2009, 45, 1433–1441. [Google Scholar] [CrossRef]
- Bouaziz, K.; Ayadi, M.; Allouche, N.; Chemtob, A. Renewable photopolymer films derived from low-grade lampante and pomace olive oils. Eur. J. Lipid Sci. Technol. 2017, 119, 1700003. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Jia, P.; Shang, Q.; Zhang, M.; Feng, G.; Liu, C.; Zhou, Y. Synthesis and application of uv-curable phosphorous-containing acrylated epoxidized soybean oil-based resins. J. Bioresour. Bioprod. 2019, 4, 9. [Google Scholar]
- Wu, Q.; Hu, Y.; Tang, J.; Zhang, J.; Wang, C.; Shang, Q.; Feng, G.; Liu, C.; Zhou, Y.; Lei, W. High-performance soybean-oil-based epoxy acrylate resins: “Green” synthesis and application in uv-curable coatings. ACS Sustain. Chem. Eng. 2018, 6, 8340–8349. [Google Scholar] [CrossRef]
- Voet, V.S.D.; Guit, J.; Loos, K. Sustainable photopolymers in 3d printing: A review on biobased, biodegradable, and recyclable alternatives. Macromol. Rapid Commun. 2021, 42, 2000475. [Google Scholar] [CrossRef]
- Lebedevaite, M.; Ostrauskaite, J.; Skliutas, E.; Malinauskas, M. Photoinitiator free resins composed of plant-derived monomers for the optical μ-3d printing of thermosets. Polymers 2019, 11, 116. [Google Scholar] [CrossRef] [Green Version]
- Miao, S.; Zhu, W.; Castro, N.J.; Nowicki, M.; Zhou, X.; Cui, H.; Fisher, J.P.; Zhang, L.G. 4d printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci. Rep. 2016, 6, 27226. [Google Scholar] [CrossRef] [Green Version]
- Guit, J.; Tavares, M.B.L.; Hul, J.; Ye, C.; Loos, K.; Jager, J.; Folkersma, R.; Voet, V.S.D. Photopolymer resins with biobased methacrylates based on soybean oil for stereolithography. ACS Appl. Polym. Mater. 2020, 2, 949–957. [Google Scholar] [CrossRef]
- Skliutas, E.; Lebedevaite, M.; Kasetaite, S.; Rekštytė, S.; Lileikis, S.; Ostrauskaite, J.; Malinauskas, M. A bio-based resin for a multi-scale optical 3d printing. Sci. Rep. 2020, 10, 9758. [Google Scholar] [CrossRef] [PubMed]
- Bail, R.; Hong, J.Y.; Chin, B.D. Effect of a red-shifted benzotriazole uv absorber on curing depth and kinetics in visible light initiated photopolymer resins for 3d printing. J. Ind. Eng. Chem. 2016, 38, 141–145. [Google Scholar] [CrossRef]
- Lantean, S.; Roppolo, I.; Sangermano, M.; Pirri, C.; Chiappone, A. Development of new hybrid acrylic/epoxy dlp-3d printable materials. Inventions 2018, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, M.; Shukla, V.; Habib, F. Development of a heat resistant uv-curable epoxy coating. Prog. Org. Coat. 2005, 53, 239–245. [Google Scholar] [CrossRef]
- Omonov, T.S.; Curtis, J.M. Plant oil-based epoxy intermediates for polymers. In Bio-Based Plant Oil Polymers and Composites; Elsevier: Amsterdam, The Netherlands, 2016; pp. 99–125. [Google Scholar]
- Khalid, M.; Salmiaton, A.; Ratnam, C.T.; Luqman, C.A. Effect of trimethylolpropane triacrylate (tmpta) on the mechanical properties of palm fiber empty fruit bunch and cellulose fiber biocomposite. J. Eng. Sci. Technol. 2008, 3, 10. [Google Scholar]
- Kunwong, D.; Sumanochitraporn, N.; Kaewpirom, S. Curing behavior of a uv-curable coating based on urethane acrylate oligomer: The influence of reactive monomers. J. Sci. Technol. 2011, 33, 201. [Google Scholar]
- Ley, C.; Carré, C.; Ibrahim, A.; Allonas, X. Application of high performance photoinitiating systems for holographic grating recording. In Holographic Materials and Optical Systems; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Kaewpirom, S.; Kunwong, D. Curing behavior and cured film performance of easy-to-clean uv-curable coatings based on hybrid urethane acrylate oligomers. J. Polym. Res. 2012, 19, 1–12. [Google Scholar] [CrossRef]
- Liu, W.; Fei, M.e.; Ban, Y.; Jia, A.; Qiu, R. Preparation and evaluation of green composites from microcrystalline cellulose and a soybean-oil derivative. Polymers 2017, 9, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, W.; Li, X.; Liu, H.; Han, L.; Zhao, Y.; Li, X. Interfacial polycondensation synthesis of optically sensitive polyurea microcapsule. J. Chem. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Wu, G.; Wang, S.; Xu, M.; Feng, X. Dynamic postpolymerization of 3d-printed photopolymer nanocomposites: Effect of cellulose nanocrystal and postcure temperature. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 935–946. [Google Scholar] [CrossRef]
- Pelletier, H.; Belgacem, N.; Gandini, A.; Ne Pelletier, H.; Belgacem, N.; Gandini, A. Acrylated vegetable oils as photocrosslinkable materials. J. Appl. Polym. Sci. 2006, 99, 4. [Google Scholar] [CrossRef]
- Adekunle, K.; Akesson, D.; Skrifvars, M. Synthesis of reactive soybean oils for use as a biobased thermoset resins in structural natural fiber composites. J. Appl. Polym. Sci. 2010, 115, 3137–3145. [Google Scholar] [CrossRef]
- Li, Z.; Chen, H.; Wang, C.; Chen, L.; Liu, J.; Liu, R. Efficient photopolymerization of thick pigmented systems using upconversion nanoparticles-assisted photochemistry. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 994–1002. [Google Scholar] [CrossRef]
- Loginos, P.; Patsidis, A.; Georgakilas, V. Uv-cured poly(ethylene glycol) diacrylate/carbon nanostructure thin films. Preparation, characterization, and electrical properties. J. Compos. Sci. 2020, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Francos, X.; Kazarian, S.G.; Ramis, X.; Serra, A. Simultaneous monitoring of curing shrinkage and degree of cure of thermosets by attenuated total reflection fourier transform infrared (atr ft-ir) spectroscopy. Appl. Spectrosc. 2013, 67, 1427–1436. [Google Scholar] [CrossRef]
- Salih, A.M.; Ahmad, M.B.; Ibrahim, N.A.; Dahlan, K.Z.H.M.; Tajau, R.; Mahmood, M.H.; Yunus, W.M.Z.W. Synthesis of radiation curable palm oil-based epoxy acrylate: Nmr and ftir spectroscopic investigations. Molecules 2015, 20, 14191–14211. [Google Scholar] [CrossRef] [Green Version]
- Navaruckiene, A.; Skliutas, E.; Kasetaite, S.; Rekstyte, S.; Raudoniene, V.; Bridziuviene, D.; Malinauskas, M.; Ostrauskaite, J. Vanillin acrylate-based resins for optical 3d printing. Polymers 2020, 12, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asmussen, S.; Vallo, C. Light absorbing products during polymerization of methacrylate monomers photoinitiated with phenyl-1,2-propanedione/amine. J. Photochem. Photobiol. A Chem. 2009, 202, 228–234. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J., Jr. Statistical mechanics of cross-linked polymer networks i. Rubberlike elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Gaidukovs, S.; Medvids, A.; Onufrijevs, P.; Grase, L. Uv-light-induced curing of branched epoxy novolac resin for coatings. Express Polym. Lett. 2018, 12, 918–929. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Valavala, P.K.; Clancy, T.C.; Wise, K.E.; Odegard, G.M. Molecular modeling of crosslinked epoxy polymers: The effect of crosslink density on thermomechanical properties. Polymer 2011, 52, 2445–2452. [Google Scholar] [CrossRef]
- Treloar, L.R.G. The Physics of Rubber Elasticity; Oxford University Press: Oxford, UK, 1975. [Google Scholar]
- Wang, J.; Chiappone, A.; Roppolo, I.; Shao, F.; Fantino, E.; Lorusso, M.; Rentsch, D.; Dietliker, K.; Pirri, C.F.; Grützmacher, H. All-in-one cellulose nanocrystals for 3D printing of nanocomposite hydrogels. Hydrogels 2018, 57, 2353–2356. [Google Scholar]
- Rengasamy, S.; Mannari, V. Development of soy-based uv-curable acrylate oligomers and study of their film properties. Prog. Org. Coat. 2013, 76, 78–85. [Google Scholar] [CrossRef]
- Barkane, A.; Platnieks, O.; Jurinovs, M.; Gaidukovs, S. Thermal stability of uv-cured vegetable oil epoxidized acrylate-based polymer system for 3d printing application. Polym. Degrad. Stab. 2020, 181, 109347. [Google Scholar] [CrossRef]
- Lu, J.; Khot, S.; Wool, R.P. New sheet molding compound resins from soybean oil. I. Synthesis and characterization. Polymer 2005, 46, 71–80. [Google Scholar] [CrossRef]
- Ahmad, F.; Yuvaraj, N.; Bajpai, P.K. Influence of reinforcement architecture on static and dynamic mechanical properties of flax/epoxy composites for structural applications. Compos. Struct. 2021, 255. [Google Scholar] [CrossRef]




| Resin | Mc (g/mol) | N, ×103 (mol/cc) |
|---|---|---|
| AESO-1 | 130 | 24.6 |
| AESO-3 | 193 | 16.4 |
| AESO-5 | 223 | 14.4 |
| AESO-7 | 149 | 21.5 |
| M-AESO-1 | 45 | 73.9 |
| M-AESO-3 | 45 | 73.9 |
| M-AESO-5 | 73 | 46.2 |
| M-AESO-7 | 269 | 12.3 |
| Resin | T (°C) at Weight Losses | Tmax (°C) | |||||
|---|---|---|---|---|---|---|---|
| 5% | 10% | 30% | 50% | 70% | 90% | ||
| AESO-1 | 358 | 386 | 426 | 541 | 480 | 519 | 390 |
| AESO-3 | 357 | 390 | 432 | 457 | 483 | 523 | 399 |
| AESO-5 | 355 | 386 | 427 | 453 | 481 | 525 | 391 |
| AESO-7 | 339 | 380 | 423 | 450 | 479 | 522 | 387 |
| M-AESO-1 | 366 | 395 | 437 | 465 | 491 | 531 | 420 |
| M-AESO-3 | 374 | 402 | 442 | 468 | 493 | 532 | 416 |
| M-AESO-5 | 357 | 391 | 435 | 463 | 489 | 529 | 415 |
| M-AESO-7 | 372 | 399 | 441 | 467 | 493 | 537 | 416 |
| Resin | Storage Modulus (MPa) | Tg (°C) | ||||||
|---|---|---|---|---|---|---|---|---|
| −40 °C | −20 °C | 0 °C | 20 °C | 40 °C | 60 °C | 80 °C | ||
| AESO-1 | 2337 | 1754 | 821 | 213 | 50 | 29 | 24 | 28 |
| AESO-3 | 1970 | 1489 | 721 | 196 | 44 | 19 | 16 | 24 |
| AESO-5 | 1996 | 1591 | 805 | 204 | 42 | 18 | 14 | 29 |
| AESO-7 | 2016 | 1627 | 879 | 233 | 45 | 23 | 21 | 24 |
| M-AESO-1 | 2273 | 1890 | 1262 | 726 | 385 | 136 | 72 | 62 |
| M-AESO-3 | 2500 | 2014 | 1210 | 572 | 245 | 108 | 72 | 50 |
| M-AESO-5 | 2280 | 1849 | 1241 | 572 | 194 | 60 | 45 | 48 |
| M-AESO-7 | 1942 | 1622 | 1004 | 416 | 156 | 51 | 12 | 47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barkane, A.; Platnieks, O.; Jurinovs, M.; Kasetaite, S.; Ostrauskaite, J.; Gaidukovs, S.; Habibi, Y. UV-Light Curing of 3D Printing Inks from Vegetable Oils for Stereolithography. Polymers 2021, 13, 1195. https://doi.org/10.3390/polym13081195
Barkane A, Platnieks O, Jurinovs M, Kasetaite S, Ostrauskaite J, Gaidukovs S, Habibi Y. UV-Light Curing of 3D Printing Inks from Vegetable Oils for Stereolithography. Polymers. 2021; 13(8):1195. https://doi.org/10.3390/polym13081195
Chicago/Turabian StyleBarkane, Anda, Oskars Platnieks, Maksims Jurinovs, Sigita Kasetaite, Jolita Ostrauskaite, Sergejs Gaidukovs, and Youssef Habibi. 2021. "UV-Light Curing of 3D Printing Inks from Vegetable Oils for Stereolithography" Polymers 13, no. 8: 1195. https://doi.org/10.3390/polym13081195










