Interaction of Diphenhydramine Hydrochloride with Cationic and Anionic Surfactants: Mixed Micellization and Binding Studies
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Surface Tension Measurement
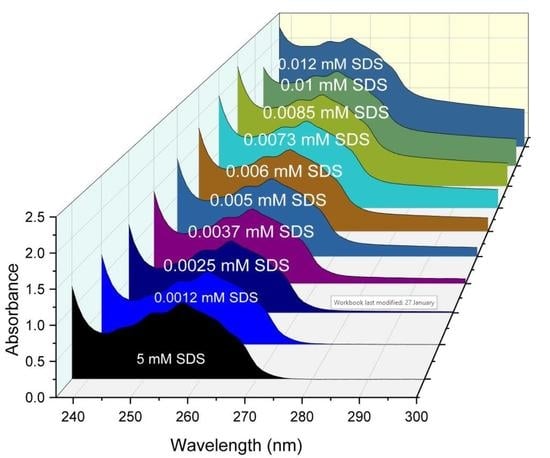
2.2.2. Electronic Absorption Measurement
3. Results and Discussion
3.1. Critical Micelle Concentration of Single and Mixed Amphiphiles
- CMC* = ideal critical micelle concentration of the mixed system
- CMC1 = critical micelle concentration of surfactant (SDS/CPC)
- CMC2 = critical micelle concentration of the drug (DPH)
- α1 = mole fraction of surfactant (SDS/CPC)
3.2. Synergistic Effects
- (1)
- β should be negative
- (2)
3.3. Surface Parameters of Drug-Surfactants Mixtures
- γ = surface tension
- Γi = surface excess
- μi = chemical potential of the ith components in the solution
- (1)
- should be negative
- (2)
3.4. Thermodynamic Parameters of Micellization
3.5. Spectroscopic Method (UV–Visible Spectroscopy)
4. Conclusions
- (1)
- Experimental CMC values are seen to be less than CMC* values, showing a negative deviation from ideality. The deviation from ideality refers to the interactions between the two amphiphiles.
- (2)
- The values micelle mole fraction of surfactants (X1) are lower than ideal values () at all mole fractions, confirm a higher contribution of the drug in the mixed micelle.
- (3)
- The calculated values of the interaction parameter (β) are negative suggesting synergistic interaction between the drug and surfactants. The SDS+DPH mixed system shows greater synergism than CPC+DPH. This can be clarified based on fact that the DPH binds tightly with SDS because of preferred electrostatic interactions amid the cationic head group of DPH and anionic head group pf SDS, which form ion pair.
- (4)
- The lower values of surface excess () for DPH+CPC mixed system than pure DPH is due to the larger hydrocarbon chain of CPC that resists the adsorption of the mixture on the surface.
- (5)
- The packing parameter (p) values of pure and mixtures are below 0.33, confirm spherical geometry.
- (6)
- The changed standard Gibbs free energy () obtained for both mixed systems are negative, suggesting the feasibility of the micellization.
- (7)
- The DPH is showing more binding affinity toward SDS (3.349 × 108) than CPC (8.469 × 107) due to the less steric hindrance caused by SDS single-chain hydrophobic group.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schreier, S.; Malheiros, S.V.; de Paula, E. Surface active drugs: Self-association and interaction with membranes and surfactants. Physicochemical and biological aspects. Biochim. Biophys. Acta (BBA) Biomembr. 2000, 1508, 210–234. [Google Scholar] [CrossRef] [Green Version]
- Causon, D.; Gettins, J.; Gormally, J.; Greenwood, R.; Natarajan, N.; Wyn-Jones, E. Ultrasonic relaxations associated with aggregation in drugs. J. Chem. Soc. Faraday Trans. 2 1981, 77, 143–151. [Google Scholar] [CrossRef]
- Yokoyama, S.; Fujino, Y.; Kawamoto, Y.; Kaneko, A.; Fujie, T. Micellization of an Aqueous Solution of Piperidolate Hydrochloride in the Presence of Acetylcholine Chloride. Chem. Pharm. Bull. 1994, 42, 1351–1353. [Google Scholar] [CrossRef] [Green Version]
- Attwood, D.; Tolley, J.A. Self-association of analgesics in aqueous solution: Association models for codeine, oxycodone, ethylmorphine and pethidine. J. Pharm. Pharmacol. 1980, 32, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Azum, N.; Ahmed, A.; Rub, M.A.; Asiri, A.M.; Alamery, S.F. Investigation of aggregation behavior of ibuprofen sodium drug under the influence of gelatin protein and salt. J. Mol. Liq. 2019, 290, 111187. [Google Scholar] [CrossRef]
- Kumar, D.; Azum, N.; Rub, M.A.; Asiri, A.M. Aggregation behavior of sodium salt of ibuprofen with conventional and gemini surfactant. J. Mol. Liq. 2018, 262, 86–96. [Google Scholar] [CrossRef]
- Rub, M.A.; Azum, N.; Khan, F.; Asiri, A.M. Aggregation of sodium salt of ibuprofen and sodium taurocholate mixture in different media: A tensiometry and fluorometry study. J. Chem. Thermodyn. 2018, 121, 199–210. [Google Scholar] [CrossRef]
- Wan-Bahdi, W.; Mwakibete, H.; Bloor, D.M.; Palepu, R.; Wyn-Jones, E. Emf and ultrasonic relaxation measurements of premicellar and micellar aggregation in the drug promethazine hydrochloride. J. Phys. Chem. 1992, 96, 918–925. [Google Scholar] [CrossRef]
- Attwood, D.; Natarajan, R. Effect of pH on the micellar properties of amphiphilic drugs in aqueous solution. J. Pharm. Pharmacol. 1981, 33, 136–140. [Google Scholar] [CrossRef]
- Attwood, D.; Florence, A.T.; Gillan, J.M.N. Micellar Properties of Drugs: Properties of Micellar Aggregates of Phenothiazines and Their Aqueous Solutions. J. Pharm. Sci. 1974, 63, 988–993. [Google Scholar] [CrossRef]
- Hwang, P.M.; Vogel, H.J. Structure-function relationships of antimicrobial peptides. Biochem. Cell Biol. 1998, 76, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, F.; López-Fontán, J.L.; Prieto, G.; Mosquera, V.; Attwood, D. Mixed micelles of structurally related antidepressant drugs. Colloid Polym. Sci. 1997, 275, 1144–1147. [Google Scholar] [CrossRef]
- Atherton, A.D.; Barry, B.W. Photon correlation spectroscopy of surface active cationic drugs. J. Pharm. Pharmacol. 1985, 37, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Rub, M.A.; Azum, N.; Asiri, A.M. Mixtures of antidepressant amphiphilic drug imipramine hydrochloride and anionic surfactant: Micellar and thermodynamic investigation. J. Phys. Org. Chem. 2018, 31, e3812. [Google Scholar] [CrossRef]
- Rub, M.A.; Azum, N.; Khan, F.; Asiri, A.M. Surface, micellar, and thermodynamic properties of antidepressant drug nortriptyline hydrochloride with TX-114 in aqueous/urea solutions. J. Phys. Org. Chem. 2017, 30, e3676. [Google Scholar] [CrossRef]
- Rub, M.A.; Khan, J.M.; Azum, N.; Asiri, A.M. Influence of antidepressant clomipramine hydrochloride drug on human serum albumin: Spectroscopic study. J. Mol. Liq. 2017, 241, 91–98. [Google Scholar] [CrossRef]
- Ruso, J.M.; Attwood, D.; Rey, C.; Taboada, P.; Mosquera, V.; Sarmiento, F. Light Scattering and NMR Studies of the Self-Association of the Amphiphilic Molecule Propranolol Hydrochloride in Aqueous Electrolyte Solutions. J. Phys. Chem. B 1999, 103, 7092–7096. [Google Scholar] [CrossRef]
- Matsuki, H.; Hashimoto, S.; Kaneshina, S.; Yamanaka, M. Surface Adsorption and Volume Behavior of Local Anesthetics. Langmuir 1994, 10, 1882–1887. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Asiri, A.M. Interaction of antipsychotic drug with novel surfactants: Micellization and binding studies. Chin. J. Chem. Eng. 2018, 26, 566–573. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Asiri, A.M.; Kashmery, H.A. Synergistic effect of an antipsychotic drug chlorpromazine hydrochloride with pluronic triblock copolymer: A physicochemical study. J. Mol. Liq. 2018, 260, 159–165. [Google Scholar] [CrossRef]
- King, S.-Y.P.; Basista, A.M.; Torosian, G. Self-Association and Solubility Behaviors of a Novel Anticancer Agent, Brequinar Sodium. J. Pharm. Sci. 1989, 78, 95–100. [Google Scholar] [CrossRef]
- Bhadoriya, S.S.; Madoriya, N. Biosurfactants: A New Pharmaceutical Additive for Solubility Enhancement and Pharmaceutical Development. Biochem. Pharmacol. Open Access 2013, 2. [Google Scholar] [CrossRef] [Green Version]
- Vinarov, Z.; Katev, V.; Radeva, D.; Tcholakova, S.; Denkov, N.D. Micellar solubilization of poorly water-soluble drugs: Effect of surfactant and solubilizate molecular structure. Drug Dev. Ind. Pharm. 2018, 44, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Klevens, H.B. Solubilization. Chem. Rev. 1950, 47, 1–74. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Yagui, C.O.; Pessoa, A., Jr.; Tavares, L.C. Micellar solubilization of drugs. J. Pharm. Pharm. Sci. 2005, 8, 147–165. [Google Scholar]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef] [Green Version]
- Attwood, D.; Udeala, O.K. Aggregation of antihistamines in aqueous solution. The effect of electrolyte on the micellar properties of some diphenylmethane derivatives. J. Pharm. Pharmacol. 1975, 27, 395–399. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, K.; Chauhan, S. Micellization properties of antihistaminic drug diphenhydramine.HCl in aqueous electrolytic solution: Conductometric and spectroscopic studies. J. Mol. Liq. 2020, 300, 112306. [Google Scholar] [CrossRef]
- Srivastava, A.; Uchiyama, H.; Wada, Y.; Hatanaka, Y.; Shirakawa, Y.; Kadota, K.; Tozuka, Y. Mixed micelles of the antihistaminic cationic drug diphenhydramine hydrochloride with anionic and non-ionic surfactants show improved solubility, drug release and cytotoxicity of ethenzamide. J. Mol. Liq. 2019, 277, 349–359. [Google Scholar] [CrossRef]
- Church, M.K. Allergy, Histamine and Antihistamines. Snake Venoms 2016, 241, 321–331. [Google Scholar]
- Sharma, R.; Mahajan, R.K. An investigation of binding ability of ionic surfactants with trifluoperazine dihydrochloride: Insights from surface tension, electronic absorption and fluorescence measurements. RSC Adv. 2012, 2, 9571–9583. [Google Scholar] [CrossRef]
- Attwood, D. Micelle formation by some antihistamines in aqueous solution. J. Pharm. Pharmacol. 1972, 24, 751–752. [Google Scholar] [CrossRef] [PubMed]
- Khatua, P.K.; Ghosh, S.; Ghosh, S.K.; Bhattacharya, S.C. Characterization of Binary Surfactant Mixtures (Cetylpyridinium Chloride and Tween 60) in an Aqueous Medium. J. Dispers. Sci. Technol. 2005, 25, 741–748. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Asiri, A.M. Bile salt–bile salt interaction in mixed monolayer and mixed micelle formation. J. Chem. Thermodyn. 2019, 128, 406–414. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Asiri, A.M. Interaction of triblock-copolymer with cationic gemini and conventional surfactants: A physicochemical study. J. Dispers. Sci. Technol. 2017, 38, 1785–1791. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Asiri, A.M. Micellization and interfacial behavior of binary and ternary mixtures in aqueous medium. J. Mol. Liq. 2016, 216, 94–98. [Google Scholar] [CrossRef]
- Khan, F.; Rub, M.A.; Azum, N.; Kumar, D.; Asiri, A.M. Interaction of an Amphiphilic Drug and Sodium Bis(2-ethylhexyl)sulfosuccinate at Low Concentrations in the Absence and Presence of Sodium Chloride. J. Solut. Chem. 2015, 44, 1937–1961. [Google Scholar] [CrossRef]
- Kumar, D.; Rub, M.A.; Azum, N.; Asiri, A.M. Mixed micellization study of ibuprofen (sodium salt) and cationic surfactant (conventional as well as gemini). J. Phys. Org. Chem. 2018, 31, e3730. [Google Scholar] [CrossRef]
- Clint, J.H. Micellization of mixed nonionic surface active agents. J. Chem. Soc. Faraday Trans. Phys. Chem. Condens. Phases 1975, 71, 1327–1334. [Google Scholar] [CrossRef]
- Holland, P.M.; Rubingh, D.N. Nonideal multicomponent mixed micelle model. J. Phys. Chem. 1983, 87, 1984–1990. [Google Scholar] [CrossRef]
- Azum, N.; Naqvi, A.Z.; Rub, M.A.; Asiri, A.M. Multi-technique approach towards amphiphilic drug-surfactant interaction: A physicochemical study. J. Mol. Liq. 2017, 240, 189–195. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Asiri, A.M.; Bawazeer, W.A. Micellar and interfacial properties of amphiphilic drug–non-ionic surfactants mixed systems: Surface tension, fluorescence and UV–vis studies. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 183–192. [Google Scholar] [CrossRef]
- Lange, H.; Beck, K.-H. Zur Mizellbildung in Mischlösungen homologer und nichthomologer Tenside. Colloid Polym. Sci. 1973, 251, 424–431. [Google Scholar] [CrossRef]
- Rub, M.A.; Azum, N.; Asiri, A.M. Binary Mixtures of Sodium Salt of Ibuprofen and Selected Bile Salts: Interface, Micellar, Thermodynamic, and Spectroscopic Study. J. Chem. Eng. Data 2017, 62, 3216–3228. [Google Scholar] [CrossRef]
- Shaheen, A.; Mir, A.W. Effect of additives on mixed micellization of a phenothiazine drug promethazine hydrochloride and an ester-based pyridinium gemini surfactant. J. Dispers. Sci. Technol. 2020, 41, 1513–1525. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Asiri, A.M. Self-association and micro-environmental properties of sodium salt of ibuprofen with BRIJ-56 under the influence of aqueous/urea solution. J. Dispers. Sci. Technol. 2017, 38, 96–104. [Google Scholar] [CrossRef]
- Zhou, Q.; Rosen, M.J. Molecular Interactions of Surfactants in Mixed Monolayers at the Air/Aqueous Solution Interface and in Mixed Micelles in Aqueous Media: The Regular Solution Approach. Langmuir 2003, 19, 4555–4562. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Asiri, A.M. Micellization and Interfacial Behavior of the Sodium Salt of Ibuprofen–BRIJ-58 in Aqueous/Brine Solutions. J. Solut. Chem. 2016, 45, 791–803. [Google Scholar] [CrossRef]
- Ghosh, S.; Chakraborty, T. Mixed Micelle Formation among Anionic Gemini Surfactant (212) and Its Monomer (SDMA) with Conventional Surfactants (C12E5and C12E8) in Brine Solution at pH 11. J. Phys. Chem. B 2007, 111, 8080–8088. [Google Scholar] [CrossRef] [PubMed]
- Charles, T. The Hydrophobic Effect: Formation of Micelles and Biological Membranes, 2nd ed.; Wiley-Interscience: New York, NY, USA, 1980. [Google Scholar]
- Rosen, M.J.; Aronson, S. Standard free energies of adsorption of surfactants at the aqueous solution/air interface from surface tension data in the vicinity of the critical micelle concentration. Colloids Surf. 1981, 3, 201–208. [Google Scholar] [CrossRef]
- Mukerjee, P. The nature of the association equilibria and hydrophobic bonding in aqueous solutions of association colloids. Adv. Colloid Interface Sci. 1967, 1, 242–275. [Google Scholar] [CrossRef]
- Sugihara, G.; Miyazono, A.; Nagadome, S.; Oida, T.; Hayashi, Y.; Ko, J.-S. Adsorption and Micelle Formation of Mixed Surfactant Systems in Water II: A Combination of Cationic Gemini-type Surfactant with MEGA-10. J. Oleo Sci. 2003, 52, 449–461. [Google Scholar] [CrossRef] [Green Version]
- Hall, D.G. Electrostatic effects in dilute solutions containing charged colloidal entities. J. Chem. Soc. Faraday Trans. 1991, 87, 3529–3535. [Google Scholar] [CrossRef]
- Benesi, H.A.; Hildebrand, J.H. A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Almgren, M.; Grieser, F.; Thomas, J.K. Dynamic and static aspects of solubilization of neutral arenes in ionic micellar solutions. J. Am. Chem. Soc. 1979, 101, 279–291. [Google Scholar] [CrossRef]





| α1 | CMC (mM) | CMC* (mM) | X1 | −β | f1 | f2 | ||
|---|---|---|---|---|---|---|---|---|
| SDS+DPH | ||||||||
| 0 | 112 ± 6.10 | |||||||
| 0.1 | 7.877 ± 1.29 | 45.5 | 0.534 | 0.635 | 7.272 | 0.206 | 0.126 | −2.75 |
| 0.2 | 6.622 ± 1.21 | 28.5 | 0.584 | 0.796 | 6.635 | 0.317 | 0.104 | |
| 0.8 | 1.239 ± 0.58 | 8.80 | 0.647 | 0.984 | 12.363 | 0.214 | 0.006 | |
| 0.9 | 1.010 ± 0.63 | 7.89 | 0.657 | 0.993 | 13.961 | 0.193 | 0.002 | |
| 1 | 7.150 ± 0.87 | |||||||
| CPC+DPH | ||||||||
| 0 | 112 ± 6.1 | |||||||
| 0.1 | 4.416 ± 1.17 | 7.65 | 0.728 | 0.938 | 3.988 | 0.744 | 0.121 | −4.92 |
| 0.3 | 1.890 ± 1.12 | 2.67 | 0.811 | 0.983 | 4.317 | 0.857 | 0.058 | |
| 0.5 | 1.037 ± 1.03 | 1.62 | 0.802 | 0.993 | 5.938 | 0.793 | 0.022 | |
| 0.7 | 0.906 ± 1.05 | 1.16 | 0.863 | 0.997 | 5.518 | 0.901 | 0.016 | |
| 0.9 | 0.703 ± 0.91 | 0.905 | 0.872 | 0.999 | 7.133 | 0.890 | 0.004 | |
| 1 | 0.815 ± 0.23 | |||||||
| α1 | 106 Γmax (mol·m−2) | Amin (nm2) | Aideal (nm2) | pC20 | γcmc (mN·m−1) | πcmc (mN·m−1) |
|---|---|---|---|---|---|---|
| SDS+DPH | ||||||
| 0 | 1.809 ± 0.21 | 0.917 ± 0.10 | 0.963 ± 0.08 | 49.710 ± 0.67 | 20.290 ± 0.67 | |
| 0.1 | 2.213 ± 0.28 | 0.750 ± 0.09 | 0.710 | 2.909 ± 0.14 | 31.350 ± 0.81 | 38.650 ± 0.81 |
| 0.2 | 2.257 ± 0.25 | 0.735 ± 0.13 | 0.699 | 3.061 ± 0.09 | 31.495 ± 0.90 | 38.505 ± 0.90 |
| 0.8 | 2.277 ± 0.27 | 0.729 ± 0.11 | 0.681 | 3.688 ± 0.11 | 30.196 ± 0.73 | 39.804 ± 0.73 |
| 0.9 | 2.328 ± 0.21 | 0.713 ± 0.06 | 0.679 | 3.818 ± 0.07 | 30.124 ± 0.43 | 39.876 ± 0.43 |
| 1 | 3.011 ± 0.23 | 0.551 ± 0.04 | 2.700 ± 0.05 | 30.208 ± 0.29 | 39.792 ± 0.29 | |
| CPC+DPH | ||||||
| 0 | 1.809 ± 0.21 | 0.917 ± 0.10 | 0.963 ± 0.08 | 49.710 ± 0.67 | 20.290 ± 0.67 | |
| 0.1 | 1.219 ± 0.23 | 1.361 ± 0.09 | 0.866 | 3.022 ± 0.09 | 35.859 ± 0.69 | 34.141 ± 0.69 |
| 0.3 | 1.243 ± 0.28 | 1.335 ± 0.15 | 0.864 | 3.410 ± 0.13 | 35.502 ± 0.73 | 34.498 ± 0.73 |
| 0.5 | 1.541 ± 0.19 | 1.077 ± 0.12 | 0.863 | 3.680 ± 0.11 | 35.362 ± 0.48 | 34.638 ± 0.48 |
| 0.7 | 1.266 ± 0.14 | 1.311 ± 0.07 | 0.859 | 3.718 ± 0.15 | 34.929 ± 0.33 | 35.071 ± 0.33 |
| 0.9 | 1.109 ± 0.22 | 1.496 ± 0.09 | 0.861 | 3.913 ± 0.08 | 35.285 ± 0.18 | 34.715 ± 0.18 |
| 1 | 1.933 ± 0.11 | 0.858 ± 0.05 | 3.920 ± 0.11 | 37.710 ± 0.15 | 32.290 ± 0.15 | |
| α1 | CS (mmol·dm−3) | X1S | −βS | P | |
|---|---|---|---|---|---|
| SDS+DPH | |||||
| 0 | 109 ± 11 | 0.23 | –3.98 | ||
| 0.1 | 1.23 ± 6.5 | 0.565 | 11.82 | 0.28 | |
| 0.2 | 0.869 ± 8.3 | 0.594 | 11.78 | 0.29 | |
| 0.8 | 0.205 ± 3.1 | 0.645 | 16.52 | 0.29 | |
| 0.9 | 0.152 ± 1.3 | 0.650 | 18.54 | 0.30 | |
| 1 | 2.04 ± 0.15 | 0.38 | |||
| CPC+DPH | |||||
| 0 | 109 ± 11 | 0.23 | –6.81 | ||
| 0.1 | 0.951 ± 6.8 | 0.860 | 4.17 | 0.15 | |
| 0.3 | 0.341 ± 7.1 | 0.897 | 4.79 | 0.16 | |
| 0.5 | 0.207 ± 3.2 | 0.907 | 5.82 | 0.20 | |
| 0.7 | 0.167 ± 0.23 | 0.976 | 4.33 | 0.16 | |
| 0.9 | 0.122 ± 0.35 | 0.940 | 7.49 | 0.14 | |
| 1 | 0.120 ± 0.17 | 0.24 | |||
| α1 | − (kj·mol−1) | −ΔGads (kj·mol−1) | Gmin (kj·mol−1) | −GEX |
|---|---|---|---|---|
| SDS+DPH | ||||
| 0 | 15.37 ± 0.15 | 26.58 ± 0.14 | 27.46 ± 2.67 | |
| 0.1 | 21.95 ± 0.18 | 39.41 ± 0.16 | 14.16 ± 2.35 | 4.484 |
| 0.2 | 22.38 ± 0.23 | 39.44 ± 0.19 | 13.94 ± 1.90 | 3.994 |
| 0.8 | 26.53 ± 0.27 | 44.01 ± 0.12 | 13.25 ± 2.08 | 6.996 |
| 0.9 | 27.04 ± 0.20 | 44.17 ± 0.24 | 12.93 ± 1.67 | 7.794 |
| 1 | 22.19 ± 0.33 | 35.41 ± 0.81 | 10.03 ± 0.90 | |
| CPC+DPH | ||||
| 0 | 15.37 ± 0.15 | 26.58 ± 0.14 | 27.46 ± 2.67 | |
| 0.1 | 23.38 ± 0.18 | 51.37 ± 0.80 | 29.39 ± 2.31 | 1.957 |
| 0.3 | 25.49 ± 0.15 | 53.23 ± 0.18 | 28.54 ± 2.39 | 1.637 |
| 0.5 | 26.97 ± 0.35 | 49.45 ± 0.99 | 22.93 ± 0.99 | 2.333 |
| 0.7 | 27.31 ± 0.31 | 55.01 ± 1.01 | 27.58 ± 1.50 | 1.616 |
| 0.9 | 27.94 ± 0.17 | 59.23 ± 0.88 | 31.80 ± 0.99 | 1.969 |
| 1 | 27.57 ± 0.57 | 44.27 ± 1.34 | 19.49 ± 1.18 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azum, N.; Rub, M.A.; Alfaifi, S.Y.; Asiri, A.M. Interaction of Diphenhydramine Hydrochloride with Cationic and Anionic Surfactants: Mixed Micellization and Binding Studies. Polymers 2021, 13, 1214. https://doi.org/10.3390/polym13081214
Azum N, Rub MA, Alfaifi SY, Asiri AM. Interaction of Diphenhydramine Hydrochloride with Cationic and Anionic Surfactants: Mixed Micellization and Binding Studies. Polymers. 2021; 13(8):1214. https://doi.org/10.3390/polym13081214
Chicago/Turabian StyleAzum, Naved, Malik Abdul Rub, Sulaiman Yahya Alfaifi, and Abdullah M. Asiri. 2021. "Interaction of Diphenhydramine Hydrochloride with Cationic and Anionic Surfactants: Mixed Micellization and Binding Studies" Polymers 13, no. 8: 1214. https://doi.org/10.3390/polym13081214
APA StyleAzum, N., Rub, M. A., Alfaifi, S. Y., & Asiri, A. M. (2021). Interaction of Diphenhydramine Hydrochloride with Cationic and Anionic Surfactants: Mixed Micellization and Binding Studies. Polymers, 13(8), 1214. https://doi.org/10.3390/polym13081214