Mechanically Sustainable Starch-Based Flame-Retardant Coatings on Polyurethane Foams
Abstract
:1. Introduction
2. Experimental
2.1. Layer-by-Layer Assembly
2.2. Measurements and Characterization
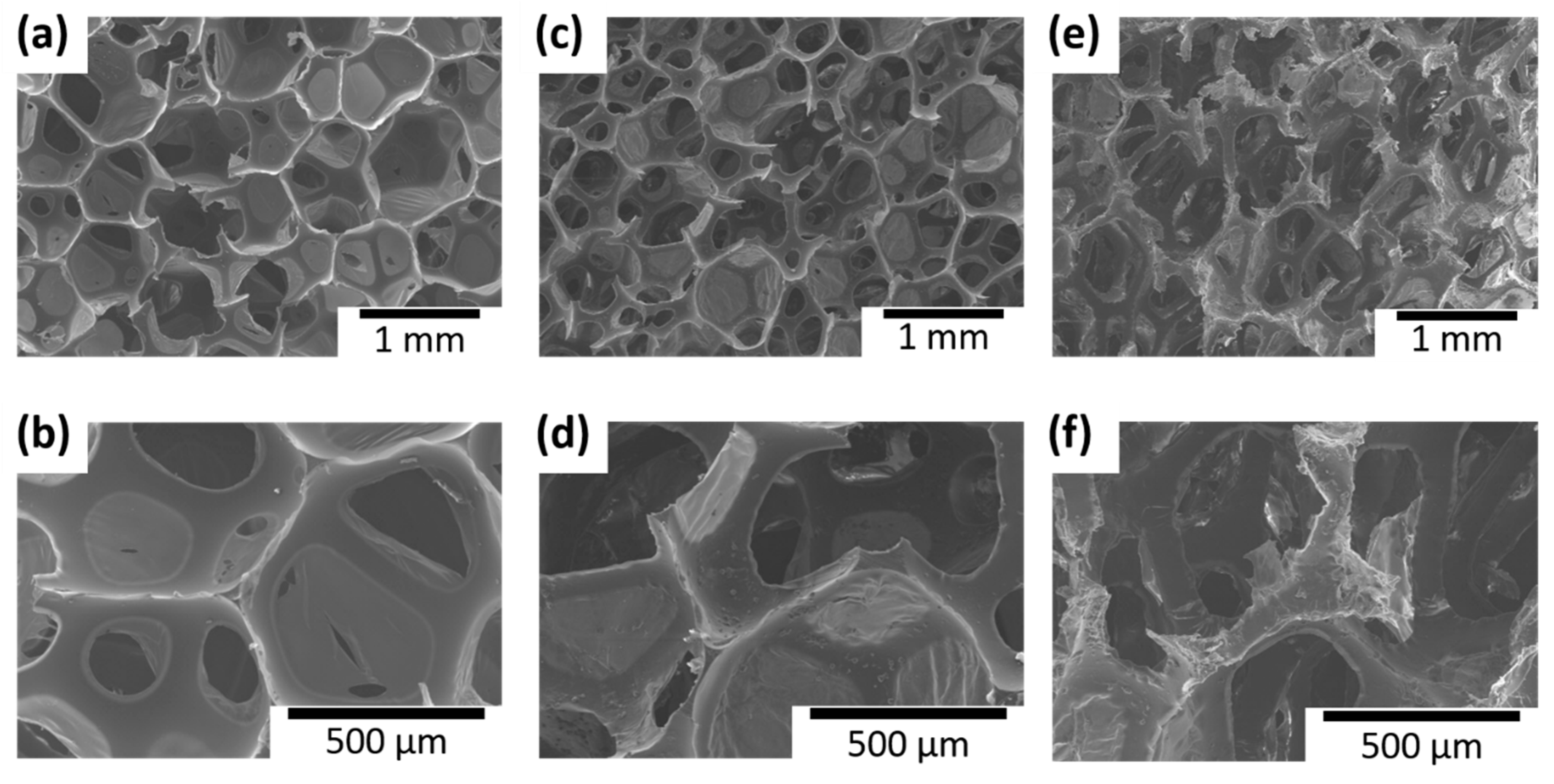
3. Results and Discussion
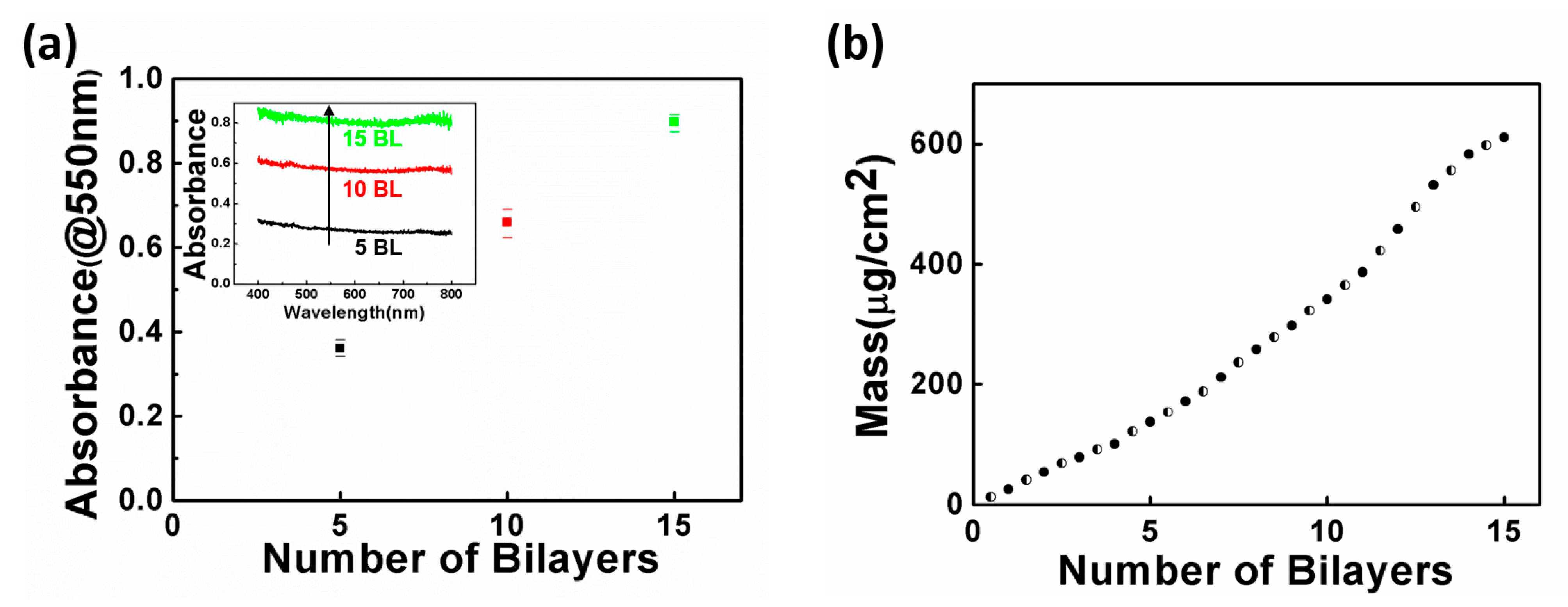
3.1. Spray-Assisted LbL Thin Film Growth
3.2. Thermal Stability
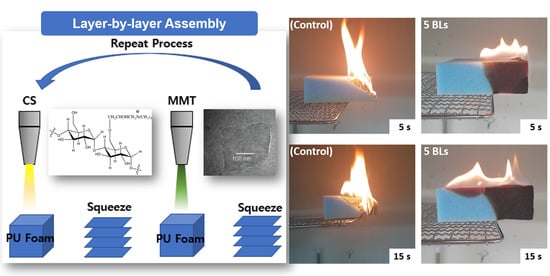
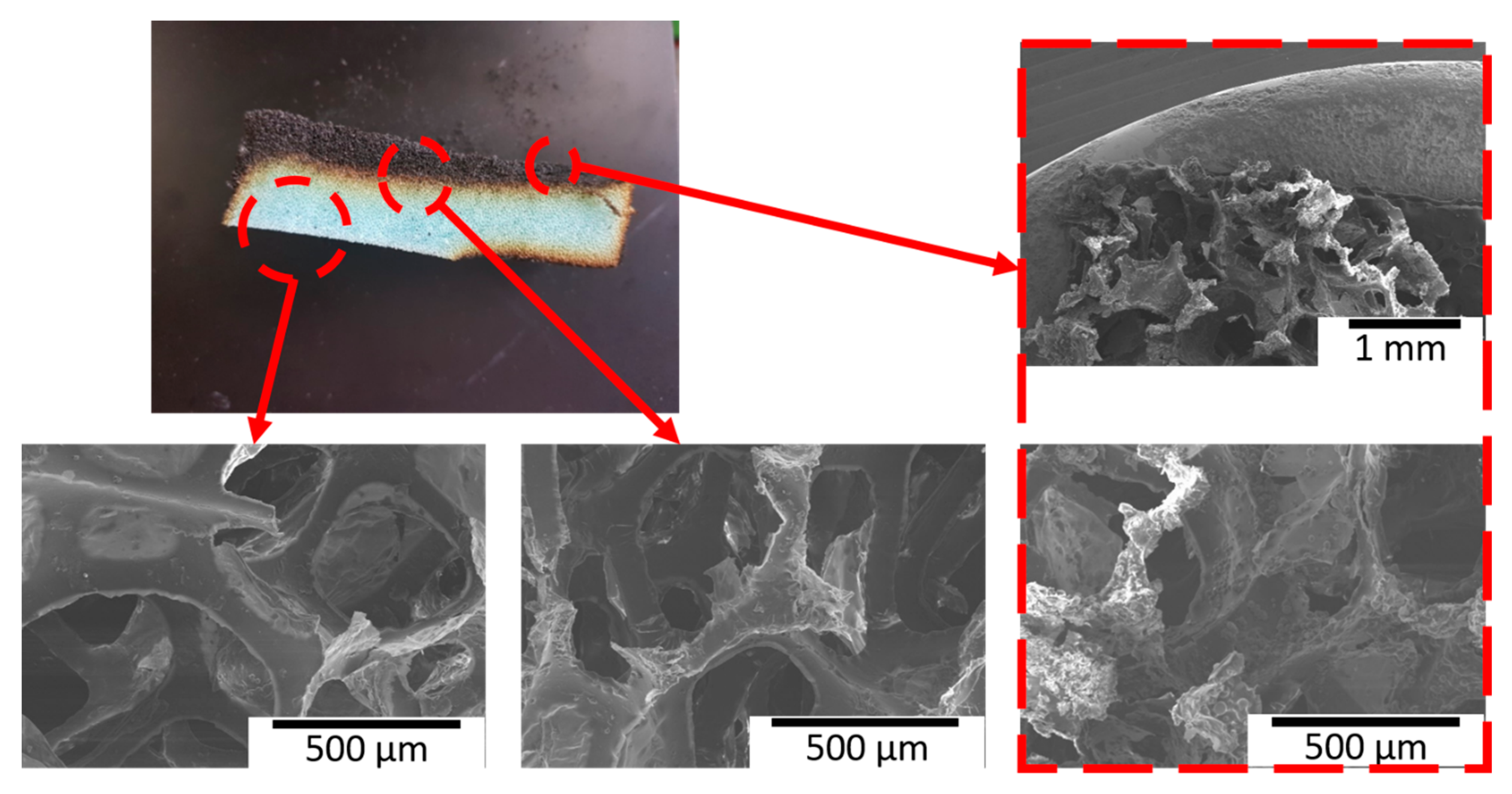
3.3. Flame Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bergman, Å.; Rydén, A.; Law, R.J.; de Boer, J.; Covaci, A.; Alaee, M.; Birnbaum, L.; Petreas, M.; Rose, M.; Sakai, S.; et al. A novel abbreviation standard for organobromine, organochlorine and organophosphorus flame retardants and some characteristics of the chemicals. Environ. Int. 2012, 49, 57–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darnerud, P.O. Toxic effects of brominated flame retardants in man and in wildlife. Environ. Int. 2003, 29, 841–853. [Google Scholar] [CrossRef]
- Lu, S.Y.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Carosio, F.; Di Blasio, A.; Alongi, J.; Malucelli, G. Green DNA-based flame retardant coatings assembled through Layer by Layer. Polymer 2013, 54, 5148–5153. [Google Scholar] [CrossRef]
- El-Shafei, A.; El-Shemy, M.; Abou-Okeil, A. Eco-friendly finishing agent for cotton fabrics to improve flame retardant and antibacterial properties. Carbohyd. Polym. 2015, 118, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Carja, I.D.; Serbezeanu, D.; Vlad-Bubulac, T.; Hamciuc, C.; Coroaba, A.; Lisa, G.; López, C.G.; Soriano, M.F.; Pérezd, V.F.; Sánchezd, M.D.R. A straightforward, eco-friendly and cost-effective approach towards flame retardant epoxy resins. J. Mater. Chem. A. 2014, 2, 16230–16241. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Xu, Y.; Wang, C.; Chu, F. Preparation and characterization of phenolic foams with eco-friendly halogen-free flame retardant. J. Therm. Anal. Calorim. 2013, 114, 1143–1151. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.T.; Wan, J.T.; Wang, D.Y. An eco-friendly way to fire retardant flexible polyurethane foam: Layer-by-layer assembly of fully bio-based substances. RSC Adv. 2014, 4, 46164–46169. [Google Scholar] [CrossRef] [Green Version]
- Sukhorukov, G.B.; Möhwald, H.; Decher, G.; Lvov, Y.M. Assembly of polyelectrolyte multilayer films by consecutively alternating adsorption of polynucleotides and polycations. Thin Solid Film. 1996, 284, 220–223. [Google Scholar] [CrossRef]
- Zhang, J.; Senger, B.; Vautier, D.; Picart, C.; Schaaf, P.; Voegel, J.C.; Lavalle, P. Natural polyelectrolyte films based on layer-by layer deposition of collagen and hyaluronic acid. Biomaterials 2005, 26, 3353–3361. [Google Scholar] [CrossRef]
- Wood, K.C.; Boedicker, J.Q.; Lynn, D.M.; Hammond, P.T. Tunable drug release from hydrolytically degradable layer-by-layer thin films. Langmuir 2005, 21, 1603–1609. [Google Scholar] [CrossRef]
- Mariani, S.; Robbiano, V.; Strambini, L.M.; Debrassi, A.; Egri, G.; Dähne, L.; Barillaro, G. Layer-by-layer biofunctionalization of nanostructured porous silicon for high-sensitivity and high-selectivity label-free affinity biosensing. Nat. Commun. 2018, 9, 5256. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Choi, D.; Hong, J. Nanostructured Polymer Thin Films Fabricated with Brush-based Layer-by-Layer Self-assembly for Site-selective Construction and Drug release. Sci. Rep. 2018, 8, 3365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, B.S.; Tang, Z.; Morabito, M.P.; Agarwal, A.; Hong, H.; Kotov, N.A. Integration of Conductivity, Transparency, and Mechanical Strength into Highly Homogeneous Layer-by-Layer Composites of Single-Walled Carbon Nanotubes for Optoelectronics. Chem. Mater. 2007, 19, 5467–5474. [Google Scholar] [CrossRef]
- Lee, J.; Ryu, J.; Youn, H.J. Conductive paper through LbL multilayering with conductive polymer: Dominant factors to increase electrical conductivity. Cellulose 2012, 19, 2153–2164. [Google Scholar] [CrossRef]
- Zhu, J.; Shim, B.S.; Prima, M.D.; Kotov, N.A. Transparent conductors from carbon nanotubes LBL-assembled with polymer dopant with π−π electron transfer. J. Am. Chem. Soc. 2011, 133, 7450–7460. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Loh, X.J. Layer-by-layer assemblies for antibacterial applications. Biomater. Sci. 2015, 3, 1505–1518. [Google Scholar] [CrossRef]
- Wu, Y.; Long, Y.; Li, Q.; Han, S.; Ma, J.; Yang, Y.; Gao, H. Layer-by-Layer (LBL) self-assembled biohybrid nanomaterials for efficient antibacterial applications. ACS Appl. Mater. Interfaces 2015, 7, 17255–17263. [Google Scholar] [CrossRef] [PubMed]
- Priolo, M.A.; Holder, K.M.; Gamboa, D.; Grunlan, J.C. Influence of clay concentration on the gas barrier of clay-polymer nanobrick wall thin film assemblies. Langmuir 2011, 27, 12106–12114. [Google Scholar] [CrossRef]
- Yang, Y.H.; Haile, M.; Park, Y.T.; Malek, F.A.; Grunlan, J.C. Super Gas Barrier of All-Polymer Multilayer Thin Films. Macromolecules 2011, 44, 1450–1459. [Google Scholar] [CrossRef]
- Chen, J.; Fu, Y.; An, Q.; Lo, S.; Huang, S.; Hung, W.; Hu, C.; Lee, K.; Laia, J. Tuning nanostructure of graphene oxide/polyelectrolyte LbL assemblies by controlling pH of GO suspension to fabricate transparent and super gas barrier films. Nanoscale 2013, 5, 9081–9088. [Google Scholar] [CrossRef]
- Nohria, R.; Khillan, R.K.; Su, Y.; Dikshit, R.; Lvov, Y.; Varahramyan, K. Humidity sensor based on ultrathin polyaniline film deposited using layer-by-layer nano-assembly. Sensor Actuat. B Chem. 2006, 114, 218–222. [Google Scholar] [CrossRef]
- Fernandesa, E.G.R.; Brazacaa, L.C.; Rodríguez-Mendez, M.L.; Saja, J.A.; Zucolotto, V. Immobilization of lutetium bisphthalocyanine in nanostructured biomimetic sensors using the LbL technique for phenol detection. Biosens Bioelectron. 2011, 26, 4715–4719. [Google Scholar] [CrossRef]
- Ferreira, M.; Fiorito, P.A.; Oliveira, O.N., Jr.; Torresi, S.I.C. Enzyme-mediated amperometric biosensors prepared with the Layer-by-Layer (LbL) adsorption technique. Biosens. Bioelectron. 2004, 19, 1611–1615. [Google Scholar] [CrossRef]
- Kim, B.; Park, S.W.; Hammond, P.T. Hydrogen-Bonding Layer-by-Layer-Assembled Biodegradable Polymeric Micelles as Drug Delivery Vehicles from Surfaces. ACS Nano 2008, 2, 386–392. [Google Scholar] [CrossRef]
- Delcea, M.; Möhwald, H.; Skirtach, A.G. Stimuli-responsive LbL capsules and nanoshells for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 730–747. [Google Scholar] [CrossRef]
- Mercato, L.L.; Ferraro, M.M.; Baldassarre, F.; Mancarella, S.; Greco, V.; Rinaldi, R.; Leporatti, S. Biological applications of LbL multilayer capsules: From drug delivery to sensing. Adv. Colloid Interface Sci. 2014, 207, 139–154. [Google Scholar] [CrossRef]
- Carosio, F.; Laufer, G.; Alongi, J.; Camino, G.; Grunlan, J.C. Layer-by-layer assembly of silica-based flame retardant thin film on PET fabric. Polym. Degrad. Stabil. 2011, 96, 745–750. [Google Scholar] [CrossRef]
- Apaydin, K.; Laachachi, A.; Ball, V.; Jimenez, M.; Bourbigot, S.; Toniazzo, V.; Ruch, D. Polyallylamine–montmorillonite as super flame retardant coating assemblies by layer-by layer deposition on polyamide. Polym. Degrad. Stabil. 2013, 98, 627–634. [Google Scholar] [CrossRef]
- Choi, K.; Seo, S.; Kwon, H.; Kim, D.; Park, Y.T. Fire protection behavior of layer-by-layer assembled starch–clay multilayers on cotton fabric. J. Mater. Sci. 2018, 53, 11433–11443. [Google Scholar] [CrossRef]
- Jang, W.; Chung, I.J.; Kim, J.; Seo, S.; Park, Y.T.; Choi, K. Improving fire resistance of cotton fabric through Layer-by-Layer assembled graphene multilayer nanocoating. J. Korean Phys. Soc. 2018, 72, 1052–1057. [Google Scholar] [CrossRef]
- Zhang, A.; Zhao, H.; Cheng, J.; Li, M.; Li, S.; Cao, M.; Wang, Y. Construction of durable eco-friendly biomass-based flame-retardant coating for cotton fabrics. Chem. Eng. J. 2021, 410, 128361. [Google Scholar] [CrossRef]
- Fang, Y.; Sun, W.; Li, J.; Liu, H.; Liu, X. Eco-friendly flame retardant and dripping-resistant of polyester/cotton blend fabrics through layer-by-layer assembly fully bio-based chitosan/phytic acid coating. Int. J. Biol. Macromol. 2021, 175, 140–146. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, C.; Li, P.; Wang, B.; Xu, Y.; Jiang, Z.; Liu, Y.; Zhu, P. A flame-retardant PET fabric coating: Flammability, anti-dripping properties, and flame-retardant mechanism. Prog. Org. Coat. 2021, 150, 105971. [Google Scholar] [CrossRef]
- Yu, Z.; Mao, M.; Li, S.; Xia, Q.; Cao, C.; Zhao, L.; Zhang, G.; Zheng, Z.; Gao, J.; Tang, L. Facile and green synthesis of mechanically flexible and flame-retardant clay/graphene oxide nanoribbon interconnected networks for fire safety and prevention. Chem. Eng. J. 2021, 405, 126620. [Google Scholar] [CrossRef]
- Rahman, F.; Langford, K.H.; Scrimshaw, M.D.; Lester, J.N. Polybrominated diphenyl ether (PBDE) flame retardants. Sci. Total Environ. 2011, 275, 1–17. [Google Scholar] [CrossRef]
- Meng, X.Y.; Ye, L.; Zhang, X.G.; Tang, P.M.; Tang, J.H.; Ji, X.; Li, Z.M. Effects of expandable graphite and ammonium polyphosphate on the flame-retardant and mechanical properties of rigid polyurethane foams. J. Appl. Polym. Sci. 2009, 114, 853–863. [Google Scholar] [CrossRef]
- Harikrishnan, G.; Singh, S.N.; Kiesel, E.; Macosko, C.W. Nanodispersions of carbon nanofiber for polyurethane foaming. Polymer 2010, 51, 3349–3353. [Google Scholar] [CrossRef]
- Chen, H.-H.; Thirumavalavan, M.; Lin, F.-Y.; Lee, J.-F. A facile approach for achieving an effective dual sorption ability of Si/SH/S grafted sodium montmorillonite. RSC Adv. 2015, 5, 57792–57803. [Google Scholar] [CrossRef]
- Chen, X.; Huo, L.; Jiao, C.; Li, S. TG–FTIR characterization of volatile compounds from flame retardant polyurethane foams materials. J. Anal. Appl. Pyrol. 2013, 100, 186–191. [Google Scholar] [CrossRef]
- Trovati, G.; Sanches, E.A.; Neto, S.C.; Mascarenhas, Y.P.; Chierice, G.O. Characterization of polyurethane resins by FTIR, TGA, and XRD. J. Appl. Polym. Sci. 2010, 115, 263–268. [Google Scholar] [CrossRef]
- König, A.; Kroke, E. Methyl-DOPO—A new flame retardant for flexible polyurethane foam. Polym. Advan. Technol. 2011, 22, 5–13. [Google Scholar] [CrossRef]

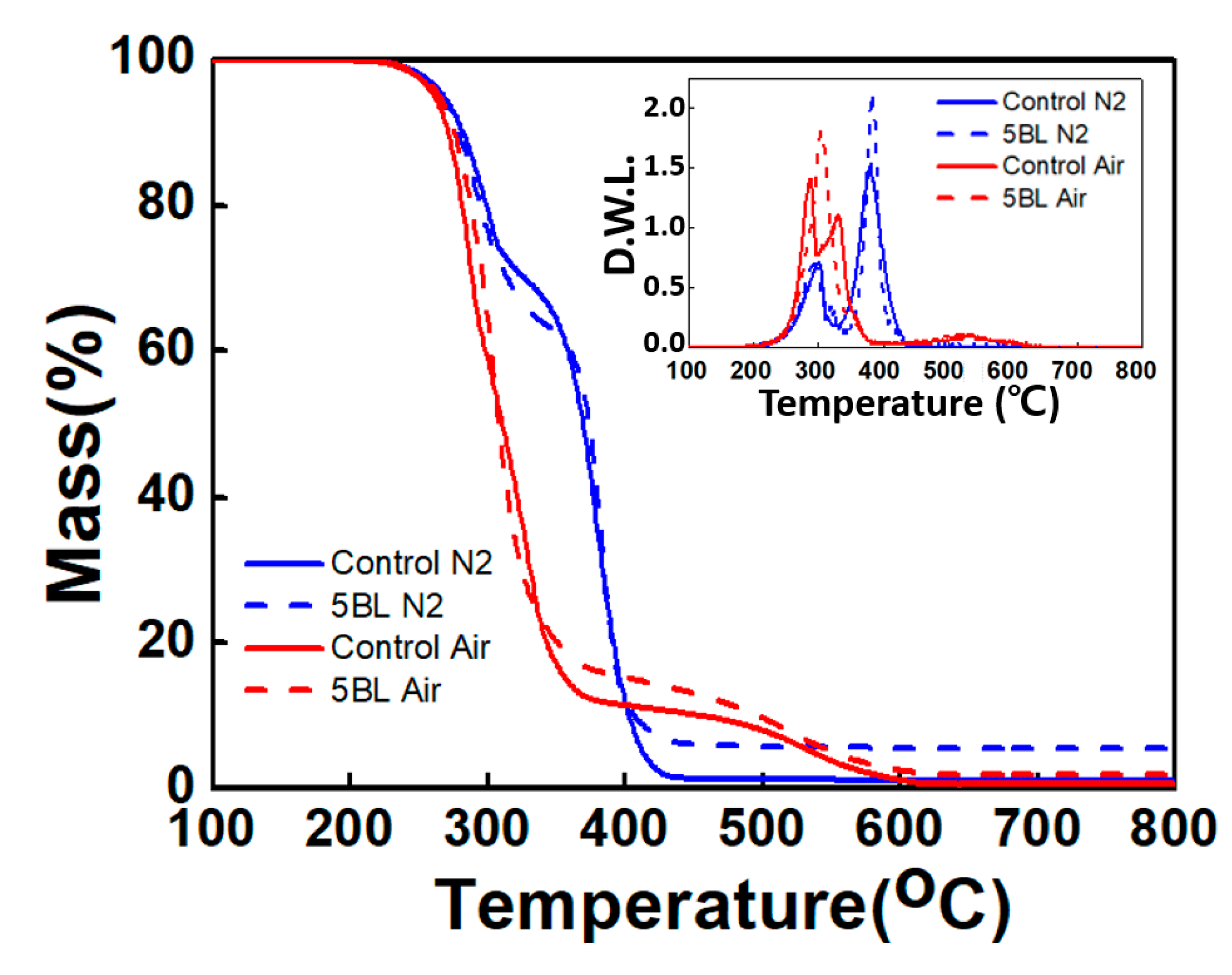


| Sample | T−2% (°C) | T−10% (°C) | Tmax1 (°C) | Tmax2 (°C) | Residue at 800 °C (%) |
|---|---|---|---|---|---|
| Under N2 | |||||
| No coating | 250 | 290 | 300 | 372 | 1.2 |
| 5 BL coating | 250 | 288 | 298 | 380 | 5.6 |
| Under air | |||||
| No coating | 245 | 275 | 291 | 332 | 0.7 |
| 5 BL coating | 248 | 282 | 302 | - | 2.0 |
| Sample | phHRR (kW/m2) | MARHE (kW/m2) | THR (MJ/m2) |
|---|---|---|---|
| Control | 190.2 | 138.5 | 42.1 |
| 5 BL-Before | 147.0 | 97.2 | 19.9 |
| 5 BL-After | 195.3 | 102.5 | 17.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, K.-W.; Kim, J.-W.; Kwon, T.-S.; Kang, S.-W.; Song, J.-I.; Park, Y.-T. Mechanically Sustainable Starch-Based Flame-Retardant Coatings on Polyurethane Foams. Polymers 2021, 13, 1286. https://doi.org/10.3390/polym13081286
Choi K-W, Kim J-W, Kwon T-S, Kang S-W, Song J-I, Park Y-T. Mechanically Sustainable Starch-Based Flame-Retardant Coatings on Polyurethane Foams. Polymers. 2021; 13(8):1286. https://doi.org/10.3390/polym13081286
Chicago/Turabian StyleChoi, Kyung-Who, Jun-Woo Kim, Tae-Soon Kwon, Seok-Won Kang, Jung-Il Song, and Yong-Tae Park. 2021. "Mechanically Sustainable Starch-Based Flame-Retardant Coatings on Polyurethane Foams" Polymers 13, no. 8: 1286. https://doi.org/10.3390/polym13081286