Influence of Ultraviolet Radiation on Mechanical Properties of a Photoinitiator Compounded High Vinyl Styrene–Butadiene–Styrene Block Copolymer
Abstract
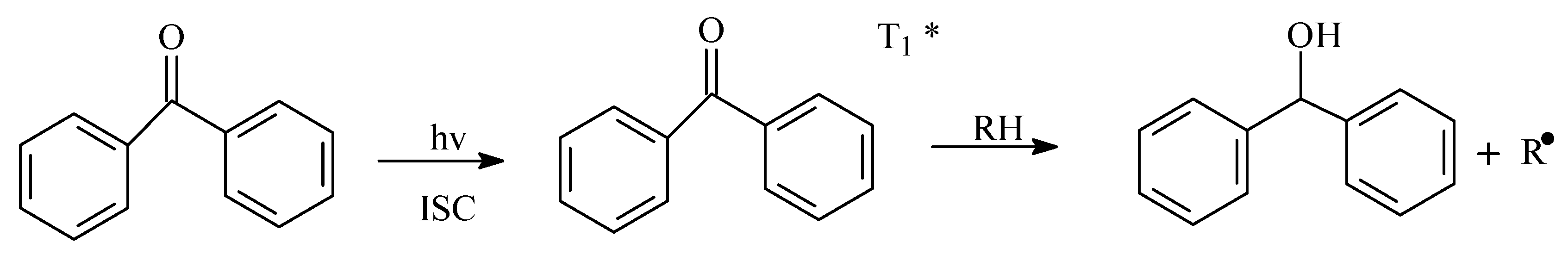
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Batches
2.3. Testing Programs
2.3.1. Mechanical Characterization
2.3.2. Spectroscopic Characterization
2.3.3. Calculation of Surface Energy by Contact Angle Method
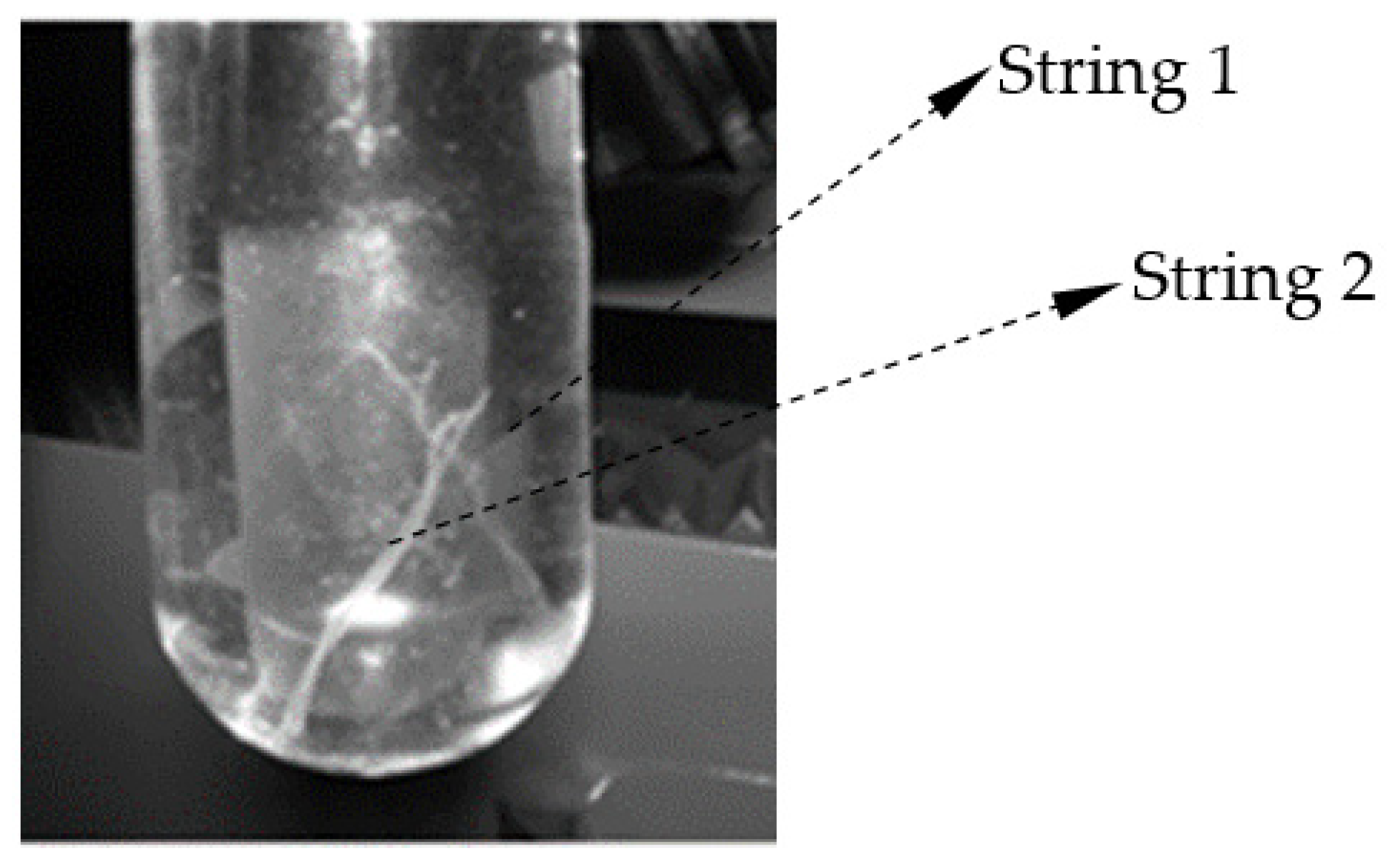
2.3.4. Morphological Studies in Raame Hart Camera
2.3.5. Scanning Electron Microscopic Studies
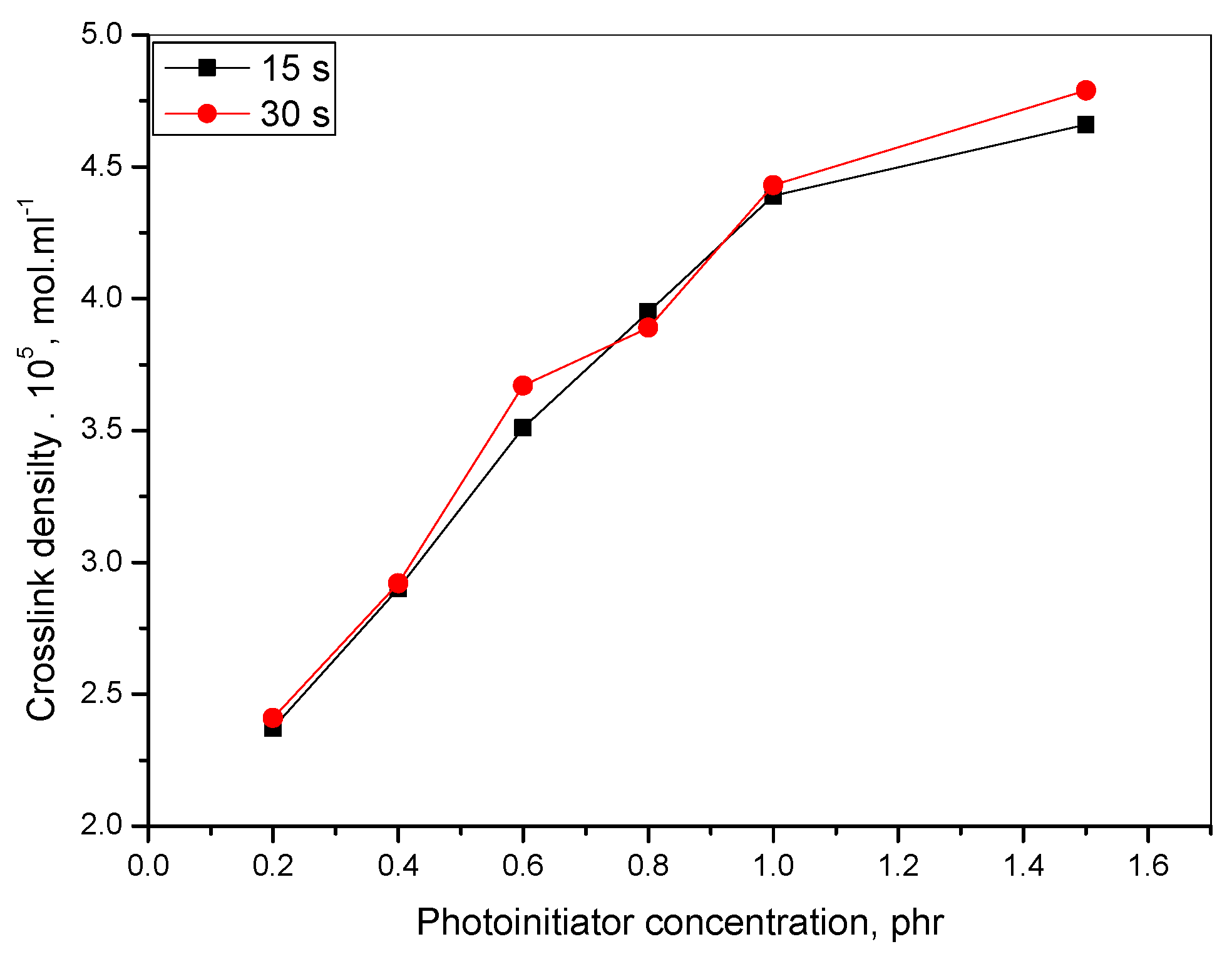
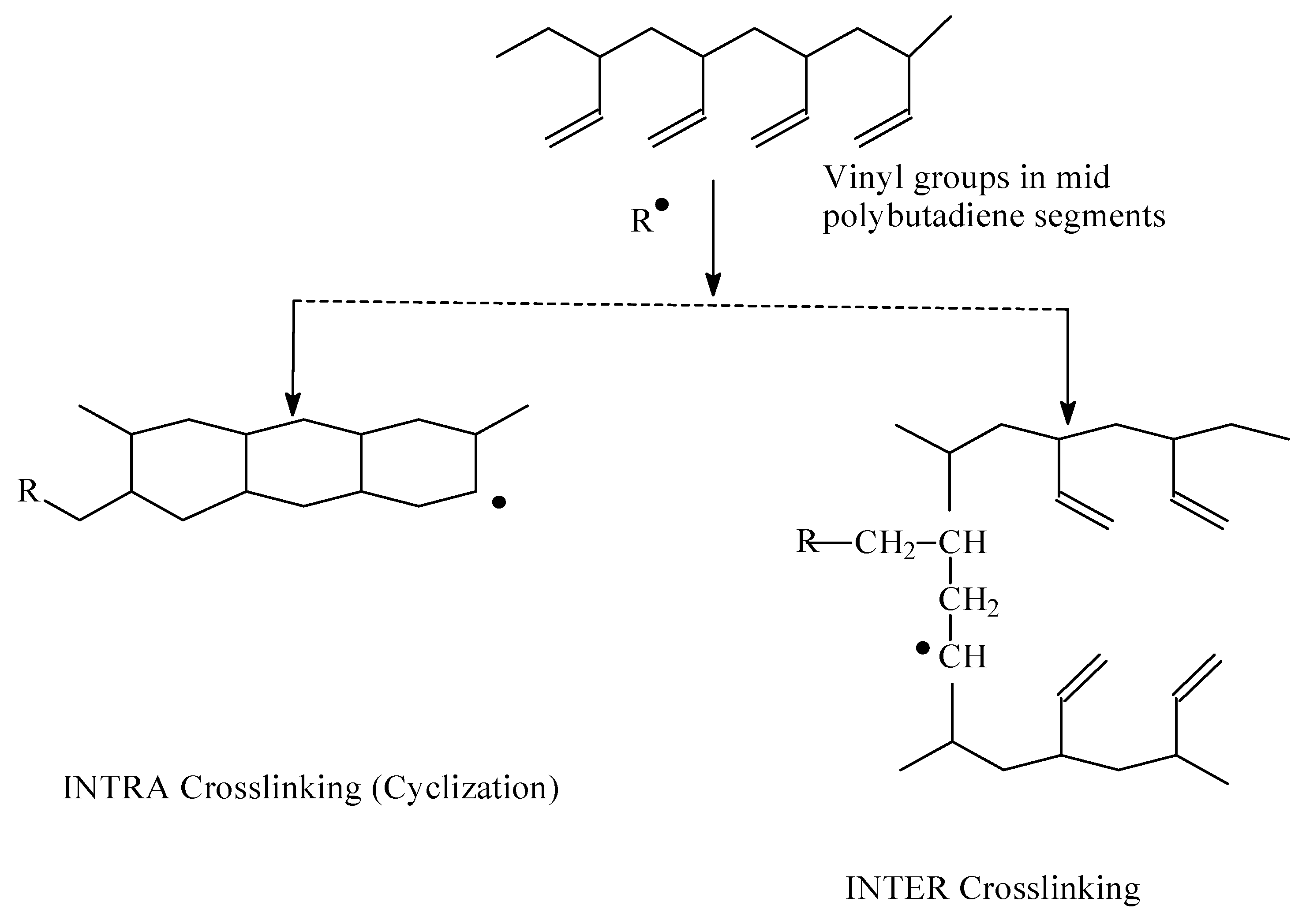
2.3.6. Crosslink Density Calculation
- ν → = number of moles of effectively elastic chains per unit volume of the polymer [mol/mL] (Overall Crosslink Density),
- Vs → = molar volume of the solvent (here cyclohexane) used [cm3/mol],
- χ → = polymer-swelling agent interaction parameter (here, 0.3) (Barton 1985) or Flory–Huggin’s parameter,
- Vr → = volume fraction of the polymer in the swollen network, expressed as Vr = 1/(Ar + 1),
- Ar → = is the ratio of the volume of absorbed solvent (cyclohexane) to that of the polymer after swelling (Flory and Rehner 1943; Naskar 2004).
3. Results and Discussion
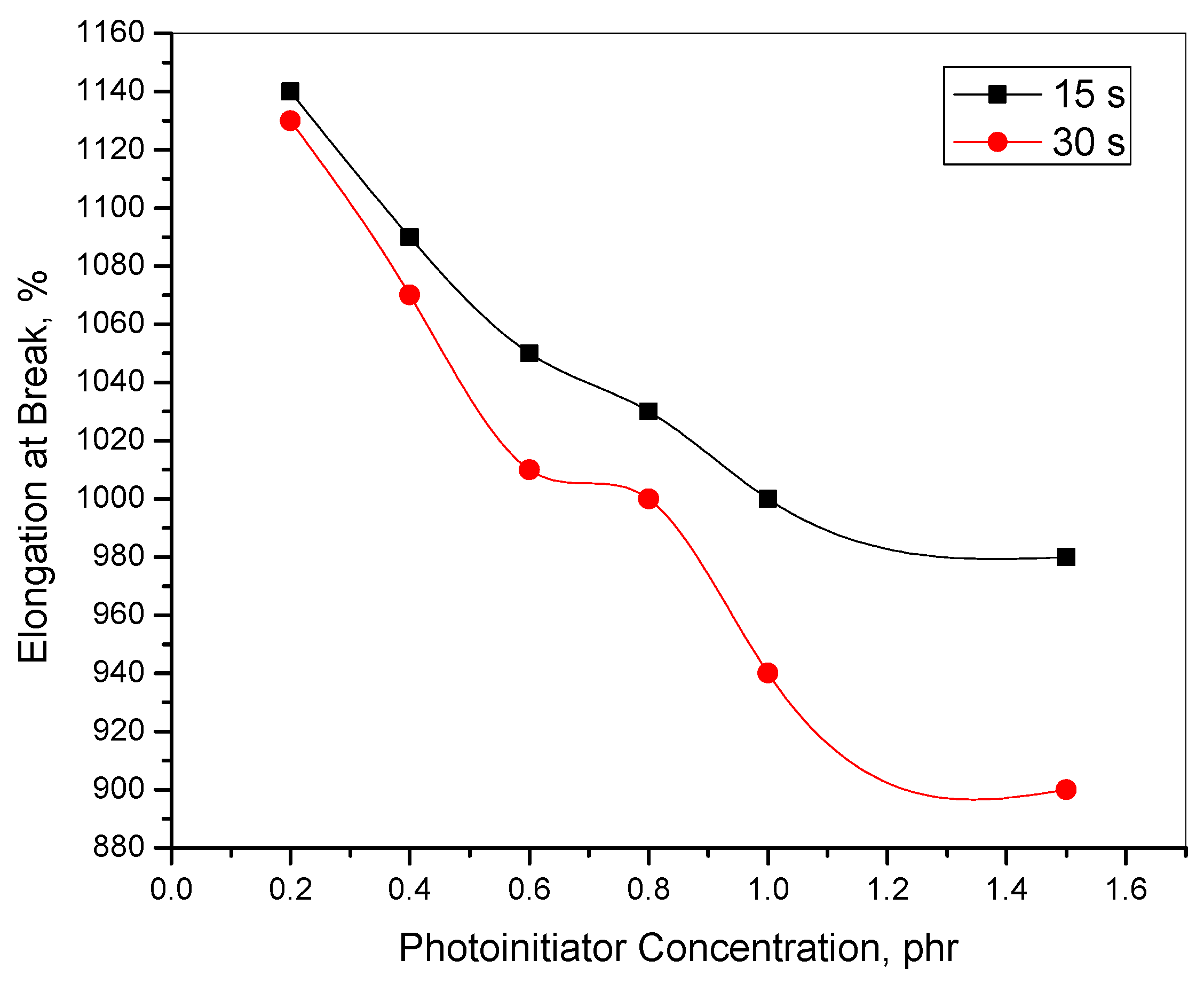
3.1. Mechanical
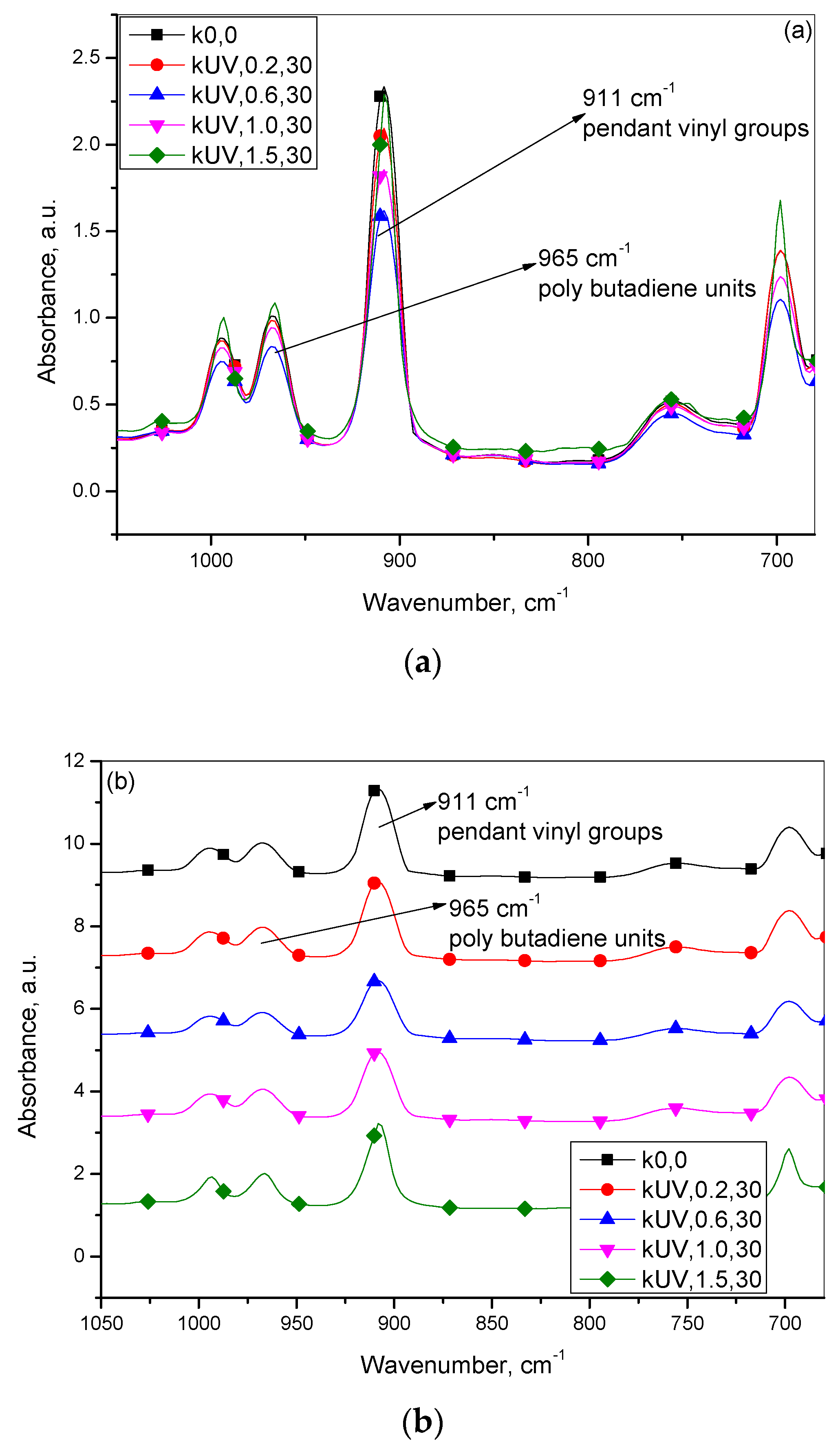
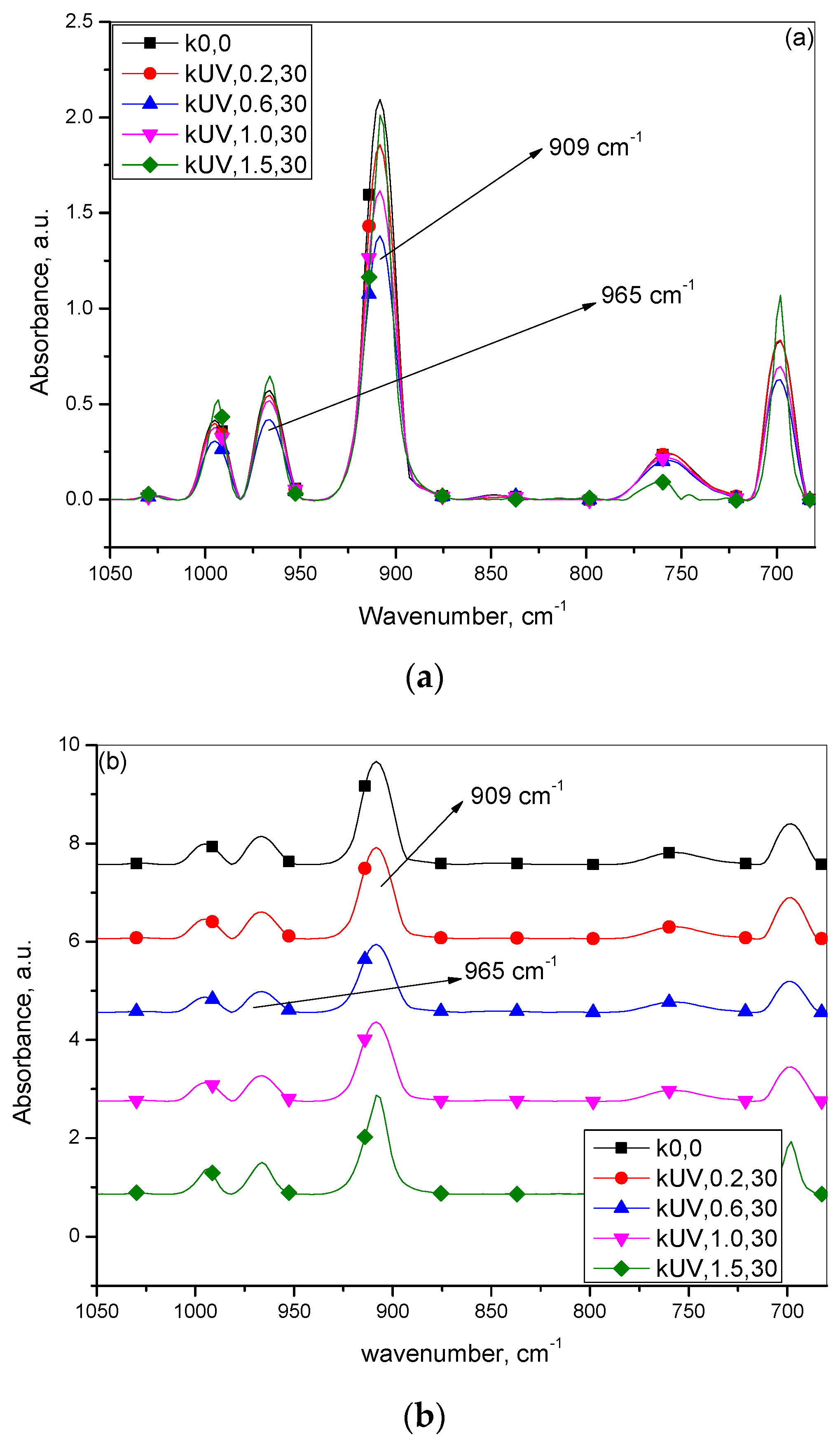
3.2. Infrared Spectroscopic Studies
3.3. Surface Phenomenon through Contact Angle Studies
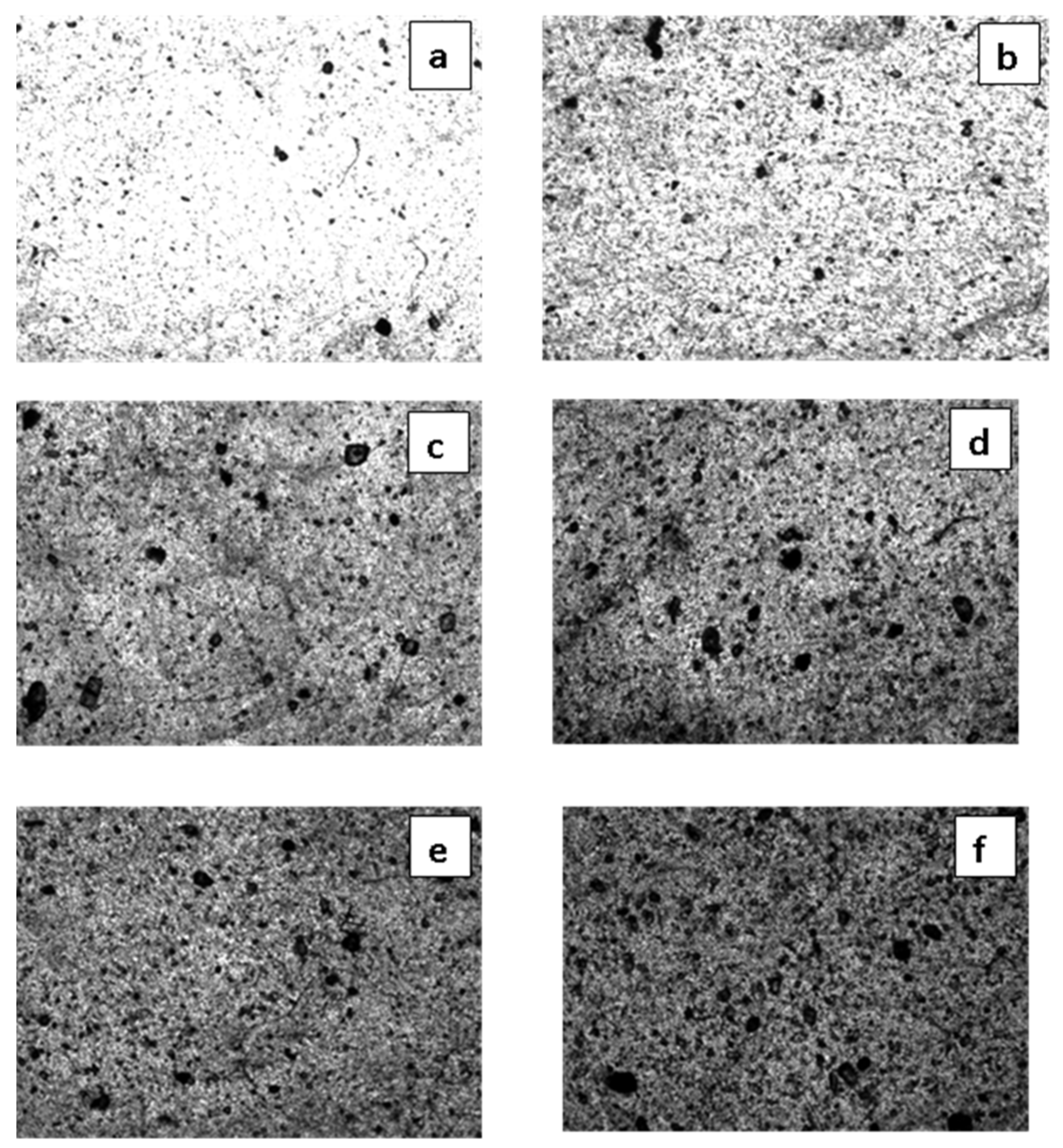
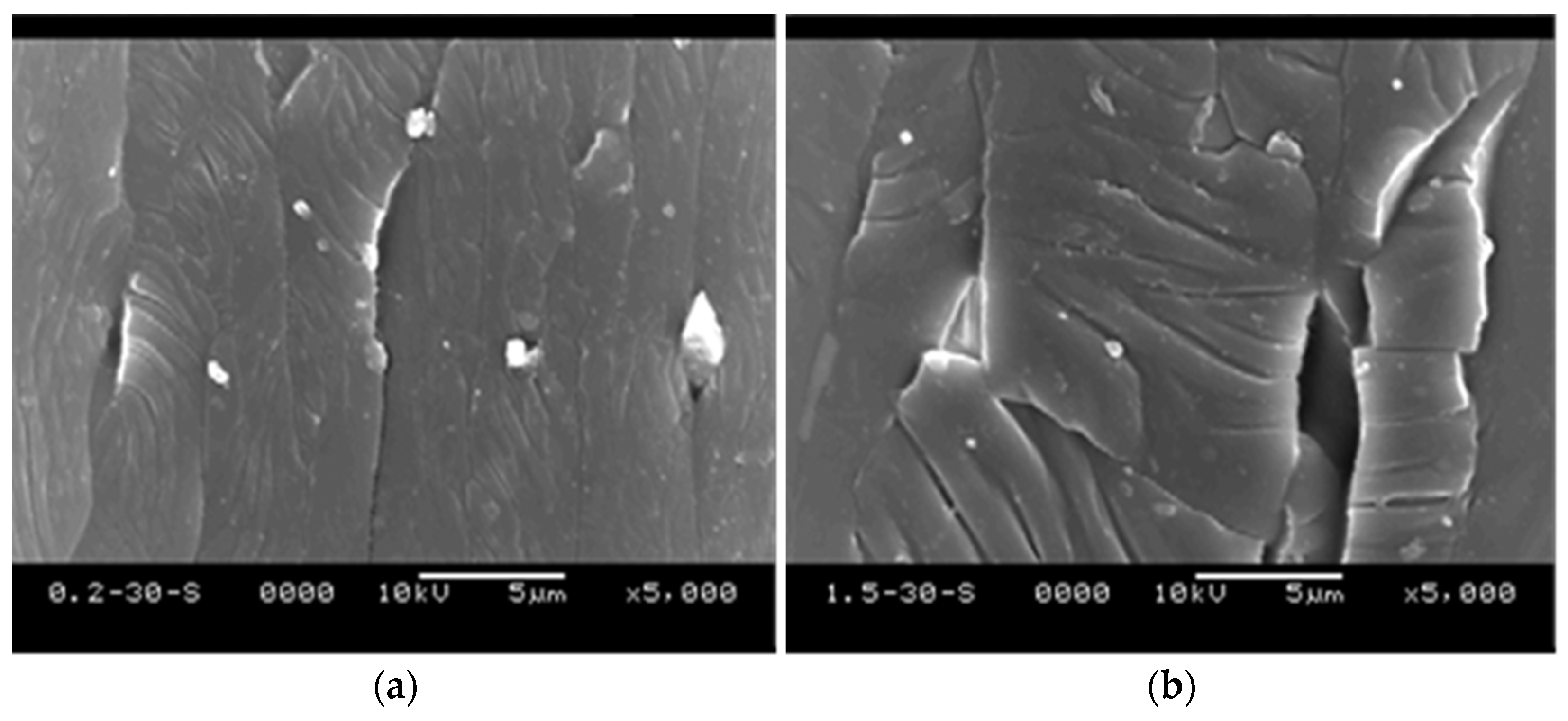
3.4. SEM Studies on the Surfaces
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Donnell, J. Radiation Curing of Polymeric Materials. In ACS Symposium Series; Hoyle, C.E., Kinstle, J.F., Eds.; American Chemical Society: Washington, DC, USA, 1990; no. 417; p. xiv + 567. [Google Scholar]
- Decker, C. Photoinitiated crosslinking polymerisation. Prog. Polym. Sci. 1996, 21, 593–650. [Google Scholar] [CrossRef]
- Scranton, A.B.; Bowman, C.N.; Peiffer, R.W. Photopolymerization: Fundamentals and Applications. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1997; no. 673. [Google Scholar]
- Green, G.E.; Stark, B.P.; Zahir, S.A. Photocross-linkable resin systems. J. Macromol. Sci. Rev. Macromol. Chem. 1982, C21, 187–273. [Google Scholar] [CrossRef]
- Reiser, A.; Egerton, P.L. Mechanism of crosslink formation in solid polyvinylcinnamate and related photopolymers. Photogr. Sci. Eng. 1979, 23, 144–150. [Google Scholar]
- Puskas, J.E.; Kaszas, G.; Kennedy, J.P. New transparent flexibleUV-cured films from polyisobutylene-polyisoprene block polymers. J. Macromol. Sci. Chem. 1991, A28, 65–80. [Google Scholar] [CrossRef]
- Crivello, J.V.; Yang, B. Synthesis and photoinitiated cationic polymerization of epoxidized elastomers. J. Macromol. Sci. Chem. 1994, A31, 517–533. [Google Scholar] [CrossRef]
- Xuan, H.L.; Decker, C. Photo-cross-linking of acryleted natural-rubber. J. Polym. Sci. Pol. Chem. 1993, 31, 769–780. [Google Scholar] [CrossRef]
- Decker, C.; LeXuan, H.; Viet, T.N.T. Photo-cross-linking of functionalized rubber. 2. Photoinitiated cationic polymerization of epoxidized liquid natural-rubber. J. Polym. Sci. Pol. Chem. 1995, 33, 2759–2772. [Google Scholar] [CrossRef]
- Decker, C.; LeXuan, H.; Viet, T.N.T. Photo-cross-linking of functionalized rubber. 3. Polymerization of multifunctional monomers in epoxidized liquid natural rubber. J. Polym. Sci. Pol. Chem. 1996, 34, 1771–1781. [Google Scholar] [CrossRef]
- Decker, C.; Viet, T.N.T.; LeXuan, H. Photo-cross-linking of functionalized rubber. 6. Cationic polymerization of epoxidized rubber. Eur. Polym. J. 1996, 32, 1319–1331. [Google Scholar] [CrossRef]
- Decker, C.; Viet, T.N.T.; LeXuan, H. Photocuring of functionalized rubbers. 4. Synthesis of rubbers with acrylate groups. Eur. Polym. J. 1996, 32, 549–557. [Google Scholar] [CrossRef]
- Decker, C.; Viet, T.N.T.; LeXuan, H. Photocuring of functionalized rubbers. 5. Radical polymerization of rubbers with acrylate groups. Eur. Polym. J. 1996, 32, 559–567. [Google Scholar] [CrossRef]
- Brochure of Kraton: Kraton Fact Sheet; K0406; Kraton Polymers US LCC: Houston, TX, USA, 2006.
- Decker, C.; Viet, T.N.T. Photocrosslinking of functionalized rubbers IX. Thiol-ene polymerization of styrene-butadiene-block-copolymers. Polymer 2000, 41, 3905–3912. [Google Scholar] [CrossRef]
- Decker, C.; Viet, T.N.T. High-speed photocrosslinking of thermoplastic styrene-butadiene elastomers. J. Appl. Polym. Sci. 2000, 77, 1902–1912. [Google Scholar] [CrossRef]
- Decker, C.; Viet, T.N.T. Photocrosslinking of functionalized rubbers, 8 The thiol-polybutadiene system. Macromol. Chem. Phys. 1999, 200, 1965–1974. [Google Scholar] [CrossRef]
- Datta, S.; Babu, R.R.; Bhardwaj, Y.K.; Sabharwal, S.; Naskar, K. Optimization of Mechanical properties of Photocrosslinked of Styrene Butadiene Styrene Block Copolymers using Statistical Experimental Design. Tpe Mag. 2011, 4, 232–241. [Google Scholar]
- Mayenez, C.; Muyldermans, X. Flexographic Printing Plates from Photocurable Elastomer Compositions. EP 0 696 761 B1 8 April 1998. [Google Scholar]
- Datta, S.; Antos, J.; Stocek, R. Smart numerical method for calculation of simple general infrared parameter identifying binary rubber blends. Polym. Test. 2017, 57, 192–202. [Google Scholar] [CrossRef]
- Datta, S.; Antos, J.; Stocek, R. Characterisation of ground tyre rubber by using combination of FT-IR numerical parameter and DTG analysis to determine the composition of ternary rubber blend. Polym. Test. 2017, 59, 208–217. [Google Scholar] [CrossRef]
- Datta, S.; Harea, D.; Harea, E.; Stocek, R. An advanced method for calculation of infrared parameter to quantitatively identify rubber grade in a multi-component rubber blend. Polym. Test. 2019, 73, 208–217. [Google Scholar] [CrossRef]
- Datta, S.; Harea, E.; Stocek, R.; Kratina, O.; Stenicka, M. Configuration of Novel Fractographic Reverse Engineering Approach Based on relationship between Spectroscopy of Ruptured Surface and Fracture Behaviour of Rubber Sample. Materials 2020, 13, 4445. [Google Scholar] [CrossRef]
- Litminov, V.M.; Dey, P.P. Spectroscopy of Rubbers and Rubbery Materials; Rapra Technology: Shawbury, UK, 2002. [Google Scholar]
- Spelt, J.K.; Neumann, A.W. Solid-surface tension—The equation of state approach and the theory of surface-tension components—theoretical and conceptual considerations. Langmuir 1987, 3, 588–591. [Google Scholar] [CrossRef]
- Hefer, A.W.; Basin, A.; Little, D.N. Bitumen Surface Energy Characterization using a Contact Angle Approach. J. Mat. Civ. Eng. 2006, 18, 757–767. [Google Scholar] [CrossRef]
- Tang, S.; Kwon, O.J.; Choi, H.S. Surface characteristics of stainless steel after an AISI 304L atmospheric pressure plasma treatment. Surf. Coat. Technol. 2005, 195, 298–306. [Google Scholar] [CrossRef]
- Deng, J.P.; Yang, W.T.; Ranby, B. Surface photografting polymerization of vinyl acetate (VAc), maleic anhydride, and their charge transfer complex. I. VAc(1). J. Appl. Polym. Sci. 2000, 77, 1513–1521. [Google Scholar] [CrossRef]
- Decker, C. Kinetic-study of light-induced polymerization by real-time UV and IR spectroscopy. J. Polym. Sci. Pol. Chem. 1992, 30, 913–928. [Google Scholar] [CrossRef]
- Basfar, A.A.; Mosnacek, J.; Shukri, T.M.; Bahattab, M.A.; Noireaux, P.; Courdreuse, A. Mechanical and thermal properties of blends of low-density polyethylene and ethylene vinyl acetate crosslinked by both dicumyl peroxide and ionizing radiation for wire and cable applications. J. Appl. Polym. Sci. 2008, 107, 642–649. [Google Scholar] [CrossRef]
- Lu, H.; Wei, M.H. On the origin of the Vogel-Fulcher-Tammann law in the thermo-responsive shape memory effect of amorphous polymers. Smart Mater. Struct. 2013, 22, 105021. [Google Scholar] [CrossRef]
- Kaczmarek, H. Changes to polymer morphology caused by UV irradiation. 1. Surface damage. Polymer 1996, 37, 189–194. [Google Scholar] [CrossRef]





| Sample Designation | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Components, phr * | k0,0 | kUV,0.2,15 | kUV,0.4,15 | kUV,0.6,15 | kUV,0.8,15 | kUV,1.0,15 | kUV,1.5,15 | kUV,0.2,30 | kUV,0.4,30 | kUV,0.6,30 | kUV,0.8,30 | kUV,1.0,30 | kUV,1.5,30 |
| SBS block copolymer | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Photoinitiator | 0 | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 | 1.5 | 0 | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 |
| Serial Number | Liquid | Reference | ||
|---|---|---|---|---|
| 1 | Formamide | 39.5 | 18.7 | 26 Hefter (06) |
| 2 | Diiodomethane | 48.5 | 2.3 | 27 Tang (2005) |
| 3 | Water | 21.8 | 51.0 | 27 Tang (2005) |
| Components | k0,0 | kUV,0.2,15 | kUV,0.4,15 | kUV,0.6,15 | kUV,0.8,15 | kUV,1.0,15 | kUV,1.5,15 | kUV,0.2,30 | kUV,0.4,30 | kUV,0.6,30 | kUV,0.8,30 | kUV,1.0,30 | kUV,1.5,30 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S-B-S | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Photoinitiator | 0 | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 | 1.5 | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 | 1.5 |
| Mechanical Properties | |||||||||||||
| Hardness, Shore A | 41 | 50 | 50 | 51 | 51 | 51 | 51 | 50 | 51 | 51 | 52 | 52 | 53 |
| T.S. *, MPa | 5.3 | 7.3 | 7.0 | 6.5 | 6.1 | 5.6 | 5.0 | 7.2 | 6.3 | 6.1 | 5.8 | 5.4 | 4.8 |
| M # 100, MPa | 0.7 | 0.9 | 1.0 | 1.2 | 1.3 | 1.3 | 1.0 | 0.7 | 0.8 | 1.0 | 1.2 | 1.3 | 0.9 |
| M200, MPa | 0.9 | 1.2 | 1.3 | 1.4 | 1.6 | 1.7 | 1.3 | 1.0 | 1.2 | 1.4 | 1.5 | 1.7 | 1.3 |
| M300, MPa | 1.2 | 1.6 | 1.7 | 1.8 | 2.2 | 2.2 | 1.7 | 1.3 | 1.6 | 1.8 | 2.2 | 2.2 | 1.7 |
| E.B. $, % | 1200 | 1140 | 1090 | 1050 | 1030 | 1000 | 980 | 1130 | 1070 | 1010 | 1000 | 940 | 900 |
| XLD &, mol∙mL−1∙105 | | 2.37 | 2.90 | 3.51 | 3.95 | 4.39 | 4.86 | 2.41 | 2.92 | 3.67 | 3.89 | 4.43 | 4.87 |
| Infrared Absorbance Peak Heights | |||
|---|---|---|---|
| Sample Designation | Vinyl, 909 cm−1 | Polubutadiene, 965 cm−1 | Vinyl/Polybutadiene |
| k0,0 | 2.0944 | 0.5703 | 3.6728 |
| kUV,0.2,30 | 1.8572 | 0.5451 | 3.4074 |
| kUV,0.6,30 | 1.3804 | 0.4197 | 3.2889 |
| kUV,1.0,30 | 1.6156 | 0.5178 | 3.1202 |
| kUV,1.5,30 | 2.0113 | 0.6456 | 3.1155 |
| Sample Designation | Water and Formamide | Formamide and Diiodomethane | Water and Diiodomethane |
|---|---|---|---|
| kUV,0.2,15 | 41.43 | 41.91 | 42.06 |
| kUV,0.4,15 | 40.68 | 40.38 | 40.29 |
| kUV,0.6,15 | 39.96 | 39.12 | 38.89 |
| kUV,0.8,15 | 37.36 | 37.27 | 37.26 |
| kUV,1.0,15 | 36.59 | 35.65 | 35.45 |
| kUV,1.5,15 | 35.05 | 34.65 | 34.55 |
| kUV,0.2,30 | 43.56 | 41.34 | 40.68 |
| kUV,0.4,30 | 40.32 | 40.06 | 39.99 |
| kUV,0.6,30 | 39.60 | 38.49 | 38.20 |
| kUV0.8,30 | 35.89 | 36.64 | 36.82 |
| kUV,1.0,30 | 36.59 | 35.29 | 35.00 |
| kUV,1.5,30 | 35.05 | 34.30 | 34.11 |
| Sample Designation | |||
|---|---|---|---|
| kUV,0.2,15 | 42.06 | 33.83 | 8.23 |
| kUV,0.4,15 | 40.29 | 32.64 | 7.65 |
| kUV,0.6,15 | 38.89 | 31.95 | 6.94 |
| kUV,0.8,15 | 37.26 | 31.50 | 5.76 |
| kUV,1.0,15 | 35.45 | 30.14 | 5.31 |
| kUV,1.5,15 | 34.55 | 28.89 | 5.66 |
| kUV,0.2,30 | 40.68 | 33.20 | 7.48 |
| kUV,0.4,30 | 39.99 | 32.88 | 7.11 |
| kUV,0.6,30 | 38.20 | 31.60 | 6.60 |
| kUV0.8,30 | 36.82 | 30.89 | 5.93 |
| kUV,1.0,30 | 35.00 | 29.51 | 5.49 |
| kUV,1.5,30 | 34.11 | 28.26 | 5.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Datta, S.; Stocek, R.; Naskar, K. Influence of Ultraviolet Radiation on Mechanical Properties of a Photoinitiator Compounded High Vinyl Styrene–Butadiene–Styrene Block Copolymer. Polymers 2021, 13, 1287. https://doi.org/10.3390/polym13081287
Datta S, Stocek R, Naskar K. Influence of Ultraviolet Radiation on Mechanical Properties of a Photoinitiator Compounded High Vinyl Styrene–Butadiene–Styrene Block Copolymer. Polymers. 2021; 13(8):1287. https://doi.org/10.3390/polym13081287
Chicago/Turabian StyleDatta, Sanjoy, Radek Stocek, and Kinsuk Naskar. 2021. "Influence of Ultraviolet Radiation on Mechanical Properties of a Photoinitiator Compounded High Vinyl Styrene–Butadiene–Styrene Block Copolymer" Polymers 13, no. 8: 1287. https://doi.org/10.3390/polym13081287
APA StyleDatta, S., Stocek, R., & Naskar, K. (2021). Influence of Ultraviolet Radiation on Mechanical Properties of a Photoinitiator Compounded High Vinyl Styrene–Butadiene–Styrene Block Copolymer. Polymers, 13(8), 1287. https://doi.org/10.3390/polym13081287






