Colorless and Transparent Polyimide Microporous Film with Excellent Physicochemical Property
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Synthesis of Microporous CPI Film
2.4. Characterization
3. Results and Discussion
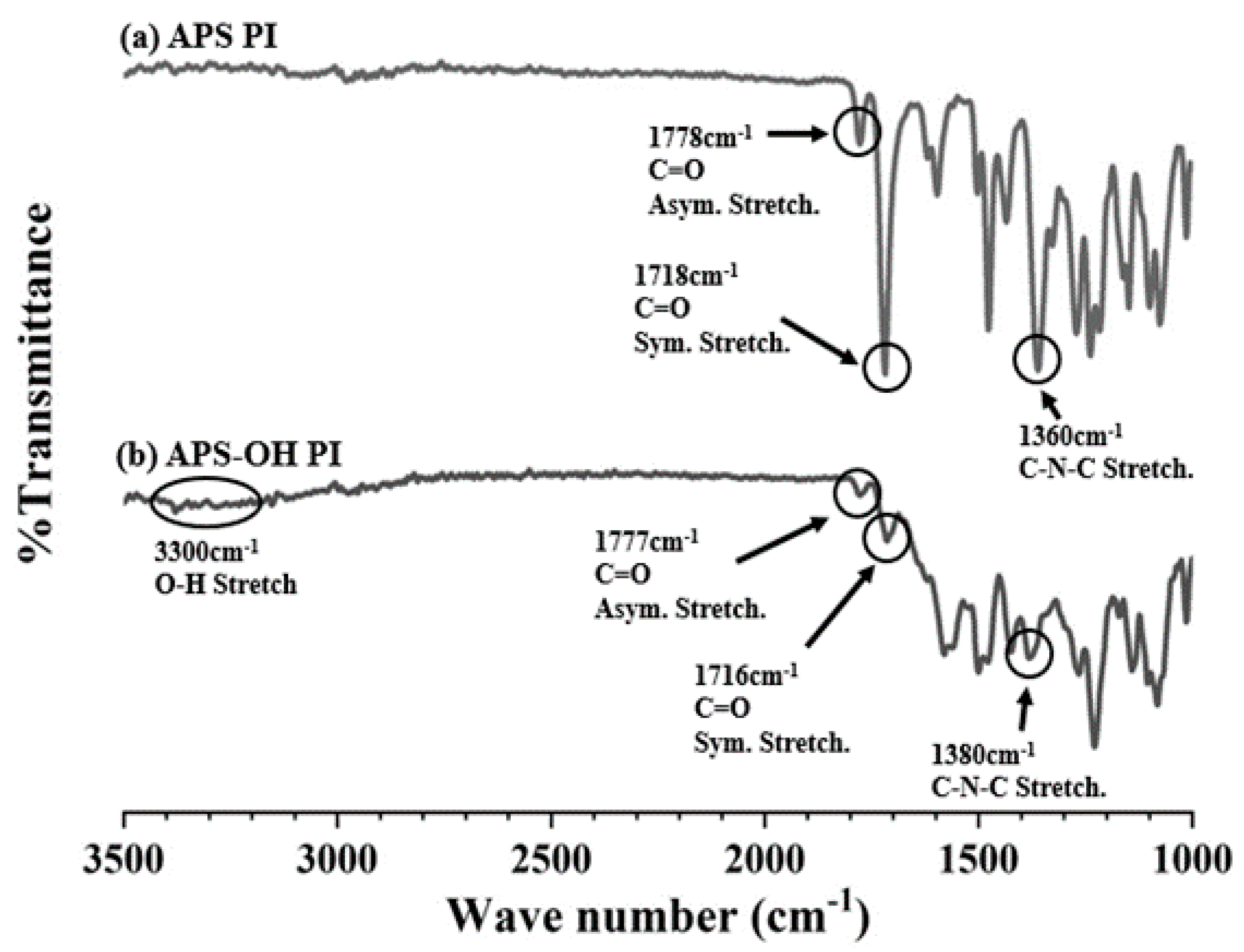
3.1. FT-IR
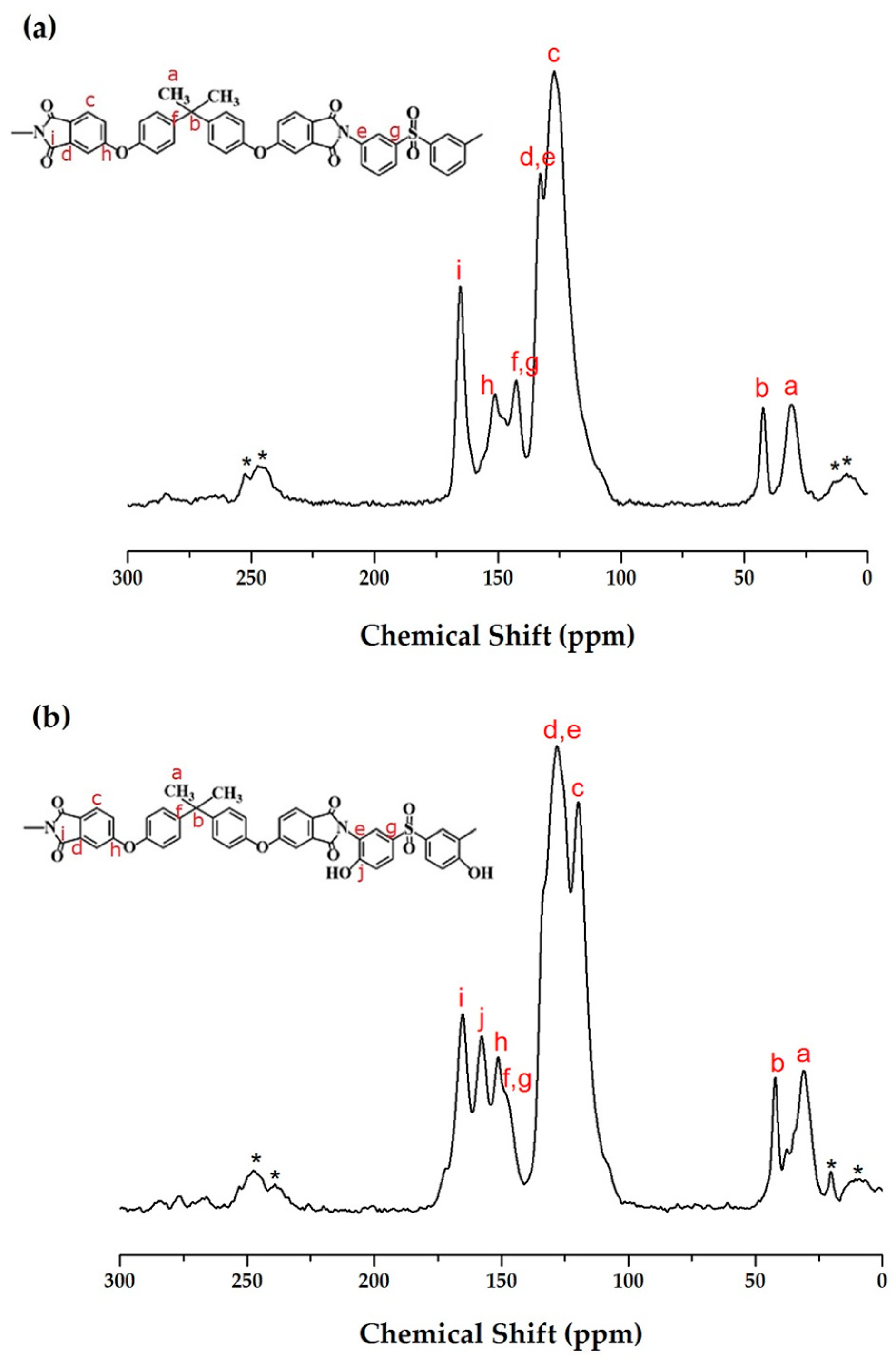
3.2. Solid State C-13 NMR
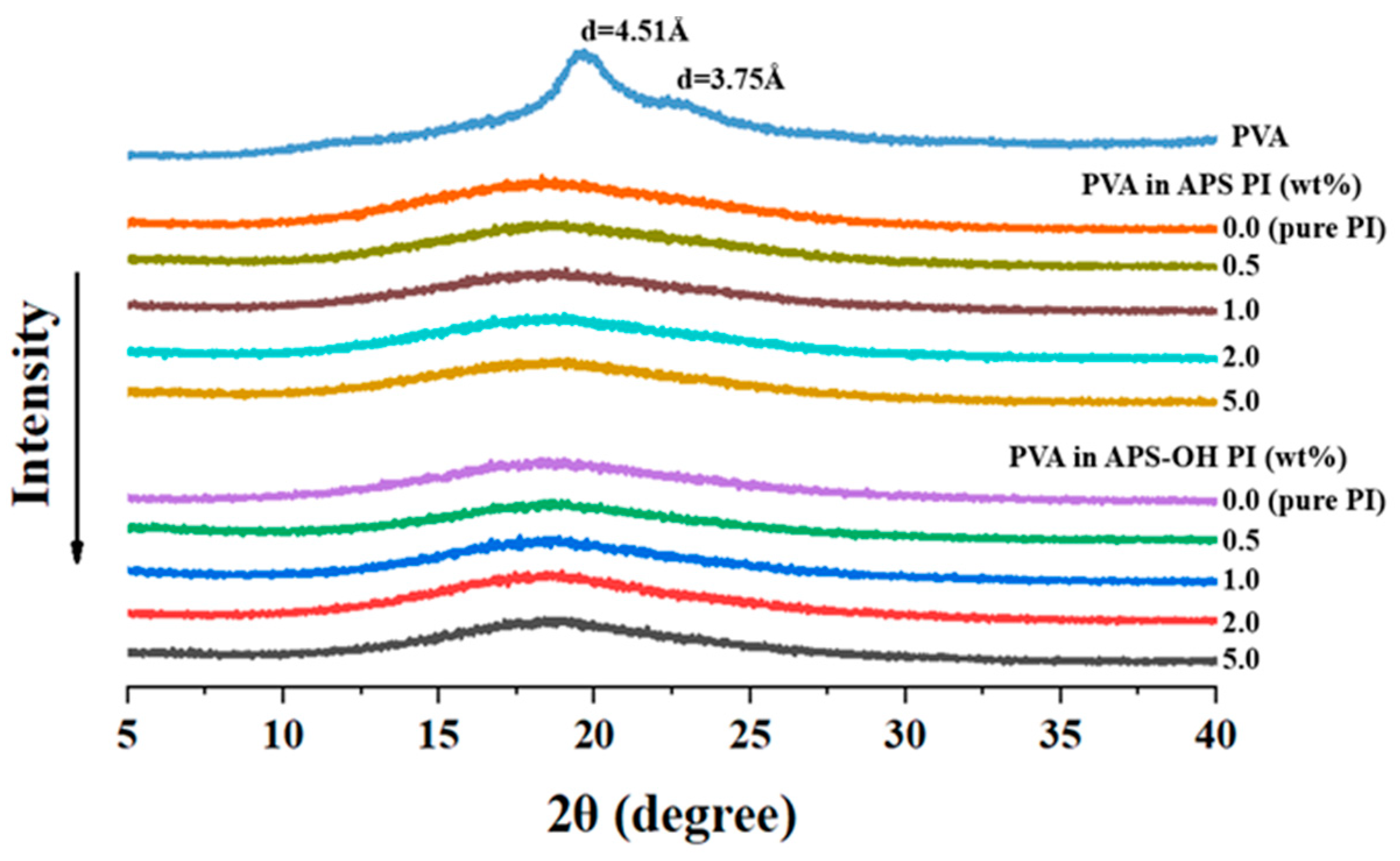
3.3. Thermal Properties
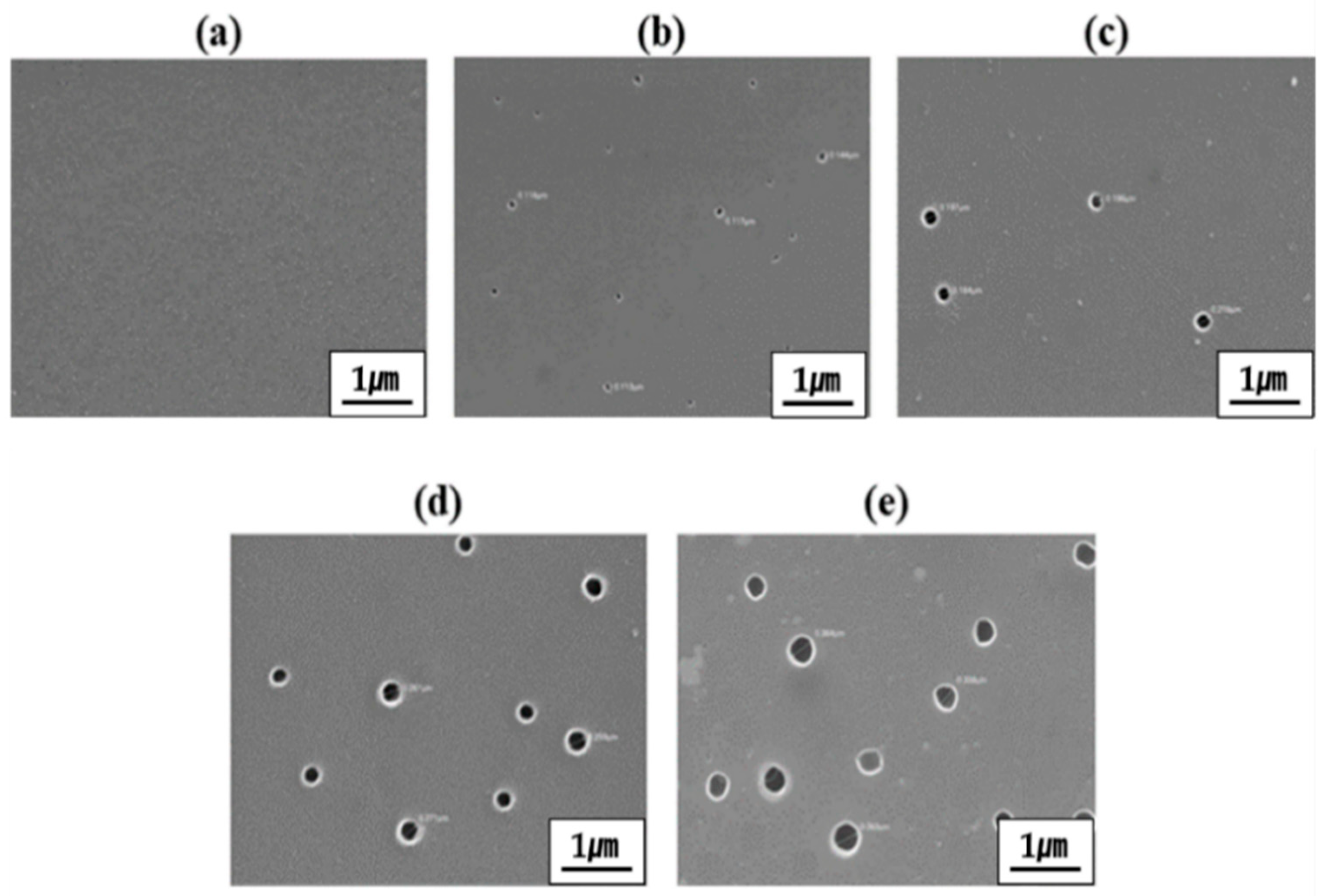
3.4. Membrane Morphology
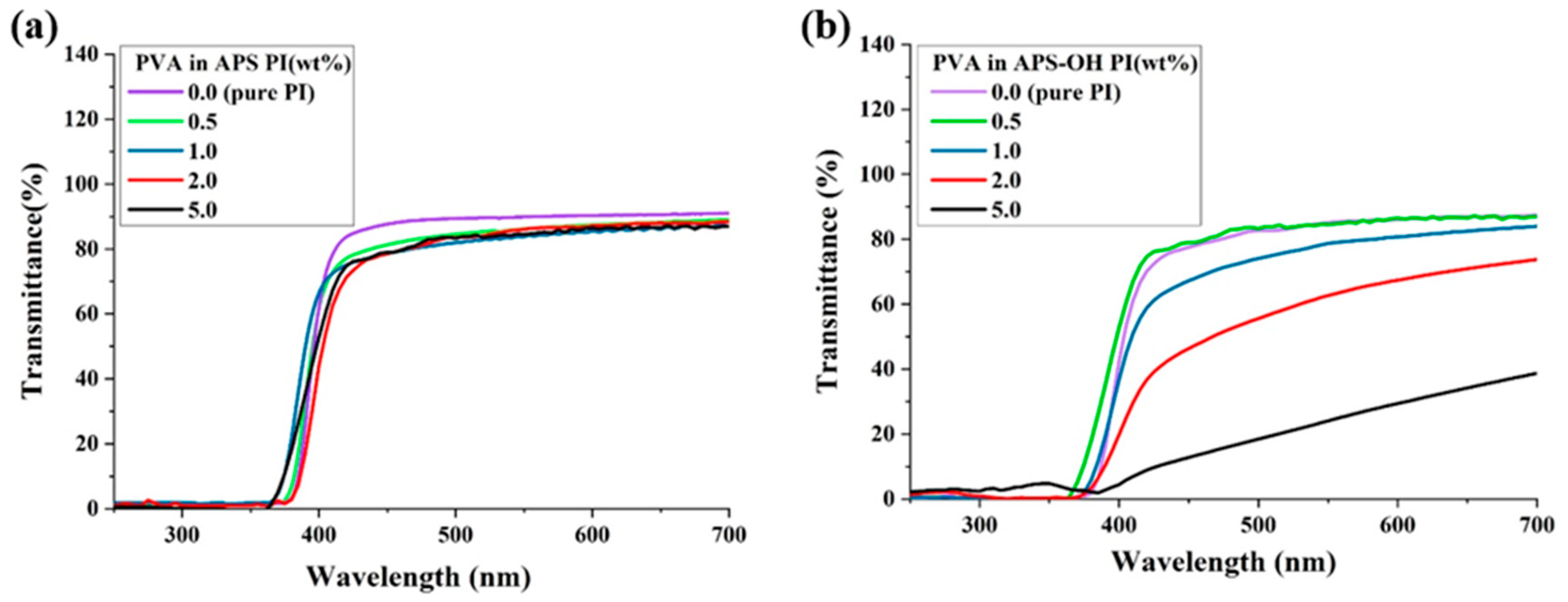
3.5. Optical Transparency
3.6. Solubility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuang, Y.; Zhang, X.; Zhou, S. Adsorption of Methylene Blue in Water onto Activated Carbon by Surfactant Modification. Water 2020, 12, 587. [Google Scholar] [CrossRef]
- Kalla, S. Use of Membrane Distillation for Oily Wastewater Treatment-A Review. J. Environ. Chem. Eng. 2021, 9, 104641. [Google Scholar] [CrossRef]
- Chi, X.Y.; Zhang, P.Y.; Guo, X.J.; Xu, Z.L. A Novel TFC Forward Osmosis (FO) Membrane Supported by Polyimide (PI) Mi-croporous Nanofiber Membrane. Appl. Surf. Sci. 2018, 427, 1–9. [Google Scholar] [CrossRef]
- Anis, S.; Hashaikeh, R.; Hilal, N. Microfiltration Membrane Processes: A Review of Research Trends Over the Past Decade. J. Water Process. Eng. 2019, 32, 100941. [Google Scholar] [CrossRef]
- Mohammad, A.; Teow, Y.; Ang, W.; Chung, Y.; Oatley-Radcliffe, D.; Hilal, N. Nanofiltration Membranes Review: Recent Advances and Future Prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Charcosset, C. Ultrafiltration, Microfiltration, Nanofiltration and Reverse Osmosis in Integrated Membrane Processes. In Integrated Membrane Systems and Processes; Wiley: Hoboken, NJ, USA, 2016; pp. 1–22. [Google Scholar]
- Dolar, D.; Košutić, K. Removal of Pharmaceuticals by Ultrafiltration (UF), Nanofiltration (NF), and Reverse Osmosis (RO). In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; Volume 62, pp. 319–344. [Google Scholar]
- Belfort, G.; Davis, R.H.; Zydney, A.L. The Behavior of Suspensions and Macromolecular Solutions in Crossflow Microfiltration. J. Membr. Sci. 1994, 96, 1–58. [Google Scholar] [CrossRef]
- Zsigmondy, R. Filter and method of producing same. U.S. Patent US1421341A, 27 June 1922. [Google Scholar]
- Zhong, J.; Sun, X.; Wang, C. Treatment of Oily Wastewater Produced from Refinery Processes Using Flocculation and Ceramic Membrane Filtration. Sep. Purif. Technol. 2003, 32, 93–98. [Google Scholar] [CrossRef]
- Das, B.; Chakrabarty, B.; Barkakati, P. Preparation and Characterization of Novel Ceramic Membranes for Micro-Filtration Applications. Ceram. Int. 2016, 42, 14326–14333. [Google Scholar] [CrossRef]
- Howe, K.J.; Clark, M.M. Fouling of Microfiltration and Ultrafiltration Membranes by Natural Waters. Environ. Sci. Technol. 2002, 36, 3571–3576. [Google Scholar] [CrossRef] [PubMed]
- Grosso, V.; Vuono, D.; Bahattab, M.A.; Di Profio, G.; Curcio, E.; Al-Jilil, S.A.; Alsubaie, F.; Alfife, M.; Nagy, J.B.; Drioli, E.; et al. Polymeric and Mixed Matrix Polyimide Membranes. Sep. Purif. Technol. 2014, 132, 684–696. [Google Scholar] [CrossRef]
- Ba, C.; Economy, J. Preparation of PMDA/ODA Polyimide Membrane for Use as Substrate in a Thermally Stable Composite Reverse Osmosis Membrane. J. Membr. Sci. 2010, 363, 140–148. [Google Scholar] [CrossRef]
- Ni, H.-J.; Liu, J.-G.; Wang, Z.-H.; Yang, S.-Y. A Review on Colorless and Optically Transparent Polyimide Films: Chemistry, Process and Engineering Applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced Polyimide Materials: Syntheses, Physical Properties and Applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.H.; Mohamad, A.B.; Al-Amiery, A.A. Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Polyvinyl Alcohol: A Review of Research Status and Use of Polyvinyl Alcohol Based Nanocomposites. Polym. Eng. Sci. 2018, 58, 2119–2132. [Google Scholar] [CrossRef]
- Boussemghoune, M.; Chikhi, M.; Balaska, F.; Ozay, Y.; Dizge, N.; Kebabi, B. Preparation of a Zirconia-Based Ceramic Mem-brane and Its Application for Drinking Water Treatment. Symmetry 2020, 12, 933. [Google Scholar] [CrossRef]
- Peng, F.; Huang, X.; Jawor, A.; Hoek, E.M.V. Transport, Structural, and Interfacial Properties of Poly (Vinyl Alcohol)–Polysulfone Composite Nanofiltration Membranes. J. Membr. Sci. 2010, 353, 169–176. [Google Scholar] [CrossRef]
- Zhang, H.; Tung, W.Y.; Li, X.; Jin, H.; Deng, R.; Chen, Y.M.; Mao, Y.; Zhu, Y. Conjugated Polymer with Dynamic and Ther-moreversible Hydrogen Bonding on the Backbone. Polymer 2020, 203, 122787. [Google Scholar] [CrossRef]
- Qiu, F.-X.; Zhou, Y.-M.; Liu, J.-Z. The Synthesis and Characteristic Study of 6FDA–6FHP–NLO Polyimide/SiO2 Nanohybrid Materials. Eur. Polym. J. 2004, 40, 713–720. [Google Scholar] [CrossRef]
- Shin, H.I.; Chang, J.-H. Transparent Polyimide/Organoclay Nanocomposite Films Containing Different Diamine Monomers. Polymers 2020, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy; Cengage Learning: Boston, MA, USA, 2008; Volume 2, pp. 15–104. [Google Scholar]
- Rusu, R.-D.; Constantin, C.-P.; Drobota, M.; Gradinaru, L.-M.; Butnaru, M.; Pislaru, M. Polyimide Films Tailored by UV Irra-diation: Surface Evaluation and Structure-Properties Relationship. Polym. Degrad. Stab. 2020, 177, 109182. [Google Scholar] [CrossRef]
- Fukukawa, K.-I.; Okazaki, M.; Sakata, Y.; Urakami, T.; Yamashita, W.; Tamai, S. Synthesis and Properties of Multi-Block Semi-alicyclic Polyimides for Thermally Stable Transparent and Low CTE Film. Polymer 2013, 54, 1053–1063. [Google Scholar] [CrossRef]
- Zhai, L.; Yang, S.; Fan, L. Preparation and Characterization of Highly Transparent and Colorless Semi-Aromatic Polyimide Films Derived from Alicyclic Dianhydride and Aromatic Diamines. Polymer 2012, 53, 3529–3539. [Google Scholar] [CrossRef]
- Kim, S.D.; Lee, S.; Heo, J.; Kim, S.Y.; Chung, I.S. Soluble Polyimides with Trifluoromethyl Pendent Groups. Polymer 2013, 54, 5648–5654. [Google Scholar] [CrossRef]
- Pouchert, C.; Behnke, J. The Aldrich Library of 13C and 1H FT NMR spectra; Aldrich Chem. Co., Inc.: Milwaukee, WI, USA, 1993. [Google Scholar]
- Kim, J.W.; Chang, J.-H. Syntheses of Colorless and Transparent Polyimide Membranes for Microfiltration. Polymers 2020, 12, 1610. [Google Scholar] [CrossRef]
- Sanders, D.F.; Guo, R.; Smith, Z.P.; Liu, Q.; Stevens, K.A.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Influence of Polyimide Precursor Synthesis Route and Ortho-Position Functional Group on Thermally Rearranged (TR) Polymer Properties: Conversion and Free Volume. Polymer 2014, 55, 1636–1647. [Google Scholar] [CrossRef]
- Scholes, C.A.; Ribeiro, C.P.; Kentish, S.E.; Freeman, B.D. Thermal Rearranged Poly (benzoxazole)/Polyimide Blended Mem-branes for CO2 Separation. Sep. Purif. Technol. 2014, 124, 134–140. [Google Scholar] [CrossRef]
- Comer, A.C.; Ribeiro, C.P.; Freeman, B.D.; Kalakkunnath, S.; Kalika, D.S. Dynamic Relaxation Characteristics of Thermally Rearranged Aromatic Polyimides. Polymer 2013, 54, 891–900. [Google Scholar] [CrossRef]
- Wang, H.; Paul, D.R.; Chung, T.-S. The Effect of Purge Environment on Thermal Rearrangement of Ortho-Functional Poly-amide and Polyimide. Polymer 2013, 54, 2324–2334. [Google Scholar] [CrossRef]
- MaryTheresa, M.P.; Eric, M.V.H. A Review of Water Treatment Membrane Nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar]
- Schäfer, A.; Schwicker, U.; Fischer, M.M.; Fane, A.G.; Waite, T.D. Microfiltration of Colloids and Natural Organic Matter. J. Membr. Sci. 2000, 171, 151–172. [Google Scholar] [CrossRef]
- Kudo, H.; Sudo, S.; Oka, T.; Hama, Y.; Oshima, A.; Washio, M.; Murakami, T. Ion-Beam Irradiation Effects on Polyimide-UV–vis and Infrared Spectroscopic Study. Radiat. Phys. Chem. 2009, 78, 1067–1070. [Google Scholar] [CrossRef]
- Jang, Y.M.; Seo, J.Y.; Chae, K.H.; Yi, M.H. Positive-Type Photosensitive Polyimide Based on a Photobase Generator Containing oxime-Urethane Groups as a Photosensitive Compound. Macromol. Res. 2006, 14, 300–305. [Google Scholar] [CrossRef]
- Shin, H.I.; Kwark, Y.-J.; Chang, J.-H. Colorless and Transparent Copolyimides and Their Nanocomposites: Thermo-Optical Properties, Morphologies, and Gas Permeabilities. Polymers 2019, 11, 585. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-P.; Su, Y.-Y. Colorless and High Organosoluble Polyimides from 2, 5-bis(3,4-Dicarboxy-Phenoxy)-t-Butylbenzene Di-Anhydride and Aromatic Bis(Ether Amine)s Bearing Pendent Trifluoromethyl Groups. Polymer 2005, 46, 5778–5788. [Google Scholar] [CrossRef]






| Samples | Temperature (°C)/Time (h)/Pressure (Torr) |
|---|---|
| PAA | 25/1/760 → 0/1/760 → 25/12/760 |
| PAA/PVA blend | 50/3/760 |
| CPI/PVA blend | 50/2/1 → 80/1/1 → 110/0.5/1 → 140/0.5/1 →
170/0.5/1 → 195/0.8/1 → 220/0.8/1 → 235/2/1 |
| PVA (wt%) | APS CPI | APS-OH CPI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thickness (μm) | Tg (°C) | TDia (°C) | wtR600b (%) | D.C c (%) | D d (μm) | Thickness (μm) | Tg (°C) | TD i (°C) | wtR600 (%) | D.C (%) | D (μm) | |
| 0 (pure CPI) | 24 | 206 | 466 | 52 | 0 | - | 20 | 240 | 335 | 66 | 0 | - |
| 0.5 | 23 | 207 | 468 | 52 | 0 | 0.21 | 21 | 237 | 331 | 67 | 0 | 0.12 |
| 1.0 | 21 | 206 | 463 | 56 | 0 | 0.29 | 24 | 236 | 335 | 67 | 0 | 0.20 |
| 2.0 | 22 | 206 | 465 | 56 | 0 | 0.66 | 22 | 237 | 329 | 65 | 0 | 0.26 |
| 5.0 | 21 | 204 | 465 | 55 | 0 | 0.86 | 22 | 238 | 328 | 67 | 0 | 0.36 |
| 100 (PVA) | - | 44 | 201 | 30 | 18 | - | - | 44 | 201 | 30 | 18 | - |
| PVA (wt%) | APS CPI | APS-OH CPI | ||||
|---|---|---|---|---|---|---|
| λ0 a (nm) | 500 nmtrans (%) | YI b | λ0 (nm) | 500 nmtrans (%) | YI | |
| 0 (pure CPI) | 390 | 88 | 2 | 370 | 83 | 5 |
| 0.5 | 385 | 84 | 2 | 370 | 81 | 6 |
| 1.0 | 370 | 81 | 2 | 380 | 74 | 7 |
| 2.0 | 380 | 87 | 3 | 390 | 56 | 9 |
| 5.0 | 375 | 82 | 2 | 390 | 18 | 10 |
| CPI | Act | CHCl3 | CH2Cl2 | DMAc | DMF | DMSO | CH3OH | NMP | Tol | THF | Py |
|---|---|---|---|---|---|---|---|---|---|---|---|
| APS | × | × | × | × | × | × | × | × | × | × | × |
| APS-OH | × | × | × | × | × | × | × | × | × | × | × |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.W.; Lee, S.J.; Choi, M.Y.; Chang, J.-H. Colorless and Transparent Polyimide Microporous Film with Excellent Physicochemical Property. Polymers 2021, 13, 1298. https://doi.org/10.3390/polym13081298
Kim JW, Lee SJ, Choi MY, Chang J-H. Colorless and Transparent Polyimide Microporous Film with Excellent Physicochemical Property. Polymers. 2021; 13(8):1298. https://doi.org/10.3390/polym13081298
Chicago/Turabian StyleKim, Jong Won, Seon Ju Lee, Moon Young Choi, and Jin-Hae Chang. 2021. "Colorless and Transparent Polyimide Microporous Film with Excellent Physicochemical Property" Polymers 13, no. 8: 1298. https://doi.org/10.3390/polym13081298
APA StyleKim, J. W., Lee, S. J., Choi, M. Y., & Chang, J.-H. (2021). Colorless and Transparent Polyimide Microporous Film with Excellent Physicochemical Property. Polymers, 13(8), 1298. https://doi.org/10.3390/polym13081298







