Dynamics & Spectroscopy with Neutrons—Recent Developments & Emerging Opportunities
Abstract
:1. Overview
2. Soft Media for Photovoltaics & Photonics
3. From Confined Polymers to Soft Supramolecular Frameworks
| Acronym | Investigation | Range | Instrument | Reference |
|---|---|---|---|---|
| MOF-5 | p–H2 | 0–20 meV | QENS | 2003 [249] |
| IRMOF-X (X = 1, 8, 11, 177) | p–H2 | 0–20 meV | QENS | 2005 [250] |
| MOF-5 | Bare material | 20–170 meV | FANS * | 2006 [291] |
| NaNi3(OH)(SIP)2 | p–H2 | 0–20 meV | QENS | 2006 [292] |
| HKUST-1 | p–H2 | 5–45 meV | FANS | 2007 [293] |
| TMBB | p–H2 | 0–30 meV | QENS | 2007 [294] |
| MIL-53 | Bare material | 50–180 meV | FANS * | 2008 [295] |
| MOF-74 | p–H2 | 5–25 meV | FANS | 2008 [296] |
| PCN-12 | p–H2 | 0–20 meV | QENS | 2008 [297] |
| PCN-6; PCN-6’ | p–H2 | 0–20 meV | QENS | 2008 [298] |
| HKUST-1 | p–H2 | 5–200 meV | FANS * | 2009 [299] |
| ZMOFs | p–H2 | 0–25 meV | QENS | 2009 [300] |
| CPO-27-M (M = Ni, Co, Mg) | p–H2 | 0–15 meV | FOCUS | 2010 [301] |
| Cr3(BTC)2 | p–H2 | 5–45 meV | FANS | 2011 [302] |
| Mg2(dobdc) | p–H2 | 5–45 meV | FANS | 2011 [303] |
| MOF-324 | p–H2 | 0–25 meV | QENS | 2012 [304] |
| rht-MOF-1 and rht-MOF-4a | p–H2 | 0–20 meV | FOCUS | 2012 [305] |
| Fe2(dobdc) and Fe2(O2)(dobdc) | p–H2 | 0–125 meV | FANS / TOSCA | 2012 [306] |
| NU-301 and NU-302 | p–H2 | 0–20 meV | FOCUS | 2013 [307] |
| HKUST-1 | p–H2 | 0–50 meV | TOSCA / MARI | 2013 [308] |
| MIL-53(Fe) | CH3OH | 0–250 meV | TOSCA * | 2013 [309] |
| rht-MOF-7 | p–H2 | 0–20 meV | TOFTOF | 2013 [310] |
| CPO-27–M (M = Mn, Cu) | p–H2 | 0–20 meV | TOFTOF / FOCUS | 2014 [311] |
| SIFSIX-2-Cu and SIFSIX-2-Cu-i | p–H2 | 0–20 meV | TOFTOF / FOCUS | 2014 [312] |
| Y-FTZB | p–H2 | 0–20 meV | TOFTOF | 2014 [313] |
| NOTT-300 | p–H2 | 0–250 meV | TOSCA * | 2014 [314] |
| ZIF-4, ZIF-7, and ZIF-8 | Bare material | 0–400 meV | TOSCA * | 2014 [264] |
| a-[Mg3(O2CH)6] | p–H2 | 0–20 meV | QENS | 2015 [315] |
| Zn(trz)(tftph) | p–H2 | 0–20 meV | TOFTOF | 2015 [316] |
| M-MOF-74 (M = Mg, Ni, Co, Zn) | p–H2 | 0–20 meV | FOCUS | 2015 [317] |
| NOTT-300 | C2H6, C2H4, and C2H2 | 0–250 meV | TOSCA * | 2015 [314] |
| M-soc-MOF-1-X (M = Fe, In; X = a, b) | p–H2 | 0–20 meV | IN5 | 2016 [318] |
| ZIF-8; ZIF-8@AC | N2 | 0–150 meV | VISION * | 2016 [266] |
| Cu(I)-MFU-4l | H2 and D2 | 0–40 meV | VISION * | 2016 [267] |
| MFM-300(In) | H2 and CH4 | 0–250 meV | TOSCA * | 2016 [268] |
| MFM-300(In) | N2, CO2, and SO2 | 0–250 meV | TOSCA * | 2016 [269] |
| ZIF-8 | D2O and CH4 | 0–200 meV | TOSCA | 2016 [266] |
| COF-1; COF-2 | p–H2 | 0–20 meV | QENS | 2017 [270] |
| ZIF-7 | N2 | 0–200 meV | VISION * | 2017 [271] |
| ZIF-8 | N2, O2, Ar, and CO | 0–150 meV | VISION | 2017 [272] |
| MFM-300(Sc) | I2 | 0–250 meV | TOSCA * | 2017 [273] |
| MFM-300(V) | CO2 | 0–250 meV | TOSCA * | 2017 [274] |
| MIL-140A | Bare material | 0–250 meV | TOSCA * | 2017 [275] |
| MFM-305 and MFM-305-CH3 | CO2 | 0–250 meV | VISION * | 2018 [276] |
| MFM-102-NO2 | C2H2 | 0–250 meV | VISION * | 2018 [277] |
| MFM-520 | NO2 | 0–250 meV | VISION * | 2019 [276] |
| Zn(MeIm)2 | Bare material | 0–500 meV | SEQUOIA | 2019 [319] |
| MFM-102-NO2 and MFM-102-NH2 | CO2 | 0–250 meV | VISION * | 2019 [278] |
| ZIF-4 | Bare material | 0–250 meV | VISION * | 2019 [279] |
| CO2 | 0–250 meV | TOSCA | 2019 [280] | |
| ZIF-4(Zn) | Bare material | 0–250 meV | TOSCA * | 2019 [281] |
| ZIF-7 | CO2 | 0–150 meV | TOSCA | 2019 [282] |
| HKUST-1 | Drug encapsulation | 0–250 meV | TOSCA * | 2019 [151] |
| MFM-126 | CO2 | 0–400 meV | TOSCA* | 2019 [283] |
| MFM-100 | Benzyl alcohol | 0–250 meV | TOSCA* | 2019 [284] |
| Pd@OX-1 | Catalytic properties | 0–400 meV | TOSCA* | 2019 [285] |
| MFM-170 | H2O and SO2 | 0–150 meV | TOSCA * | 2019 [286] |
| Y-shp-MOF-5 and Cr-soc-MOF-1 | D2O and CH4 | 0–300 meV | VISION * | 2020 [287] |
| MIL-100 (Fe) | Drug Encapsulation | 0–250 meV | TOSCA | 2020 [288] |
| MFM-520 | D2O, CO2, and SO2 | 0–250 meV | TOSCA * | 2020 [289] |
| MFM-300(M) (M = Al, Fe, VIII, VIV) | NH3 | 0–200 meV | TOSCA / VISION * | 2021 [290] |
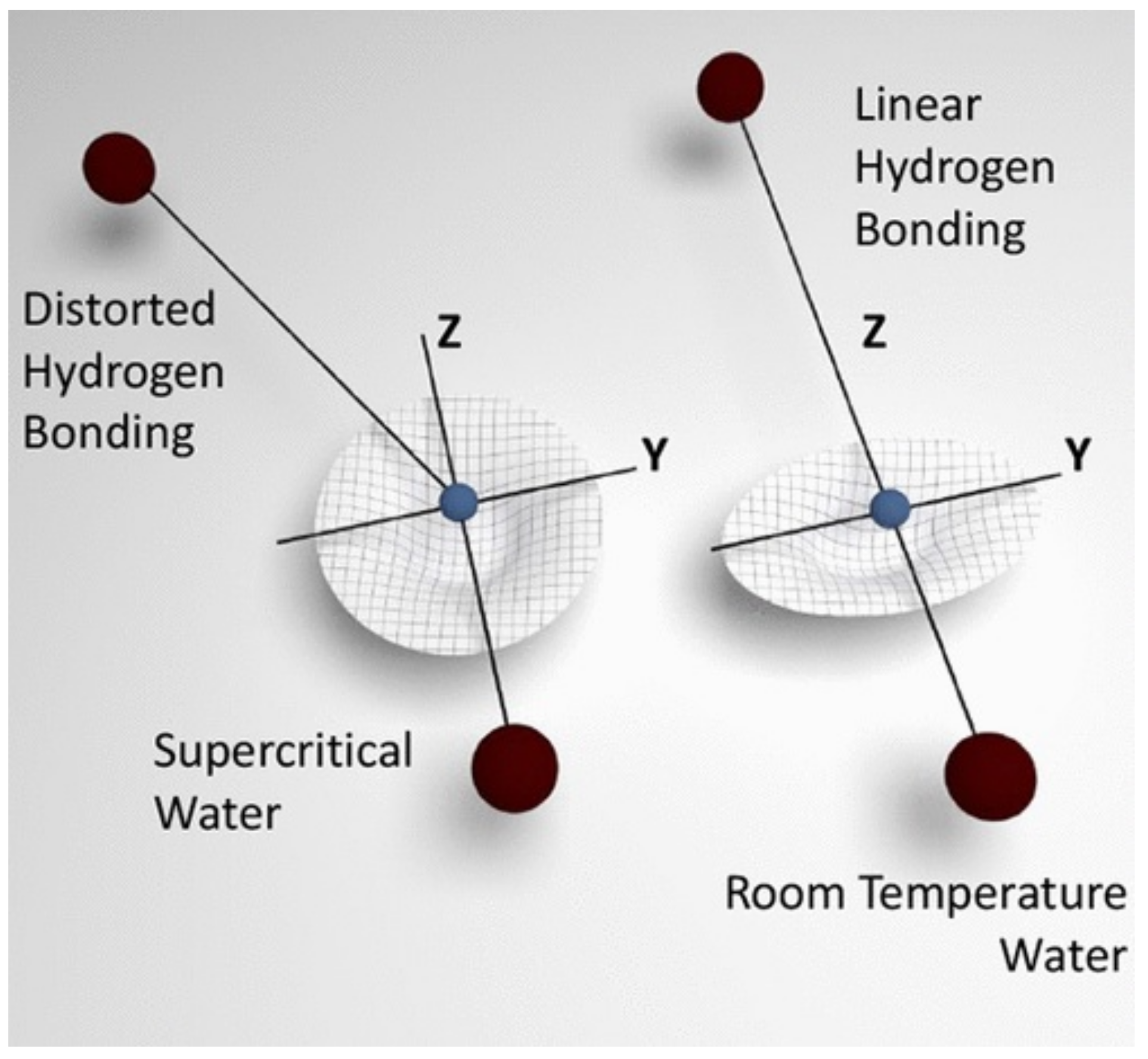
4. Back to Basics: Water




5. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersen, K.; Argyriou, D.; Jackson, A.; Houston, J.; Henry, P.; Deen, P.; Toft-Petersen, R.; Beran, P.; Strobl, M.; Arnold, T.; et al. The Instrument Suite of the European Spallation Source. Nucl. Instrum. Methods Phys. Res. 2020, 957, 163402. [Google Scholar] [CrossRef]
- Carpenter, J.M. The Development of Compact Neutron Sources. Nat. Rev. Phys. 2019, 1, 177–179. [Google Scholar] [CrossRef]
- Ott, F.; Menelle, A.; Alba-Simionesco, C. The SONATE Project, a French CANS for Materials Sciences Research. EPJ Web Conf. 2020, 231, 01004. [Google Scholar] [CrossRef]
- Otake, Y. RIKEN Accelerator-driven Compact Neutron Systems. EPJ Web Conf. 2020, 231, 01009. [Google Scholar] [CrossRef]
- Zakalek, P.; Cronert, T.; Baggemann, J.; Doege, P.E.; Rimmler, M.; Voigt, J.; Mauerhofer, E.; Rücker, U.; Gutberlet, T.; Podlech, H.; et al. High-brilliance Neutron Source Project. J. Phys. Conf. Ser. 2020, 1401, 012010. [Google Scholar] [CrossRef]
- Anderson, I.; Andreani, C.; Carpenter, J.; Festa, G.; Gorini, G.; Loong, C.K.; Senesi, R. Research Opportunities with Compact Accelerator-driven Neutron Sources. Phys. Rep. 2016, 654, 1–58. [Google Scholar] [CrossRef] [Green Version]
- De Vicente, J.P.; Sordo, F.; Perlado, J.M.; Bermejo, F.J.; Fernandez-Alonso, F. Guiding Criteria for Instrument Design at Long-pulse Neutron Sources. J. Phys. Conf. Ser. 2015, 663, 012011. [Google Scholar] [CrossRef]
- Sordo, F.; Fernandez-Alonso, F.; Terrón, S.; Magán, M.; Ghiglino, A.; Martinez, F.; Bermejo, F.; Perlado, J. Baseline Design of a Low Energy Neutron Source at ESS-Bilbao. Phys. Procedia 2014, 60, 125–137. [Google Scholar] [CrossRef] [Green Version]
- De Vicente, J.; Fernandez-Alonso, F.; Sordo, F.; Bermejo, F. Neutrons at ESS-Bilbao: From Production to Utilisation; Technical Report RAL-TR-2013-016; Rutherford Appleton Laboratory: Oxfordshire, UK, 2013. [Google Scholar]
- Skarzynski, T. Collecting Data in the Home Laboratory: Evolution of X-ray Sources, Detectors and Working Practices. Acta Crystallogr. D 2013, 69, 1283–1288. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Alonso, F.; Price, D. (Eds.) Neutron Scattering—Fundamentals; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Fernandez-Alonso, F.; Price, D. (Eds.) Neutron Scattering—Magnetic and Quantum Phenomena; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Fernandez-Alonso, F.; Price, D. (Eds.) Neutron Scattering—Applications in Biology, Chemistry, and Materials Science; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Richter, D.; Monkenbusch, M.; Arbe, A.; Colmenero, J. Neutron Spin Echo in Polymer Systems; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar] [CrossRef]
- Krutyeva, M.; Wischnewski, A.; Monkenbusch, M.; Willner, L.; Maiz, J.; Mijangos, C.; Arbe, A.; Colmenero, J.; Radulescu, A.; Holderer, O.; et al. Effect of Nanoconfinement on Polymer Dynamics: Surface Layers and Interphases. Phys. Rev. Lett. 2013, 110. [Google Scholar] [CrossRef] [Green Version]
- Richter, D.; Monkenbusch, M.; Allgeier, J.; Arbe, A.; Colmenero, J.; Farago, B.; Bae, Y.C.; Faust, R. From Rouse Dynamics to Local Relaxation: A Neutron Spin Echo Study on Polyisobutylene Melts. J. Chem. Phys. 1999, 111, 6107–6120. [Google Scholar] [CrossRef]
- Colmenero, J.; Mukhopadhyay, R.; Alegría, A.; Frick, B. Quantum Rotational Tunneling of Methyl Groups in Polymers. Phys. Rev. Lett. 1998, 80, 2350–2353. [Google Scholar] [CrossRef]
- Zorn, R.; Frick, B.; Fetters, L.J. Quasielastic Neutron Scattering Study of the Methyl Group Dynamics in Polyisoprene. J. Chem. Phys. 2002, 116, 845–853. [Google Scholar] [CrossRef] [Green Version]
- Colmenero, J.; Arbe, A. Segmental Dynamics in Miscible Polymer Blends: Recent Results and Open Questions. Soft Matter 2007, 3, 1474. [Google Scholar] [CrossRef]
- Colmenero, J.; Arbe, A. Recent Progress on Polymer Dynamics by Neutron Scattering: From Simple Polymers to Complex Materials. J. Polym. Sci. B Polym. Phys. 2012, 51, 87–113. [Google Scholar] [CrossRef] [Green Version]
- Bhowmik, D.; Pomposo, J.A.; Juranyi, F.; Sakai, V.G.; Zamponi, M.; Arbe, A.; Colmenero, J. Investigation of a Nanocomposite of 75 wt % Poly(methyl methacrylate) Nanoparticles with 25 wt % Poly(ethylene oxide) Linear Chains: A Quasielatic Neutron Scattering, Calorimetric, and WAXS Study. Macromolecules 2014, 47, 3005–3016. [Google Scholar] [CrossRef]
- Arbe, A.; Nilsen, G.J.; Stewart, J.R.; Alvarez, F.; Sakai, V.G.; Colmenero, J. Coherent Structural Relaxation of Water from Meso- to Intermolecular Scales Measured using Neutron Spectroscopy with Polarization Analysis. Phys. Rev. Res. 2020, 2. [Google Scholar] [CrossRef] [Green Version]
- Fischer, E.W. Studies of Structure and Dynamics of Solid Polymers by Elastic and Inelastic Neutron Scattering. Pure Appl. Chem. 1978, 50, 1319–1341. [Google Scholar] [CrossRef]
- Higgins, J. Neutron Scattering from Polymers: Five Decades of Developing Possibilities. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Berrod, Q.; Lagrené, K.; Ollivier, J.; Zanotti, J.M. Inelastic and Quasi-elastic Neutron Scattering. Application to Soft-matter. EPJ Web Conf. 2018, 188, 05001. [Google Scholar] [CrossRef]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro-Claro, P.J.A.; Rudić, S.; Silvestre, A.J.D.; Vaz, P.D.; Sousa, A.F. Inside PEF: Chain Conformation and Dynamics in Crystalline and Amorphous Domains. Macromolecules 2018, 51, 3515–3526. [Google Scholar] [CrossRef]
- Lambri, O.A.; Giordano, E.D.V.; Bonifacich, F.G.; Jiménez-Ruiz, M.; Lambri, M.A.; Sánchez, F.A.; Pérez-Landazábal, J.I.; García, J.Á.; Boschetti, C.E.; Recarte, V.; et al. Changes in the Crystalline Degree in Neutron Irradiated EPDM Viewed Through Infrared Spectroscopy and Inelastic Neutron Scattering. Matéria (Rio de Janeiro) 2018, 23. [Google Scholar] [CrossRef]
- Nolasco, M.M.; Araujo, C.F.; Thiyagarajan, S.; Rudić, S.; Vaz, P.D.; Silvestre, A.J.D.; Ribeiro-Claro, P.J.A.; Sousa, A.F. Asymmetric Monomer, Amorphous Polymer? Structure–Property Relationships in 2, 4-FDCA and 2, 4-PEF. Macromolecules 2020, 53, 1380–1387. [Google Scholar] [CrossRef]
- Moreno, A.J.; Alegría, A.; Colmenero, J.; Frick, B. Methyl Group Dynamics in Poly(methyl methacrylate): From Quantum Tunneling to Classical Hopping. Macromolecules 2001, 34, 4886–4896. [Google Scholar] [CrossRef]
- Colmenero, J.; Moreno, A.J.; Alegría, A. Neutron Scattering Investigations on Methyl Group Dynamics in Polymers. Prog. Polym. Sci. 2005, 30, 1147–1184. [Google Scholar] [CrossRef] [Green Version]
- Goracci, G.; Arbe, A.; Alegría, A.; Sakai, V.G.; Rudić, S.; Schneider, G.J.; Lohstroh, W.; Juranyi, F.; Colmenero, J. Influence of Solvent on Poly(2-(Dimethylamino)Ethyl Methacrylate) Dynamics in Polymer-Concentrated Mixtures: A Combined Neutron Scattering, Dielectric Spectroscopy, and Calorimetric Study. Macromolecules 2015, 48, 6724–6735. [Google Scholar] [CrossRef] [Green Version]
- Annis, B.K.; Lohse, D.J.; Trouw, F. Observation of Boson Peaks by Inelastic Neutron Scattering in Polyolefins. J. Chem. Phys. 1999, 111, 1699–1704. [Google Scholar] [CrossRef]
- Etienne, S.; David, L.; Dianoux, A.; Saviot, L.; Duval, E. Effect of Aging on the Boson Peak and Relaxation Processes in a Glassy Polymer. J. Non-Cryst. Solids 2002, 307–310, 109–113. [Google Scholar] [CrossRef]
- Hong, L.; Begen, B.; Kisliuk, A.; Alba-Simionesco, C.; Novikov, V.N.; Sokolov, A.P. Pressure and Density Dependence of the Boson Peak in Polymers. Phys. Rev. B 2008, 78. [Google Scholar] [CrossRef]
- Zorn, R.; Yin, H.; Lohstroh, W.; Harrison, W.; Budd, P.M.; Pauw, B.R.; Böhning, M.; Schönhals, A. Anomalies in the Low Frequency Vibrational Density of States for a Polymer with Intrinsic Microporosity—The Boson Peak of PIM-1. Phys. Chem. Chem. Phys. 2018, 20, 1355–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomoshige, N.; Mizuno, H.; Mori, T.; Kim, K.; Matubayasi, N. Boson Peak, Elasticity, and Glass Transition Temperature in Polymer Glasses: Effects of the Rigidity of Chain Bending. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Kanaya, T.; Nishida, K.; Tsukushi, I.; Shibata, K. Inelastic Neutron Scattering Study of Low Energy Excitations in Polymer Thin Films. Phys. Rev. Lett. 2005, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanaya, T.; Miyazaki, T.; Inoue, R.; Nishida, K. Thermal Expansion and Contraction of Polymer Thin Films. Phys. Status Solidi B 2005, 242, 595–606. [Google Scholar] [CrossRef]
- Kanaya, T.; Inoue, R.; Kawashima, K.; Miyazaki, T.; Tsukushi, I.; Shibata, K.; Matsuba, G.; Nishida, K.; Hino, M. Glassy Dynamics and Heterogeneity of Polymer Thin Films. J. Phys. Soc. Jpn. 2009, 78, 041004. [Google Scholar] [CrossRef]
- Andreani, C.; Krzystyniak, M.; Romanelli, G.; Senesi, R.; Fernandez-Alonso, F. Electron-volt Neutron Spectroscopy: Beyond Fundamental Systems. Adv. Phys. 2017, 66, 1–73. [Google Scholar] [CrossRef]
- Evans, A.C.; Mayers, J.; Timms, D.N.; Cooper, M.J. Deep Inelastic Neutron Scattering in the Study of Atomic Momentum Distributions. Z. Naturforsch. A 1993, 48, 425–432. [Google Scholar] [CrossRef]
- Krzystyniak, M.; Seel, A.G.; Richards, S.E.; Gutmann, M.J.; Fernandez-Alonso, F. Mass-selective Neutron Spectroscopy Beyond the Proton. J. Phys. Conf. Ser. 2014, 571, 012002. [Google Scholar] [CrossRef] [Green Version]
- Krzystyniak, M.; Drużbicki, K.; Fernandez-Alonso, F. Nuclear Dynamics in the Metastable Phase of the Solid Acid Caesium Hydrogen Sulfate. Phys. Chem. Chem. Phys. 2015, 17, 31287–31296. [Google Scholar] [CrossRef] [Green Version]
- Syrykh, G.F.; Stolyarov, A.A.; Krzystyniak, M.; Romanelli, G.; Sadykov, R.A. Temperature Dependence of the Kinetic Energy in the Zr40Be60 Amorphous Alloy. JETP Lett. 2017, 105, 591–594. [Google Scholar] [CrossRef]
- Krzystyniak, M.; Syrykh, G.; Stolyarov, A.; Sadykov, R.A.; Armstrong, J.; da Silva, I.; Romanelli, G.; Fernandez-Alonso, F. Mass-selective Neutron Spectroscopy of Glassy versus Polycrystalline Structures in Binary Mixtures of Beryllium and Zirconium. J. Phys. Conf. Ser. 2018, 1055, 012004. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, J.; Krzystyniak, M.; Romanelli, G.; Parker, S.F.; Drużbicki, K.; Fernandez-Alonso, F. Fractal Dimension as a Scaling Law for Nuclear Quantum Effects: A Neutron Compton Scattering Study on Carbon Allotropes. J. Phys. Conf. Ser. 2018, 1055, 012007. [Google Scholar] [CrossRef]
- Krzystyniak, M.; Gutmann, M.J.; Romanelli, G.; Trenikhina, Y.; Romanenko, A.; Fernandez-Alonso, F. Nitrogen Doping and the Performance of Superconducting Radio-frequency Niobium Cavities: Insights from Neutron Diffraction and Neutron Compton Scattering. J. Phys. Conf. Ser. 2018, 1055, 012006. [Google Scholar] [CrossRef]
- Ding, H.; Liu, Q.; Wang, X.; Fan, X.; Krzystyniak, M.; Glandut, N.; Li, C. Effects of Boron Addition on the Microstructure and Properties of in situ Synthesis TiC-Reinforced Cu Ti C Composites. J. Alloys Compd. 2018, 766, 66–73. [Google Scholar] [CrossRef]
- Krzystyniak, M.; Drużbicki, K.; Rudić, S.; Fabian, M. Positional, Isotopic Mass and Force Constant Disorder in Molybdate Glasses and Their Parent Metal Oxides as Observed by Neutron Diffraction and Compton Scattering. J. Phys. Commun. 2020, 4, 095027. [Google Scholar] [CrossRef]
- Senesi, R.; Andreani, C.; Bowden, Z.; Colognesi, D.; Degiorgi, E.; Fielding, A.; Mayers, J.; Nardone, M.; Norris, J.; Praitano, M.; et al. VESUVIO: A Novel Instrument for Performing Spectroscopic Studies in Condensed Matter With eV Neutrons at the ISIS Facility. Phys. B Condens. Matter 2000, 276–278, 200–201. [Google Scholar] [CrossRef]
- Andreani, C.; Pietropaolo, A.; Senesi, R.; Gorini, G.; Tardocchi, M.; Bracco, A.; Rhodes, N.; Schooneveld, E. Electron-volt Spectroscopy at a Pulsed Neutron Source Using a Resonance Detector Technique. Nucl. Instrum. Methods Phys. Res. 2002, 481, 509–520. [Google Scholar] [CrossRef] [Green Version]
- Pietropaolo, A.; Andreani, C.; D’Agelo, A.; Gorini, G.; Imberti, S.; Rhodes, N.; Schoneveld, E.M.; Senesi, R.; Tardocchi, M. The Resonance Detector Spectrometer for Neutron Spectroscopy in the eV Energy Region. In Capture Gamma-Ray Spectroscopy and Related Topics; World Scientific: Singapore, 2003. [Google Scholar] [CrossRef]
- Andreani, C.; Colognesi, D.; Degiorgi, E.; Filabozzi, A.; Nardone, M.; Pace, E.; Pietropaolo, A.; Senesi, R. Double Difference Method in Deep Inelastic Neutron Scattering on the VESUVIO Spectrometer. Nucl. Instrum. Methods Phys. Res. 2003, 497, 535–549. [Google Scholar] [CrossRef] [Green Version]
- Mayers, J.; Tomkinson, J.; Abdul-Redah, T.; Stirling, W.; Andreani, C.; Senesi, R.; Nardone, M.; Colognesi, D.; Degiorgi, E. VESUVIO—the Double Difference Inverse Geometry Spectrometer at ISIS. Phys. B Condens. Matter 2004, 350, E659–E662. [Google Scholar] [CrossRef] [Green Version]
- Imberti, S.; Andreani, C.; Garbuio, V.; Gorini, G.; Pietropaolo, A.; Senesi, R.; Tardocchi, M. Resolution of the VESUVIO Spectrometer for High-energy Inelastic Neutron Scattering Experiments. Nucl. Instrum. Methods Phys. Res. 2005, 552, 463–476. [Google Scholar] [CrossRef] [Green Version]
- Gorini, G.; Festa, G.; Andreani, C. Epithermal Neutron Instrumentation at ISIS. J. Phys. Conf. Ser. 2014, 571, 012005. [Google Scholar] [CrossRef] [Green Version]
- Romanelli, G.; Krzystyniak, M.; Senesi, R.; Raspino, D.; Boxall, J.; Pooley, D.; Moorby, S.; Schooneveld, E.; Rhodes, N.J.; Andreani, C.; et al. Characterisation of the Incident Beam and Current Diffraction Capabilities on the VESUVIO Spectrometer. Meas. Sci. Technol. 2017, 28, 095501. [Google Scholar] [CrossRef]
- Ulpiani, P.; Romanelli, G.; Arcidiacono, L.; Onorati, D.; Festa, G.; Krzystyniak, M.; Schooneveld, E.; Fernandez-Alonso, F.; Andreani, C.; Senesi, R. Enhancement of Counting Statistics and Noise Reduction in the Forward-scattering Detectors on the VESUVIO Spectrometer. J. Phys. Conf. Ser. 2018, 1055, 012008. [Google Scholar] [CrossRef] [Green Version]
- Krzystyniak, M.; Romanelli, G.; Fabian, M.; Gutmann, M.; Festa, G.; Arcidiacono, L.; Gigg, M.; Drużbicki, K.; Andreani, C.; Senesi, R.; et al. VESUVIO: The Current Testbed for a Next-generation Epithermal Neutron Spectrometer. J. Phys. Conf. Ser. 2018, 1021, 012026. [Google Scholar] [CrossRef]
- Senesi, R.; Kolesnikov, A.I.; Andreani, C. Measurement of Proton Momentum Distributions Using a Direct Geometry Instrument. J. Phys. Conf. Ser. 2014, 571, 012007. [Google Scholar] [CrossRef] [Green Version]
- Stock, C.; Cowley, R.A.; Taylor, J.W.; Bennington, S.M. High-energy Neutron Scattering from Hydrogen Using a Direct Geometry Spectrometer. Phys. Rev. B 2010, 81. [Google Scholar] [CrossRef] [Green Version]
- Mayers, J.; Gidopoulos, N.I.; Adams, M.A.; Reiter, G.; Andreani, C.; Senesi, R. Comment on “High-energy Neutron Scattering from Hydrogen Using a Direct Geometry Spectrometer”. Phys. Rev. B 2011, 84. [Google Scholar] [CrossRef] [Green Version]
- ESS Technical Design Report. Available online: https://docdb01.esss.lu.se/DocDB/0002/000274/015/TDR_online_ver_all.pdf (accessed on 28 April 2021).
- Poglitsch, A.; Weber, D. Dynamic Disorder in Methylammoniumtrihalogenoplumbates (II) Observed by Millimeter-wave Spectroscopy. J. Chem. Phys. 1987, 87, 6373–6378. [Google Scholar] [CrossRef]
- Angelis, F.D. Celebrating 10 Years of Perovskite Photovoltaics. ACS Energy Lett. 2019, 4, 853–854. [Google Scholar] [CrossRef] [Green Version]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Gonzalez-Pedro, V.; Juarez-Perez, E.J.; Arsyad, W.S.; Barea, E.M.; Fabregat-Santiago, F.; Mora-Sero, I.; Bisquert, J. General Working Principles of CH3NH3PbX3 Perovskite Solar Cells. Nano Lett. 2014, 14, 888–893. [Google Scholar] [CrossRef]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef]
- Bisquert, J.; Juarez-Perez, E.J. The Causes of Degradation of Perovskite Solar Cells. J. Phys. Chem. Lett. 2019, 10, 5889–5891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aranda, C.; Guerrero, A.; Bisquert, J. Crystalline Clear or Not: Beneficial and Harmful Effects of Water in Perovskite Solar Cells. ChemPhysChem 2019, 20, 2587–2599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alaei, A.; Circelli, A.; Yuan, Y.; Yang, Y.; Lee, S.S. Polymorphism in Metal Halide Perovskites. Mater. Adv. 2021, 2, 47–63. [Google Scholar] [CrossRef]
- Sun, S.; Isikgor, F.H.; Deng, Z.; Wei, F.; Kieslich, G.; Bristowe, P.D.; Ouyang, J.; Cheetham, A.K. Factors Influencing the Mechanical Properties of Formamidinium Lead Halides and Related Hybrid Perovskites. ChemSusChem 2017, 10, 3740–3745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Fang, Y.; Kieslich, G.; White, T.J.; Cheetham, A.K. Mechanical Properties of Organic–inorganic Halide Perovskites, CH3NH3PbX3(X = I, Br and Cl), by Nanoindentation. J. Mater. Chem. A 2015, 3, 18450–18455. [Google Scholar] [CrossRef]
- Tan, J.C.; Cheetham, A.K. Mechanical Properties of Hybrid Inorganic–organic Framework Materials: Establishing Fundamental Structure–property Relationships. Chem. Soc. Rev. 2011, 40, 1059. [Google Scholar] [CrossRef]
- Drużbicki, K.; Lavén, R.; Armstrong, J.; Malavasi, L.; Fernandez-Alonso, F.; Karlsson, M. Cation Dynamics and Structural Stabilization in Formamidinium Lead Iodide Perovskites. J. Phys. Chem. Lett. 2021, 3503–3508. [Google Scholar] [CrossRef]
- Li, M.; Liu, T.; Wang, Y.; Yang, W.; Lü, X. Pressure Responses of Halide Perovskites with Various Compositions, Dimensionalities, and Morphologies. Matter Radiat. Extremes 2020, 5, 018201. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Gong, J.; Hu, Q.; Capitani, F.; Celeste, A.; Hattori, T.; Sano-Furukawa, A.; Li, N.; Yang, W.; Liu, G.; et al. Suppressed Lattice Disorder for Large Emission Enhancement and Structural Robustness in Hybrid Lead Iodide Perovskite Discovered by High-Pressure Isotope Effect. Adv. Funct. Mater. 2020, 31, 2009131. [Google Scholar] [CrossRef]
- Tan, S.; Yavuz, I.; Weber, M.H.; Huang, T.; Chen, C.H.; Wang, R.; Wang, H.C.; Ko, J.H.; Nuryyeva, S.; Xue, J.; et al. Shallow Iodine Defects Accelerate the Degradation of α-Phase Formamidinium Perovskite. Joule 2020, 4, 2426–2442. [Google Scholar] [CrossRef]
- Fabini, D.H.; Hogan, T.; Evans, H.A.; Stoumpos, C.C.; Kanatzidis, M.G.; Seshadri, R. Dielectric and Thermodynamic Signatures of Low-Temperature Glassy Dynamics in the Hybrid Perovskites CH3NH3PbI3 and HC(NH2)2PbI3. J. Phys. Chem. Lett. 2016, 7, 376–381. [Google Scholar] [CrossRef] [Green Version]
- Fabini, D.H.; Siaw, T.A.; Stoumpos, C.C.; Laurita, G.; Olds, D.; Page, K.; Hu, J.G.; Kanatzidis, M.G.; Han, S.; Seshadri, R. Universal Dynamics of Molecular Reorientation in Hybrid Lead Iodide Perovskites. J. Am. Chem. Soc. 2017, 139, 16875–16884. [Google Scholar] [CrossRef] [Green Version]
- Francisco-López, A.; Charles, B.; Alonso, M.I.; Garriga, M.; Campoy-Quiles, M.; Weller, M.T.; Goñi, A.R. Phase Diagram of Methylammonium/Formamidinium Lead Iodide Perovskite Solid Solutions from Temperature-Dependent Photoluminescence and Raman Spectroscopies. J. Phys. Chem. C 2020, 124, 3448–3458. [Google Scholar] [CrossRef]
- Swainson, I.; Hammond, R.; Soullière, C.; Knop, O.; Massa, W. Phase transitions in the perovskite methylammonium lead bromide, CH3ND3PbBr3. J. Solid State Chem. 2003, 176, 97–104. [Google Scholar] [CrossRef]
- Baikie, T.; Barrow, N.S.; Fang, Y.; Keenan, P.J.; Slater, P.R.; Piltz, R.O.; Gutmann, M.; Mhaisalkar, S.G.; White, T.J. A Combined Single Crystal Neutron/X-ray Diffraction and Solid-state Nuclear Magnetic Resonance Study of the Hybrid Perovskites CH3NH3PbX3 (X = I, Br and Cl). J. Mater. Chem. A 2015, 3, 9298–9307. [Google Scholar] [CrossRef]
- Weller, M.T.; Weber, O.J.; Frost, J.M.; Walsh, A. Cubic Perovskite Structure of Black Formamidinium Lead Iodide, α-[HC(NH2)2]PbI3, at 298 K. J. Phys. Chem. Lett. 2015, 6, 3209–3212. [Google Scholar] [CrossRef]
- Weller, M.T.; Weber, O.J.; Henry, P.F.; Pumpo, A.M.D.; Hansen, T.C. Complete structure and cation orientation in the perovskite photovoltaic methylammonium lead iodide between 100 and 352 K. Chem. Commun. 2015, 51, 4180–4183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitfield, P.S.; Herron, N.; Guise, W.E.; Page, K.; Cheng, Y.Q.; Milas, I.; Crawford, M.K. Structures, Phase Transitions and Tricritical Behavior of the Hybrid Perovskite Methyl Ammonium Lead Iodide. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.; Foley, B.J.; Park, C.; Brown, C.M.; Harriger, L.W.; Lee, J.; Ruff, J.; Yoon, M.; Choi, J.J.; Lee, S.H. Entropy-driven Structural Transition and Kinetic Trapping in Formamidinium Lead Iodide Perovskite. Sci. Adv. 2016, 2, e1601650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comin, R.; Crawford, M.K.; Said, A.H.; Herron, N.; Guise, W.E.; Wang, X.; Whitfield, P.S.; Jain, A.; Gong, X.; McGaughey, A.J.H.; et al. Lattice Dynamics and the Nature of Structural Transitions in Organolead Halide Perovskites. Phys. Rev. B 2016, 94. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Oswald, I.W.H.; Wang, X.; McCandless, G.T.; Chan, J.Y. Orientation of Organic Cations in Hybrid Inorganic–Organic Perovskite CH3NH3PbI3 from Subatomic Resolution Single Crystal Neutron Diffraction Structural Studies. Cryst. Growth Des. 2016, 16, 2945–2951. [Google Scholar] [CrossRef]
- Minns, J.L.; Zajdel, P.; Chernyshov, D.; van Beek, W.; Green, M.A. Structure and Interstitial Iodide Migration in Hybrid Perovskite Methylammonium Lead Iodide. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Weber, O.J.; Ghosh, D.; Gaines, S.; Henry, P.F.; Walker, A.B.; Islam, M.S.; Weller, M.T. Phase Behavior and Polymorphism of Formamidinium Lead Iodide. Chem. Mater. 2018, 30, 3768–3778. [Google Scholar] [CrossRef]
- Mozur, E.M.; Trowbridge, J.C.; Maughan, A.E.; Gorman, M.J.; Brown, C.M.; Prisk, T.R.; Neilson, J.R. Dynamical Phase Transitions and Cation Orientation-Dependent Photoconductivity in CH(NH2)2PbBr3. ACS Mater. Lett. 2019, 1, 260–264. [Google Scholar] [CrossRef]
- Franz, A.; Többens, D.M.; Lehmann, F.; Kärgell, M.; Schorr, S. The Influence of Deuteration on the Crystal Structure of Hybrid Halide Perovskites: A Temperature-dependent Neutron Diffraction Study of FAPbBr3. Acta Crystallogr. B 2020, 76, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breternitz, J.; Tovar, M.; Schorr, S. Twinning in MAPbI3 at Room Temperature Uncovered Through Laue Neutron Diffraction. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Keen, D.A.; Gutmann, M.J.; Wilson, C.C. SXD – the single-crystal diffractometer at the ISIS spallation neutron source. J. Appl. Crystallogr. 2006, 39, 714–722. [Google Scholar] [CrossRef]
- Koetzle, T.F.; Bau, R.; Hoffmann, C.; Piccoli, P.M.B.; Schultz, A.J. Topaz: A Single-crystal Diffractometer for the Spallation Neutron Source. Acta Crystallogr. A 2006, 62, s116. [Google Scholar] [CrossRef]
- Ohhara, T.; Kiyanagi, R.; Oikawa, K.; Kaneko, K.; Kawasaki, T.; Tamura, I.; Nakao, A.; Hanashima, T.; Munakata, K.; Moyoshi, T.; et al. SENJU: A new time-of-flight single-crystal neutron diffractometer at J-PARC. J. Appl. Crystallogr. 2016, 49, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, A.J. Neutron Diffraction – Recent Applications to Chemical Structure Determination. Aust. J. Chem. 2011, 64, 869. [Google Scholar] [CrossRef]
- Raventós, M.; Tovar, M.; Medarde, M.; Shang, T.; Strobl, M.; Samothrakitis, S.; Pomjakushina, E.; Grünzweig, C.; Schmidt, S. Laue Three Dimensional Neutron Diffraction. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Szafrański, M.; Katrusiak, A. Photovoltaic Hybrid Perovskites under Pressure. J. Phys. Chem. Lett. 2017, 8, 2496–2506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postorino, P.; Malavasi, L. Pressure-Induced Effects in Organic–Inorganic Hybrid Perovskites. J. Phys. Chem. Lett. 2017, 8, 2613–2622. [Google Scholar] [CrossRef] [PubMed]
- Capitani, F.; Marini, C.; Caramazza, S.; Postorino, P.; Garbarino, G.; Hanfland, M.; Pisanu, A.; Quadrelli, P.; Malavasi, L. High-pressure Behavior of Methylammonium Lead Iodide (MAPbI3) Hybrid Perovskite. J. Appl. Phys. 2016, 119, 185901. [Google Scholar] [CrossRef] [Green Version]
- Jaffe, A.; Lin, Y.; Beavers, C.M.; Voss, J.; Mao, W.L.; Karunadasa, H.I. High-Pressure Single-Crystal Structures of 3D Lead-Halide Hybrid Perovskites and Pressure Effects on their Electronic and Optical Properties. ACS Cent. Sci. 2016, 2, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Szafrański, M.; Katrusiak, A. Mechanism of Pressure-Induced Phase Transitions, Amorphization, and Absorption-Edge Shift in Photovoltaic Methylammonium Lead Iodide. J. Phys. Chem. Lett. 2016, 7, 3458–3466. [Google Scholar] [CrossRef] [Green Version]
- Swainson, I.P.; Tucker, M.G.; Wilson, D.J.; Winkler, B.; Milman, V. Pressure Response of an Organic-Inorganic Perovskite: Methylammonium Lead Bromide. Chem. Mater. 2007, 19, 2401–2405. [Google Scholar] [CrossRef]
- Wiedemann, D.; Breternitz, J.; Paley, D.W.; Schorr, S. Hybrid Perovskite at Full Tilt: Structure and Symmetry Relations of the Incommensurately Modulated Phase of Methylammonium Lead Bromide, MAPbBr3. J. Phys. Chem. Lett. 2021, 2358–2362. [Google Scholar] [CrossRef]
- Kong, L.; Gong, J.; Hu, Q.; Capitani, F.; Celeste, A.; Hattori, T.; Sano-Furukawa, A.; Li, N.; Yang, W.; Liu, G.; et al. Halide Perovskites: Suppressed Lattice Disorder for Large Emission Enhancement and Structural Robustness in Hybrid Lead Iodide Perovskite Discovered by High-Pressure Isotope Effect. Adv. Funct. Mater. 2021, 31, 2170057. [Google Scholar] [CrossRef]
- Mozur, E.M.; Neilson, J.R. Cation Dynamics in Hybrid Halide Perovskites. arXiv 2020, arXiv:2012.05115. [Google Scholar]
- Manley, M.E.; Hong, K.; Yin, P.; Chi, S.; Cai, Y.; Hua, C.; Daemen, L.L.; Hermann, R.P.; Wang, H.; May, A.F.; et al. Giant Isotope Effect on Phonon Dispersion and Thermal Conductivity in Methylammonium Lead Iodide. Sci. Adv. 2020, 6, eaaz1842. [Google Scholar] [CrossRef] [PubMed]
- Gold-Parker, A.; Gehring, P.M.; Skelton, J.M.; Smith, I.C.; Parshall, D.; Frost, J.M.; Karunadasa, H.I.; Walsh, A.; Toney, M.F. Acoustic Phonon Lifetimes Limit Thermal Transport in Methylammonium Lead Iodide. Proc. Natl. Acad. Sci. USA 2018, 115, 11905–11910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weadock, N.J.; Gehring, P.M.; Gold-Parker, A.; Smith, I.C.; Karunadasa, H.I.; Toney, M.F. Test of the Dynamic-Domain and Critical Scattering Hypotheses in Cubic Methylammonium Lead Triiodide. Phys. Rev. Lett. 2020, 125. [Google Scholar] [CrossRef]
- Songvilay, M.; Bari, M.; Ye, Z.G.; Xu, G.; Gehring, P.M.; Ratcliff, W.D.; Schmalzl, K.; Bourdarot, F.; Roessli, B.; Stock, C. Lifetime-shortened acoustic phonons and static order at the Brillouin zone boundary in the organic-inorganic perovskite CH3NH3PbCl3. Phys. Rev. Mater. 2018, 2. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Létoublon, A.; Paofai, S.; Raymond, S.; Ecolivet, C.; Rufflé, B.; Cordier, S.; Katan, C.; Saidaminov, M.I.; Zhumekenov, A.A.; et al. Elastic Softness of Hybrid Lead Halide Perovskites. Phys. Rev. Lett. 2018, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Even, J.; Paofai, S.; Bourges, P.; Letoublon, A.; Cordier, S.; Durand, O.; Katan, C. Carrier scattering processes and low energy phonon spectroscopy in hybrid perovskites crystals. In Physics, Simulation, and Photonic Engineering of Photovoltaic Devices V; Freundlich, A., Lombez, L., Sugiyama, M., Eds.; SPIE: Bellingham, WA, USA, 2016. [Google Scholar] [CrossRef] [Green Version]
- Létoublon, A.; Paofai, S.; Rufflé, B.; Bourges, P.; Hehlen, B.; Michel, T.; Ecolivet, C.; Durand, O.; Cordier, S.; Katan, C.; et al. Elastic Constants, Optical Phonons, and Molecular Relaxations in the High Temperature Plastic Phase of the CH3NH3PbBr3 Hybrid Perovskite. J. Phys. Chem. Lett. 2016, 7, 3776–3784. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.C.; Paofai, S.; Létoublon, A.; Ollivier, J.; Raymond, S.; Hehlen, B.; Rufflé, B.; Cordier, S.; Katan, C.; Even, J.; et al. Direct Evidence of Weakly Dispersed and Strongly Anharmonic Optical Phonons in Hybrid Perovskites. Commun. Phys. 2020, 3. [Google Scholar] [CrossRef]
- Swainson, I.P.; Stock, C.; Parker, S.F.; Eijck, L.V.; Russina, M.; Taylor, J.W. From Soft Harmonic Phonons to Fast Relaxational Dynamics in CH3NH3PbBr3. Phys. Rev. B 2015, 92. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.L.; Parker, S.F.; García, I.R.; Mukhopadhyay, S.; Sakai, V.G.; Stock, C. Molecular Orientational Melting Within a Lead-halide Octahedron Framework: The Order-disorder Transition in CH3NH3PbBr3. Phys. Rev. B 2017, 96. [Google Scholar] [CrossRef] [Green Version]
- Songvilay, M.; Wang, Z.; Sakai, V.G.; Guidi, T.; Bari, M.; Ye, Z.G.; Xu, G.; Brown, K.L.; Gehring, P.M.; Stock, C. Decoupled molecular and inorganic framework dynamics in CH3NH3PbCl3. Phys. Rev. Mater. 2019, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Hu, X.; Chen, T.; Abernathy, D.L.; Kajimoto, R.; Nakamura, M.; Kofu, M.; Foley, B.J.; Yoon, M.; Choi, J.J.; et al. Temporally Decoherent and Spatially Coherent Vibrations in Metal Halide Perovskites. Phys. Rev. B 2020, 102. [Google Scholar] [CrossRef]
- Li, B.; Kawakita, Y.; Liu, Y.; Wang, M.; Matsuura, M.; Shibata, K.; Ohira-Kawamura, S.; Yamada, T.; Lin, S.; Nakajima, K.; et al. Polar Rotor Scattering as Atomic-level Origin of Low Mobility and Thermal Conductivity of Perovskite CH3NH3PbI3. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Schuck, G.; Lehmann, F.; Ollivier, J.; Mutka, H.; Schorr, S. Influence of Chloride Substitution on the Rotational Dynamics of Methylammonium in MAPbI3–xClxPerovskites. J. Phys. Chem. C 2019, 123, 11436–11446. [Google Scholar] [CrossRef] [Green Version]
- Beecher, A.N.; Semonin, O.E.; Skelton, J.M.; Frost, J.M.; Terban, M.W.; Zhai, H.; Alatas, A.; Owen, J.S.; Walsh, A.; Billinge, S.J.L. Direct Observation of Dynamic Symmetry Breaking above Room Temperature in Methylammonium Lead Iodide Perovskite. ACS Energy Lett. 2016, 1, 880–887. [Google Scholar] [CrossRef]
- Ma, H.; Ma, Y.; Wang, H.; Slebodnick, C.; Alatas, A.; Urban, J.J.; Tian, Z. Experimental Phonon Dispersion and Lifetimes of Tetragonal CH3NH3PbI3 Perovskite Crystals. J. Phys. Chem. Lett. 2018, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Heyer, D.; Buchenau, U.; Stamm, M. Determination of elastic shear constants of polyethylene at room temperature by inelastic neutron scattering. J. Polym. Sci. Polym. Phys. Ed. 1984, 22, 1515–1527. [Google Scholar] [CrossRef]
- Yang, J.; Wen, X.; Xia, H.; Sheng, R.; Ma, Q.; Kim, J.; Tapping, P.; Harada, T.; Kee, T.W.; Huang, F.; et al. Acoustic-optical Phonon Up-conversion and Hot-phonon Bottleneck in Lead-halide Perovskites. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Handa, T.; Yamada, T.; Nagai, M.; Kanemitsu, Y. Phonon, Thermal, and Thermo-optical Properties of Halide Perovskites. Phys. Chem. Chem. Phys. 2020, 22, 26069–26087. [Google Scholar] [CrossRef]
- Sendner, M.; Nayak, P.K.; Egger, D.A.; Beck, S.; Müller, C.; Epding, B.; Kowalsky, W.; Kronik, L.; Snaith, H.J.; Pucci, A.; et al. Optical phonons in methylammonium lead halide perovskites and implications for charge transport. Mater. Horizons 2016, 3, 613–620. [Google Scholar] [CrossRef] [Green Version]
- Herz, L.M. Charge-Carrier Mobilities in Metal Halide Perovskites: Fundamental Mechanisms and Limits. ACS Energy Lett. 2017, 2, 1539–1548. [Google Scholar] [CrossRef]
- Herz, L.M. How Lattice Dynamics Moderate the Electronic Properties of Metal-Halide Perovskites. J. Phys. Chem. Lett. 2018, 9, 6853–6863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanigan-Atkins, T.; He, X.; Krogstad, M.J.; Pajerowski, D.M.; Abernathy, D.L.; Xu, G.N.M.N.; Xu, Z.; Chung, D.Y.; Kanatzidis, M.G.; Rosenkranz, S.; et al. Two-dimensional Overdamped Fluctuations of the Soft Perovskite Lattice in CsPbBr3. Nat. Mater. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, F.; Meggiolaro, D.; Mosconi, E.; Angelis, F.D. Charge Localization, Stabilization, and Hopping in Lead Halide Perovskites: Competition between Polaron Stabilization and Cation Disorder. ACS Energy Lett. 2019, 4, 2013–2020. [Google Scholar] [CrossRef] [Green Version]
- Leguy, A.M.A.; Goñi, A.R.; Frost, J.M.; Skelton, J.; Brivio, F.; Rodríguez-Martínez, X.; Weber, O.J.; Pallipurath, A.; Alonso, M.I.; Campoy-Quiles, M.; et al. Dynamic Disorder, Phonon Lifetimes, and the Assignment of Modes to the Vibrational Spectra of Methylammonium Lead Halide Perovskites. Phys. Chem. Chem. Phys. 2016, 18, 27051–27066. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Hunger, J.; Cánovas, E.; Karakus, M.; Mics, Z.; Grechko, M.; Turchinovich, D.; Parekh, S.H.; Bonn, M. Direct observation of mode-specific phonon-band gap coupling in methylammonium lead halide perovskites. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Foley, B.J.; Ipek, B.; Tyagi, M.; Copley, J.R.D.; Brown, C.M.; Choi, J.J.; Lee, S.H. Rotational Dynamics of Organic Cations in the CH3NH3PbI3 Perovskite. Phys. Chem. Chem. Phys. 2015, 17, 31278–31286. [Google Scholar] [CrossRef] [Green Version]
- Parker, S.F.; Ramirez-Cuesta, A.J.; Albers, P.W.; Lennon, D. The use of Direct Geometry Spectrometers in Molecular Spectroscopy. J. Phys. Conf. Ser. 2014, 554, 012004. [Google Scholar] [CrossRef]
- Parker, S.F.; Fernandez-Alonso, F.; Ramirez-Cuesta, A.J.; Tomkinson, J.; Rudic, S.; Pinna, R.S.; Gorini, G.; Castañon, J.F. Recent and future developments on TOSCA at ISIS. J. Phys. Conf. Ser. 2014, 554, 012003. [Google Scholar] [CrossRef]
- Pinna, R.S.; Rudić, S.; Capstick, M.J.; McPhail, D.J.; Pooley, D.E.; Howells, G.D.; Gorini, G.; Fernandez-Alonso, F. Detailed Characterisation of the Incident Neutron Beam on the TOSCA Spectrometer. Nucl. Instrum. Methods Phys. Res. A 2017, 870, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Pinna, R.S.; Rudić, S.; Parker, S.F.; Armstrong, J.; Zanetti, M.; Škoro, G.; Waller, S.P.; Zacek, D.; Smith, C.A.; Capstick, M.J.; et al. The Neutron Guide Upgrade of the TOSCA Spectrometer. Nucl. Instrum. Methods Phys. Res. A 2018, 896, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Pinna, R.S.; Zanetti, M.; Rudić, S.; Parker, S.; Armstrong, J.; Waller, S.; Zacek, D.; Smith, C.; Harrison, S.; Gorini, G.; et al. The TOSCA Spectrometer at ISIS: The Guide Upgrade and Beyond. J. Phys. Conf. Ser. 2018, 1021, 012029. [Google Scholar] [CrossRef]
- Zanetti, M.; Bellissima, S.; del Rosso, L.; Masi, F.; Chowdhury, M.; Bonis, A.D.; Fresco, L.D.; Scatigno, C.; Armstrong, J.; Rudić, S.; et al. Neutronic developments on TOSCA and VESPA: Progress to date. Phys. B Condens. Matter. 2019, 562, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Seeger, P.A.; Daemen, L.L.; Larese, J.Z. Resolution of VISION, a Crystal-analyzer Spectrometer. Nucl. Instrum. Methods Phys. Res. 2009, 604, 719–728. [Google Scholar] [CrossRef]
- Ivanov, A.; Jimenéz-Ruiz, M.; Kulda, J. IN1-LAGRANGE – the new ILL Instrument to Explore Vibration Dynamics of Complex Materials. J. Phys. Conf. Ser. 2014, 554, 012001. [Google Scholar] [CrossRef] [Green Version]
- Drużbicki, K.; Pinna, R.S.; Rudić, S.; Jura, M.; Gorini, G.; Fernandez-Alonso, F. Unexpected Cation Dynamics in the Low-Temperature Phase of Methylammonium Lead Iodide: The Need for Improved Models. J. Phys. Chem. Lett. 2016, 7, 4701–4709. [Google Scholar] [CrossRef] [Green Version]
- Demmel, F.; McPhail, D.; Crawford, J.; Maxwell, D.; Pokhilchuk, K.; Garcia-Sakai, V.; Mukhopadyay, S.; Telling, M.; Bermejo, F.; Skipper, N.; et al. Opening the Terahertz Window on the OSIRIS Spectrometer. EPJ Web Conf. 2015, 83, 03003. [Google Scholar] [CrossRef] [Green Version]
- Bernard, G.M.; Wasylishen, R.E.; Ratcliffe, C.I.; Terskikh, V.; Wu, Q.; Buriak, J.M.; Hauger, T. Methylammonium Cation Dynamics in Methylammonium Lead Halide Perovskites: A Solid-State NMR Perspective. J. Phys. Chem. A 2018, 122, 1560–1573. [Google Scholar] [CrossRef] [Green Version]
- Supercritical Water. Chem. Eng. News 1991, 69, 26–39. [CrossRef]
- Bryce, D.L. NMR crystallography: Structure and properties of materials from solid-state nuclear magnetic resonance observables. IUCrJ 2017, 4, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Drużbicki, K.; Pajzderska, A.; Chudoba, D.; Jenczyk, J.; Jarek, M.; Mielcarek, J.; Wa̧sicki, J. Elucidating the Structure of Ranitidine Hydrochloride Form II: Insights from Solid-State Spectroscopy and ab initio Simulations. Cryst. Growth Des. 2018, 18, 4671–4681. [Google Scholar] [CrossRef]
- Trzeciak, K.; Kazmierski, S.; Druzbicki, K.; Potrzebowski, M.J. Mapping of Guest Localization in Mesoporous Silica Particles by Solid-state NMR and ab initio Modelling: New Insights into Benzoic Acid and p-Fluorobenzoic Acid Embedded in MCM-41 via Ball Milling. J. Phys. Chem. C 2021, in press. [Google Scholar]
- Souza, B.E.; Rudić, S.; Titov, K.; Babal, A.S.; Taylor, J.D.; Tan, J.C. Guest–host Interactions of Nanoconfined Anti-cancer Drug in Metal–organic Framework Exposed by Terahertz Dynamics. Chem. Commun. 2019, 55, 3868–3871. [Google Scholar] [CrossRef] [PubMed]
- Łuczyńska, K.; Drużbicki, K.; Runka, T.; Pałka, N.; Węsicki, J. Vibrational Response of Felodipine in the THz Domain: Optical and Neutron Spectroscopy Versus Plane-Wave DFT Modeling. J. Infrared Millim. Terahertz Waves 2019, 41, 1301–1336. [Google Scholar] [CrossRef] [Green Version]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First Principles Methods Using CASTEP. Z. Kristallogr. Cryst. Mater. 2005, 220. [Google Scholar] [CrossRef] [Green Version]
- Refson, K.; Tulip, P.R.; Clark, S.J. Variational Density-functional Perturbation Theory for Dielectrics and Lattice Dynamics. Phys. Rev. B 2006, 73. [Google Scholar] [CrossRef] [Green Version]
- Giannozzi, P.; Baseggio, O.; Bonfà, P.; Brunato, D.; Car, R.; Carnimeo, I.; Cavazzoni, C.; de Gironcoli, S.; Delugas, P.; Ruffino, F.F.; et al. Quantum ESPRESSO Toward the Exascale. J. Chem. Phys. 2020, 152, 154105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonze, X.; Jollet, F.; Araujo, F.A.; Adams, D.; Amadon, B.; Applencourt, T.; Audouze, C.; Beuken, J.M.; Bieder, J.; Bokhanchuk, A.; et al. Recent developments in the ABINIT software package. Comput. Phys. Commun. 2016, 205, 106–131. [Google Scholar] [CrossRef] [Green Version]
- Hafner, J.; Kresse, G. The Vienna AB-Initio Simulation Program VASP: An Efficient and Versatile Tool for Studying the Structural, Dynamic, and Electronic Properties of Materials. In Properties of Complex Inorganic Solids; Springer: New York, NY, USA, 1997; pp. 69–82. [Google Scholar] [CrossRef]
- Enkovaara, J.; Rostgaard, C.; Mortensen, J.J.; Chen, J.; Dułak, M.; Ferrighi, L.; Gavnholt, J.; Glinsvad, C.; Haikola, V.; Hansen, H.A.; et al. Electronic Structure Calculations with GPAW: A Real-space Implementation of the Projector Augmented-wave Method. J. Phys. Condens. Matter 2010, 22, 253202. [Google Scholar] [CrossRef]
- Blum, V.; Gehrke, R.; Hanke, F.; Havu, P.; Havu, V.; Ren, X.; Reuter, K.; Scheffler, M. ab initio Molecular Simulations with Numeric Atom-centered Orbitals. Comput. Phys. Commun. 2009, 180, 2175–2196. [Google Scholar] [CrossRef] [Green Version]
- Shang, H.; Carbogno, C.; Rinke, P.; Scheffler, M. Lattice Dynamics Calculations Based on Density-functional Perturbation Theory in Real Space. Comput. Phys. Commun. 2017, 215, 26–46. [Google Scholar] [CrossRef]
- García, A.; Papior, N.; Akhtar, A.; Artacho, E.; Blum, V.; Bosoni, E.; Brandimarte, P.; Brandbyge, M.; Cerdá, J.I.; Corsetti, F.; et al. Siesta: Recent Developments and Applications. J. Chem. Phys. 2020, 152, 204108. [Google Scholar] [CrossRef] [PubMed]
- Dovesi, R.; Erba, A.; Orlando, R.; Zicovich-Wilson, C.M.; Civalleri, B.; Maschio, L.; Rérat, M.; Casassa, S.; Baima, J.; Salustro, S.; et al. Quantum-mechanical Condensed Matter Simulations with CRYSTAL. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1360. [Google Scholar] [CrossRef]
- Balasubramani, S.G.; Chen, G.P.; Coriani, S.; Diedenhofen, M.; Frank, M.S.; Franzke, Y.J.; Furche, F.; Grotjahn, R.; Harding, M.E.; Hättig, C.; et al. TURBOMOLE: Modular Program Suite for ab initio Quantum-chemical and Condensed-matter Simulations. J. Chem. Phys. 2020, 152, 184107. [Google Scholar] [CrossRef]
- Kühne, T.D.; Iannuzzi, M.; Ben, M.D.; Rybkin, V.V.; Seewald, P.; Stein, F.; Laino, T.; Khaliullin, R.Z.; Schütt, O.; Schiffmann, F.; et al. CP2K: An Electronic Structure and Molecular Dynamics Software Package—Quickstep: Efficient and Accurate Electronic Structure Calculations. J. Chem. Phys. 2020, 152, 194103. [Google Scholar] [CrossRef] [PubMed]
- Blaha, P.; Schwarz, K.; Tran, F.; Laskowski, R.; Madsen, G.K.H.; Marks, L.D. WIEN2k: An APWlo Program for Calculating the Properties of Solids. J. Chem. Phys. 2020, 152, 074101. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst, J.K.; Sharma, S.; Nordström, L.; Cricchio, F. Available online: elk.sourceforge.net (accessed on 28 April 2021).
- Parlinski, K.; Li, Z.Q.; Kawazoe, Y. First-Principles Determination of the Soft Mode in Cubic ZrO2. Phys. Rev. Lett. 1997, 78, 4063–4066. [Google Scholar] [CrossRef]
- Baroni, S.; Giannozzi, P.; Testa, A. Green’s-function Approach to Linear Response in Solids. Phys. Rev. Lett. 1987, 58, 1861–1864. [Google Scholar] [CrossRef]
- Gonze, X. Perturbation Expansion of Variational Principles at Arbitrary Order. Phys. Rev. A 1995, 52, 1086–1095. [Google Scholar] [CrossRef]
- Gonze, X. Adiabatic Density-Functional Perturbation Theory. Phys. Rev. A 1995, 52, 1096–1114. [Google Scholar] [CrossRef]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Chaput, L.; Togo, A.; Tanaka, I.; Hug, G. Phonon-phonon Interactions in Transition Metals. Phys. Rev. B 2011, 84. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shang, S.L.; Fang, H.; Liu, Z.K.; Chen, L.Q. First-principles Calculations of Lattice Dynamics and Thermal Properties of Polar Solids. NPJ Comput. Mater. 2016, 2. [Google Scholar] [CrossRef]
- Lloyd-Williams, J.H.; Monserrat, B. Lattice Dynamics and Electron-phonon Coupling Calculations Using Nondiagonal Supercells. Phys. Rev. B 2015, 92. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Cuesta, A. aCLIMAX 4.0.1, The new version of the software for analyzing and interpreting INS spectra. Comput. Phys. Commun. 2004, 157, 226–238. [Google Scholar] [CrossRef]
- Dymkowski, K.; Parker, S.F.; Fernandez-Alonso, F.; Mukhopadhyay, S. AbINS: The Modern Software for INS Interpretation. Phys. B Condens. Matter 2018, 551, 443–448. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Daemen, L.L.; Kolesnikov, A.I.; Ramirez-Cuesta, A.J. Simulation of Inelastic Neutron Scattering Spectra Using OCLIMAX. J. Chem. Theory Comput. 2019, 15, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Farhi, E. iFit: A Simple Library to Analyze Data. Available online: http://ifit.mccode.org/Models_Phonons.html (accessed on 28 April 2021).
- Euphonic. Available online: https://euphonic.readthedocs.io/en/latest/ (accessed on 28 April 2021).
- Parlinski, K. PhononA. Available online: http://www.computingformaterials.com/ (accessed on 28 April 2021).
- Kieslich, G.; Skelton, J.M.; Armstrong, J.; Wu, Y.; Wei, F.; Svane, K.L.; Walsh, A.; Butler, K.T. Hydrogen Bonding versus Entropy: Revealing the Underlying Thermodynamics of the Hybrid Organic–Inorganic Perovskite [CH3NH3]PbBr3. Chem. Mater. 2018, 30, 8782–8788. [Google Scholar] [CrossRef] [Green Version]
- Brehm, M.; Thomas, M.; Gehrke, S.; Kirchner, B. TRAVIS—A Free Analyzer for Trajectories from Molecular Simulation. J. Chem. Phys. 2020, 152, 164105. [Google Scholar] [CrossRef] [Green Version]
- Goret, G.; Aoun, B.; Pellegrini, E. MDANSE: An Interactive Analysis Environment for Molecular Dynamics Simulations. J. Chem. Inf. Model. 2017, 57, 1–5. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Kolesnikov, A.I.; Ramirez-Cuesta, A.J. Simulation of Inelastic Neutron Scattering Spectra Directly from Molecular Dynamics Trajectories. J. Chem. Theory Comput. 2020, 16, 7702–7708. [Google Scholar] [CrossRef] [PubMed]
- Fransson, E.; Slabanja, M.; Erhart, P.; Wahnström, G. dynasor—A Tool for Extracting Dynamical Structure Factors and Current Correlation Functions from Molecular Dynamics Simulations. Adv. Theory Simul. 2021, 4, 2000240. [Google Scholar] [CrossRef]
- Carreras, A.; Togo, A.; Tanaka, I. DynaPhoPy: A code for extracting phonon quasiparticles from molecular dynamics simulations. Comput. Phys. Commun. 2017, 221, 221–234. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhang, D.B.; Sun, T.; Wentzcovitch, R. phq: A Fortran code to compute phonon quasiparticle properties and dispersions. Comput. Phys. Commun. 2019, 243, 110–120. [Google Scholar] [CrossRef] [Green Version]
- Parker, S.F.; Leich, V.; Hönig, J.; Albers, P.W. Investigation of Commercial Graphenes. ChemistryOpen 2020, 9, 1060–1064. [Google Scholar] [CrossRef]
- Pontiroli, D.; Aramini, M.; Gaboardi, M.; Mazzani, M.; Sanna, S.; Caracciolo, F.; Carretta, P.; Cavallari, C.; Rols, S.; Tatti, R.; et al. Tracking the Hydrogen Motion in Defective Graphene. J. Phys. Chem. C 2014, 118, 7110–7116. [Google Scholar] [CrossRef]
- Natkaniec, I.; Sheka, E.F.; Drużbicki, K.; Hołderna-Natkaniec, K.; Gubin, S.P.; Buslaeva, E.Y.; Tkachev, S.V. Computationally Supported Neutron Scattering Study of Parent and Chemically Reduced Graphene Oxide. J. Phys. Chem. C 2015, 119, 18650–18662. [Google Scholar] [CrossRef]
- Cavallari, C.; Pontiroli, D.; Jiménez-Ruiz, M.; Johnson, M.; Aramini, M.; Gaboardi, M.; Parker, S.F.; Riccó, M.; Rols, S. Hydrogen Motions in Defective Graphene: The Role of Surface Defects. Phys. Chem. Chem. Phys. 2016, 18, 24820–24824. [Google Scholar] [CrossRef]
- Cavallari, C.; Rols, S.; Fischer, H.E.; Brunelli, M.; Gaboardi, M.; Magnani, G.; Riccò, M.; Pontiroli, D. Neutron Scattering Study of Nickel Decorated Thermally Exfoliated Graphite Oxide. Int. J. Hydrogen Energy 2019, 44, 30999–31007. [Google Scholar] [CrossRef]
- Vottero, E.; Carosso, M.; Jiménez-Ruiz, M.; Pellegrini, R.; Groppo, E.; Piovano, A. How do the Graphenic Domains Terminate in Activated Carbons and Carbon-supported Metal Catalysts? Carbon 2020, 169, 357–369. [Google Scholar] [CrossRef]
- Blackburn, J.L.; Engtrakul, C.; Bult, J.B.; Hurst, K.; Zhao, Y.; Xu, Q.; Parilla, P.A.; Simpson, L.J.; Rocha, J.d.R.; Hudson, M.R.; et al. Spectroscopic Identification of Hydrogen Spillover Species in Ruthenium-Modified High Surface Area Carbons by Diffuse Reflectance Infrared Fourier Transform Spectroscopy. J. Phys. Chem. C 2012, 116, 26744–26755. [Google Scholar] [CrossRef]
- Tsao, C.S.; Liu, Y.; Chuang, H.Y.; Tseng, H.H.; Chen, T.Y.; Chen, C.H.; Yu, M.S.; Li, Q.; Lueking, A.; Chen, S.H. Hydrogen Spillover Effect of Pt-Doped Activated Carbon Studied by Inelastic Neutron Scattering. J. Phys. Chem. Lett. 2011, 2322–2325. [Google Scholar] [CrossRef]
- Cavallari, C.; Pontiroli, D.; Jiménez-Ruiz, M.; Ivanov, A.; Mazzani, M.; Gaboardi, M.; Aramini, M.; Brunelli, M.; Riccò, M.; Rols, S. Hydrogen on Graphene Investigated by Inelastic Neutron Scattering. J. Phys. Conf. Ser. 2014, 554, 012009. [Google Scholar] [CrossRef] [Green Version]
- Drużbicki, K.; Natkaniec, I. Vibrational Properties of Water Retained in Graphene Oxide. Chem. Phys. Lett. 2014, 600, 106–111. [Google Scholar] [CrossRef]
- Romanelli, G.; Senesi, R.; Zhang, X.; Loh, K.P.; Andreani, C. Probing the Effects of 2D Confinement on Hydrogen Dynamics in Water and Ice Adsorbed in Graphene Oxide Sponges. Phys. Chem. Chem. Phys. 2015, 17, 31680–31684. [Google Scholar] [CrossRef] [Green Version]
- Romanelli, G.; Liscio, A.; Senesi, R.; Zamboni, R.; Treossi, E.; Liscio, F.; Giambastiani, G.; Palermo, V.; Fernandez-Alonso, F.; Andreani, C. Soft Confinement of Water in Graphene-oxide Membranes. Carbon 2016, 108, 199–203. [Google Scholar] [CrossRef]
- Osti, N.C.; Naguib, M.; Ganeshan, K.; Shin, Y.K.; Ostadhossein, A.; van Duin, A.C.T.; Cheng, Y.; Daemen, L.L.; Gogotsi, Y.; Mamontov, E.; et al. Influence of Metal Ions Intercalation on the Vibrational Dynamics of Water Confined Between MXene Layers. Phys. Rev. Mater. 2017, 1. [Google Scholar] [CrossRef]
- Barroso-Bujans, F.; Fernandez-Alonso, F.; Colmenero, J. Neutron Spectroscopy as a Probe of Macromolecular Structure and Dynamics under Extreme Spatial Confinement. J. Phys. Conf. Ser. 2014, 549, 012009. [Google Scholar] [CrossRef]
- Barroso-Bujans, F.; Fernandez-Alonso, F.; Cerveny, S.; Parker, S.F.; Alegría, A.; Colmenero, J. Polymers Under Extreme Two-dimensional Confinement: Poly(ethylene oxide) in Graphite Oxide. Soft Matter 2011, 7, 7173. [Google Scholar] [CrossRef] [Green Version]
- Barroso-Bujans, F.; Fernandez-Alonso, F.; Cerveny, S.; Arrese-Igor, S.; Alegría, A.; Colmenero, J. Two-Dimensional Subnanometer Confinement of Ethylene Glycol and Poly(ethylene oxide) by Neutron Spectroscopy: Molecular Size Effects. Macromolecules 2012, 45, 3137–3144. [Google Scholar] [CrossRef]
- Barroso-Bujans, F.; Fernandez-Alonso, F.; Pomposo, J.A.; Cerveny, S.; Alegría, A.; Colmenero, J. Macromolecular Structure and Vibrational Dynamics of Confined Poly(ethylene oxide): From Subnanometer 2D-Intercalation into Graphite Oxide to Surface Adsorption onto Graphene Sheets. ACS Macro Lett. 2012, 1, 550–554. [Google Scholar] [CrossRef] [Green Version]
- Barroso-Bujans, F.; Palomino, P.; Cerveny, S.; Fernandez-Alonso, F.; Rudić, S.; Alegría, A.; Colmenero, J.; Enciso, E. Confinement of Poly(ethylene oxide) in the Nanometer-scale Pores of Resins and Carbon Nanoparticles. Soft Matter 2013, 9, 10960. [Google Scholar] [CrossRef]
- Vilela, C.; Freire, C.S.R.; Araújo, C.; Rudić, S.; Silvestre, A.J.D.; Vaz, P.D.; Ribeiro-Claro, P.J.A.; Nolasco, M.M. Understanding the Structure and Dynamics of Nanocellulose-Based Composites with Neutral and Ionic Poly(methacrylate) Derivatives Using Inelastic Neutron Scattering and DFT Calculations. Molecules 2020, 25, 1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilela, C.; Cordeiro, D.M.; Boas, J.V.; Barbosa, P.; Nolasco, M.; Vaz, P.D.; Rudić, S.; Ribeiro-Claro, P.; Silvestre, A.J.; Oliveira, V.B.; et al. Poly(4-styrene Sulfonic Acid)/Bacterial Cellulose Membranes: Electrochemical Performance in a Single-chamber Microbial Fuel Cell. Bioresour. Technol. Rep. 2020, 9, 100376. [Google Scholar] [CrossRef]
- Parker, S.F.; Shah, S. Characterisation of Hydration Water in Nafion Membrane. RSC Adv. 2021, 11, 9381–9385. [Google Scholar] [CrossRef]
- Lovell, A.; Fernandez-Alonso, F.; Skipper, N.T.; Refson, K.; Bennington, S.M.; Parker, S.F. Quantum Delocalization of Molecular Hydrogen in Alkali-Graphite Intercalates. Phys. Rev. Lett. 2008, 101, 126101. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Alonso, F.; Bermejo, F.J.; Cabrillo, C.; Loutfy, R.O.; Leon, V.; Saboungi, M.L. Nature of the Bound States of Molecular Hydrogen in Carbon Nanohorns. Phys. Rev. Lett. 2007, 98, 215503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzystyniak, M.; Romanelli, G.; Fernandez-Alonso, F. Non-destructive Quantitation of Hydrogen via Mass-resolved Neutron Spectroscopy. Analyst 2019, 144, 3936–3941. [Google Scholar] [CrossRef] [PubMed]
- Schur, D.V.; Zaginaichenko, S.Y.; Savenko, A.F.; Bogolepov, V.A.; Anikina, N.S.; Zolotarenko, A.; Matysina, Z.A.; Veziroglu, T.N.; Skryabina, N.E. Experimental Evaluation of Total Hydrogen Capacity for Fullerite C60. Int. J. Hydrogen Energy 2011, 36, 1143–1151. [Google Scholar] [CrossRef]
- Mauron, P.; Remhof, A.; Bliersbach, A.; Borgschulte, A.; Züttel, A.; Sheptyakov, D.; Gaboardi, M.; Choucair, M.; Pontiroli, D.; Aramini, M.; et al. Reversible Hydrogen Absorption in Sodium Intercalated Fullerenes. Int. J. Hydrogen Energy 2012, 37, 14307–14314. [Google Scholar] [CrossRef]
- Gaboardi, M.; Amadé, N.S.; Aramini, M.; Milanese, C.; Magnani, G.; Sanna, S.; Riccò, M.; Pontiroli, D. Extending the Hydrogen Storage Limit in Fullerene. Carbon 2017, 120, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Mauron, P.; Gaboardi, M.; Pontiroli, D.; Remhof, A.; Riccò, M.; Züttel, A. Hydrogen Desorption Kinetics in Metal Intercalated Fullerides. J. Phys. Chem. C 2015, 119, 1714–1719. [Google Scholar] [CrossRef]
- Amadè, N.S.; Pontiroli, D.; Maidich, L.; Riccò, M.; Gaboardi, M.; Magnani, G.; Carretta, P.; Sanna, S. Molecular and Ionic Dynamics in NaxLi6–xC60. J. Phys. Chem. C 2017, 121, 6554–6560. [Google Scholar] [CrossRef]
- Gaboardi, M.; Amadè, N.S.; Riccò, M.; Milanese, C.; Girella, A.; Gioventù, M.; Fernandez-Alonso, F. Synthesis and Characterization of Mixed Sodium and Lithium Fullerides for Hydrogen Storage. Int. J. Hydrogen Energy 2018, 43, 16766–16773. [Google Scholar] [CrossRef] [Green Version]
- Mauron, P.; Gaboardi, M.; Remhof, A.; Bliersbach, A.; Sheptyakov, D.; Aramini, M.; Vlahopoulou, G.; Giglio, F.; Pontiroli, D.; Riccò, M.; et al. Hydrogen Sorption in Li12C60. J. Phys. Chem. C 2013, 117, 22598–22602. [Google Scholar] [CrossRef]
- Gaboardi, M.; Duyker, S.; Milanese, C.; Magnani, G.; Peterson, V.K.; Pontiroli, D.; Sharma, N.; Riccò, M. In Situ Neutron Powder Diffraction of Li6C60 for Hydrogen Storage. J. Phys. Chem. C 2015, 119, 19715–19721. [Google Scholar] [CrossRef]
- Aramini, M.; Gaboardi, M.; Vlahopoulou, G.; Pontiroli, D.; Cavallari, C.; Milanese, C.; Riccò, M. Muon Spin Relaxation Reveals the Hydrogen Storage Mechanism in Light Alkali Metal Fullerides. Carbon 2014, 67, 92–97. [Google Scholar] [CrossRef]
- Giglio, F.; Pontiroli, D.; Gaboardi, M.; Aramini, M.; Cavallari, C.; Brunelli, M.; Galinetto, P.; Milanese, C.; Riccò, M. Li12C60: A Lithium Clusters Intercalated Fulleride. Chem. Phys. Lett. 2014, 609, 155–160. [Google Scholar] [CrossRef]
- Gaboardi, M.; Cavallari, C.; Magnani, G.; Pontiroli, D.; Rols, S.; Riccò, M. Hydrogen Storage Mechanism and Lithium Dynamics in Li12C60 investigated by μSR. Carbon 2015, 90, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Maidich, L.; Pontiroli, D.; Gaboardi, M.; Lenti, S.; Magnani, G.; Riva, G.; Carretta, P.; Milanese, C.; Marini, A.; Riccò, M.; et al. Investigation of Li and H Dynamics in Li6C60 and Li6C60Hy. Carbon 2016, 96, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Sarzi Amadè, N.; Gaboardi, M.; Magnani, G.; Riccò, M.; Pontiroli, D.; Milanese, C.; Girella, A.; Carretta, P.; Sanna, S. H and Li dynamics in Li12C60 and Li12C60Hy. Int. J. Hydrogen Energy 2017, 42, 22544–22550. [Google Scholar] [CrossRef] [Green Version]
- Gaboardi, M.; Milanese, C.; Magnani, G.; Girella, A.; Pontiroli, D.; Cofrancesco, P.; Marini, A.; Riccò, M. Optimal Hydrogen Storage in Sodium Substituted Lithium Fullerides. Phys. Chem. Chem. Phys. 2017, 19, 21980–21986. [Google Scholar] [CrossRef] [Green Version]
- Scaravonati, S.; Magnani, G.; Gaboardi, M.; Allodi, G.; Riccò, M.; Pontiroli, D. Electrochemical Intercalation of Fullerene and Hydrofullerene with Sodium. Carbon 2018, 130, 11–18. [Google Scholar] [CrossRef]
- Aramini, M.; Magnani, G.; Pontiroli, D.; Milanese, C.; Girella, A.; Bertoni, G.; Gaboardi, M.; Zacchini, S.; Marini, A.; Riccò, M. Nickel Addition to Optimize the Hydrogen Storage Performance of Lithium Intercalated Fullerides. Mater. Res. Bull. 2020, 126, 110848. [Google Scholar] [CrossRef]
- Sartori, S.; Guzik, M.N.; Knudsen, K.D.; Sørby, M.H.; Teprovich, J.A.; Zidan, R.; Hauback, B.C. Stability and Phase Formation in the (Li/Na)6C60-H Systems Studied by Neutron Scattering. J. Phys. Chem. C 2018, 122, 18346–18355. [Google Scholar] [CrossRef] [Green Version]
- Ward, P.A.; Teprovich, J.A.; Compton, R.; Schwartz, V.; Veith, G.M.; Zidan, R. Evaluation of the physi- and chemisorption of hydrogen in alkali (Na, Li) doped fullerenes. Int. J. Hydrogen Energy 2015, 40, 2710–2716. [Google Scholar] [CrossRef]
- Yoshida, A.; Okuyama, T.; Terada, T.; Naito, S. Reversible hydrogen storage/release phenomena on lithium fulleride (LinC60) and their mechanistic investigation by solid-state NMR spectroscopy. J. Mater. Chem. 2011, 21, 9480. [Google Scholar] [CrossRef]
- Teprovich, J.A.; Wellons, M.S.; Lascola, R.; Hwang, S.J.; Ward, P.a.; Compton, R.N.; Zidan, R. Synthesis and characterization of a lithium-doped fullerane (Lix-C60-Hy) for reversible hydrogen storage. Nano Lett. 2012, 12, 582–589. [Google Scholar] [CrossRef]
- Teprovich, J.A.; Knight, D.A.; Peters, B.; Zidan, R. Comparative study of reversible hydrogen storage in alkali-doped fulleranes. J. Alloy. Compd. 2013, 580, S364–S367. [Google Scholar] [CrossRef]
- Pontiroli, D.; D’Alessio, D.; Gaboardi, M.; Magnani, G.; Milanese, C.; Duyker, S.G.; Peterson, V.K.; Sharma, N.; Riccò, M. Ammonia-storage in Lithium Intercalated Fullerides. J. Mater. Chem. A 2015, 3, 21099–21105. [Google Scholar] [CrossRef] [Green Version]
- Durand, P.; Dubitsky, Y.; Rosseinsky, M.J.; Zaopo, A. Expanded fullerides and electron localisation—Lithium-rich ammoniated C(60) phases. Dalton Trans. 2004, 3137–3143. [Google Scholar] [CrossRef]
- Fullagar, W.; Reynolds, P.; White, J. Lithium and sodium fullerides prepared in liquid ammonia. Solid State Commun. 1997, 104, 23–27. [Google Scholar] [CrossRef]
- Aramini, M.; Milanese, C.; Pontiroli, D.; Gaboardi, M.; Girella, A.; Bertoni, G.; Riccò, M. Addition of Transition Metals to Lithium Intercalated Fullerides Enhances Hydrogen Storage Properties. Int. J. Hydrogen Energy 2014, 39, 2124–2131. [Google Scholar] [CrossRef]
- Eklund, P.C.; Rao, A.M. (Eds.) Fullerene Polymers and Fullerene Polymer Composites; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar] [CrossRef]
- Rols, S.; Cambedouzou, J.; Bantignies, J.L.; Rachdi, F.; Sauvajol, J.L.; Agafonov, V.; Rakhmanina, A.V.; Davydov, V.A.; Hennion, B.; Kahn, R. Lattice Dynamics of Pressure-polymerized Phases of C60: A Neutron Scattering Investigation. Phys. Rev. B 2004, 70. [Google Scholar] [CrossRef]
- Rols, S.; Bantignies, J.L.; Maurin, D.; Sauvajol, J.L.; Agafonov, V.; Rakhmanina, A.V.; Davydov, V.A. Low-Frequency Phonons in High-Pressure High-Temperature C60 Polymers. Fuller. Nanotub. Carbon Nanostructures 2005, 12, 263–268. [Google Scholar] [CrossRef]
- Cambedouzou, J.; Rols, S.; Almairac, R.; Sauvajol, J.L.; Kataura, H.; Schober, H. Low-frequency Excitations of C60 Chains Inserted Inside Single-walled Carbon Nanotubes. Phys. Rev. B 2005, 71. [Google Scholar] [CrossRef] [Green Version]
- Pontiroli, D.; Aramini, M.; Gaboardi, M.; Mazzani, M.; Gorreri, A.; Riccò, M.; Margiolaki, I.; Sheptyakov, D. Ionic conductivity in the Mg intercalated fullerene polymer Mg2C60. Carbon 2013, 51, 143–147. [Google Scholar] [CrossRef]
- Kubozono, Y.; Takabayashi, Y.; Kambe, T.; Fujiki, S.; Kashino, S.; Emura, S. Structure and physical properties of Na4C60 under ambient and high pressures. Phys. Rev. B 2001, 63, 045418. [Google Scholar] [CrossRef] [Green Version]
- Pekker, S.; Jánossy, A.; Mihaly, L.; Chauvet, O.; Carrard, M.; Forró, L. Single-Crystalline (KC60)n: A Conducting Linear Alkali Fulleride Polymer. Science 1994, 265, 1077–1078. [Google Scholar] [CrossRef]
- Stephens, P.W.; Bortel, G.; Faigel, G.; Tegze, M.; Jánossy, A.; Pekker, S.; Oszlanyi, G.; Forró, L. Polymeric fullerene chains in RbC60 and KC60. Nature 1994, 370, 636–639. [Google Scholar] [CrossRef]
- Chauvet, O.; Oszlànyi, G.; Forro, L.; Stephens, P.; Tegze, M.; Faigel, G.; Jànossy, A. Quasi-one-dimensional electronic structure in orthorhombic RbC60. Phys. Rev. Lett. 1994, 72, 2721–2724. [Google Scholar] [CrossRef] [PubMed]
- Rols, S.; Pontiroli, D.; Cavallari, C.; Gaboardi, M.; Aramini, M.; Richard, D.; Johnson, M.R.; Zanotti, J.M.; Suard, E.; Maccarini, M.; et al. Structure and Dynamics of the Fullerene Polymer Li4C60 Studied with Neutron Scattering. Phys. Rev. B 2015, 92. [Google Scholar] [CrossRef] [Green Version]
- Rols, S.; Pontiroli, D.; Aramini, M.; Gaboardi, M.; Cavallari, C.; Riccò, M.; Suard, E.; Johnson, M.R.; Richard, D. Lattice Dynamics of the Ionic Superconductor Li4C60. Inelastic Neutron Scattering and Powder Averaged Lattice Dynamics (PALD) investigations. Acta Crystallogr. A 2016, 72, s76. [Google Scholar] [CrossRef] [Green Version]
- Riccò, M.; Belli, M.; Mazzani, M.; Pontiroli, D.; Quintavalle, D.; Jánossy, A.; Csányi, G. Superionic Conductivity in the Li4C60 Fulleride Polymer. Phys. Rev. Lett. 2009, 102, 145901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosi, N.L. Hydrogen Storage in Microporous Metal-Organic Frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowsell, J.L.C.; Eckert, J.; Yaghi, O.M. Characterization of H2Binding Sites in Prototypical Metal-Organic Frameworks by Inelastic Neutron Scattering. J. Am. Chem. Soc. 2005, 127, 14904–14910. [Google Scholar] [CrossRef]
- Ye, S.; Xu, M.; Bacic, Z.; Lawler, R.; Turro, N.J. Quantum Dynamics of a Hydrogen Molecule Inside an Anisotropic Open-Cage Fullerene: Coupled Translation-Rotation Eigenstates and Comparison with Inelastic Neutron Scattering Spectroscopy. J. Phys. Chem. A 2010, 114, 9936–9947. [Google Scholar] [CrossRef]
- Horsewill, A.J.; Panesar, K.S.; Rols, S.; Johnson, M.R.; Murata, Y.; Komatsu, K.; Mamone, S.; Danquigny, A.; Cuda, F.; Maltsev, S.; et al. Quantum Translator-Rotator: Inelastic Neutron Scattering of Dihydrogen Molecules Trapped inside Anisotropic Fullerene Cages. Phys. Rev. Lett. 2009, 102. [Google Scholar] [CrossRef] [Green Version]
- Horsewill, A.J.; Goh, K.; Rols, S.; Ollivier, J.; Johnson, M.R.; Levitt, M.H.; Carravetta, M.; Mamone, S.; Murata, Y.; Chen, J.Y.C.; et al. Quantum Rotation and Translation of Hydrogen Molecules Encapsulated Inside C60: Temperature Dependence of Inelastic Neutron Scattering Spectra. Philos. Trans. R. Soc. A 2013, 371, 20110627. [Google Scholar] [CrossRef]
- Horsewill, A.J.; Panesar, K.S.; Rols, S.; Ollivier, J.; Johnson, M.R.; Carravetta, M.; Mamone, S.; Levitt, M.H.; Murata, Y.; Komatsu, K.; et al. Inelastic Neutron Scattering Investigations of the Quantum Molecular Dynamics of a H2 Molecule Entrapped Inside a Fullerene Cage. Phys. Rev. B 2012, 85. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Alonso, F.; Cabrillo, C.; Fernández-Perea, R.; Bermejo, F.J.; González, M.A.; Mondelli, C.; Farhi, E. Solid para-Hydrogen as the Paradigmatic Quantum Crystal: Three Observables Probed by Ultrahigh-resolution Neutron Spectroscopy. Phys. Rev. B 2012, 86. [Google Scholar] [CrossRef] [Green Version]
- Cabrillo, C.; Fernández-Alonso, F.; Fernández-Perea, R.; Bermejo, F.J.; González, M.A.; Mondelli, C.; Farhi, E. Crystallization of para-Hydrogen: A Quantum Phase Transition at Finite Temperature? J. Phys. Conf. Ser. 2015, 663, 012006. [Google Scholar] [CrossRef]
- Xu, M.; Jiménez-Ruiz, M.; Johnson, M.R.; Rols, S.; Ye, S.; Carravetta, M.; Denning, M.S.; Lei, X.; Bačić, Z.; Horsewill, A.J. Confirming a Predicted Selection Rule in Inelastic Neutron Scattering Spectroscopy: The Quantum Translator-Rotator H2 Entrapped Inside C60. Phys. Rev. Lett. 2014; 113. [Google Scholar] [CrossRef] [Green Version]
- Easun, T.L.; Moreau, F.; Yan, Y.; Yang, S.; Schröder, M. Structural and Dynamic Studies of Substrate Binding in Porous Metal–organic Frameworks. Chem. Soc. Rev. 2017, 46, 239–274. [Google Scholar] [CrossRef] [Green Version]
- Kibble, M.G.; Ramirez-Cuesta, A.J.; Goodway, C.M.; Evans, B.E.; Kirichek, O. Hydrogen Gas Sample Environment for TOSCA. J. Phys. Conf. Ser. 2014, 554, 012006. [Google Scholar] [CrossRef] [Green Version]
- Redfern, L.R.; Farha, O.K. Mechanical properties of metal–organic frameworks. Chem. Sci. 2019, 10, 10666–10679. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, A.U.; Boutin, A.; Fuchs, A.H.; Coudert, F.X. Anisotropic Elastic Properties of Flexible Metal-Organic Frameworks: How Soft are Soft Porous Crystals? Phys. Rev. Lett. 2012, 109. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.C.; Civalleri, B.; Lin, C.C.; Valenzano, L.; Galvelis, R.; Chen, P.F.; Bennett, T.D.; Mellot-Draznieks, C.; Zicovich-Wilson, C.M.; Cheetham, A.K. Exceptionally Low Shear Modulus in a Prototypical Imidazole-Based Metal-Organic Framework. Phys. Rev. Lett. 2012, 108. [Google Scholar] [CrossRef] [Green Version]
- Widmer, R.N.; Lampronti, G.I.; Chibani, S.; Wilson, C.W.; Anzellini, S.; Farsang, S.; Kleppe, A.K.; Casati, N.P.M.; MacLeod, S.G.; Redfern, S.A.T.; et al. Rich Polymorphism of a Metal–Organic Framework in Pressure–Temperature Space. J. Am. Chem. Soc. 2019, 141, 9330–9337. [Google Scholar] [CrossRef] [Green Version]
- Ryder, M.; Civalleri, B.; Bennett, T.; Henke, S.; Rudić, S.; Cinque, G.; Fernandez-Alonso, F.; Tan, J.C. Identifying the Role of Terahertz Vibrations in Metal-Organic Frameworks: From Gate-Opening Phenomenon to Shear-Driven Structural Destabilization. Phys. Rev. Lett. 2014, 113. [Google Scholar] [CrossRef] [Green Version]
- Fairen-Jimenez, D.; Moggach, S.A.; Wharmby, M.T.; Wright, P.A.; Parsons, S.; D’uren, T. Opening the Gate: Framework Flexibility in ZIF-8 Explored by Experiments and Simulations. J. Am. Chem. Soc. 2011, 133, 8900–8902. [Google Scholar] [CrossRef] [Green Version]
- Casco, M.E.; Cheng, Y.Q.; Daemen, L.L.; Fairen-Jimenez, D.; Ramos-Fernández, E.V.; Ramirez-Cuesta, A.J.; Silvestre-Albero, J. Gate-opening Effect in ZIF-8: The First Experimental Proof using Inelastic Neutron Scattering. Chem. Commun. 2016, 52, 3639–3642. [Google Scholar] [CrossRef] [Green Version]
- Weinrauch, I.; Savchenko, I.; Denysenko, D.; Souliou, S.M.; Kim, H.H.; Tacon, M.L.; Daemen, L.L.; Cheng, Y.; Mavrandonakis, A.; Ramirez-Cuesta, A.J.; et al. Capture of Heavy Hydrogen Isotopes in a Metal-organic Framework with Active Cu(I) Sites. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savage, M.; da Silva, I.; Johnson, M.; Carter, J.H.; Newby, R.; Suyetin, M.; Besley, E.; Manuel, P.; Rudić, S.; Fitch, A.N.; et al. Observation of Binding and Rotation of Methane and Hydrogen within a Functional Metal–Organic Framework. J. Am. Chem. Soc. 2016, 138, 9119–9127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savage, M.; Cheng, Y.; Easun, T.L.; Eyley, J.E.; Argent, S.P.; Warren, M.R.; Lewis, W.; Murray, C.; Tang, C.C.; Frogley, M.D.; et al. Selective Adsorption of Sulfur Dioxide in a Robust Metal-Organic Framework Material. Adv. Mater. 2016, 28, 8705–8711. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; Forrest, K.A.; Mostrom, M.; Hunt, J.R.; Furukawa, H.; Eckert, J.; Space, B. The Rotational Dynamics of H2 Adsorbed in Covalent Organic Frameworks. Phys. Chem. Chem. Phys. 2017, 19, 13075–13082. [Google Scholar] [CrossRef]
- Cuadrado-Collados, C.; Fernández-Català, J.; Fauth, F.; Cheng, Y.Q.; Daemen, L.L.; Ramirez-Cuesta, A.J.; Silvestre-Albero, J. Understanding the Breathing Phenomena in Nano-ZIF-7 upon Gas Adsorption. J. Mater. Chem. A 2017, 5, 20938–20946. [Google Scholar] [CrossRef] [Green Version]
- Casco, M.E.; Fernández-Catalá, J.; Cheng, Y.; Daemen, L.; Ramirez-Cuesta, A.J.; Cuadrado-Collados, C.; Silvestre-Albero, J.; Ramos-Fernandez, E.V. Understanding ZIF-8 Performance upon Gas Adsorption by Means of Inelastic Neutron Scattering. ChemistrySelect 2017, 2, 2750–2753. [Google Scholar] [CrossRef]
- Zhang, X.; da Silva, I.; Godfrey, H.G.W.; Callear, S.K.; Sapchenko, S.A.; Cheng, Y.; Vitórica-Yrezábal, I.; Frogley, M.D.; Cinque, G.; Tang, C.C.; et al. Confinement of Iodine Molecules into Triple-Helical Chains within Robust Metal–Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 16289–16296. [Google Scholar] [CrossRef]
- Lu, Z.; Godfrey, H.G.W.; da Silva, I.; Cheng, Y.; Savage, M.; Tuna, F.; McInnes, E.J.L.; Teat, S.J.; Gagnon, K.J.; Frogley, M.D.; et al. Modulating Supramolecular Binding of Carbon Dioxide in a Redox-active Porous Metal-organic Framework. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Ryder, M.; de Voorde, B.V.; Civalleri, B.; Bennett, T.D.; Mukhopadhyay, S.; Cinque, G.; Fernandez-Alonso, F.; Vos, D.D.; Rudić, S.; Tan, J.C. Detecting Molecular Rotational Dynamics Complementing the Low-Frequency Terahertz Vibrations in a Zirconium-Based Metal-Organic Framework. Phys. Rev. Lett. 2017, 118. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; da Silva, I.; Kolokolov, D.I.; Han, X.; Li, J.; Smith, G.; Cheng, Y.; Daemen, L.L.; Morris, C.G.; Godfrey, H.G.W.; et al. Post-synthetic Modulation of the Charge Distribution in a Metal–organic Framework for Optimal Binding of Carbon Dioxide and Sulfur Dioxide. Chem. Sci. 2019, 10, 1472–1482. [Google Scholar] [CrossRef]
- Duong, T.D.; Sapchenko, S.A.; da Silva, I.; Godfrey, H.G.W.; Cheng, Y.; Daemen, L.L.; Manuel, P.; Ramirez-Cuesta, A.J.; Yang, S.; Schröder, M. Optimal Binding of Acetylene to a Nitro-Decorated Metal–Organic Framework. J. Am. Chem. Soc. 2018, 140, 16006–16009. [Google Scholar] [CrossRef] [Green Version]
- Duong, T.D.; Sapchenko, S.A.; da Silva, I.; Godfrey, H.G.W.; Cheng, Y.; Daemen, L.L.; Manuel, P.; Frogley, M.D.; Cinque, G.; Ramirez-Cuesta, A.J.; et al. Observation of Binding of Carbon Dioxide to Nitro-decorated Metal–organic Frameworks. Chem. Sci. 2020, 11, 5339–5346. [Google Scholar] [CrossRef]
- Gandara-Loe, J.; Missyul, A.; Fauth, F.; Daemen, L.L.; Cheng, Y.Q.; Ramirez-Cuesta, A.J.; Ravikovitch, P.I.; Silvestre-Albero, J. New Insights into the Breathing Phenomenon in ZIF-4. J. Mater. Chem. A 2019, 7, 14552–14558. [Google Scholar] [CrossRef]
- Doan, H.; Cheng, F.; Dyirakumunda, T.; Elsegood, M.; Chin, J.; Rowe, O.; Redshaw, C.; Ting, V. Using Supercritical CO2 in the Preparation of Metal-Organic Frameworks: Investigating Effects on Crystallisation. Crystals 2019, 10, 17. [Google Scholar] [CrossRef] [Green Version]
- Butler, K.T.; Vervoorts, P.; Ehrenreich, M.G.; Armstrong, J.; Skelton, J.M.; Kieslich, G. Experimental Evidence for Vibrational Entropy as Driving Parameter of Flexibility in the Metal–Organic Framework ZIF-4(Zn). Chem. Mater. 2019, 31, 8366–8372. [Google Scholar] [CrossRef]
- Zhao, P.; Fang, H.; Mukhopadhyay, S.; Li, A.; Rudić, S.; McPherson, I.J.; Tang, C.C.; Fairen-Jimenez, D.; Tsang, S.C.E.; Redfern, S.A.T. Structural Dynamics of a Metal–organic Framework Induced by CO2 Migration in its Non-uniform Porous Structure. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef]
- Humby, J.D.; Benson, O.; Smith, G.L.; Argent, S.P.; da Silva, I.; Cheng, Y.; Rudić, S.; Manuel, P.; Frogley, M.D.; Cinque, G.; et al. Host–guest Selectivity in a Series of Isoreticular Metal–organic Frameworks: Observation of Acetylene-to-alkyne and Carbon Dioxide-to-amide Interactions. Chem. Sci. 2019, 10, 1098–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, X.; Lyu, K.; Li, L.; Li, J.; Kimberley, L.; Wang, B.; Liu, L.; Cheng, Y.; Frogley, M.D.; Rudić, S.; et al. Integration of Mesopores and Crystal Defects in Metal-organic Frameworks via Templated Electrosynthesis. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef]
- Titov, K.; Eremin, D.B.; Kashin, A.S.; Boada, R.; Souza, B.E.; Kelley, C.S.; Frogley, M.D.; Cinque, G.; Gianolio, D.; Cibin, G.; et al. OX-1 Metal–Organic Framework Nanosheets as Robust Hosts for Highly Active Catalytic Palladium Species. ACS Sustain. Chem. Eng. 2019, 7, 5875–5885. [Google Scholar] [CrossRef]
- Smith, G.L.; Eyley, J.E.; Han, X.; Zhang, X.; Li, J.; Jacques, N.M.; Godfrey, H.G.W.; Argent, S.P.; McPherson, L.J.M.; Teat, S.J.; et al. Reversible Coordinative Binding and Separation of Sulfur Dioxide in a Robust Metal–organic Framework with Open Copper Sites. Nat. Mater. 2019, 18, 1358–1365. [Google Scholar] [CrossRef]
- Cuadrado-Collados, C.; Mouchaham, G.; Daemen, L.; Cheng, Y.; Ramirez-Cuesta, A.; Aggarwal, H.; Missyul, A.; Eddaoudi, M.; Belmabkhout, Y.; Silvestre-Albero, J. Quest for an Optimal Methane Hydrate Formation in the Pores of Hydrolytically Stable Metal–Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 13391–13397. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.E.; Möslein, A.F.; Titov, K.; Taylor, J.D.; Rudić, S.; Tan, J.C. Green Reconstruction of MIL-100 (Fe) in Water for High Crystallinity and Enhanced Guest Encapsulation. ACS Sustain. Chem. Eng. 2020, 8, 8247–8255. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Z.; Han, X.; Zhang, X.; Yan, Y.; Li, W.; Smith, G.L.; Cheng, Y.; MPherson, L.J.M.; Teat, S.J.; et al. Guest-Controlled Incommensurate Modulation in a Meta-Rigid Metal–Organic Framework Material. J. Am. Chem. Soc. 2020, 142, 19189–19197. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lu, W.; Chen, Y.; da Silva, I.; Li, J.; Lin, L.; Li, W.; Sheveleva, A.M.; Godfrey, H.G.W.; Lu, Z.; et al. High Ammonia Adsorption in MFM-300 Materials: Dynamics and Charge Transfer in Host–Guest Binding. J. Am. Chem. Soc. 2021, 143, 3153–3161. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yildirim, T. Lattice Dynamics of Metal-organic Frameworks: Neutron Inelastic Scattering and First-principles Calculations. Phys. Rev. B 2006, 74. [Google Scholar] [CrossRef] [Green Version]
- Forster, P.M.; Eckert, J.; Heiken, B.D.; Parise, J.B.; Yoon, J.W.; Jhung, S.H.; Chang, J.S.; Cheetham, A.K. Adsorption of Molecular Hydrogen on Coordinatively Unsaturated Ni(II) Sites in a Nanoporous Hybrid Material. J. Am. Chem. Soc. 2006, 128, 16846–16850. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Brown, C.; Neumann, D.; Peterson, V.; Kepert, C. Inelastic Neutron Scattering of H2 adsorbed in HKUST-1. J. Alloys Compd. 2007, 446–447, 385–388. [Google Scholar] [CrossRef]
- Liu, Y.; Eubank, J.; Cairns, A.; Eckert, J.; Kravtsov, V.; Luebke, R.; Eddaoudi, M. Assembly of Metal–Organic Frameworks (MOFs) Based on Indium-Trimer Building Blocks: A Porous MOF with soc Topology and High Hydrogen Storage. Angew. Chem. Int. Ed. 2007, 46, 3278–3283. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Her, J.H.; Dailly, A.; Ramirez-Cuesta, A.J.; Neumann, D.A.; Brown, C.M. Reversible Structural Transition in MIL-53 with Large Temperature Hysteresis. J. Am. Chem. Soc. 2008, 130, 11813–11818. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kabbour, H.; Brown, C.M.; Neumann, D.A.; Ahn, C.C. Increasing the Density of Adsorbed Hydrogen with Coordinatively Unsaturated Metal Centers in Metal-Organic Frameworks. Langmuir 2008, 24, 4772–4777. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Ma, S.; Forster, P.; Yuan, D.; Eckert, J.; López, J.; Murphy, B.; Parise, J.; Zhou, H.C. Enhancing H2Uptake by “Close-Packing” Alignment of Open Copper Sites in Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2008, 47, 7263–7266. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Eckert, J.; Forster, P.M.; Yoon, J.W.; Hwang, Y.K.; Chang, J.S.; Collier, C.D.; Parise, J.B.; Zhou, H.C. Further Investigation of the Effect of Framework Catenation on Hydrogen Uptake in Metal-Organic Frameworks. J. Am. Chem. Soc. 2008, 130, 15896–15902. [Google Scholar] [CrossRef]
- Brown, C.M.; Liu, Y.; Yildirim, T.; Peterson, V.K.; Kepert, C.J. Hydrogen Adsorption in HKUST-1: A Combined Inelastic Neutron Scattering and First-principles Study. Nanotechnology 2009, 20, 204025. [Google Scholar] [CrossRef] [PubMed]
- Nouar, F.; Eckert, J.; Eubank, J.F.; Forster, P.; Eddaoudi, M. Zeolite-likeMetal-Organic Frameworks (ZMOFs) as Hydrogen Storage Platform: Lithium and Magnesium Ion-Exchange and H2-(rho-ZMOF) Interaction Studies. J. Am. Chem. Soc. 2009, 131, 2864–2870. [Google Scholar] [CrossRef] [PubMed]
- Dietzel, P.D.C.; Georgiev, P.A.; Eckert, J.; Blom, R.; Strässle, T.; Unruh, T. Interaction of Hydrogen with Accessible Metal Sites in the Metal–organic frameworks M2(dhtp) (CPO-27-M;M = Ni, Co, Mg). Chem. Commun. 2010, 46, 4962. [Google Scholar] [CrossRef]
- Sumida, K.; Her, J.H.; Dincă, M.; Murray, L.J.; Schloss, J.M.; Pierce, C.J.; Thompson, B.A.; FitzGerald, S.A.; Brown, C.M.; Long, J.R. Neutron Scattering and Spectroscopic Studies of Hydrogen Adsorption in Cr3(BTC)2—A Metal-Organic Framework with Exposed Cr2 Sites. J. Phys. Chem. C 2011, 115, 8414–8421. [Google Scholar] [CrossRef]
- Sumida, K.; Brown, C.M.; Herm, Z.R.; Chavan, S.; Bordiga, S.; Long, J.R. Hydrogen Storage Properties and Neutron Scattering Studies of Mg2(dobdc)—A Metal–organic Framework with Open Mg2Adsorption Sites. Chem. Commun. 2011, 47, 1157–1159. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Park, K.S.; Furukawa, H.; Eckert, J.; Knobler, C.B.; Yaghi, O.M. Hydrogen Storage in New Metal–Organic Frameworks. J. Phys. Chem. C 2012, 116, 13143–13151. [Google Scholar] [CrossRef]
- Eubank, J.F.; Nouar, F.; Luebke, R.; Cairns, A.J.; Wojtas, L.; Alkordi, M.; Bousquet, T.; Hight, M.R.; Eckert, J.; Embs, J.P.; et al. On Demand: The Singular rht Net, an Ideal Blueprint for the Construction of a Metal-Organic Framework (MOF) Platform. Angew. Chem. Int. Ed. 2012, 51, 10099–10103. [Google Scholar] [CrossRef]
- Queen, W.L.; Bloch, E.D.; Brown, C.M.; Hudson, M.R.; Mason, J.A.; Murray, L.J.; Ramirez-Cuesta, A.J.; Peterson, V.K.; Long, J.R. Hydrogen Adsorption in the Metal–organic Frameworks Fe2(dobdc) and Fe2(O2)(dobdc). Dalton Trans. 2012, 41, 4180. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, M.B.; Getman, R.B.; Lee, J.Y.; Roberts, J.M.; Sarjeant, A.A.; Scheidt, K.A.; Georgiev, P.A.; Embs, J.P.; Eckert, J.; Farha, O.K.; et al. A Zwitterionic Metal–organic Framework with Free Carboxylic Acid Sites that Exhibits Enhanced Hydrogen Adsorption Energies. CrystEngComm 2013, 15, 9408. [Google Scholar] [CrossRef]
- Callear, S.K.; Ramirez-Cuesta, A.J.; David, W.I.; Millange, F.; Walton, R.I. High-resolution Inelastic Neutron Scattering and Neutron Powder Diffraction Study of the Adsorption of Dihydrogen by the Cu(II) Metal–organic Framework Material HKUST-1. Chem. Phys. 2013, 427, 9–17. [Google Scholar] [CrossRef]
- Munn, A.S.; Ramirez-Cuesta, A.J.; Millange, F.; Walton, R.I. Interaction of Methanol with the Flexible Metal-organic Framework MIL-53(Fe) Observed by Inelastic Neutron Scattering. Chem. Phys. 2013, 427, 30–37. [Google Scholar] [CrossRef]
- Pham, T.; Forrest, K.A.; Eckert, J.; Georgiev, P.A.; Mullen, A.; Luebke, R.; Cairns, A.J.; Belmabkhout, Y.; Eubank, J.F.; McLaughlin, K.; et al. Investigating the Gas Sorption Mechanism in an rht-Metal–Organic Framework through Computational Studies. J. Phys. Chem. C 2013, 118, 439–456. [Google Scholar] [CrossRef]
- Rosnes, M.H.; Opitz, M.; Frontzek, M.; Lohstroh, W.; Embs, J.P.; Georgiev, P.A.; Dietzel, P.D.C. Intriguing Differences in Hydrogen Adsorption in CPO-27 Materials Induced by Metal Substitution. J. Mater. Chem. A 2015, 3, 4827–4839. [Google Scholar] [CrossRef]
- Nugent, P.; Pham, T.; McLaughlin, K.; Georgiev, P.A.; Lohstroh, W.; Embs, J.P.; Zaworotko, M.J.; Space, B.; Eckert, J. Dramatic Effect of Pore Size Reduction on the Dynamics of Hydrogen Adsorbed in Metal–organic Materials. J. Mater. Chem. A 2014, 2, 13884. [Google Scholar] [CrossRef]
- Pham, T.; Forrest, K.A.; Georgiev, P.A.; Lohstroh, W.; Xue, D.X.; Hogan, A.; Eddaoudi, M.; Space, B.; Eckert, J. A High Rotational Barrier for Physisorbed Hydrogen in an fcu-metal–organic Framework. Chem. Commun. 2014, 50, 14109–14112. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Ramirez-Cuesta, A.J.; Schröder, M. Inelastic Neutron Scattering Study of Binding of para-Hydrogen in an Ultra-microporous Metal–organic Framework. Chem. Phys. 2014, 428, 111–116. [Google Scholar] [CrossRef]
- Pham, T.; Forrest, K.A.; Falcão, E.H.L.; Eckert, J.; Space, B. Exceptional H2 Sorption Characteristics in a Mg2-based Metal–organic Framework With Small Pores: Insights from Experimental and Theoretical Studies. Phys. Chem. Chem. Phys. 2016, 18, 1786–1796. [Google Scholar] [CrossRef]
- Forrest, K.A.; Pham, T.; Georgiev, P.A.; Pinzan, F.; Cioce, C.R.; Unruh, T.; Eckert, J.; Space, B. Investigating H2 Sorption in a Fluorinated Metal–Organic Framework with Small Pores Through Molecular Simulation and Inelastic Neutron Scattering. Langmuir 2015, 31, 7328–7336. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; Forrest, K.A.; Banerjee, R.; Orcajo, G.; Eckert, J.; Space, B. Understanding the H2Sorption Trends in the M-MOF-74 Series (M = Mg, Ni, Co, Zn). J. Phys. Chem. C 2014, 119, 1078–1090. [Google Scholar] [CrossRef]
- Cairns, A.J.; Eckert, J.; Wojtas, L.; Thommes, M.; Wallacher, D.; Georgiev, P.A.; Forster, P.M.; Belmabkhout, Y.; Ollivier, J.; Eddaoudi, M. Gaining Insights on the H2–Sorbent Interactions: Robust soc-MOF Platform as a Case Study. Chem. Mater. 2016, 28, 7353–7361. [Google Scholar] [CrossRef]
- Rosen, P.F.; Calvin, J.J.; Dickson, M.S.; Katsenis, A.D.; Friščić, T.; Navrotsky, A.; Ross, N.L.; Kolesnikov, A.I.; Woodfield, B.F. Heat Capacity and Thermodynamic Functions of Crystalline Forms of the Metal-organic Framework Zinc 2-Methylimidazolate, Zn(MeIm)2. J. Chem. Thermodyn. 2019, 136, 160–169. [Google Scholar] [CrossRef]
- Vanpoucke, D.E.P.; Lejaeghere, K.; Speybroeck, V.V.; Waroquier, M.; Ghysels, A. Mechanical Properties from Periodic Plane Wave Quantum Mechanical Codes: The Challenge of the Flexible Nanoporous MIL-47(V) Framework. J. Phys. Chem. C 2015, 119, 23752–23766. [Google Scholar] [CrossRef] [Green Version]
- Salzmann, C.G.; Radaelli, P.G.; Mayer, E.; Finney, J.L. Ice XV: A New Thermodynamically Stable Phase of Ice. Phys. Rev. Lett. 2009, 103. [Google Scholar] [CrossRef] [Green Version]
- Gasser, T.M.; Thoeny, A.V.; Fortes, A.D.; Loerting, T. Structural Characterization of Ice XIX as the Second Polymorph Related to Ice VI. Nat. Commun. 2021, 12. [Google Scholar] [CrossRef]
- Salzmann, C.G. Advances in the Experimental Exploration of Water’s Phase Diagram. J. Chem. Phys. 2019, 150, 060901. [Google Scholar] [CrossRef] [PubMed]
- Wollan, E.O.; Davidson, W.L.; Shull, C.G. Neutron Diffraction Study of the Structure of Ice. Phys. Rev. 1949, 75, 1348–1352. [Google Scholar] [CrossRef]
- Rahman, A.; Stillinger, F.H. Molecular Dynamics Study of Liquid Water. J. Chem. Phys. 1971, 55, 3336–3359. [Google Scholar] [CrossRef]
- Soper, A. The Radial Distribution Functions of Water and Ice from 220 to 673 K and at Pressures up to 400 MPa. Chem. Phys. 2000, 258, 121–137. [Google Scholar] [CrossRef]
- Soper, A.; Phillips, M. A New Determination of the Structure of Water at 25 °C. Chem. Phys. 1986, 107, 47–60. [Google Scholar] [CrossRef]
- Rosu-Finsen, A.; Amon, A.; Armstrong, J.; Fernandez-Alonso, F.; Salzmann, C.G. Deep-Glassy Ice VI Revealed with a Combination of Neutron Spectroscopy and Diffraction. J. Phys. Chem. Lett. 2020, 11, 1106–1111. [Google Scholar] [CrossRef] [Green Version]
- Li, J. Inelastic Neutron Scattering Studies of Hydrogen Bonding in Ices. J. Chem. Phys. 1996, 105, 6733–6755. [Google Scholar] [CrossRef]
- Li, J.; Kolesnikov, A. Neutron Spectroscopic Investigation of Dynamics of Water Ice. J. Mol. Liq. 2002, 100, 1–39. [Google Scholar] [CrossRef]
- Klotz, S.; Strässle, T.; Salzmann, C.G.; Philippe, J.; Parker, S.F. Incoherent Inelastic Neutron Scattering Measurements on Ice VII: Are There Two Kinds of Hydrogen Bonds in Ice? EPL 2005, 72, 576–582. [Google Scholar] [CrossRef]
- Andreani, C.; Romanelli, G.; Parmentier, A.; Senesi, R.; Kolesnikov, A.I.; Ko, H.Y.; Andrade, M.F.C.; Car, R. Hydrogen Dynamics in Supercritical Water Probed by Neutron Scattering and Computer Simulations. J. Phys. Chem. Lett. 2020, 11, 9461–9467. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, A.I.; Reiter, G.F.; Choudhury, N.; Prisk, T.R.; Mamontov, E.; Podlesnyak, A.; Ehlers, G.; Seel, A.G.; Wesolowski, D.J.; Anovitz, L.M. Quantum Tunneling of Water in Beryl: A New State of the Water Molecule. Phys. Rev. Lett. 2016, 116. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, A.I.; Reiter, G.F.; Prisk, T.R.; Krzystyniak, M.; Romanelli, G.; Wesolowski, D.J.; Anovitz, L.M. Inelastic and Deep Inelastic Neutron Spectroscopy of Water Molecules under Ultra-confinement. J. Phys. Conf. Ser. 2018, 1055, 012002. [Google Scholar] [CrossRef]
- Komatsu, K.; Noritake, F.; Machida, S.; Sano-Furukawa, A.; Hattori, T.; Yamane, R.; Kagi, H. Partially Ordered State of Ice XV. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Loerting, T. Order and Disorder—A Story about Ice. Available online: https://chemistrycommunity.nature.com/posts/order-and-disorder-a-story-about-ice (accessed on 28 April 2021).
- Gasser, T.M.; Thoeny, A.V.; Plaga, L.J.; Köster, K.W.; Etter, M.; Böhmer, R.; Loerting, T. Experiments Indicating a Second Hydrogen Ordered Phase of Ice VI. Chem. Sci. 2018, 9, 4224–4234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosu-Finsen, A.; Salzmann, C.G. Origin of the Low-temperature Endotherm of Acid-doped Ice VI: New Hydrogen-ordered Phase of Ice or Deep Glassy States? Chem. Sci. 2019, 10, 515–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, G.; Pan, D. A Closer Look at Supercritical Water. Proc. Natl. Acad. Sci. USA 2013, 110, 6250–6251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakaboylu, O.; Harinck, J.; Smit, K.; de Jong, W. Supercritical Water Gasification of Biomass: A Literature and Technology Overview. Energies 2015, 8, 859–894. [Google Scholar] [CrossRef]
- Oka, Y.; Koshizuka, S. Supercritical-pressure, Once-through Cycle Light Water Cooled Reactor Concept. J. Nucl. Sci. Technol. 2001, 38, 1081–1089. [Google Scholar] [CrossRef]
- Deguchi, S.; Tsujii, K. Supercritical Water: A Fascinating Medium for Soft Matter. Soft Matter 2007, 3, 797. [Google Scholar] [CrossRef]
- Boero, M.; Terakura, K.; Ikeshoji, T.; Liew, C.C.; Parrinello, M. Water at Supercritical Conditions: A First Principles Study. J. Chem. Phys. 2001, 115, 2219–2227. [Google Scholar] [CrossRef]
- Mayers, J.; Andreani, C.; Baciocco, G. Initial State Effects in Deep Inelastic Neutron Scattering. Phys. Rev. B 1989, 39, 2022–2028. [Google Scholar] [CrossRef]
- Andreani, C.; Colognesi, D.; Degiorgi, E.; Ricci, M.A. Proton Dynamics in Supercritical Water. J. Chem. Phys. 2001, 115, 11243–11248. [Google Scholar] [CrossRef] [Green Version]
- Blostein, J.; Dawidowski, J.; Granada, J. On the Analysis of Deep Inelastic Neutron Scattering Experiments for Light Nuclei. Phys. B Condens. Matter 2001, 304, 357–367. [Google Scholar] [CrossRef]
- Andreani, C.; Colognesi, D.; Mayers, J.; Reiter, G.F.; Senesi, R. Measurement of Momentum Distribution of Light Atoms and Molecules in Condensed Matter Systems Using Inelastic Neutron Scattering. Adv. Phys. 2005, 54, 377–469. [Google Scholar] [CrossRef]
- Pantalei, C.; Pietropaolo, A.; Senesi, R.; Imberti, S.; Andreani, C.; Mayers, J.; Burnham, C.; Reiter, G. Proton Momentum Distribution of Liquid Water from Room Temperature to the Supercritical Phase. Phys. Rev. Lett. 2008, 100. [Google Scholar] [CrossRef] [Green Version]
- Andreani, C.; Senesi, R.; Krzystyniak, M.; Romanelli, G.; Fernandez-Alonso, F. Atomic Quantum Dynamics in Materials Research. In Neutron Scattering—Applications in Biology, Chemistry, and Materials Science; Elsevier: Amsterdam, The Netherlands, 2017; pp. 403–457. [Google Scholar] [CrossRef]
- Stern, J.; Loerting, T. Crystallisation of the Amorphous Ices in the Intermediate Pressure Regime. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Car, R.; Parrinello, M. Unified Approach for Molecular Dynamics and Density-Functional Theory. Phys. Rev. Lett. 1985, 55, 2471–2474. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Han, J.; Wang, H.; Car, R.; E, W. Deep Potential Molecular Dynamics: A Scalable Model with the Accuracy of Quantum Mechanics. Phys. Rev. Lett. 2018, 120. [Google Scholar] [CrossRef] [Green Version]
- Behler, J.; Parrinello, M. Generalized Neural-Network Representation of High-Dimensional Potential-Energy Surfaces. Phys. Rev. Lett. 2007, 98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, M.; Wu, X.; Wang, H.; Weinan, E.; Car, R. Deep Neural Network for the Dielectric Response of Insulators. Phys. Rev. B 2020, 102. [Google Scholar] [CrossRef]
- Sommers, G.M.; Andrade, M.F.C.; Zhang, L.; Wang, H.; Car, R. Raman Spectrum and Polarizability of Liquid Water from Deep Neural Networks. Phys. Chem. Chem. Phys. 2020, 22, 10592–10602. [Google Scholar] [CrossRef]
- Schütt, K.T.; Gastegger, M.; Tkatchenko, A.; Müller, K.R.; Maurer, R.J. Unifying Machine Learning and Quantum Chemistry with a Deep Neural Network for Molecular Wavefunctions. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Schran, C.; Behler, J.; Marx, D. Automated Fitting of Neural Network Potentials at Coupled Cluster Accuracy: Protonated Water Clusters as Testing Ground. J. Chem. Theory Comput. 2019, 16, 88–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chmiela, S.; Sauceda, H.E.; Müller, K.R.; Tkatchenko, A. Towards Exact Molecular Dynamics Simulations with Machine-learned Force Fields. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Ruzsinszky, A.; Perdew, J.P. Strongly Constrained and Appropriately Normed Semilocal Density Functional. Phys. Rev. Lett. 2015, 115. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Remsing, R.C.; Zhang, Y.; Sun, Z.; Ruzsinszky, A.; Peng, H.; Yang, Z.; Paul, A.; Waghmare, U.; Wu, X.; et al. Accurate First-principles Structures and Energies of Diversely Bonded Systems from an Efficient Density Functional. Nat. Chem. 2016, 8, 831–836. [Google Scholar] [CrossRef]
- Car, R. Fixing Jacob’s Ladder. Nat. Chem. 2016, 8, 820–821. [Google Scholar] [CrossRef]
- Gill, P.M.W. Obituary: Density Functional Theory (1927–1993). Aust. J. Chem. 2001, 54, 661. [Google Scholar] [CrossRef]
- Chen, M.; Ko, H.Y.; Remsing, R.C.; Andrade, M.F.C.; Santra, B.; Sun, Z.; Selloni, A.; Car, R.; Klein, M.L.; Perdew, J.P.; et al. Ab initio Theory and Modeling of Water. Proc. Natl. Acad. Sci. USA 2017, 114, 10846–10851. [Google Scholar] [CrossRef] [Green Version]
- LaCount, M.D.; Gygi, F. Ensemble First-principles Molecular Dynamics Simulations of Water Using the SCAN meta-GGA Density Functional. J. Chem. Phys. 2019, 151, 164101. [Google Scholar] [CrossRef] [PubMed]
- Pestana, L.R.; Mardirossian, N.; Head-Gordon, M.; Head-Gordon, T. ab initio Molecular Dynamics Simulations of Liquid Water using High Quality meta-GGA Functionals. Chem. Sci. 2017, 8, 3554–3565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, A.; Ciardi, G.; Sidler, D.; Hamm, P.; Shalit, A. Impact of Nuclear Quantum Effects on the Structural Inhomogeneity of Liquid Water. Proc. Natl. Acad. Sci. USA 2019, 116, 2458–2463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceriotti, M.; Fang, W.; Kusalik, P.G.; McKenzie, R.H.; Michaelides, A.; Morales, M.A.; Markland, T.E. Nuclear Quantum Effects in Water and Aqueous Systems: Experiment, Theory, and Current Challenges. Chem. Rev. 2016, 116, 7529–7550. [Google Scholar] [CrossRef] [PubMed]
- Novikov, V.N.; Sokolov, A.P. Quantum Effects in Dynamics of Water and Other Liquids of Light Molecules. Eur. Phys. J. E 2017, 40. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, D.M.; Manolopoulos, D.E.; Pipolo, S.; Laage, D.; Hynes, J.T. Nuclear Quantum Effects in Water Reorientation and Hydrogen-Bond Dynamics. J. Phys. Chem. Lett. 2017, 8, 2602–2607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauceda, H.E.; Vassilev-Galindo, V.; Chmiela, S.; Müller, K.R.; Tkatchenko, A. Dynamical Strengthening of Covalent and Non-covalent Molecular Interactions by Nuclear Quantum Effects at Finite Temperature. Nat. Commun. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Markland, T.E.; Ceriotti, M. Nuclear Quantum Effects Enter the Mainstream. Nat. Rev. Chem. 2018, 2. [Google Scholar] [CrossRef] [Green Version]
- Balan, E.; Lazzeri, M.; Delattre, S.; Méheut, M.; Refson, K.; Winkler, B. Anharmonicity of Inner-OH Stretching Modes in Hydrous Phyllosilicates: Assessment from First-principles Frozen-phonon Calculations. Phys. Chem. Miner. 2007, 34, 621–625. [Google Scholar] [CrossRef]
- Lin, C.Y.; George, M.W.; Gill, P.M.W. EDF2: A Density Functional for Predicting Molecular Vibrational Frequencies. Aust. J. Chem. 2004, 57, 365. [Google Scholar] [CrossRef] [Green Version]
- Zhong, K.; Yu, C.C.; Dodia, M.; Bonn, M.; Nagata, Y.; Ohto, T. Vibrational Mode Frequency Correction of Liquid Water in Density Functional Theory Molecular Dynamics Simulations With van der Waals Correction. Phys. Chem. Chem. Phys. 2020, 22, 12785–12793. [Google Scholar] [CrossRef]
- Pestana, L.R.; Marsalek, O.; Markland, T.E.; Head-Gordon, T. The Quest for Accurate Liquid Water Properties from First Principles. J. Phys. Chem. Lett. 2018, 9, 5009–5016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsalek, O.; Markland, T.E. Quantum Dynamics and Spectroscopy of ab initio Liquid Water: The Interplay of Nuclear and Electronic Quantum Effects. J. Phys. Chem. Lett. 2017, 8, 1545–1551. [Google Scholar] [CrossRef] [Green Version]
- Elton, D.C.; Fernández-Serra, M. The Hydrogen-bond Network of Water Supports Propagating Optical Phonon-like Modes. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Rekik, N.; Suleiman, J.; Blaise, P.; Wojcik, M.J.; Flakus, H.T.; Nakajima, T. Electrical Anharmonicity in Hydrogen Bonded Systems: Complete Interpretation of the IR spectra of the Cl–H Stretching Band in the Gaseous (CH3)2OHCl complex. Phys. Chem. Chem. Phys. 2017, 19, 5917–5931. [Google Scholar] [CrossRef]
- Ivanov, S.D.; Witt, A.; Shiga, M.; Marx, D. Communications: On Artificial Frequency Shifts in Infrared Spectra Obtained from Centroid Molecular Dynamics: Quantum Liquid Water. J. Chem. Phys. 2010, 132, 031101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trenins, G.; Willatt, M.J.; Althorpe, S.C. Path-integral Dynamics of Water Using Curvilinear Centroids. J. Chem. Phys. 2019, 151, 054109. [Google Scholar] [CrossRef] [Green Version]
- Habershon, S.; Manolopoulos, D.E. Zero Point Energy Leakage in Condensed Phase Dynamics: An Assessment of Quantum Simulation Methods for Liquid Water. J. Chem. Phys. 2009, 131, 244518. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Ceriotti, M.; Manolopoulos, D.E. How to Remove the Spurious Resonances from Ring Polymer Molecular Dynamics. J. Chem. Phys. 2014, 140, 234116. [Google Scholar] [CrossRef] [Green Version]
- Benson, R.L.; Trenins, G.; Althorpe, S.C. Which Quantum Statistics–classical Dynamics Method is Best for Water? Faraday Discuss. 2020, 221, 350–366. [Google Scholar] [CrossRef]
- Lin, L.; Morrone, J.A.; Car, R.; Parrinello, M. Displaced Path Integral Formulation for the Momentum Distribution of Quantum Particles. Phys. Rev. Lett. 2010, 105. [Google Scholar] [CrossRef] [Green Version]
- Morrone, J.A.; Srinivasan, V.; Sebastiani, D.; Car, R. Proton Momentum Distribution in Water: An Open Path Integral Molecular Dynamics Study. J. Chem. Phys. 2007, 126, 234504. [Google Scholar] [CrossRef]
- Poltavsky, I.; Tkatchenko, A. Modeling Quantum Nuclei with Perturbed Path Integral Molecular Dynamics. Chem. Sci. 2016, 7, 1368–1372. [Google Scholar] [CrossRef] [Green Version]
- Poltavsky, I.; Kapil, V.; Ceriotti, M.; Kim, K.S.; Tkatchenko, A. Accurate Description of Nuclear Quantum Effects with High-Order Perturbed Path Integrals (HOPPI). J. Chem. Theory Comput. 2020, 16, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Leal, L.; Kolesnikov, A.I. Analysis of the Time-of-Flight Neutron Scattering Cross-section Data for Light Water Measured at the SEQUOIA Spectrometer, Spallation Neutron Source (SNS). EPJ Web Conf. 2020, 239, 14007. [Google Scholar] [CrossRef]
- Li, X.Z.; Walker, B.; Michaelides, A. Quantum Nature of the Hydrogen Bond. Proc. Natl. Acad. Sci. USA 2011, 108, 6369–6373. [Google Scholar] [CrossRef] [Green Version]
- Senesi, R.; Flammini, D.; Kolesnikov, A.I.; Murray, É.D.; Galli, G.; Andreani, C. The Quantum Nature of the OH Stretching Mode in Ice and Water Probed by Neutron Scattering Experiments. J. Chem. Phys. 2013, 139, 074504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parmentier, A.; Shephard, J.J.; Romanelli, G.; Senesi, R.; Salzmann, C.G.; Andreani, C. Evolution of Hydrogen Dynamics in Amorphous Ice with Density. J. Phys. Chem. Lett. 2015, 6, 2038–2042. [Google Scholar] [CrossRef]
- Kapil, V.; Cuzzocrea, A.; Ceriotti, M. Anisotropy of the Proton Momentum Distribution in Water. J. Phys. Chem. B 2018, 122, 6048–6054. [Google Scholar] [CrossRef] [Green Version]
- Ceriotti, M.; Parrinello, M.; Markland, T.E.; Manolopoulos, D.E. Efficient Stochastic Thermostatting of Path Integral Molecular Dynamics. J. Chem. Phys. 2010, 133, 124104. [Google Scholar] [CrossRef] [Green Version]
- Ceriotti, M.; Bussi, G.; Parrinello, M. Colored-Noise Thermostats à la Carte. J. Chem. Theory Comput. 2010, 6, 1170–1180. [Google Scholar] [CrossRef] [Green Version]
- Ceriotti, M.; Manolopoulos, D.E.; Parrinello, M. Accelerating the Convergence of Path Integral Dynamics with a Generalized Langevin Equation. J. Chem. Phys. 2011, 134, 084104. [Google Scholar] [CrossRef]
- Marsalek, O.; Markland, T.E. ab initio Molecular Dynamics with Nuclear Quantum Effects at Classical Cost: Ring Polymer Contraction for Density Functional Theory. J. Chem. Phys. 2016, 144, 054112. [Google Scholar] [CrossRef] [Green Version]
- Kapil, V.; Rossi, M.; Marsalek, O.; Petraglia, R.; Litman, Y.; Spura, T.; Cheng, B.; Cuzzocrea, A.; Meißner, R.H.; Wilkins, D.M.; et al. i-PI 2.0: A universal force engine for advanced molecular simulations. Comput. Phys. Commun. 2019, 236, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Ceriotti, M.; More, J.; Manolopoulos, D.E. i-PI: A Python Interface for ab initio Path Integral Molecular Dynamics Simulations. Comput. Phys. Commun. 2014, 185, 1019–1026. [Google Scholar] [CrossRef] [Green Version]
- Romanelli, G.; Ceriotti, M.; Manolopoulos, D.E.; Pantalei, C.; Senesi, R.; Andreani, C. Direct Measurement of Competing Quantum Effects on the Kinetic Energy of Heavy Water upon Melting. J. Phys. Chem. Lett. 2013, 4, 3251–3256. [Google Scholar] [CrossRef] [Green Version]
- Morrone, J.A.; Lin, L.; Car, R. Tunneling and Delocalization Effects in Hydrogen Bonded Systems: A Study in Position and Momentum space. J. Chem. Phys. 2009, 130, 204511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flammini, D.; Pietropaolo, A.; Senesi, R.; Andreani, C.; McBride, F.; Hodgson, A.; Adams, M.A.; Lin, L.; Car, R. Spherical Momentum Distribution of the Protons in Hexagonal Ice from Modeling of Inelastic Neutron Scattering Data. J. Chem. Phys. 2012, 136, 024504. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.; Ceriotti, M.; Manolopoulos, D.E. Nuclear Quantum Effects in H and OH– Diffusion along Confined Water Wires. J. Phys. Chem. Lett. 2016, 7, 3001–3007. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Behler, J.; Ceriotti, M. Nuclear Quantum Effects in Water at the Triple Point: Using Theory as a Link Between Experiments. J. Phys. Chem. Lett. 2016, 7, 2210–2215. [Google Scholar] [CrossRef] [PubMed]
- Gaiduk, A.P.; Gygi, F.; Galli, G. Density and Compressibility of Liquid Water and Ice from First-Principles Simulations with Hybrid Functionals. J. Phys. Chem. Lett. 2015, 6, 2902–2908. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Engel, E.A.; Behler, J.; Dellago, C.; Ceriotti, M. ab initio Thermodynamics of Liquid and Solid Water. Proc. Natl. Acad. Sci. USA 2019, 116, 1110–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Wang, H.; Car, R.; Weinan, E. The Phase Diagram of a Deep Potential Water Model. arXiv 2020, arXiv:2102.04804. [Google Scholar]
- Reinhardt, A.; Cheng, B. Quantum-mechanical Exploration of the Phase Diagram of Water. Nat. Commun. 2021, 12. [Google Scholar] [CrossRef]
- Bokdam, M.; Lahnsteiner, J.; Ramberger, B.; Schäfer, T.; Kresse, G. Assessing Density Functionals Using Many Body Theory for Hybrid Perovskites. Phys. Rev. Lett. 2017, 119. [Google Scholar] [CrossRef] [PubMed]
- Lahnsteiner, J.; Kresse, G.; Heinen, J.; Bokdam, M. Finite-temperature Structure of the MAPbI3 perovskite: Comparing Density Functional Approximations and Force Fields to Experiment. Phys. Rev. Mater. 2018, 2. [Google Scholar] [CrossRef] [Green Version]
- Jinnouchi, R.; Lahnsteiner, J.; Karsai, F.; Kresse, G.; Bokdam, M. Phase Transitions of Hybrid Perovskites Simulated by Machine-Learning Force Fields Trained on the Fly with Bayesian Inference. Phys. Rev. Lett. 2019, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapil, V.; Wieme, J.; Vandenbrande, S.; Lamaire, A.; Speybroeck, V.V.; Ceriotti, M. Modeling the Structural and Thermal Properties of Loaded Metal–Organic Frameworks. An Interplay of Quantum and Anharmonic Fluctuations. J. Chem. Theory Comput. 2019, 15, 3237–3249. [Google Scholar] [CrossRef] [Green Version]
- Melillo, J.H.; Cerveny, S. Isotope Effect on the Dynamics of Hydrophilic Solutions at Supercooled Temperatures. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; pp. 263–281. [Google Scholar] [CrossRef]
- Markland, T.E.; Morrone, J.A.; Berne, B.J.; Miyazaki, K.; Rabani, E.; Reichman, D.R. Quantum fluctuations can promote or inhibit glass formation. Nat. Phys. 2011, 7, 134–137. [Google Scholar] [CrossRef] [Green Version]
- Gainaru, C.; Agapov, A.L.; Fuentes-Landete, V.; Amann-Winkel, K.; Nelson, H.; Köster, K.W.; Kolesnikov, A.I.; Novikov, V.N.; Richert, R.; Böhmer, R.; et al. Anomalously Large Isotope Effect in the Glass Transition of Water. Proc. Natl. Acad. Sci. USA 2014, 111, 17402–17407. [Google Scholar] [CrossRef] [Green Version]
- Shulumba, N.; Hellman, O.; Minnich, A.J. Lattice Thermal Conductivity of Polyethylene Molecular Crystals from First-Principles Including Nuclear Quantum Effects. Phys. Rev. Lett. 2017, 119. [Google Scholar] [CrossRef] [Green Version]
- Jakowski, J.; Huang, J.; Garashchuk, S.; Luo, Y.; Hong, K.; Keum, J.; Sumpter, B.G. Deuteration as a Means to Tune Crystallinity of Conducting Polymers. J. Phys. Chem. Lett. 2017, 8, 4333–4340. [Google Scholar] [CrossRef]
- Chang, D.; Li, T.; Li, L.; Jakowski, J.; Huang, J.; Keum, J.K.; Lee, B.; Bonnesen, P.V.; Zhou, M.; Garashchuk, S.; et al. Selectively Deuterated Poly(ϵ-caprolactone)s: Synthesis and Isotope Effects on the Crystal Structures and Properties. Macromolecules 2018, 51, 9393–9404. [Google Scholar] [CrossRef]
- Cai, W.; Dunuwille, M.; He, J.; Taylor, T.V.; Hinton, J.K.; MacLean, M.C.; Molaison, J.J.; dos Santos, A.M.; Sinogeikin, S.; Deemyad, S. Deuterium Isotope Effects in Polymerization of Benzene under Pressure. J. Phys. Chem. Lett. 2017, 8, 1856–1864. [Google Scholar] [CrossRef]







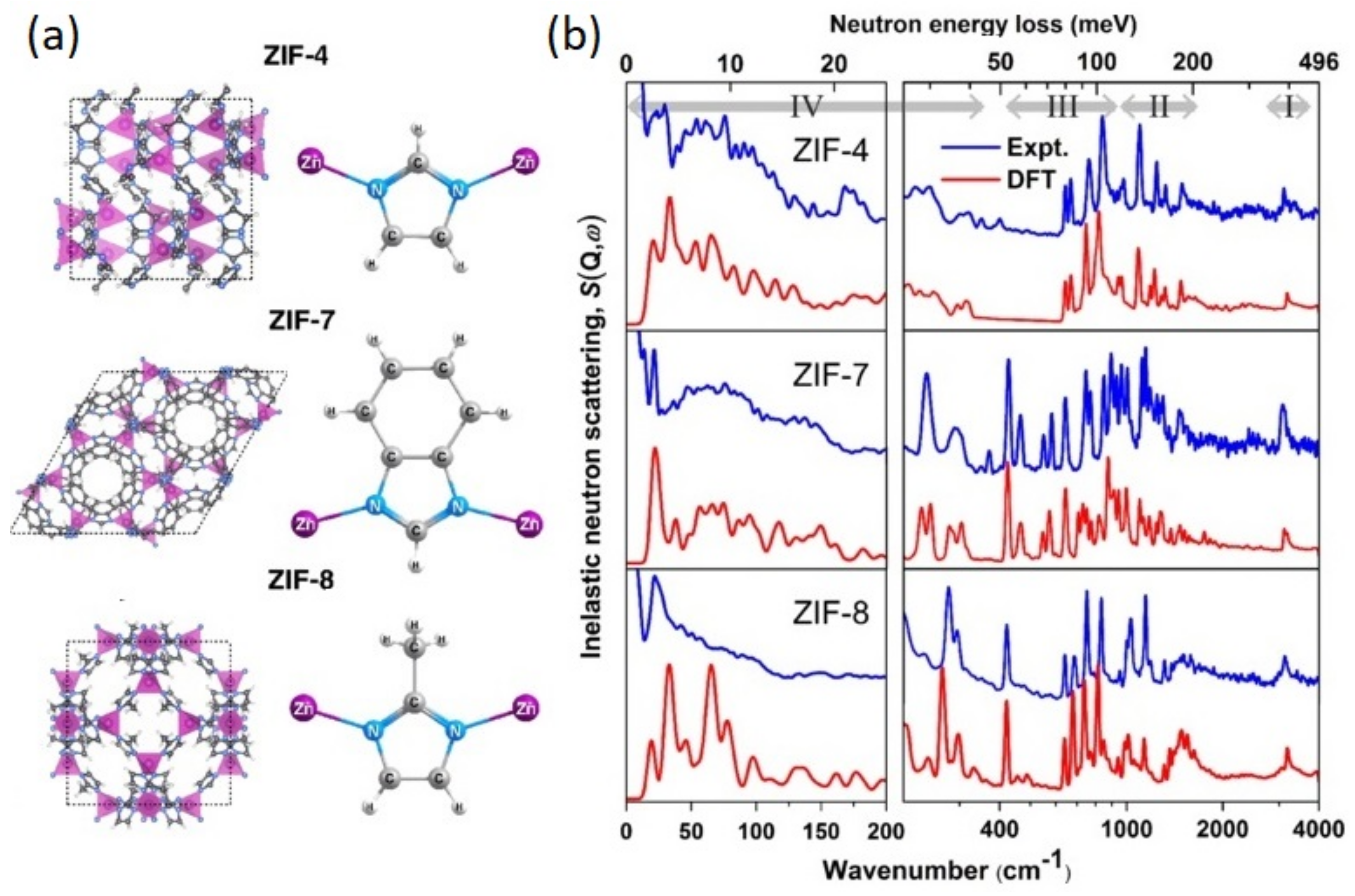


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drużbicki, K.; Gaboardi, M.; Fernandez-Alonso, F. Dynamics & Spectroscopy with Neutrons—Recent Developments & Emerging Opportunities. Polymers 2021, 13, 1440. https://doi.org/10.3390/polym13091440
Drużbicki K, Gaboardi M, Fernandez-Alonso F. Dynamics & Spectroscopy with Neutrons—Recent Developments & Emerging Opportunities. Polymers. 2021; 13(9):1440. https://doi.org/10.3390/polym13091440
Chicago/Turabian StyleDrużbicki, Kacper, Mattia Gaboardi, and Felix Fernandez-Alonso. 2021. "Dynamics & Spectroscopy with Neutrons—Recent Developments & Emerging Opportunities" Polymers 13, no. 9: 1440. https://doi.org/10.3390/polym13091440






