Moisture Absorption Characteristics of Nanoparticle-Doped Silicone Rubber and Its Influence Mechanism on Electrical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
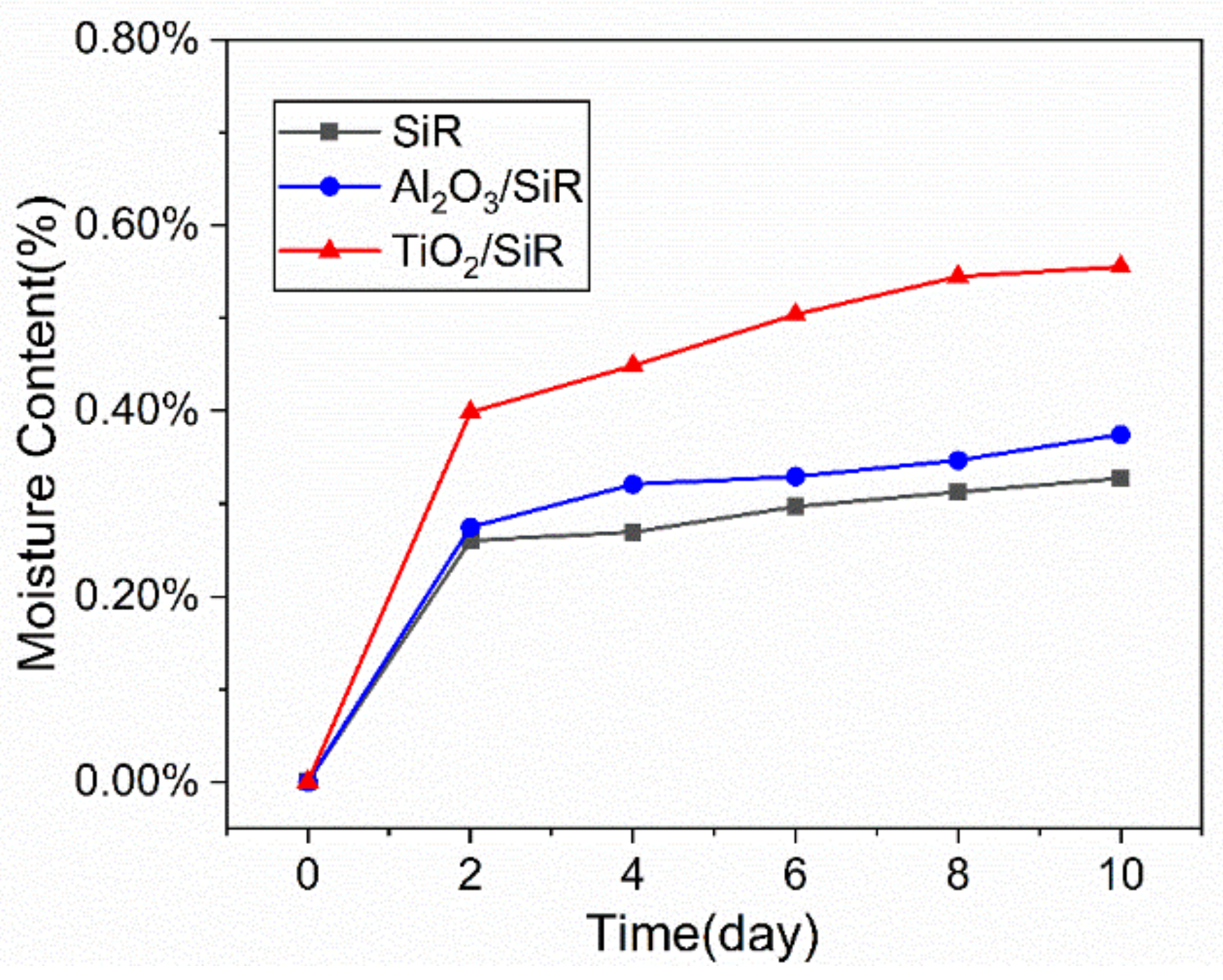
2.2. Moisture Absorption Experiment
2.3. Characterization
2.4. DC Breakdown Strength
2.5. Space Charge Distribution
2.6. Density Functional Theory and Trap Calculation
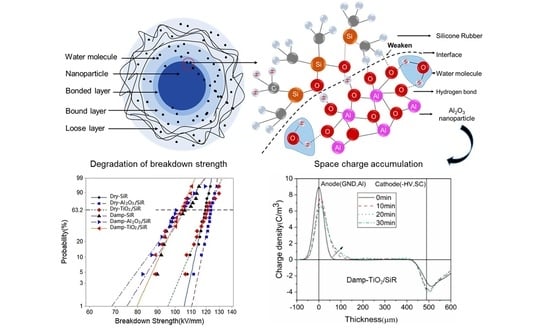
3. Results
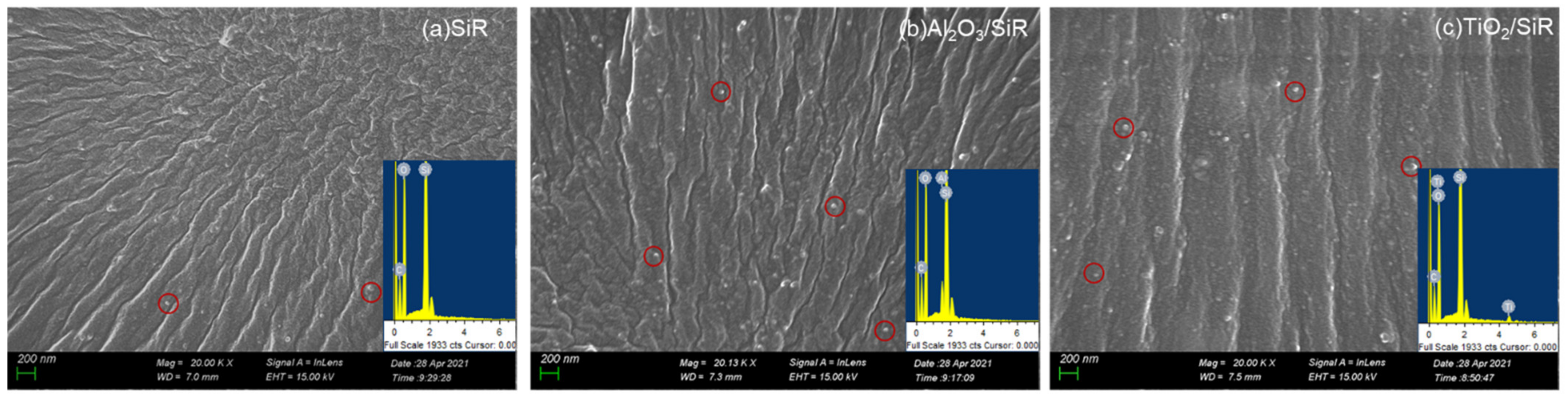
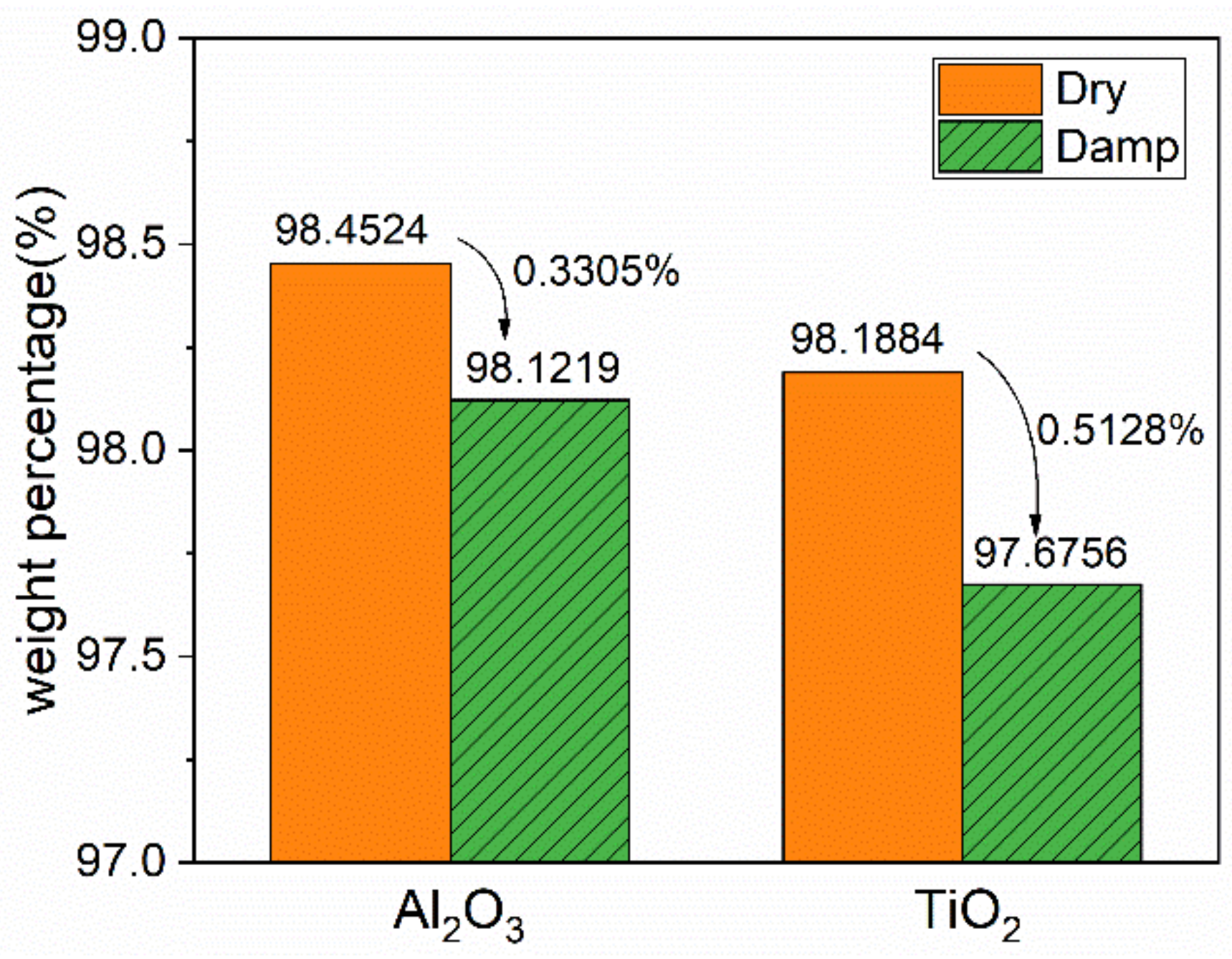
3.1. Characterization and Moisture Absorption
3.2. Space Charge Characteristics
3.3. DC Breakdown Strength
3.4. Trap Depth and Electronic Orbital Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fukawa, M.; Kawai, T.; Okano, Y.; Sakuma, S.; Asai, S.; Kanaoka, M.; Yamanouchi, H. Development of 500-kV XLPE cables and accessories for long distance underground transmission line. III. Electrical properties of 500-kV cables. IEEE Trans. Power Del. 1996, 11, 627–634. [Google Scholar] [CrossRef]
- Kaminaga, K.; Ichiahara, M.; Jinno, M.; Fujii, O.; Fukunaga, S.; Kobayashi, M.; Watanabe, K. Development of 500-kV XLPE Cables and Accessories for Long-Distance Underground Transmission Lines Part V: Long-Term Performance for 500-kV XLPE Cables and Joints. IEEE Power Eng. Rev. 1996, 11, 1185–1194. [Google Scholar]
- Maekawa, Y.; Watanabe, C.; Asano, M.; Murata, Y.; Katakai, S.; Shimada, M. Development of 500 kV XLPE Insulated DC Cable. IEEJ Trans. Power Energy 2001, 121, 390–398. [Google Scholar] [CrossRef]
- Dang, Z.M.; Xia, Y.J.; Zha, J.W.; Yuan, J.K.; Bai, J. Preparation and dielectric properties of surface modified TiO2/silicone rubber nanocomposites. Mater. Lett. 2011, 65, 3430–3432. [Google Scholar] [CrossRef]
- Fei, Y.J.; Zhang, Y.X.; Zhou, Y.X. Thermo characteristics of Silicone rubber and its effects on operational reliability of extra-high voltage cable accessories. Adv. Technol. Elect. Eng. Energy 2014, 33, 30–34. [Google Scholar]
- Cao, Y.; Irwin, P.C.; Younsi, K. The future of nanodielectrics in the electrical power industry. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 797–807. [Google Scholar]
- Tanaka, T.; Montanari, G.C.; Mulhaupt, R. Polymer nanocomposites as dielectrics and electrical insulation-perspectives for processing technologies, material characterization and future applications. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 763–784. [Google Scholar] [CrossRef]
- Tanaka, T. Dielectric Nanocomposites with Insulating Properties. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 914–928. [Google Scholar] [CrossRef]
- In-Yup, J.; Baek, J.B. Nanocomposites Derived from Polymers and Inorganic Nanoparticles. Materials 2010, 3, 3654–3674. [Google Scholar]
- Calebrese, C.; Hui, L.; Schadler, L.S.; Nelson, J.K. A review on the importance of nanocomposite processing to enhance electrical insulation. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 938–945. [Google Scholar] [CrossRef]
- Nanqiang, S.; Qingguo, C.; Xinzhe, W. Preparation and Dielectric Properties of SiC/LSR Nanocomposites for Insulation of High Voltage Direct Current Cable Accessories. Materials 2018, 11, 403. [Google Scholar]
- Chen, Q.; Xi, B.; Zhang, J.; Wang, X.; Yang, H.J. Dielectric and space charge characteristics of nano-modified liquid silicone rubber for high-voltage DC cable accessories. Mater. Sci. Mater. Electron. 2020, 31, 16819–16829. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Nie, H.; Zhang, Y.X.; Zhang, L.; Lu, Y. Conductivity Current and Electric Breakdown Properties of Thermally Aged Nano Silicone Rubber. In Proceedings of the International Electrical and Energy Conference (CIEEC), Beijing, China, 7–9 September 2019. [Google Scholar]
- Han, Z.; Garrett, R. Overview of Polymer Nanocomposites as Dielectrics and Electrical Insulation Materials for Large High Voltage Rotating Machines. In Proceedings of the International Conference on Nanotechnology, Stockholm, Sweden, 24–27 June 2013. [Google Scholar]
- Hui, L.; Schadler, L.S.; Nelson, J.K. The influence of moisture on the electrical properties of crosslinked polyethylene/silica nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 641–653. [Google Scholar] [CrossRef]
- Radoń, A.; Włodarczyk, P.J. Influence of water on the dielectric properties, electrical conductivity and microwave absorption properties of amorphous yellow dextrin. Cellulose 2019, 11, 403. [Google Scholar] [CrossRef] [Green Version]
- Fabiani, D.; Montanari, G.; Testa, L. Effect of aspect ratio and water contamination on the electric properties of nanostructured insulating materials. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 221–230. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.M.; Zhao, H.X.; Ming, Z.; Zhang, W.L. Influence of Moisture Absorption on the DC Conduction and Space Charge Property of MgO/LDPE Nanocomposite. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1957–1964. [Google Scholar]
- Chi, X.H.; Liu, W.F.; Li, S.T.; Zhang, X.H. The Effect of Humidity on Dielectric Properties of PP-Based Nano-Dielectric. Materials 2019, 12, 1378. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Khani, M.M.; Krentz, T.M.; Huang, Y.H.; Zhou, Y.X.; Benicewicz, B.C.; Nelson, J.K.; Schadler, L.S. Suppression of space charge in crosslinked polyethylene filled with poly(stearyl methacrylate)-grafted SiO2 nanoparticles. Appl. Phys. Lett. 2017, 110, 132903. [Google Scholar] [CrossRef] [Green Version]
- Seidl, A.; Görling, A.; Vogl, P.; Majewski, J.A.; Levy, M. Generalized Kohn-Sham schemes and the band-gap problem. Phys. Rev. B 1996, 53, 3764–3774. [Google Scholar] [CrossRef]
- Hybertsen, M.S.; Louie, S.G. Electron correlation in semiconductors and insulators: Band gaps and quasiparticle energies. Phys. Rev B Condens Matter. 1986, 34, 5390–5413. [Google Scholar] [CrossRef]
- Meunier, M.; Quirke, N.; Aslanides, A. Molecular modeling of electron traps in polymer insulators: Chemical defects and impurities. J. Chem. Phys. 2001, 115, 2876–2881. [Google Scholar] [CrossRef]
- Anta, J.A.; Marcelli, G.; Meunier, M.; Quirke, N. Models of electron trapping and transport in polyethylene: Current-voltage characteristics. J. Appl. Phys. 2002, 92, 1002–1008. [Google Scholar] [CrossRef]
- Nakayama, N.; Hayashi, T. Preparation of TiO2 nanoparticles surface-modified by both carboxylic acid and amine: Dispersibility and stabilization in organic solvents. Colloid. Surf. A Physicochem. Eng. Asp. 2008, 317, 543–550. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Guo, S.W.; Nie, Q.; Liu, R.; Hou, F. Influences of Nano-alumina on the Space Charge Behavior of Silicone Rubber. High Voltage Eng. 2010, 36, 1605–1611. [Google Scholar]
- Takada, T.; Hayase, Y.; Tanaka, Y. Space charge trapping in electrical potential well caused by permanent and induced dipoles for LDPE/MgO nanocomposite. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 152–160. [Google Scholar] [CrossRef]
- Mazzanti, G.; Montanari, G.C.; Alison, J.M. A space-charge based method for the estimation of apparent mobility and trap depth as markers for insulation degradation-theoretical basis and experimental validation. IEEE Trans. Dielectr. Electr. Insul. 2003, 8, 187–197. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Wang, Y.S.; Zahn, M.; Wang, N.H.; Sun, Q.H. Morphology Effects on Space Charge Characteristics of Low Density Polyethylene. Jpn. J. Appl. Phys. 2011, 50, 017101. [Google Scholar] [CrossRef] [Green Version]
- Veena, M.G.; Renukappa, N.M.; Raj, J.M.; Ranganathaiah, C.; Shivakumar, K.N. Characterization of nanosilica-filled epoxy composites for electrical and insulation applications. J. Appl. Polym. Sci. 2011, 121, 2752–2760. [Google Scholar] [CrossRef]
- Teng, C.Y.; Zhou, Y.X.; Li, S.H.; Zhang, L.; Zhao, L. Regulation of temperature resistivity characteristics of insulating epoxy composite by incorporating positive temperature coefficient material. IEEE Tarns. Dielectr. Electr. Insul. 2020, 27, 512–520. [Google Scholar] [CrossRef]
- Tanaka, T.; Kozako, M.; Fuse, N.; Ohki, Y. Proposal of a Multi-core Model for Polymer Nanocomposite Dielectrics. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 669–681. [Google Scholar] [CrossRef]
- Plesa, I.; Notingher, P.V.; Schlogl, S.; Sumereder, C.; Muhr, M. Properties of Polymer Composites Used in High-Voltage Applications. Polymers 2016, 8, 173. [Google Scholar] [CrossRef]
- Muroga, S.; Muramoto, Y.; Shimizu, N. Influence of H2O molecules on electrical tree initiation in silicone rubber. In Proceedings of the Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Cancun, Mexico, 16–19 October 2011; pp. 784–787. [Google Scholar]
- Peebles, L.H. Durability of Structural Adhesives; Kinloch, A.J., Ed.; Applied Science Publishers: London, UK; New York, NY, USA, 1983. [Google Scholar]


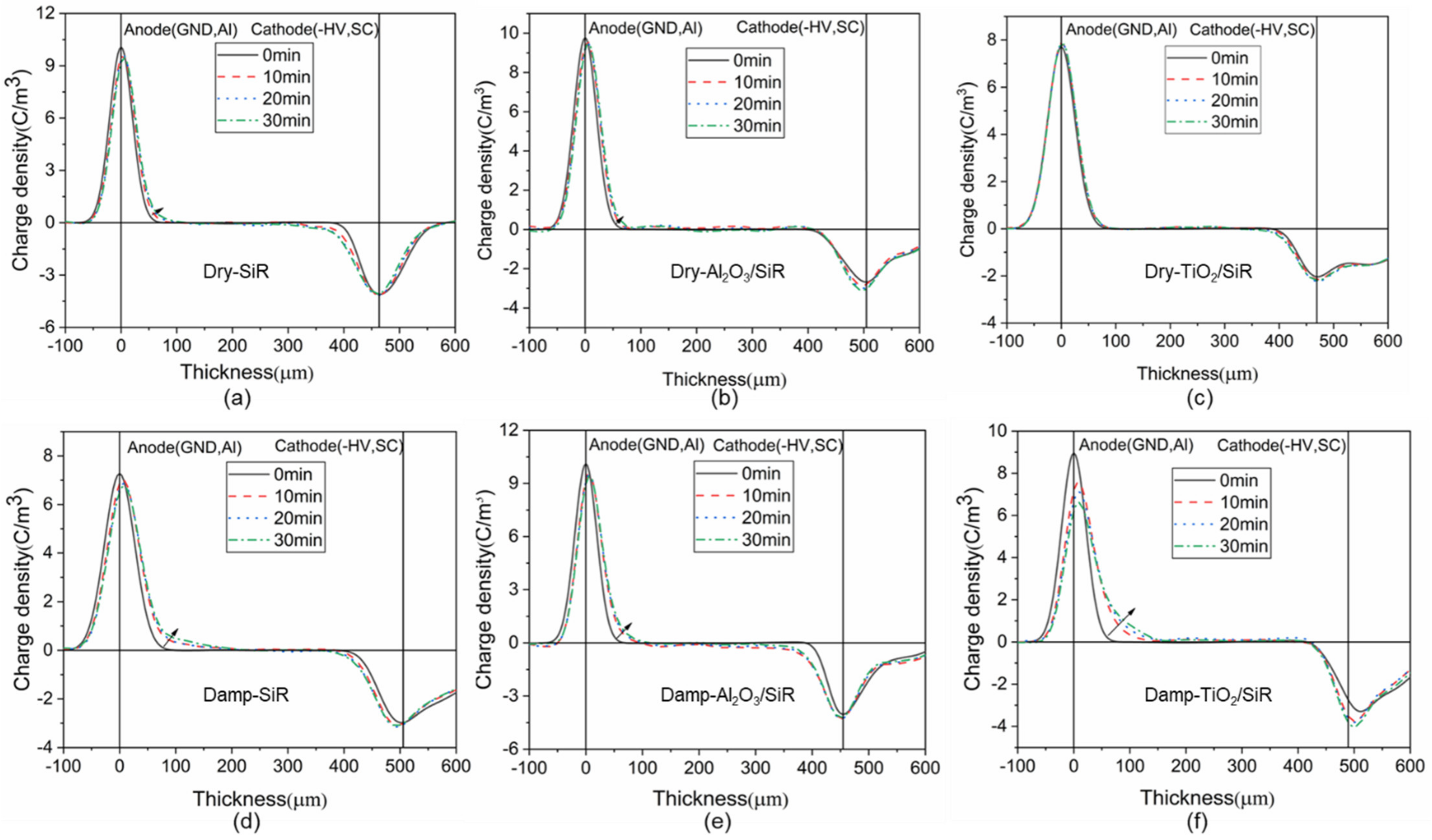

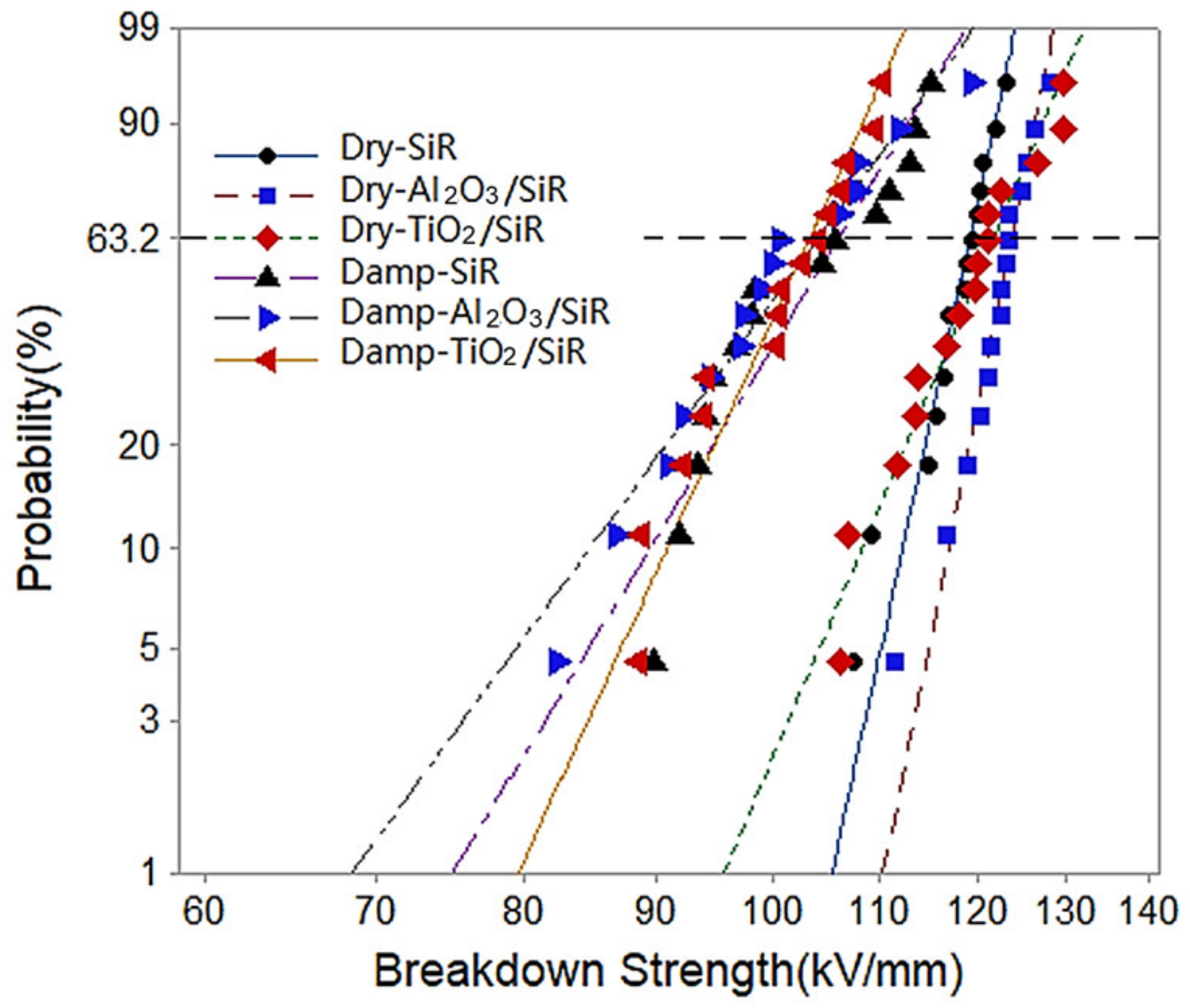

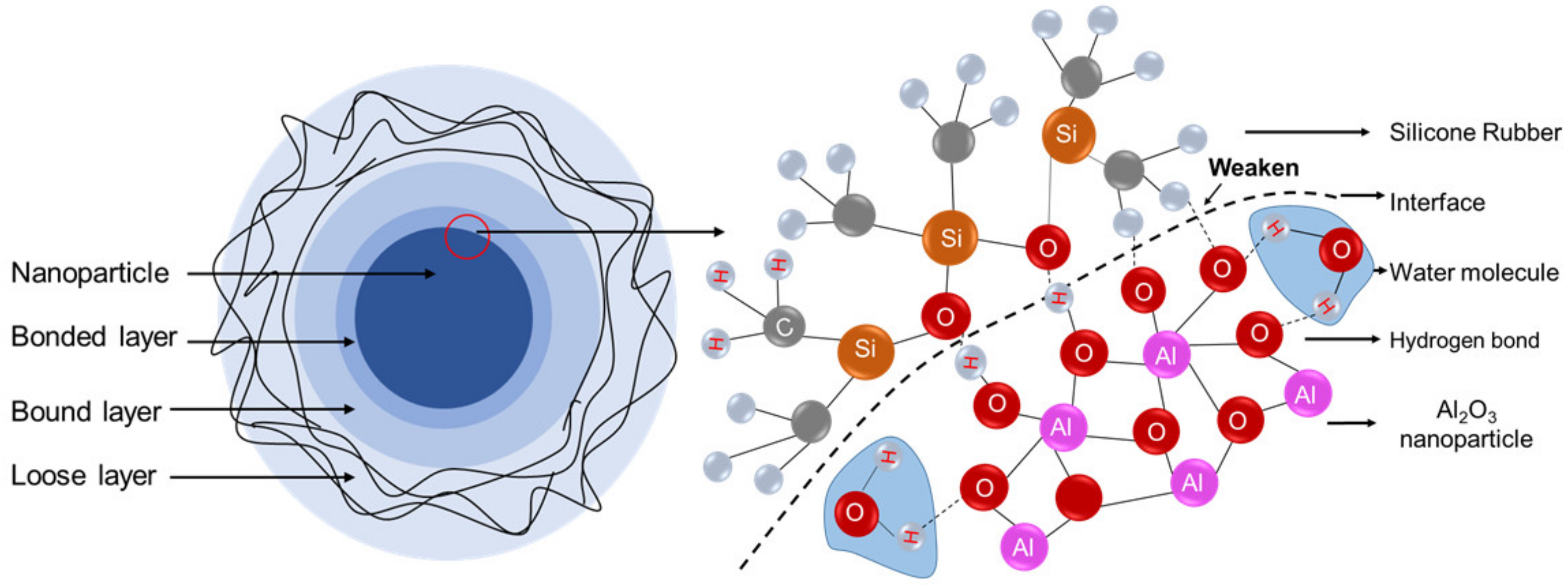
| Sets | Dry Sample (kV/mm) | Damp Samples (kV/mm) | Drop Rate |
|---|---|---|---|
| SiR | 119.2 | 105.9 | 11.16% |
| Al2O3/SiR | 123.7 | 104.0 | 15.93% |
| TiO2/SiR | 121.7 | 103.2 | 15.20% |
| Sets | ||||
|---|---|---|---|---|
| Reference(SiR) | −440.76 | −440.26 | −0.50 | Null |
| Defect(H2O/SiR) | −494.36 | −494.25 | −0.11 | 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Zhang, Y.; Zhou, Y.; Huang, X. Moisture Absorption Characteristics of Nanoparticle-Doped Silicone Rubber and Its Influence Mechanism on Electrical Properties. Polymers 2021, 13, 1474. https://doi.org/10.3390/polym13091474
Zhu X, Zhang Y, Zhou Y, Huang X. Moisture Absorption Characteristics of Nanoparticle-Doped Silicone Rubber and Its Influence Mechanism on Electrical Properties. Polymers. 2021; 13(9):1474. https://doi.org/10.3390/polym13091474
Chicago/Turabian StyleZhu, Xiaoqian, Yunxiao Zhang, Yuanxiang Zhou, and Xin Huang. 2021. "Moisture Absorption Characteristics of Nanoparticle-Doped Silicone Rubber and Its Influence Mechanism on Electrical Properties" Polymers 13, no. 9: 1474. https://doi.org/10.3390/polym13091474
APA StyleZhu, X., Zhang, Y., Zhou, Y., & Huang, X. (2021). Moisture Absorption Characteristics of Nanoparticle-Doped Silicone Rubber and Its Influence Mechanism on Electrical Properties. Polymers, 13(9), 1474. https://doi.org/10.3390/polym13091474