Strategic Possibility Routes of Recycled PET
Abstract
:1. Introduction
2. Physical Properties PET
3. The Common Recycling of PET
- (i)
- Water Contaminant
- (ii)
- Coloring Contaminant
- (iii)
- Acetaldehyde
- (iv)
- Heavy Metal Contamination
3.1. Conventional Recycling PET
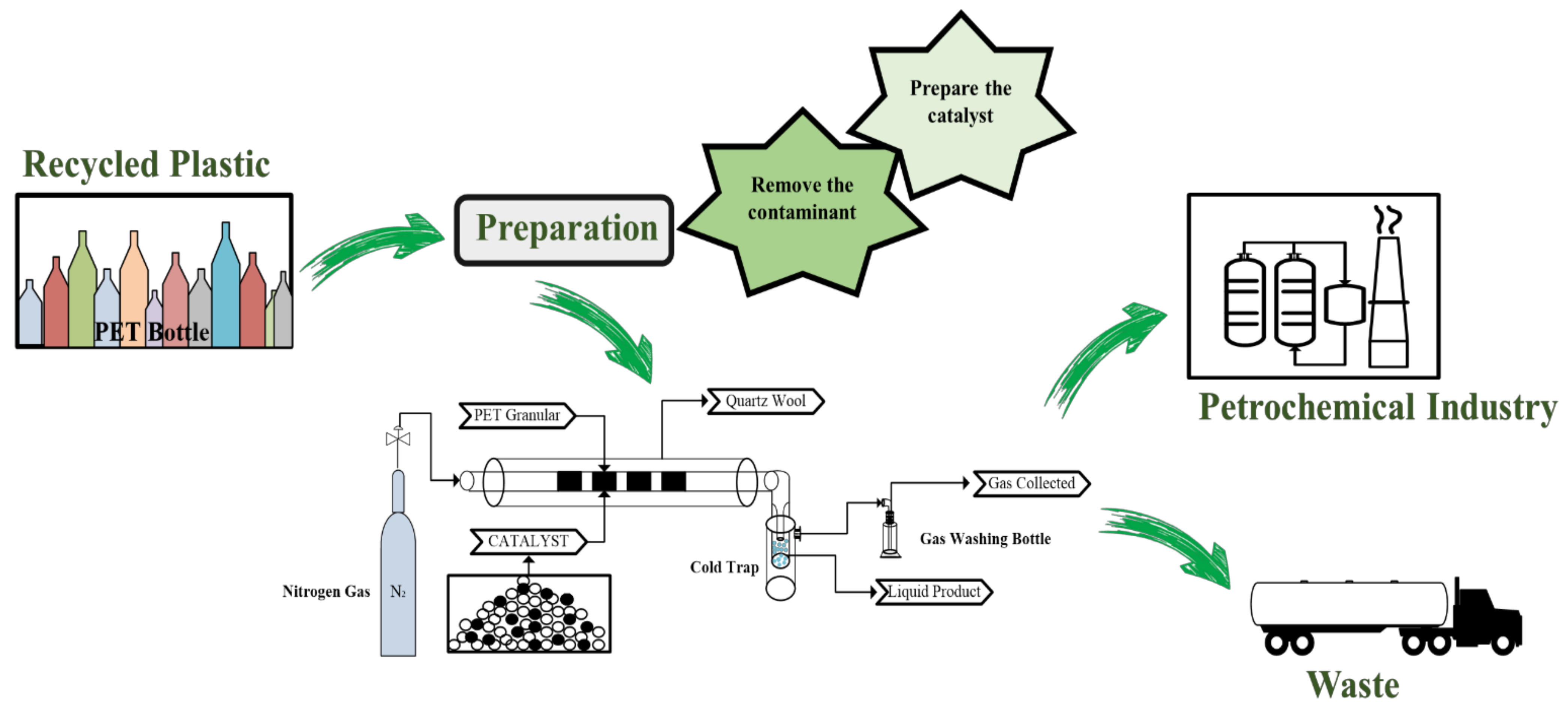
3.2. PET Super-Clean Recycling Processes Based on Pellets
4. The Mechanical Recycling of PET
- a.
- Extrusion molding: The small particle of plastic solid waste is melted and extruded through by single or twin screws to get the molded product—applying extrusion moldings such as pipes, sheets, film and wire covering.
- b.
- Injection molding: The melted polymer is injected into a mold to solidify form to obtain the product desired. The implementation of this process, for instance, washbowls, buckets, bumpers and pallets.
- c.
- Blow molding: Air is applied in the blow molding. The extrusion or injection molded is clamped in a mold with air to make bottles or containers.
- d.
- Vacuum molding: Mild heat layer is sandwiched in the mold, and there is a space between the layer and mold sealed. The vacuum moldings are used for several products, e.g., cups and trays.
- e.
- Inflation molding: The melted polymer is inflated into a cylinder to form a film by extrusion molding—the implementation of inflation molding such as shopping bags [44].
Contamination Levels and Maximum Consumer Exposure from Food Packages Made from rPET
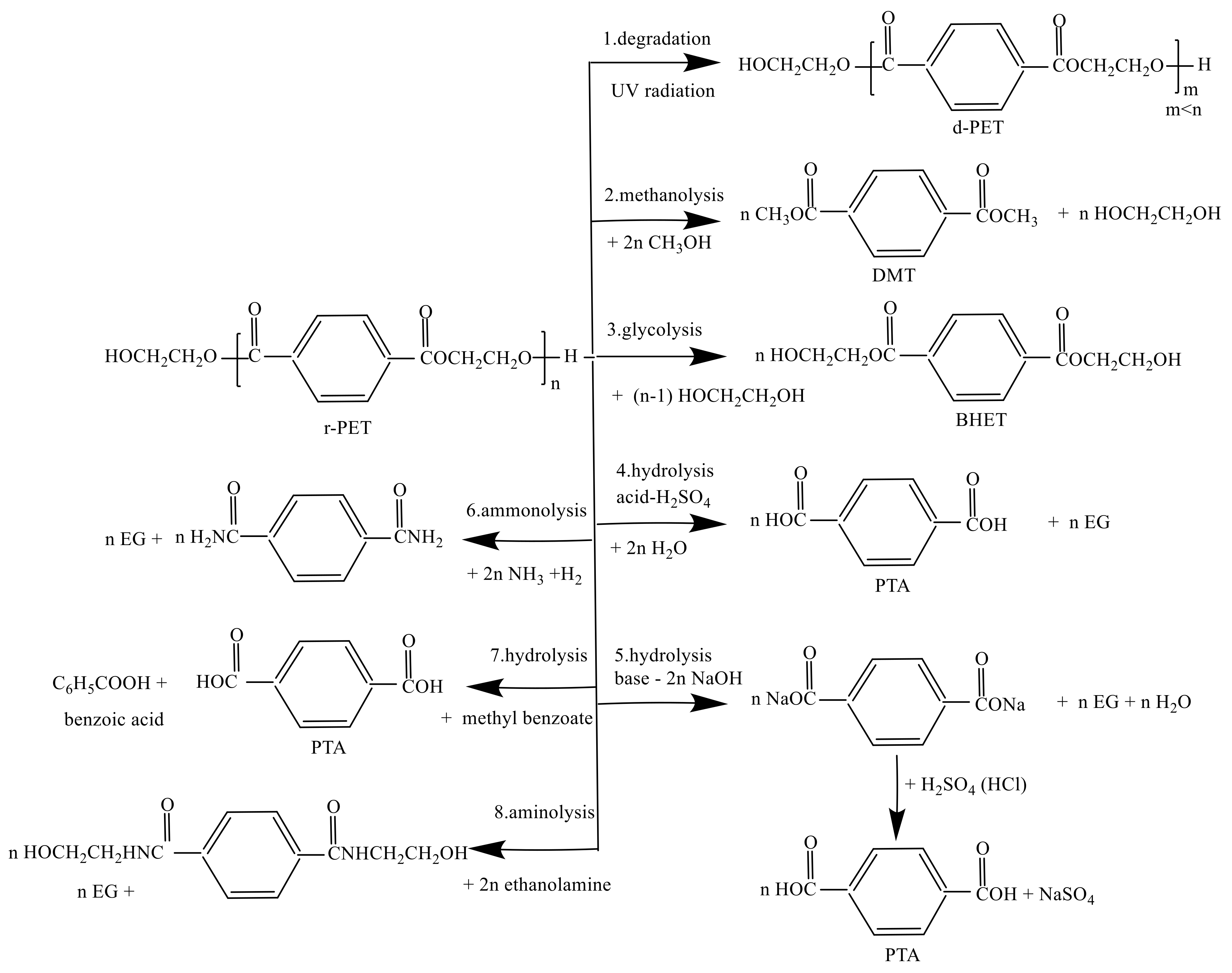
5. The Chemical Recycling of PET
5.1. Recycle PET Using Degradation Process/Pyrolysis
5.2. PET Recycling by Hydrolysis
5.2.1. PET Recycling by Alkaline Hydrolysis
5.2.2. PET Recycling by Acid Hydrolysis
5.3. PET Recycling by Methanolysis
5.4. PET Recycling by Glycolysis
5.5. PET Recycling by Aminolysis and Ammonolysis
6. The Recent Development of PET Chemical Recycling
6.1. PET Recycling Using Microwave Irradiation
6.2. PET Recycling Using Ionic Liquid
6.3. PET Recycling Using Phase–Transfer Catalysis
6.4. PET Recycling Using Nautral or Biomass-Based Catalyst
6.5. PET Recycling Using an Enzymatic Catalyst
6.6. PET Recycling Using Methanolysis–Hydrolysis
6.7. PET Recycling Using Glycolysis–Methanolysis
6.8. PET Recycling Using Glycolysis–Hydrolysis
6.9. PET Recycling Using Steam Hydrolysis
6.10. PET Recycling Using Solid-State Hydrolysis
7. Factor for Recycling PET
7.1. Crystllility of PET
7.2. Particle Size of PET
7.3. Effect of Amount of Catalyst
7.4. Downstream Treatment
7.4.1. After Hydrolysis
7.4.2. After Glycolysis
7.5. Complex of Plastics
8. Kinetic and Modeling Analysis of Chemical Recycling PET
9. Reactor of Recycling PET and Application of rPET
9.1. Reactor and Product Distribution
9.2. Application of Product after PET Upcycling
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Notation
| PET | polyethylene terephthalate |
| BHET | bis (2-hydroxyethyl terephthalate) |
| DBTP | dibuthyl terephthalate |
| DMT | dimethyl terephthalate |
| EG | ethylene glycol |
| HEB | 2-hydroxyethyl benzoate |
| MDI | diphenylmethane diisocyanate |
| MHET | methyl-(2-hydroxyethyl) terephthalate |
| rBHET | recycled bis (2-hydroxyethyl terephthalate) |
| rPET | recycled polyethylene terephthalate |
| rTPA | recycled terephthalic acid |
| TBAB | tetrabutylammonium bromide |
| TBAI | tetrabutylammonium iodide |
| TDI | 2,4-Toluene diisocyanate |
| TOMAB | trioctyl ammonium bromide |
| TPA | terephthalic acid |
| X | conversion of PET |
| Wo | initial weight sample |
| W | weight t during pyrolysis |
| We | weight the end of the reaction |
| Ea | activation energy |
| A | preexponential factor |
| T | temperature |
References
- Ubeda, S.; Aznar, M.; Nerín, C. Determination of oligomers in virgin and recycled polyethylene terephthalate (PET) samples by UPLC-MS-QTOF. Anal. Bioanal. Chem. 2018, 410, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- Welle, F. Food Law Compliance of Poly(ethylene Terephthalate) (PET) Food Packaging Materials. In Food Additives and Packaging; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2014; Volume 1162, pp. 167–195. [Google Scholar]
- Li, B.; Wang, Z.-W.; Lin, Q.-B.; Hu, C.-Y. Study of the Migration of Stabilizer and Plasticizer from Polyethylene Terephthalate into Food Simulants. J. Chromatogr. Sci. 2016, 54, 939–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begley, T.H.; Biles, J.E.; Cunningham, C.; Piringer, O. Migration of a UV stabilizer from polyethylene terephthalate (PET) into food simulants. Food Addit. Contam. 2004, 21, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, P.T. Waste Treatment and Disposal; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Le, D.K.; Ng, G.N.; Koh, H.W.; Zhang, X.; Thai, Q.B.; Phan-Thien, N.; Duong, H.M. Methyltrimethoxysilane-coated recycled polyethylene terephthalate aerogels for oil spill cleaning applications. Mater. Chem. Phys. 2020, 239, 122064. [Google Scholar] [CrossRef]
- Administration, F.D. Points to Consider for the Use of Recycled Plastics: Food Packaging, Chemistry Considerations; FDA Division of Food Chemistry and Technology Publication: Washington, DC, USA, 1992; Volume 410. [Google Scholar]
- Nikles, D.E.; Farahat, M.S. New Motivation for the Depolymerization Products Derived from Poly(Ethylene Terephthalate) (PET) Waste: A Review. Macromol. Mater. Eng. 2005, 290, 13–30. [Google Scholar] [CrossRef]
- Jankauskaite, V.; Macijauskas, G.; Lygaitis, R. Polyethylene terephthalate waste recycling and application possibilities: A review. Mater. Sci. (Medzg.) 2008, 14, 119–127. [Google Scholar]
- Lau, W.W.Y.; Shiran, Y.; Bailey, R.M.; Cook, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating scenarios toward zero plastic pollution. Science 2020, 369, 1455–1461. [Google Scholar] [CrossRef]
- Chowdhury, S.; Maniar, A.T.; Suganya, O. Polyethylene Terephthalate (PET) Waste as Building Solution. Int. J. Chem. Environ. Biol. Sci. 2013, 1, 2320–4087. [Google Scholar]
- Alaloul, W.S.; John, V.O.; Musarat, M.A. Mechanical and Thermal Properties of Interlocking Bricks Utilizing Wasted Polyethylene Terephthalate. Int. J. Concr. Struct. Mater. 2020, 14, 1–11. [Google Scholar] [CrossRef]
- Jankauskaite, V. Recycled Polyethylene Terephthalate Waste for Different Application Solutions. Environ. Res. Eng. Manag. 2016, 72, 5–7. [Google Scholar] [CrossRef]
- Sanches, R.A.; Takamune, K.; Guimarães, B.; Alonso, R.; Baruque-Ramos, J.; de Held, M.S.B.; Marcicano, J.P.P. Wearbility Analysis of knited fabrics produced with colored organic cotton. Bamboo rayon, corn, recycled pet/cotton and recycled pet/polyester. Am. Int. J. Contemp. Res. 2014, 4, 28–37. [Google Scholar]
- Shen, L.; Worrell, E.; Patel, M.K. Open-loop recycling: A LCA case study of PET bottle-to-fibre recycling. Resour. Conserv. Recycl. 2010, 55, 34–52. [Google Scholar] [CrossRef]
- Kumartasli, S.; Avinc, O. Important Step in Sustainability: Polyethylene Terephthalate Recycling and the Recent Developments; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–19. [Google Scholar]
- Othmer, K. Encyclopedia of Chemical Technology; Radiation Curing; Wiley – Interscience: New York, NY, USA, 1982; Volume 15, pp. 607–624. [Google Scholar]
- Visakh, P.; Liang, M. Poly (Ethylene Terephthalate) Based Blends, Composites and Nanocomposites; William Andrew: Norwich, NY, USA, 2015. [Google Scholar]
- Forrest, M. Recycling of Polyethylene Terephthalate; Smithers Rapra: Shrewsbury, UK, 2016. [Google Scholar]
- Awaja, F.; Pavel, D. Recycling of PET. Eur. Polym. J. 2005, 41, 1453–1477. [Google Scholar] [CrossRef]
- Brydson, J.A. Plastics Materials; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Al-Sabagh, A.M.; Yehia, F.Z.; Eshaq, G.; Rabie, A.M.; ElMetwally, A.E. Greener routes for recycling of polyethylene terephthalate. Egypt. J. Pet. 2016, 25, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Zein, R.; Ibrahim, M. Production of Polyethylene Terephthalate. Cairo, Egypt. 2010. Available online: https://www.researchgate.net/publication/265112530_PET_Production/ (accessed on 2 May 2021).
- Giannotta, G.; Po’, R.; Cardi, N.; Tampellini, E.; Occhiello, E.; Garbassi, F.; Nicolais, L. Processing effects on poly(ethylene terephthalate) from bottle scraps. Polym. Eng. Sci. 1994, 34, 1219–1223. [Google Scholar] [CrossRef]
- Dimitrov, N.; Kratofil Krehula, L.; Ptiček Siročić, A.; Hrnjak-Murgić, Z. Analysis of recycled PET bottles products by pyrolysis-gas chromatography. Polym. Degrad. Stab. 2013, 98, 972–979. [Google Scholar] [CrossRef]
- La Mantia, F.P. Recycling of PVC and Mixed Plastic Waste; ChemTec Publishing: Toronto, ON, Canada, 1996. [Google Scholar]
- Seo, K.S.; Cloyd, J.D. Kinetics of hydrolysis and thermal degradation of polyester melts. J. Appl. Polym. Sci. 1991, 42, 845–850. [Google Scholar] [CrossRef]
- Bacha, C.; Dauchya, X.; Chagnonc, M.-C.; Etienneb, S. Chemical migration in drinking water stored in polyethylene terephthalate (PET) bottles: A source of controversy. Water Res. 2012, 46, 571–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, N.; Robin, J.J.; Boutevin, B. Study of thermal and mechanical properties of virgin and recycled poly(ethylene terephthalate) before and after injection molding. Eur. Polym. J. 2000, 36, 2075–2080. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, S.H. Poly (ethylene terephthalate) recycling for high value added textiles. Fash. Text. 2014, 1, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Whitt, M.; Vorst, K.; Brown, W.; Baker, S.; Gorman, L. Survey of heavy metal contamination in recycled polyethylene terephthalate used for food packaging. J. Plast. Film Sheeting 2013, 29, 163–173. [Google Scholar] [CrossRef]
- Welle, F. Twenty years of PET bottle to bottle recycling—An overview. Resour. Conserv. Recycl. 2011, 55, 865–875. [Google Scholar] [CrossRef]
- Okan, M.; Aydin, H.M.; Barsbay, M. Current approaches to waste polymer utilization and minimization: A review. J. Chem. Technol. Biotechnol. 2019, 94, 8–21. [Google Scholar] [CrossRef] [Green Version]
- Geueke, B.; Groh, K.; Muncke, J. Food packaging in the circular economy: Overview of chemical safety aspects for commonly used materials. J. Clean. Prod. 2018, 193, 491–505. [Google Scholar] [CrossRef]
- Khoonkari, M.; Haghighi, A.H.; Sefidbakht, Y.; Shekoohi, K.; Ghaderian, A. Chemical recycling of PET wastes with different catalysts. Int. J. Polym. Sci. 2015, 2015, 124524. [Google Scholar] [CrossRef] [Green Version]
- Feil, A.; Pretz, T. Mechanical recycling of packaging waste. In Plastic Waste and Recycling; Elsevier: Amsterdam, The Netherlands, 2020; pp. 283–319. [Google Scholar]
- Terzini, R.; Campbell, W.R. Method and Apparatus for Processing Municipal Waste. U.S. Patent 6945484B1, 20 September 2005. [Google Scholar]
- Haberle, W. Comminution Device for Scrap Plastics. U.S. Patent 3960335A, 1 June 1976. [Google Scholar]
- Doppstadt, F. Comminuting Device. U.S. Patent 20200129988A1, 30 April 2020. [Google Scholar]
- Riemann, H.-H.; Sonnenschein, H.; Skaletz, H. Rotary Drum Screen for Waste Material and Its Method of Operation. U.S. Patent 4509697A, 9 April 1985. [Google Scholar]
- Zia, K.M.; Bhatti, H.N.; Bhatti, I.A. Methods for polyurethane and polyurethane composites, recycling and recovery: A review. React. Funct. Polym. 2007, 67, 675–692. [Google Scholar] [CrossRef]
- Rahimi, A.; García, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar] [CrossRef]
- Tullo, A.H. Plastic Has a Problem; Is Chemical Recycling the Solution? Available online: https://cen.acs.org/environment/recycling/Plastic-problem-chemical-recycling-solution/97/i39 (accessed on 24 March 2021).
- Franz, R.; Mauer, A.; Welle, F. European survey on post-consumer poly(ethylene terephthalate) (PET) materials to determine contamination levels and maximum consumer exposure from food packages made from recycled PET. Food Addit. Contam. 2004, 21, 265–286. [Google Scholar] [CrossRef]
- Kalita, N.K.; Kalamdhad, A.; Katiyar, V. Recent trends and advances in the biodegradation of conventional plastics. In Advances in Sustainable Polymers; Springer: Berlin/Heidelberg, Germany, 2020; pp. 389–404. [Google Scholar]
- Zimmermann, W. Biocatalytic recycling of polyethylene terephthalate plastic. Philos. Trans. R. Soc. A 2020, 378. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Moore, C.J.; Vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- Dias, D.S.; Crespi, M.S.; Ribeiro, C.A.; Kobelnik, M. Evaluation of the thermal decomposition of blends prepared with poly(3-hydroxybutyrate) (PHB) and recyclable ethylene poly-terephthalate (RPET). J. Therm. Anal. Calorim. 2021, 143, 3447–3457. [Google Scholar] [CrossRef]
- Raheem, A.B.; Noor, Z.Z.; Hassan, A.; Hamid, M.K.A.; Samsudin, S.A.; Sabeen, A.H. Current developments in chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: A review. J. Clean. Prod. 2019, 225, 1052–1064. [Google Scholar] [CrossRef]
- Myren, T.H.T.; Stinson, T.A.; Mast, Z.J.; Huntzinger, C.G.; Luca, O.R. Chemical and Electrochemical Recycling of End-Use Poly(ethylene terephthalate) (PET) Plastics in Batch, Microwave and Electrochemical Reactors. Molecules 2020, 25, 2742. [Google Scholar] [CrossRef]
- Pulido, B.A.; Habboub, O.S.; Aristizabal, S.L.; Szekely, G.; Nunes, S.P. Recycled Poly(ethylene terephthalate) for High Temperature Solvent Resistant Membranes. ACS Appl. Polym. Mater. 2019, 1, 2379–2387. [Google Scholar] [CrossRef] [Green Version]
- Mirjalili, A.; Dong, B.; Pena, P.; Ozkan, C.S.; Ozkan, M. Upcycling of polyethylene terephthalate plastic waste to microporous carbon structure for energy storage. Energy Storage 2020, 2, e201. [Google Scholar] [CrossRef]
- Spychaj, T.; Fabrycy, E.; Spychaj, S.; Kacperski, M. Aminolysis and aminoglycolysis of waste poly(ethylene terephthalate). J. Mater. Cycles Waste Manag. 2001, 3, 24–31. [Google Scholar]
- Pohjakallio, M.; Vuorinen, T. Chemical routes for recycling—dissolving, catalytic, and thermochemical technologies. In Plastic Waste and Recycling; Elsevier: Amsterdam, The Netherlands, 2020; pp. 359–384. [Google Scholar]
- Meys, R.; Frick, F.; Westhues, S.; Sternberg, A.; Klankermayer, J.; Bardow, A. Towards a circular economy for plastic packaging wastes–the environmental potential of chemical recycling. Resour. Conserv. Recycl. 2020, 162, 105010. [Google Scholar] [CrossRef]
- FENC. New Process Recycles Any PET Waste. Available online: http://news.fenc.com/news_detail.aspx?lang=en&id=4557 (accessed on 4 March 2021).
- gr3n. Technological Breakthough. Available online: http://gr3n-recycling.com/#tab-id-9 (accessed on 4 March 2021).
- JEPLAN. BRING Technology™. Available online: https://www.jeplan.co.jp/en/technology/ (accessed on 4 March 2021).
- Garbo. CHEMPET PROJECT. Available online: http://www.garbosrl.net/chempet/?lang=en (accessed on 4 March 2021).
- Ifp. Contribution of Chemistry to Plastics Recycling. Available online: https://www.ifpenergiesnouvelles.com/innovation-and-industry/our-expertise/climate-environment-and-circular-economy/plastics-recycling (accessed on 4 March 2021).
- Ioniqa. Ioniqa’s Circular Solution. Available online: https://ioniqa.com/applications/ (accessed on 4 March 2021).
- Perpetual. Technology. Available online: https://www.perpetual-global.com/manufacturing/#polygenta (accessed on 4 March 2021).
- Poseidon Plastics. Bringing an End to Single Use Plastic. Available online: http://poseidonplastics.com/ (accessed on 4 March 2021).
- Eastman. Eastman Offers Innovative Recycling Technology for Polyesters. Available online: https://www.eastman.com/Company/News_Center/2019/Pages/Eastman-offers-innovative-recycling-technology-for-polyesters.aspx (accessed on 4 March 2021).
- Loop Indystries. Revolutionary Technology. Available online: https://www.loopindustries.com/en/ (accessed on 4 March 2021).
- DePoly. Simple Sustainable Recycling. Available online: https://www.depoly.ch/ (accessed on 4 March 2021).
- Carbios. Biorecycling. Available online: https://carbios.fr/en/technology/biorecycling/ (accessed on 4 March 2021).
- Punkkinen, H.; Oasmaa, A.; Laatikainen-Luntama, J.; Nieminen, M.; Laine-Ylijoki, J. Thermal Conversion of Plasticcontaining Waste: A Review; ARVI Research Report; Solution Architect for Global Bioeconomy & Cleantech Opportunities: Helsinki, Finland, 2017; pp. 525–538. [Google Scholar]
- López, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.; Adrados, A. Pyrolysis of municipal plastic wastes: Influence of raw material composition. Waste Manag. 2010, 30, 620–627. [Google Scholar] [CrossRef]
- Singh, R.; Ruj, B.; Sadhukhan, A.; Gupta, P. A TG-FTIR investigation on the co-pyrolysis of the waste HDPE, PP, PS and PET under high heating conditions. J. Energy Inst. 2020, 93, 1020–1035. [Google Scholar] [CrossRef]
- Çepelioğullar, Ö.; Pütün, A.E. Utilization of two different types of plastic wastes from daily and industrial life. J. Selcuk Univ. Nat. Appl. Sci. 2013, 2, 694–706. [Google Scholar]
- Du, S.; Valla, J.A.; Parnas, R.S.; Bollas, G.M. Conversion of Polyethylene Terephthalate Based Waste Carpet to Benzene-Rich Oils through Thermal, Catalytic, and Catalytic Steam Pyrolysis. ACS Sustain. Chem. Eng. 2016, 4, 2852–2860. [Google Scholar] [CrossRef]
- Özsin, G.; Pütün, A.E. A comparative study on co-pyrolysis of lignocellulosic biomass with polyethylene terephthalate, polystyrene, and polyvinyl chloride: Synergistic effects and product characteristics. J. Clean. Prod. 2018, 205, 1127–1138. [Google Scholar] [CrossRef]
- Chattopadhyay, J.; Pathak, T.; Srivastava, R.; Singh, A. Catalytic co-pyrolysis of paper biomass and plastic mixtures (HDPE (high density polyethylene), PP (polypropylene) and PET (polyethylene terephthalate)) and product analysis. Energy 2016, 103, 513–521. [Google Scholar] [CrossRef]
- Hoyle, W.; Karsa, D.R. Chemical Aspects of Plastics Recycling; Amer Chemical Society: Washington, DC, USA, 1997; Volume 199. [Google Scholar]
- Tukker, A.; De Groot, H.; Simons, L.; Wiegersma, S. Chemical Recycling of Plastic Waste (PVC and Other Resins). European Commission, DG III, Final Report; STB-99-55 Final; TNO: Delft, The Netherlands, 1999. [Google Scholar]
- Al-Salem, S.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- Park, C.; Kim, S.; Kwon, Y.; Jeong, C.; Cho, Y.; Lee, C.-G.; Jung, S.; Choi, K.-Y.; Lee, J. Pyrolysis of polyethylene terephthalate over carbon-supported Pd catalyst. Catalysts 2020, 10, 496. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, C.; Onwudili, J.A.; Meng, A.; Zhang, Y.; Williams, P.T. Polycyclic aromatic hydrocarbons (PAH) formation from the pyrolysis of different municipal solid waste fractions. Waste Manag. 2015, 36, 136–146. [Google Scholar] [CrossRef]
- Kim, S.; Park, C.; Lee, J. Reduction of polycyclic compounds and biphenyls generated by pyrolysis of industrial plastic waste by using supported metal catalysts: A case study of polyethylene terephthalate treatment. J. Hazard. Mater. 2020, 392, 122464. [Google Scholar] [CrossRef]
- Gbeddy, G.; Goonetilleke, A.; Ayoko, G.A.; Egodawatta, P. Transformation and degradation of polycyclic aromatic hydrocarbons (PAHs) in urban road surfaces: Influential factors, implications and recommendations. Environ. Pollut. 2020, 257, 113510. [Google Scholar] [CrossRef]
- Jia, H.; Ben, H.; Luo, Y.; Wang, R. Catalytic fast pyrolysis of poly (ethylene terephthalate)(PET) with zeolite and nickel chloride. Polymers 2020, 12, 705. [Google Scholar] [CrossRef] [Green Version]
- Stanica-Ezeanu, D.; Matei, D. Natural depolymerization of waste poly(ethylene terephthalate) by neutral hydrolysis in marine water. Sci. Rep. 2021, 11, 4431. [Google Scholar] [CrossRef]
- Sinha, V.; Patel, M.R.; Patel, J.V. PET waste management by chemical recycling: A review. J. Polym. Environ. 2010, 18, 8–25. [Google Scholar] [CrossRef]
- Kao, C.Y.; Cheng, W.H.; Wan, B.Z. Investigation of alkaline hydrolysis of polyethylene terephthalate by differential scanning calorimetry and thermogravimetric analysis. J. Appl. Polym. Sci. 1998, 70, 1939–1945. [Google Scholar] [CrossRef]
- Paszun, D.; Spychaj, T. Chemical recycling of poly (ethylene terephthalate). Ind. Eng. Chem. Res. 1997, 36, 1373–1383. [Google Scholar] [CrossRef]
- Hirota, Y.; Hayashi, K.; Kawanishi, T.; Takiguchi, N. Effect of pH on hydrolysis of biodegradable polyethylene terephthalate. J. Chem. Eng. Jpn. 2020, 53, 267–272. [Google Scholar] [CrossRef]
- Kozlow, N.; Korotusko, G.; Kashinskii, A.; Gavrilenco, N. Preparation of terephthalic and benzoic acid by simultaneous alkaline hydrolysis of wastes from PET and methyl benzoate. Vesti Acad. Nauk Bssr Ser. Khim. Nauk 1984, 5, 91–93. [Google Scholar]
- Pitat, J.; Holcik, V.; Bacak, M. A method of processing waste of polyethylene terephthalate by hydrolysis. Gb Pat. 1959, 822, 834. [Google Scholar]
- Yoshioka, T.; Ota, M.; Okuwaki, A. Conversion of a used poly (ethylene terephthalate) bottle into oxalic acid and terephthalic acid by oxygen oxidation in alkaline solutions at elevated temperatures. Ind. Eng. Chem. Res. 2003, 42, 675–679. [Google Scholar] [CrossRef]
- Sharma, R.K.; Bakhshi, N.N. Catalytic upgrading of pyrolysis oil. Energy Fuels 1993, 7, 306–314. [Google Scholar] [CrossRef]
- Yoshioka, T.; Motoki, T.; Okuwaki, A. Kinetics of hydrolysis of poly (ethylene terephthalate) powder in sulfuric acid by a modified shrinking-core model. Ind. Eng. Chem. Res. 2001, 40, 75–79. [Google Scholar] [CrossRef]
- Karayannidis, G.; Chatziavgoustis, A.; Achilias, D. Poly (ethylene terephthalate) recycling and recovery of pure terephthalic acid by alkaline hydrolysis. Adv. Polym. Technol. J. Polym. Process. Inst. 2002, 21, 250–259. [Google Scholar] [CrossRef]
- Chandra, R.; Adab, A. Rubber & Plastic Waste: Recycling, Reuse and Future Demand. CDB Publishers: New Delhi, India, 1994. [Google Scholar]
- Kim, B.K.; Hwang, G.C.; Bae, S.Y.; Yi, S.C.; Kumazawa, H. Depolymerization of polyethyleneterephthalate in supercritical methanol. J. Appl. Polym. Sci. 2001, 81, 2102–2108. [Google Scholar] [CrossRef]
- Pham, D.D.; Cho, J. Low-energy catalytic methanolysis of poly(ethyleneterephthalate). Green Chem. 2021, 23, 511–525. [Google Scholar] [CrossRef]
- Xi, G.; Lu, M.; Sun, C. Study on depolymerization of waste polyethylene terephthalate into monomer of bis (2-hydroxyethyl terephthalate). Polym. Degrad. Stab. 2005, 87, 117–120. [Google Scholar] [CrossRef]
- Güçlü, G.; Kasgöz, A.; Özbudak, S.; Özgümüs¸, S.; Orbay, M. Glycolysis of poly(ethylene terephthalate) wastes in xylene. J. Appl. Polym. Sci. 1998, 69, 2311–2319. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Duque-Ingunza, I.; de Rivas, B.; Flores-Giraldo, L.; Gutiérrez-Ortiz, J.I. Kinetics of catalytic glycolysis of PET wastes with sodium carbonate. Chem. Eng. J. 2011, 168, 312–320. [Google Scholar] [CrossRef]
- Troev, K.; Grancharov, G.; Tsevi, R.; Gitsov, I. A novel catalyst for the glycolysis of poly (ethylene terephthalate). J. Appl. Polym. Sci. 2003, 90, 1148–1152. [Google Scholar] [CrossRef]
- Liu, B.; Fu, W.; Lu, X.; Zhou, Q.; Zhang, S. Lewis acid–base synergistic catalysis for polyethylene terephthalate degradation by 1, 3-Dimethylurea/Zn (OAc) 2 deep eutectic solvent. ACS Sustain. Chem. Eng. 2018, 7, 3292–3300. [Google Scholar] [CrossRef]
- Guo, Z.; Adolfsson, E.; Tam, P.L. Nanostructured micro particles as a low-cost and sustainable catalyst in the recycling of PET fiber waste by the glycolysis method. Waste Manag. 2021, 126, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Inada, S.; Sato, K. Processes for the Purification of Bis(2-hydroxyethyl)terephthalate. U.S. Patent 7211193B2, 1 May 2007. [Google Scholar]
- ASPEN PLUS V11, M.; Aspen Technology Inc.: Cambridge, MA, USA, 2020.
- Raheem, A.B.; Hassana, A.B.; Noor, Z.Z.; Samsudina, S.B.; Hamida, M.A.; Belloa, A.; Oladokuna, O.; Sabeena, A.L.H.; Shamiria, A. Process Simulation of Bis (2- hydroxyethyl) terephthalate and Its Recovery Using Two–stage Evaporation Systems. Chem. Eng. Trans. 2018, 63, 655–660. [Google Scholar]
- Goh, H.W.; Ali, S.; Abdullah, N.; Idris, A. Time, Temperature and Amount of Distilled Water Effects on the Purity and Yield of Bis(2-hydroxyethyl) Terephthalate Purification System. Bull. Chem. React. Eng. Catal. 2015, 10, 143–154. [Google Scholar] [CrossRef]
- Goh, H.W.; Salmiaton, A.; Abdullah, N.; Idris, A. Process Simulation of Two-stage Evaporation and Crystallization Systems for Bis(2-hydroxyethyl) terephthalate recovery. J. Appl. Sci. 2012, 12, 1547–1555. [Google Scholar] [CrossRef]
- Pilati, F.; Toselli, M.; Stramigioli, C.; Baldi, G.; Capra, M.; Osella, M.; BavaPilone, G. Process to Prepare Bis (2-hydroxyethyl) Terephthalate. European Patent EP0723951A1, 31 July 1996. [Google Scholar]
- Gupta, P.; Bhandari, S. 6-Chemical Depolymerization of PET Bottles via Ammonolysis and Aminolysis. In Recycling of Polyethylene Terephthalate Bottles; Thomas, S., Rane, V.A., Kanny, K., Abitha, V.K., Thomas, M.G., Eds.; William Andrew Publishing: Norwich, NY, USA, 2019. [Google Scholar]
- Ghorbantabar, S.; Ghiass, M.; Yaghobi, N.; Bouhendi, H. Investigation of conventional analytical methods for determining conversion of polyethylene terephthalate waste degradation via aminolysis process. J. Mater. Cycles Waste Manag. 2021, 23, 526–536. [Google Scholar] [CrossRef]
- Bäckström, E.; Odelius, K.; Hakkarainen, M. Ultrafast microwave assisted recycling of PET to a family of functional precursors and materials. Eur. Polym. J. 2021, 151, 110441. [Google Scholar] [CrossRef]
- Demarteau, J.; Olazabal, I.; Jehanno, C.; Sardon, H. Aminolytic upcycling of poly(ethylene terephthalate) wastes using a thermally-stable organocatalyst. Polym. Chem. 2020, 11, 4875–4882. [Google Scholar] [CrossRef]
- Parrott, M. Chemical Recycling of Polyethylene Terephthalate by Microwave Irradiation. U.S. Patent 2749736, 23 March 2020. [Google Scholar]
- Hajek, M.; Sobek, J.; Brustman, J. Method for the Chemical Depolymerization of Waste Polyethylene Terephthalate. U.S. Patent 20100133088A1, 3 June 2010. [Google Scholar]
- Alnaqbi, M.A.; Mohsin, M.A.; Busheer, R.M.; Haik, Y. Microwave assisted glycolysis of poly(ethylene terephthalate) catalyzed by 1-butyl-3-methylimidazolium bromide ionic liquid. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Liu, N.; Ma, Y.S.; Shu, K.W.; Wu, B.; Zhang, D. Catalysis investigation of PET Depolymerization with Br{ø}nsted acidic ionic liquid under microwave irradiation. Adv. Mater. Res. 2014, 893, 23–26. [Google Scholar] [CrossRef]
- Ikenaga, K.; Inoue, T.; Kusakabe, K. Hydrolysis of PET by combining direct microwave heating with high pressure. Procedia Eng. 2016, 148, 314–318. [Google Scholar] [CrossRef] [Green Version]
- Cano, I.; Martin, C.; Fernandes, J.A.; Lodge, R.W.; Dupont, J.; Casado-Carmona, F.A.; Lucena, R.; Cardenas, S.; Sans, V.; de Pedro, I. Paramagnetic ionic liquid-coated SiO2@ Fe3O4 nanoparticles—The next generation of magnetically recoverable nanocatalysts applied in the glycolysis of PET. Appl. Catal. B Environ. 2020, 260, 118110. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Li, Z.; Zhang, X.; Zhang, S.; Zhang, Y. Glycolysis of poly (ethylene terephthalate) catalyzed by ionic liquids. Eur. Polym. J. 2009, 45, 1535–1544. [Google Scholar] [CrossRef]
- Wang, H.; Yan, R.; Li, Z.; Zhang, X.; Zhang, S. Fe-containing magnetic ionic liquid as an effective catalyst for the glycolysis of poly (ethylene terephthalate). Catal. Commun. 2010, 11, 763–767. [Google Scholar] [CrossRef]
- Dupont, J.; de Souza, R.F.; Suarez, P. Ionic liquid (molten salt) phase organometallic catalysis. Chem. Rev. 2002, 102, 3667–3692. [Google Scholar] [CrossRef]
- Kosmulski, M.; Gustafsson, J.; Rosenholm, J.B. Thermal stability of low temperature ionic liquids revisited. Thermochim. Acta 2004, 412, 47–53. [Google Scholar] [CrossRef]
- Matsumoto, H.; Yanagida, M.; Tanimoto, K.; Nomura, M.; Kitagawa, Y.; Miyazaki, Y. Highly conductive room temperature molten salts based on small trimethylalkylammonium cations and bis (trifluoromethylsulfonyl) imide. Chem. Lett. 2000, 29, 922–923. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Liu, Y.; Zhang, X.; Zhang, S. Degradation of poly (ethylene terephthalate) using ionic liquids. Green Chem. 2009, 11, 1568–1575. [Google Scholar] [CrossRef]
- Nunes, C.S.; da Silva, M.J.V.; da Silva, D.C.; dos Reis Freitas, A.; Rosa, F.A.; Rubira, A.F.; Muniz, E.C. PET depolymerisation in supercritical ethanol catalysed by [Bmim][BF 4]. RSC Adv. 2014, 4, 20308–20316. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Yehia, F.Z.; Eissa, A.M.F.; Moustafa, M.E.; Eshaq, G.; Rabie, A.M.; ElMetwally, A.E. Cu- and Zn-acetate-containing ionic liquids as catalysts for the glycolysis of poly(ethylene terephthalate). Polymer Degradation and Stability 2014, 110, 364–377. [Google Scholar] [CrossRef]
- Yue, Q.F.; Wang, C.X.; Zhang, L.N.; Ni, Y.; Jin, Y.X. Glycolysis of poly (ethylene terephthalate)(PET) using basic ionic liquids as catalysts. Polym. Degrad. Stabil. 2011, 96, 399–403. [Google Scholar] [CrossRef]
- Naik, S.D.; Doraiswamy, L.J.A.J. Phase transfer catalysis: Chemistry and engineering. Aiche J. 1998, 44, 612–646. [Google Scholar] [CrossRef]
- Starks, C.; Liotta, C.M. Halpern in Phase-Transfer Catalysis: Fundamentals, Applications and Industrial Perspectives; Chapman & Hall: New York, NY, USA, 1994. [Google Scholar]
- Yang, H.-M.; Wu, H.-S. Interfacial mechanism and kinetics of phase-transfer catalysis. Catal. Rev. 2003, 45, 463–540. [Google Scholar] [CrossRef]
- Khalaf, H.I.; Hasan, O.A. Effect of quaternary ammonium salt as a phase transfer catalyst for the microwave depolymerization of polyethylene terephthalate waste bottles. Chem. Eng. J. 2012, 192, 45–48. [Google Scholar] [CrossRef]
- López-Fonseca, R.; González-Marcos, M.P.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I. Chemical recycling of PET by alkaline hydrolysis in the presence of quaternary phosphonium and ammonium salts as phase transfer catalysts. In Waste Management and the Environment IV; WIT Press: Southampton, UK, 2008; pp. 511–520. [Google Scholar] [CrossRef] [Green Version]
- Kosmidis, V.A.; Achilias, D.S.; Karayannidis, G.P. Poly(ethylene terephthalate) Recycling and Recovery of Pure Terephthalic Acid. Kinetics of a Phase Transfer Catalyzed Alkaline Hydrolysis. Macromol. Mater. Eng. 2001, 286, 640–647. [Google Scholar] [CrossRef]
- Lalhmangaihzuala, S.; Laldinpuii, Z.; Lalmuanpuia, C.; Vanlaldinpuia, K. Glycolysis of Poly (Ethylene Terephthalate) Using Biomass-Waste Derived Recyclable Heterogeneous Catalyst. Polymers 2020, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Rorrer, N.A.; Nicholson, S.; Carpenter, A.; Biddy, M.J.; Grundl, N.J.; Beckham, G.T. Combining Reclaimed PET with Bio-based Monomers Enables Plastics Upcycling. Joule 2019, 3, 1006–1027. [Google Scholar] [CrossRef] [Green Version]
- Štrukil, V. Highly Efficient Solid-State Hydrolysis of Waste Polyethylene Terephthalate by Mechanochemical Milling and Vapor-Assisted Aging. ChemSusChem 2021, 14, 330–338. [Google Scholar] [CrossRef]
- Kawai, F.; Kawabata, T.; Oda, M. Current knowledge on enzymatic PET degradation and its possible application to waste stream management and other fields. Appl. Microbiol. Biotechnol. 2019, 103, 4253–4268. [Google Scholar] [CrossRef] [Green Version]
- Ion, S.; Voicea, S.; Sora, C.; Gheorghita, G.; Tudorache, M.; Parvulescu, V.I. Sequential biocatalytic decomposition of BHET as valuable intermediator of PET recycling strategy. Catal. Today 2021, 366, 177–184. [Google Scholar] [CrossRef]
- Barth, M.; Oeser, T.; Wei, R.; Then, J.; Schmidt, J.; Zimmermann, W. Effect of hydrolysis products on the enzymatic degradation of polyethylene terephthalate nanoparticles by a polyester hydrolase from Thermobifida fusca. Biochem. Eng. J. 2015, 93, 222–228. [Google Scholar] [CrossRef]
- Tournier, V.; Topham, C.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.-L.; Texier, H.; Gavalda, S. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef]
- Wei, R.; Oeser, T.; Schmidt, J.; Meier, R.; Barth, M.; Then, J.; Zimmermann, W. Engineered bacterial polyester hydrolases efficiently degrade polyethylene terephthalate due to relieved product inhibition. Biotechnol. Bioeng. 2016, 113, 1658–1665. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly (ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Sharon, C.; Sharon, M. Studies on biodegradation of polyethylene terephthalate: A synthetic polymer. J. Microbiol. Biotechnol. Res. 2012, 2, 248–257. [Google Scholar]
- Nimchua, T.; Eveleigh, D.E.; Sangwatanaroj, U.; Punnapayak, H. Screening of tropical fungi producing polyethylene terephthalate-hydrolyzing enzyme for fabric modification. J. Ind. Microbiol. Biotechnol. 2008, 35, 843. [Google Scholar] [CrossRef] [PubMed]
- Ribitsch, D.; Acero, E.H.; Przylucka, A.; Zitzenbacher, S.; Marold, A.; Gamerith, C.; Tscheließnig, R.; Jungbauer, A.; Rennhofer, H.; Lichtenegger, H. Enhanced cutinase-catalyzed hydrolysis of polyethylene terephthalate by covalent fusion to hydrophobins. Appl. Environ. Microbiol. 2015, 81, 3586–3592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.T.; Kim, J.K.; Cha, H.G.; Kang, M.J.; Lee, H.S.; Khang, T.U.; Yun, E.J.; Lee, D.-H.; Song, B.K.; Park, S.J.; et al. Biological valorization of poly(ethylene terephthalate) monomers for upcycling waste PET. ACS Sustain. Chem. Eng. 2019, 7, 19396–19406. [Google Scholar] [CrossRef]
- Biundo, A.; Reich, J.; Ribitsch, D.; Guebitz, G.M. Synergistic effect of mutagenesis and truncation to improve a polyesterase from Clostridium botulinum for polyester hydrolysis. Sci. Rep. 2018, 8, 3745. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.M.; Wang, L.Y.; Zhu, H.Y. Enzymatic Degradation of Polyester-Nanoparticles by Lipases and Adsorption of Lipases on the Polyester-Nanoparticles. Adv. Mater. Res. 2011, 418-420, 2302–2307. [Google Scholar] [CrossRef]
- Wei, R.; Oeser, T.; Then, J.; Kühn, N.; Barth, M.; Schmidt, J.; Zimmermann, W. Functional characterization and structural modeling of synthetic polyester-degrading hydrolases from Thermomonospora curvata. AMB Express 2014, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.; Da, S.; Silva, N.; Matamá, T.; Araújo, R.; Martins, M.; Chen, S.; Chen, J.; Wu, J.; Casal, M. Engineered Thermobifida fusca cutinase with increased activity on polyester substrates. Biotechnol. J. 2011, 6, 1230–1239. [Google Scholar] [CrossRef]
- Herrero Acero, E.; Ribitsch, D.; Steinkellner, G.; Gruber, K.; Greimel, K.; Eiteljoerg, I.; Trotscha, E.; Wei, R.; Zimmermann, W.; Zinn, M. Enzymatic surface hydrolysis of PET: Effect of structural diversity on kinetic properties of cutinases from Thermobifida. Macromolecules 2011, 44, 4632–4640. [Google Scholar] [CrossRef] [Green Version]
- Ribitsch, D.; Acero, E.H.; Greimel, K.; Eiteljoerg, I.; Trotscha, E.; Freddi, G.; Schwab, H.; Guebitz, G.M. Characterization of a new cutinase from Thermobifida alba for PET-surface hydrolysis. Biocatal. Biotransform. 2012, 30, 2–9. [Google Scholar] [CrossRef]
- Ribitsch, D.; Heumann, S.; Trotscha, E.; Herrero Acero, E.; Greimel, K.; Leber, R.; Birner-Gruenberger, R.; Deller, S.; Eiteljoerg, I.; Remler, P. Hydrolysis of polyethyleneterephthalate by p-nitrobenzylesterase from Bacillus subtilis. Biotechnol. Prog. 2011, 27, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.; Yamato, S.; Kanaya, E.; Kim, J.-J.; Koga, Y.; Takano, K.; Kanaya, S. Isolation of a novel cutinase homolog with polyethylene terephthalate-degrading activity from leaf-branch compost by using a metagenomic approach. Appl. Environ. Microbiol. 2012, 78, 1556–1562. [Google Scholar] [CrossRef] [Green Version]
- Tiso, T.; Narancic, T.; Wei, R.; Pollet, E.; Beagan, N.; Schröder, K.; Honak, A.; Jiang, M.; Kenny, S.T.; Wierckx, N.; et al. Bio-upcycling of Polyethylene Terephthalate; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2020. [Google Scholar]
- Herrero Acero, E.; Ribitsch, D.; Dellacher, A.; Zitzenbacher, S.; Marold, A.; Steinkellner, G.; Gruber, K.; Schwab, H.; Guebitz, G.M. Surface engineering of a cutinase from Thermobifida cellulosilytica for improved polyester hydrolysis. Biotechnol. Bioeng. 2013, 110, 2581–2590. [Google Scholar] [CrossRef]
- Wang, X.; Lu, D.; Jönsson, L.; Hong, F. Preparation of a PET-hydrolyzing lipase from Aspergillus Oryzae by the addition of bis (2-hydroxyethyl) terephthalate to the culture medium and enzymatic modification of PET fabrics. Eng. Life Sci. 2008, 8, 268–276. [Google Scholar] [CrossRef]
- Barth, M.; Wei, R.; Oeser, T.; Then, J.; Schmidt, J.; Wohlgemuth, F.; Zimmermann, W. Enzymatic hydrolysis of polyethylene terephthalate films in an ultrafiltration membrane reactor. J. Membr. Sci. 2015, 494, 182–187. [Google Scholar] [CrossRef]
- Ribitsch, D.; Herrero Acero, E.; Greimel, K.; Dellacher, A.; Zitzenbacher, S.; Marold, A.; Rodriguez, R.D.; Steinkellner, G.; Gruber, K.; Schwab, H. A new esterase from Thermobifida halotolerans hydrolyses polyethylene terephthalate (PET) and polylactic acid (PLA). Polymers 2012, 4, 617–629. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P. Feedstock Recycling of Plastic Wastes; Royal Society of Chemistry: Cambridge, UK, 2007. [Google Scholar]
- Genta, M.; Yano, F.; Kondo, Y.; Matsubara, W.; Oomoto, S. Development of chemical recycling process for post-consumer PET bottles by methanolysis in supercritical methanol. Tech. Rev 2003, 40, 1–4. [Google Scholar]
- Goje, A.; Thakur, S.; Patil, T.M.; Mishra, S. Glycolytic aminolysis of poly (ethylene terephthalate) waste for recovery of value-added comonomer at atmospheric pressure. J. Appl. Polym. Sci. 2003, 90, 3437–3444. [Google Scholar] [CrossRef]
- Thomas, S.; Rane, A.V.; Kanny, K.; Abitha, V.; Thomas, M.G. Recycling of Polyethylene Terephthalate Bottles; William Andrew: Norwich, NY, USA, 2018. [Google Scholar]
- Padhan, R.K.; Sreeram, A. Chemical depolymerization of PET bottles via combined chemolysis methods. In Recycling of Polyethylene Terephthalate Bottles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–147. [Google Scholar]
- Rogers, M.E.; Long, T.E. Synthetic Methods in Step-Growth Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Kim, B.-K.; Kim, D.; Cho, Y.; Han, M. Chemical recycling of poly (ethylene terephthalate) using a new hybrid process. J. Chem. Eng. Jpn. 2008, 41, 923–928. [Google Scholar] [CrossRef]
- George, N.; Kurian, T. Recent developments in the chemical recycling of postconsumer poly(ethylene terephthalate) waste. Ind. Eng. Chem. Res. 2014, 53, 14185–14198. [Google Scholar]
- Doerr, M.L. Depolymerization of Condensation Polymers Involving a Pre-Molecular Weight Reduction Step. U.S. Patent 4620032A, 28 October 1986. [Google Scholar]
- Güçlü, G.; Yalçınyuva, T.; Özgümüş, S.; Orbay, M. Simultaneous glycolysis and hydrolysis of polyethylene terephthalate and characterization of products by differential scanning calorimetry. Polymer 2003, 44, 7609–7616. [Google Scholar] [CrossRef]
- Aguado, A.; Martínez, L.; Becerra, L.; Arieta-araunabeña, M.; Arnaiz, S.; Asueta, A.; Robertson, I. Chemical depolymerisation of PET complex waste: Hydrolysis vs. glycolysis. J. Mater. Cycles Waste Manag. 2013, 16, 201–210. [Google Scholar] [CrossRef]
- Mandoki, J.W. Depolymerization of Condensation Polymers. U.S. Patent 4605762A, 12 August 1986. [Google Scholar]
- Rosen, B.I. Preparation of Purified Terephthalic Acid from Waste Polyethylene Terephthalate. U.S. Patent 5,095,145A, 10 March 1992. [Google Scholar]
- Čolnik, M.; Knez, Ž.; Škerget, M. Sub- and supercritical water for chemical recycling of polyethylene terephthalate waste. Chem. Eng. Sci. 2021, 233, 116389. [Google Scholar] [CrossRef]
- Benzaria, J.; Durif-Varambon, B.; Dawans, F.; Gaillard, J.-B. Process for Recovery of Alkali Metal or Alkali Earth Metal Terephtalate and Alkylene Glycol from Alkylene. Polyterephtalates. Patent EP 0597751A1, 21 January 1998. [Google Scholar]
- Pudack, C.; Stepanski, M.; Fässler, P. PET Recycling–Contributions of crystallization to sustainability. Chem. Ing. Tech. 2020, 92, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Ravindranath, K.; Mashelkar, R.A. Polyethylene terephthalate—I. Chemistry, thermodynamics and transport properties. Chem. Eng. Sci. 1986, 41, 2197–2214. [Google Scholar] [CrossRef]
- Elamri, A.; Zdiri, K.; Harzallah, O.; Lallam, A. Progress in Polyethylene Terephthalate Recycling; Nova Science Publishers: New York, NY, USA, 2017. [Google Scholar]
- Demirel, B.; Yaraş, A.; Elçiçek, H. Crystallization behavior of PET materials. BAÜ Fen Bil. Enst. Dergisi Cilt 2021, 13, 26–35. [Google Scholar]
- Carta, D.; Cao, G.; D’Angeli, C. Chemical recycling of poly (ethylene terephthalate)(PET) by hydrolysis and glycolysis. Environ. Sci. Pollut. Res. 2003, 10, 390–394. [Google Scholar] [CrossRef]
- Wan, B.-Z.; Kao, C.-Y.; Cheng, W.-H. Kinetics of Depolymerization of Poly(ethylene terephthalate) in a Potassium Hydroxide Solution. Ind. Eng. Chem. Res. 2001, 40, 509–514. [Google Scholar] [CrossRef]
- Kint, D.P.R.; Martinez de Ilarduya, A.; Bou, J.J.; Muñoz-Guerra, S. Poly(ethylene terephthalate) copolymers containing nitroterephthalic units. III. Methanolytic degradation. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2276–2285. [Google Scholar] [CrossRef]
- Imran, M.; Kim, D.H.; Al-Masry, W.A.; Mahmood, A.; Hassan, A.; Haider, S.; Ramay, S.M. Manganese-, cobalt-, and zinc-based mixed-oxide spinels as novel catalysts for the chemical recycling of poly(ethylene terephthalate) via glycolysis. Polym. Degrad. Stab. 2013, 98, 904–915. [Google Scholar] [CrossRef]
- Hayward, D.W.; Witham, D.L. Method for Preparing Extremely Small Particles of Recycled Polyethylene Terephthalate. U.S. Patent 7,380,735 B2, 3 June 2008. [Google Scholar]
- Ügdüler, S.; Van Geem, K.M.; Denolf, R.; Roosen, M.; Mys, N.; Ragaert, K.; De Meester, S. Towards closed-loop recycling of multilayer and coloured PET plastic waste by alkaline hydrolysis. Green Chem. 2020, 22, 5376–5394. [Google Scholar] [CrossRef]
- Mancini, S.D.; Zanin, M. Post Consumer Pet Depolymerization by Acid Hydrolysis. Polym. Plast. Technol. Eng. 2007, 46, 135–144. [Google Scholar] [CrossRef]
- Simonaitis, T.; Barkauskas, R.; Jankauskaitė, V. Adhesive composition with poly(ethylene terephthalate) waste. In Proceedings of the 4th Central European Conference, Lichtenfels, Germany, 26–29 March 2015; pp. 1–3. [Google Scholar]
- Mehrabzadeh, M.; Shodjaei, S.; Khosravi, M. Chemical recycling of polyethylene terephthalate. Polym. J. Iran. 2000, 9, pp. 37–40. Available online: https://www.sid.ir/en/journal/ViewPaper.aspx?ID=33912 (accessed on 30 April 2021).
- Chen, F.; Zhou, Q.; Bu, R.; Yang, F.; Li, W. Kinetics of poly(ethylene terephthalate) fiber glycolysis in ethylene glycol. Fibers Polym. 2015, 16, 1213–1219. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, C.-Y.; Lo, Y.-W.; Mao, C.-F.; Liao, W.-T. Studies of glycolysis of poly(ethylene terephthalate) recycled from postconsumer soft-drink bottles. II. Factorial experimental design. J. Appl. Polym. Sci. 2001, 80, 956–962. [Google Scholar] [CrossRef]
- Bae, D.; Roh, H.D. Process for Manufacturing Terephthalic. Acid. Patent EP0873296B1, 29 August 1995. [Google Scholar]
- Singh, S.; Sharma, S.; Umar, A.; Mehta, S.K.; Bhatti, M.S.; Kansal, S.K. Recycling of Waste Poly (ethylene terephthalate) Bottles by Alkaline Hydrolysis and Recovery of Pure Nanospindle-Shaped Terephthalic Acid. J. Nanosci. Nanotechnol. 2018, 18, 5804–5809. [Google Scholar] [CrossRef]
- Brems, A.; Baeyens, J.; Vandecasteele, C.; Dewil, R. Polymeric cracking of waste polyethylene terephthalate to chemicals and energy. J. Air Waste Manag. Assoc. 2011, 61, 721–731. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Q.; Lei, Y.; Guo, W.; Xu, Y. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis. Polym. Degrad. Stab. 2008, 93, 90–98. [Google Scholar] [CrossRef]
- Damayanti; Wu, H. S. Pyrolysis kinetic of alkaline and dealkaline lignin using catalyst. J. Polym. Res. 2017, 25, 7. [Google Scholar]
- Reeb, J.E.; Michael, R. Moisture content by the oven-dry method for industrial testing. In Proceedings of the Western Dry Kiln Association, Portland, OR, USA, 10–11 May 1999; pp. 66–74. [Google Scholar]
- Cooney, J.D.; Day, M.; Wiles, D.M. Thermal degradation of poly(ethylene terephthalate): A kinetic analysis of thermogravimetric data. J. Appl. Polym. Sci. 1983, 28, 2887–2902. [Google Scholar] [CrossRef]
- Jenekhe, S.A.; Lin, J.W.; Sun, B. Kinetics of the thermal degradation of polyethylene terephthalate. Thermochim. Acta 1983, 61, 287–299. [Google Scholar] [CrossRef]
- Campanelli, J.R.; Kamal, M.R.; Cooper, D.G. A kinetic study of the hydrolytic degradation of polyethylene terephthalate at high temperatures. J. Appl. Polym. Sci. 1993, 48, 443–451. [Google Scholar] [CrossRef]
- Chen, J.Y.; Ou, C.F.; Hu, Y.C.; Lin, C.C. Depolymerization of poly(ethylene terephthalate) resin under pressure. J. Appl. Polym. Sci. 1991, 42, 1501–1507. [Google Scholar] [CrossRef] [Green Version]
- Baliga, S.; Wong, W.T. Depolymerization of poly(ethylene terephthalate) recycled from post-consumer soft-drink bottles. J. Polym. Sci. Part A Polym. Chem. 1989, 27, 2071–2082. [Google Scholar] [CrossRef]
- Niksiar, A.; Faramarzi, A.H.; Sohrabi, M. Kinetic study of polyethylene terephthalate (PET) pyrolysis in a spouted bed reactor. J. Anal. Appl. Pyrolysis 2015, 113, 419–425. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, T.R. Kinetics of hydrolysis of polyethylene terephthalate pellets in nitric acid. J. Appl. Polym. Sci. 2003, 87, 1781–1783. [Google Scholar] [CrossRef]
- Chen, J.W.; Chen, L.W.; Cheng, W.H. Kinetics of glycolysis of polyethylene terephthalate with zinc catalyst. Polym. Int. 1999, 48, 885–888. [Google Scholar] [CrossRef]
- Genta, M.; Iwaya, T.; Sasaki, M.; Goto, M.; Hirose, T. Depolymerization Mechanism of Poly(ethylene terephthalate) in Supercritical Methanol. Ind. Eng. Chem. Res. 2005, 44, 3894–3900. [Google Scholar] [CrossRef]
- Goje, A.S.; Mishra, S. Chemical Kinetics, Simulation, and Thermodynamics of Glycolytic Depolymerization of Poly(ethylene terephthalate) Waste with Catalyst Optimization for Recycling of Value Added Monomeric Products. Macromol. Mater. Eng. 2003, 288, 326–336. [Google Scholar] [CrossRef]
- Farzi, A.; Dehnad, A.; Fotouhi, A.F. Biodegradation of polyethylene terephthalate waste using Streptomyces species and kinetic modeling of the process. Biocatal. Agric. Biotechnol. 2019, 17, 25–31. [Google Scholar] [CrossRef]
- Shahi, A.; Roozbehani, B.; Mirdrikvand, M. Pyrolysis of Polyethylene Terephthalate Granules in Presence of Lewis Brønsted Acid Sites Catalysts. Res. Sq. 2021. [Google Scholar]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic pyrolysis of plastic waste: A review. Process Safety and Environmental Protection 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Zhang, S.; Song, X.; Zhang, D.; Tian, Y. Kinetics of the hydrolytic depolymerization of poly(ethylene terephthalate) under microwave irradiation. Polym. J. 2011, 43, 811. [Google Scholar] [CrossRef]
- Kang, M.J.; Yu, H.J.; Jegal, J.; Kim, H.S.; Cha, H.G. Depolymerization of PET into terephthalic acid in neutral media catalyzed by the ZSM-5 acidic catalyst. Chem. Eng. J. 2020, 398, 125655. [Google Scholar] [CrossRef]
- Yoshioka, T.; Okayama, N.; Okuwaki, A. Kinetics of Hydrolysis of PET Powder in Nitric Acid by a Modified Shrinking-Core Model. Ind. Eng. Chem. Res. 1998, 37, 336–340. [Google Scholar] [CrossRef]
- Mishra, S.; Goje, A.S.; Zope, V.S. Chemical Recycling, Kinetics, and Thermodynamics of Poly (Ethylene Terephthalate) (PET) Waste Powder by Nitric Acid Hydrolysis. Polym. React. Eng. 2003, 11, 79–99. [Google Scholar] [CrossRef]
- López-Fonseca, R.; González-Marcos, M.P.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I. A kinetic study of the depolymerisation of poly(ethylene terephthalate) by phase transfer catalysed alkaline hydrolysis. J. Chem. Technol. Biotechnol. 2009, 84, 92–99. [Google Scholar] [CrossRef]
- Campanelli, J.R.; Kamal, M.R.; Cooper, D.G. Kinetics of glycolysis of poly(ethylene terephthalate) melts. J. Appl. Polym. Sci. 1994, 54, 1731–1740. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Yehia, F.Z.; Eshaq, G.; ElMetwally, A.E. Ionic Liquid-Coordinated Ferrous Acetate Complex Immobilized on Bentonite As a Novel Separable Catalyst for PET Glycolysis. Ind. Eng. Chem. Res. 2015, 54, 12474–12481. [Google Scholar] [CrossRef]
- Goje, A.; Chauhan, Y.; Mishra, S. Chemical recycling, kinetics, and thermodynamics of alkaline depolymerization of waste polyesters. Polym. Plast. Technol. Eng. 2004, 43, 95–120. [Google Scholar] [CrossRef]
- Mishra, S.; Goje, A.S. Kinetic and thermodynamic study of methanolysis of poly(ethylene terephthalate) waste powder. Polym. Int. 2003, 52, 337–342. [Google Scholar] [CrossRef]
- Bai, B.; Liu, Y.; Zhang, H.; Zhou, F.; Han, X.; Wang, Q.; Jin, H. Experimental investigation on gasification characteristics of polyethylene terephthalate (PET) microplastics in supercritical water. J Fuel 2020, 262, 116630. [Google Scholar] [CrossRef]
- Williams, P.T.; Slaney, E. Analysis of products from the pyrolysis and liquefaction of single plastics and waste plastic mixtures. Resour. Conserv. Recycl. 2007, 51, 754–769. [Google Scholar] [CrossRef]
- Sarker, M.; Kabir, A.; Rashid, M.M.; Molla, M.; Mohammad, A.D. Waste Polyethylene Terephthalate (PETE-1)Conversioninto Liquid Fuel. J. Fundam. Renew. Energy Appl. 2011, 1, R101202. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Li, L.; Yu, S.; Xie, C.; Liu, F. Butanol alcoholysis reaction of polyethylene terephthalate using acidic ionic liquid as catalyst. J. Appl. Polym. Sci. 2013, 130, 1840–1844. [Google Scholar] [CrossRef]
- Kurokawa, H.; Ohshima, M.-a.; Sugiyama, K.; Miura, H. Methanolysis of polyethylene terephthalate (PET) in the presence of aluminium tiisopropoxide catalyst to form dimethyl terephthalate and ethylene glycol. Polym. Degrad. Stab. 2003, 79, 529–533. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, Y.; Xiang, H.; Xu, Y.; Li, Y. Study on methanolytic depolymerization of PET with supercritical methanol for chemical recycling. Polym. Degrad. Stab. 2002, 75, 185–191. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Redhwi, H.H.; Achilias, D.S. Recycling of poly (ethylene terephthalate) waste through methanolic pyrolysis in a microwave reactor. J. Anal. Appl. Pyrolysis 2012, 98, 214–220. [Google Scholar] [CrossRef]
- Zhang, L. Kinetics of hydrolysis of poly (ethylene terephthalate) wastes catalyzed by dual functional phase transfer catalyst: A mechanism of chain-end scission. Eur. Polym. J. 2014, 60, 1–5. [Google Scholar] [CrossRef]
- Sharma, V.; Parashar, P.; Srivastava, P.; Kumar, S.; Agarwal, D.D.; Richharia, N. Recycling of Waste PET-Bottles Using Dimethyl Sulfoxide and Hydrotalcite Catalyst. Appl. Polym. Sci 2013, 129, 1513–1519. [Google Scholar] [CrossRef]
- Paliwal, N.R.; Mungray, A.K. Ultrasound assisted alkaline hydrolysis of poly (ethylene terephthalate) in presence of phase transfer catalyst. Polym. Degrad. Stab. 2013, 98, 2094–2101. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Achilias, D.S.; Redhwi, H.H.; Bikiaris, D.N.; Katsogiannis, K.A.G.; Karayannidis, G.P. Hydrolytic depolymerization of PET in a microwave reactor. Macromol. Mater. Eng. 2010, 295, 575–584. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, D.; An, L.; Zhang, H.; Tian, Y. Hydrolytic Depolymerization of poly(ethylene terephthalate) under microwave irradiation. J. Appl. Polym. Sci. 2004, 95, 719–723. [Google Scholar]
- Valh, J.V.; Vončina, B.; Lobnik, A.; Zemljič, L.F.; Škodič, L.; Vajnhandl, S. Conversion of polyethylene terephthalate to high-quality terephthalic acid by hydrothermal hydrolysis: The study of process parameters. Text. Res. J. 2020, 90, 1446–1461. [Google Scholar] [CrossRef]
- Lee, S.-C.; Sze, Y.-W.; Lin, C.-C. Polyurethanes synthesized from polyester polyols derived from PET waste. II. Thermal properties. J. Appl. Polym. Sci. 1994, 52, 869–873. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Chou, Y.-L.; Yang, H.-C.; Chen, C.-W.; Rwei, S.-P. Synthesis and Characterization of Thermoplastic Poly(Ester Amide)s Elastomer (TPEaE) Obtained from Recycled PET. J. Renew. Mater. 2021, 9, 867–880. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Ranganathan, P.; Lee, Y.-H.; Rwei, S.-P. New Strategy and Polymer Design to Synthesize Polyamide 66 (PA66) Copolymers with Aromatic Moieties from Recycled PET (rPET). ACS Sustain. Chem. Eng. 2021, 9, 3518–3528. [Google Scholar] [CrossRef]
- Ashoor, A.S.; Kareem, M.M.; Al-Baiati, M.N. Improved asphalt binder using recycle polyethylene terephthalate polymer. IOP Conf. Ser. Mater. Sci. Eng. 2019, 571, 012094. [Google Scholar] [CrossRef] [Green Version]
- Spósito, F.A.; Higuti, R.T.; Tashima, M.M.; Akasaki, J.L.; Melges, J.L.P.; Assunção, C.C.; Bortoletto, M.; Silva, R.G.; Fioriti, C.F. Incorporation of PET wastes in rendering mortars based on Portland cement/hydrated lime. J. Build. Eng. 2020, 32, 101506. [Google Scholar] [CrossRef]
- Jung, K.-W.; Kim, J.-H.; Choi, J.-W. Synthesis of magnetic porous carbon composite derived from metal-organic framework using recovered terephthalic acid from polyethylene terephthalate (PET) waste bottles as organic ligand and its potential as adsorbent for antibiotic tetracycline hydrochloride. Compos. Part B Eng. 2020, 187, 107867. [Google Scholar]
- Starosta, W.; Łyczko, K. Appicatons of Terephthalic Acid Recovered from Waste PET Bottles for Synthesis of Valuable Metal-Oranic Framework Materials; Michalik, J., Godlewska-Para, E., Eds.; Institute of Nuclear Chemistry and Technology: Warszawa, Poland, 2019. [Google Scholar]
- Hronec, M.; Fulajtárová, K. Terephthalic acid from waste PET: An efficient and reusable catalyst for xylose conversion into furfural. Catal. Today 2019, 324, 27–32. [Google Scholar] [CrossRef]






| Company | Reaction | Country/Region | Ref |
|---|---|---|---|
| FENC | hydrolysis | Taiwan | [58] |
| Gr3n | hydrolysis | Switzerland | [59] |
| JEPLAN (PRT) | glycolysis | Japan | [60] |
| Garbo | glycolysis | Italy | [61] |
| IFPEN | glycolysis | France | [62] |
| Ioniqa | glycolysis | Netherlands | [63] |
| PerPETual | glycolysis | UK | [64] |
| Poseidon Plastics | glycolysis | UK | [65] |
| Eastman | methanolysis | USA | [66] |
| Loop industries | methanolysis | Canada | [67] |
| DePoly | Switzerland | [68] | |
| Carbios | enzyme | French | [69] |
| Agiylx | pyrolysis | USA | [45] |
| Pyrowave | microwave radiation | USA | [45] |
| Type of Catalyst | Reactor | Time of Reaction, min | Molar Ratio Catalyst to PET | Particle Size | Temperature °C | YieldBHET % | Ref |
|---|---|---|---|---|---|---|---|
| Na2CO3 | Batch | 60 | 1:100 | 0.25 mm | 196 | 78.8 | [101] |
| Na2SO4 | Batch | 60 | 1:380 | 0.25 mm | 196 | 19.6 | [101] |
| NaHCO3 | Batch | 60 | 1:190 | 0.25 mm | 196 | 60.2 | [101] |
| Titanium (IV)-phosphate with EG | Necked flask | 150 | 0.3:0.13 | NA | 190–200 | 97.5 | [102] |
| Zn(OOCCH3)2 with EG | Necked flask | 150 | 0.3:0.13 | NA | 190–200 | 62.8 | [102] |
| di-n-propylamine with EG | Necked flask | 90 | 1:4 | NA | 160 | 73.7 | [103] |
| di-n-butylamine with EG | Necked flask | 90 | 1:4 | NA | 160 | 76.8 | [103] |
| N-propylamine with EG | Necked flask | 90 | 1:4 | NA | 160 | 72.8 | [103] |
| diisopropylamine | Necked flask | 90 | 1:4 | NA | 160 | 70.1 | [103] |
| γ-Fe2O3nanoparticles | NA | 60–80 | 1:8 (wt%) | >50 µm–<10 µm | 255–300 | 80–90 | [104] |
| Fe3O4-boosted MWCNT | NA | 120 | 1:19 (wt%) | >50 µm–<10 µm | 190 | 100 | [104] |
| Mg-Al-O@Fe3O4 | NA | 90 | 1:199 (wt%) | >50 µm–<10 µm | 240 | 80 | [104] |
| Zinc Acetete | NA | 480 | 1:99 (wt%) | >50 µm–<10 µm | 198 | 75 | [104] |
| Type of Enzyme | Microorganism | Materials | Temperature (°C) | Product | Ref |
|---|---|---|---|---|---|
| Cbotu_EstA | Clostridium botulinum ATCC3502 | PET film | 50 | TPA,MHET | [149] |
| Lipases | Candida cylindracea | PET nanoparticels | 40 | 1,2-Ethandiol, TPA | [150] |
| Tcur0390 | Thermomonospora curvata DSM43183 | PET nanoparticels | 50 | NA | [151] |
| Hydrolase TfH | Thermobifida fusca DSM43793 | PET pellete | 44–55 | TPA, EG | [152] |
| TfCut1 | Thermobifida fusca KW3 | PET nanospheres | 55–65 | HEB, MHET, BHET, Benzoic acid | [153] |
| Tha_Cut1 | Thermobifida alba DSM43185 | PET | 50 | TPA, Benzoic acid, HEB, MHET | [154] |
| MHETase and PETase | Ideonella sakaiensis | PET | 30 | MHET | [154] |
| BsEstB | Bacillus subtilis 4P3-11 | PET | 40–45 | TPA, Benzoic acid, MHET | [155] |
| LCC cutinase | Leaf-branch compost | Amorphous PET | 50–70 | MHET, TPA, Benzoic acid | [156] |
| Polymer polyhydroxyalkanoate | Pseudomonas | PET | 70 | EG,TPA | [157] |
| Hydroxyalkanoyloxy-alkanoate | Pseudomonas | PET | 70 | EG,TPA | [157] |
| Thc_Cut2 | T. cellulosilytica | PET films | 50 | MHET, TPA, BA, HEB | [158] |
| Arg29Asn | Mutant The_Cut2 | PET films | 50 | MHET, TPA, BA, HEB | [158] |
| Lipases | Aspergillus oryzae CCUG 33812 | PET fabric | 55 | BHET,TPA | [159] |
| Gln65Glu | Mutant The_Cut2 | PET films | 50 | MHET, TPA, BA, HEB | [158] |
| TfCut2 | Thermobifida fusca KW3 | PET films | 60 | MHET | [160] |
| Thh_Est-esterase | Thermobifida halotolerans | 3PET model substrate | 50 | MHET,TPA, BA, HEB | [161] |
| PET Catalyst | Method | T (°C) | k | k2 | K | A | Ea (Kj·mol−1) | YBHET, % | YDMT, % | YTPA, % | Ref | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PET | P | 373–443 448–503 | 6.4 × 1025/min 9.31 × 1011/min | 347.4 172.6 | [73] | |||||||
| PET | P | 390 | 48 × 1021/min | 323.8 | [209,210] | |||||||
| PET | P | 202.1 | [198] | |||||||||
| PET | P | 198.6 | [199] | |||||||||
| PET | H2O | H | 180 | 0.0037 | 378 | [208,211] | ||||||
| flake | H2O | H | 265 | 0.352 g PET/(mol min) | 0.664 | 55.7 | [200] | |||||
| PET | H2@ZSM-5-25 | H | 230 | 0.1787 | 1.2 | 98.5 | [212] | |||||
| PET | H2O without catalyst | H | 230 | 0.0755 | 19.4 | [212] | ||||||
| flakes | NaOH | H | 200 | 0.1900 | 99 | 97.9 | [95] | |||||
| flakes | KOH | H | 160 | 2.24 × 10−6 L/(min cm2) | 419 L/(min cm2) | 69 | 90.9 | [182] | ||||
| PET | HNO3 | H | 100 | 0.4800 | 101.3 | [213] | ||||||
| PET | HNO3 | H | 88 | 1.9 × 10−2 L/(min cm2) | 0.042 | 659 L/(min cm2) | 88.3 | [214] | ||||
| Flake < 2 | sulfuric acid | H | 2.0 × 1011 g.cm/(mol min) | 99.7 | 90 | [187] | ||||||
| PET | TBAB | Hydrolysis | 60–80 | 0.1100 | 75 | 99.0 | [215] | |||||
| flake | 3Bu6DPB, NaOH | H | 90 | 0.2 L/(mol h) | 1.3 × 109 L/(mol h) | 68.2 | >90 | [134] | ||||
| flake < 6 | TOMAB NaOH | H | 80 | 83 | 92 | [135] | ||||||
| flake 3 × 5 | marine water | H | 205 | 5.33 × 107/h | 75 | 96.0 | [85] | |||||
| flake | ZnAc | G | 265 | 6.67 × 103 L/mol/min | 92.0 | [216] | ||||||
| pellet | Ionic liquid | G | 190 | 0.16/min | 51.6 | [217] | ||||||
| PET | EG | G | 99783/min | 46.2 | [207] | |||||||
| pellet 0.25 | Na2CO3 | G | 196 | 3.767 L2/mol2/h | 0.36 L/mol | 185 | 80 | [101] | ||||
| fiber | Zn/Al | G | 196 | 79.3 | 92 | [190] | ||||||
| PET | KOH | M | 65 | 0.0132 | 0.0246 | 127.6 | 96.0 | 96.0 | [218] | |||
| PET | CH3OH | M | 270 | 0.0033/s | 0.0008/s | [206] | ||||||
| BHET | CH3OH | M | 25 | 0.0017/day | 0.0025/ day | 56 | [183] | |||||
| PET | CH3OH | M | 140 | 1.4 × 10−3 g PET/(mol min) | 107.1 g PET/(mol min) | 95.31 | [219] | |||||
| PET | CH3OH | M | 66.5 | 80 | [98] | |||||||
| Type of Reaction | Reactor | T (°C) | Catalyst | Yield (%) | Ref |
|---|---|---|---|---|---|
| Pyrolysis | Parr mini betch top reactor | 500 | NA | 15 | [221] |
| Liquefaction | Parr mini betch top reactor | 500 | NA | Single ring aromatic 11.1% 2nd ring polycyclic aromatic hydrocarbons 13.9% 3rd ring polycyclic aromatic hydrocarbons 7.7% | [221] |
| Pyrolysis | Boiling Flask | 405 | Ca(OH)2 | ring aro Single matic 8.2% 2nd ring polycyclic aromatic hydrocarbons 11.4% 3rd ring polycyclic aromatic hydrocarbons 2.4% | [222] |
| Alcoholysis | Autoclave | 205 | Blank | DBTP (0%); EG (0%) | [223] |
| Alcoholysis | Autoclave | 205 | ZnCl2 | DBTP (87.5%); EG (88.1%) | [223] |
| Alcoholysis | Autoclave | 205 | Zn(CH3COO)2 | DBTP (88.4%); EG (88.6%) | [223] |
| Alcoholysis | Autoclave | 205 | Ti(CH3CH2CH2CH2O)4 | DBTP (89.7%); EG (89.9%) | [223] |
| Alcoholysis | Autoclave | 205 | H2SO4 | DBTP (67.3%); EG (67.2%) | [223] |
| Alcoholysis | Autoclave | 205 | (HO3S-(CH2)3-NEt3)Cl | DBTP (25.8%); EG (26.1%) | [223] |
| Alcoholysis | Autoclave | 205 | (HO3S-(CH2)3-NEt3)Cl-ZnCl2 (x = 0.67) | DBTP (95.3%); EG (95.7%) | [223] |
| Alcoholysis | Autoclave | 205 | (HO3S-(CH2)3-NEt3)Cl-FeCl3 (x = 0.67) | DBTP (85.8%); EG (85.7%) | [223] |
| Alcoholysis | Autoclave | 205 | (HO3S-(CH2)3-NEt3)Cl-FeCl2 (x = 0.67) | DBTP (85.5%); EG (85.6%) | [223] |
| Alcoholysis | Autoclave | 205 | (HO3S-(CH2)3-NEt3)Cl-CuCl2 (x = 0.67) | DBTP (77.8%); EG (77.9%) | [223] |
| Methanolysis | Autoclave | 200 | Alumunium triisopropoxide | DMT (88%) | [224] |
| Methanolysis | Autoclave | 250–270 | Zinc Acetate | DMT (60–95%) | [225] |
| Methanolysis | Microwave | 160–200 | Zinc Acetate | DMT | [226] |
| Hydrolysis | Vessel | 115–145 | ((CH3)3N(C16H33))3(PW12O40) | TPA(90%) | [227] |
| Hydrolysis | Autoclave | 70–95 | TOMAB | TPA(98%) | [95] |
| Hydrolysis | 190 | Hydrotalcite | TPA(99%) | [228] | |
| Hydrolysis | Microwave | 90–98 | TBAB | TPA(99%) | [133] |
| Alkaline Hydrolysis | Bottle neck | 90 | TBAI | TPA(90%) | [229] |
| Hydrolysis | Microwave | 180 | TOMAB | TPA(180%) | [230] |
| Aminolytic | NA | 225–227 (m.p) | EA (pKa: 9.5) | BHETA (93%) | [114] |
| Aminolytic | NA | 133–135 (m.p) | AEE (pKa:9.62) | BHEETA(92%) | [114] |
| Aminolytic | NA | 152–154 (m.p) | AEAE (pka:9.62 and 6.49) | BHEAETA (55%) | [114] |
| Aminolytic | NA | 220–222 (m.p) | AMP (pka:9.88) | BHMPTA (60%) | [114] |
| Aminolytic | NA | 174–176 (m.p) | NMEA (pka:9.95) | BHEDMTA (87%) | [114] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damayanti; Wu, H.-S. Strategic Possibility Routes of Recycled PET. Polymers 2021, 13, 1475. https://doi.org/10.3390/polym13091475
Damayanti, Wu H-S. Strategic Possibility Routes of Recycled PET. Polymers. 2021; 13(9):1475. https://doi.org/10.3390/polym13091475
Chicago/Turabian StyleDamayanti, and Ho-Shing Wu. 2021. "Strategic Possibility Routes of Recycled PET" Polymers 13, no. 9: 1475. https://doi.org/10.3390/polym13091475
APA StyleDamayanti, & Wu, H.-S. (2021). Strategic Possibility Routes of Recycled PET. Polymers, 13(9), 1475. https://doi.org/10.3390/polym13091475







