Preparation and Characterization of an Injectable and Photo-Responsive Chitosan Methacrylate/Graphene Oxide Hydrogel: Potential Applications in Bone Tissue Adhesion and Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chitosan Methacrylation
2.3. Synthesis of Graphene Oxide
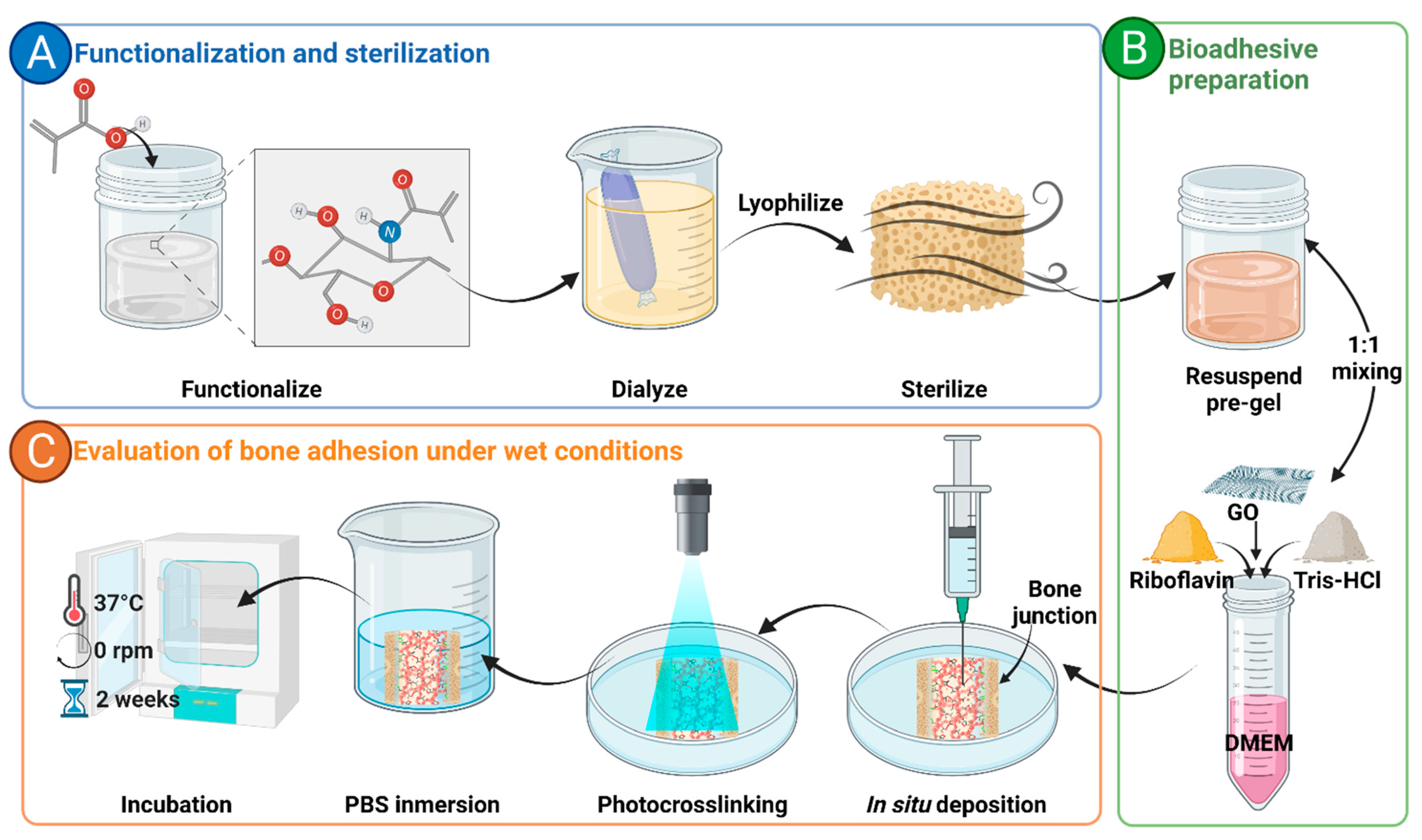
2.4. Preparation of the Bioadhesive
2.5. Rheological Evaluation
2.6. GO Dispersion
2.7. Morphological Analysis of Hydrogels
2.8. Qualitative Adhesion Test
2.9. Mechanical Adhesion
2.10. Texture Analysis
2.11. Swelling and Degradation
2.12. Hemolysis and Platelet Aggregation
2.13. Cytotoxicity
2.14. Finite Element Analyses of Bioadhesive-Repaired Bones
2.15. Statistical Analysis
3. Results and Discussion
3.1. Chemical Functionalization of Chitosan
3.2. Synthesis of Graphene Oxide
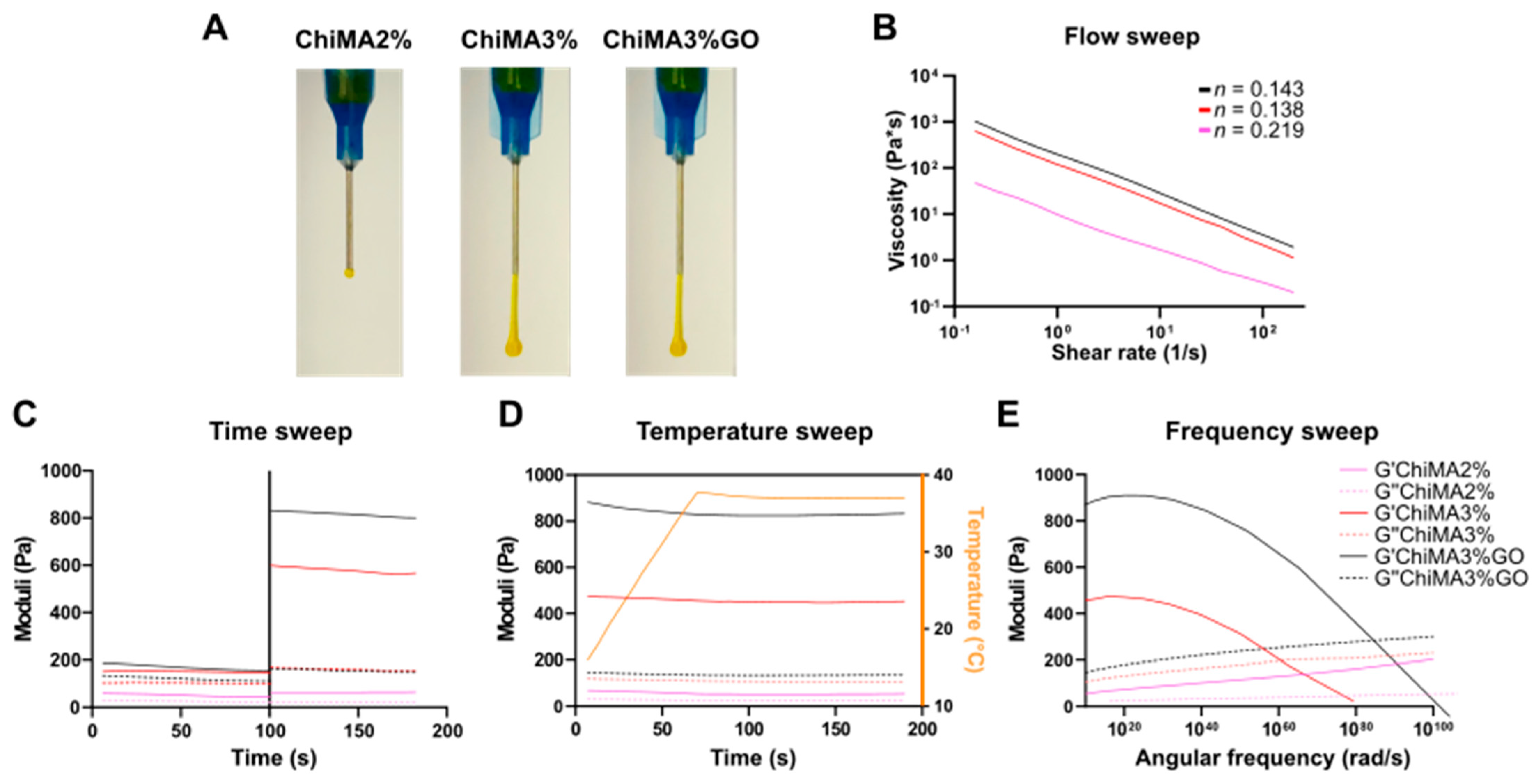
3.3. Rheological Evaluation
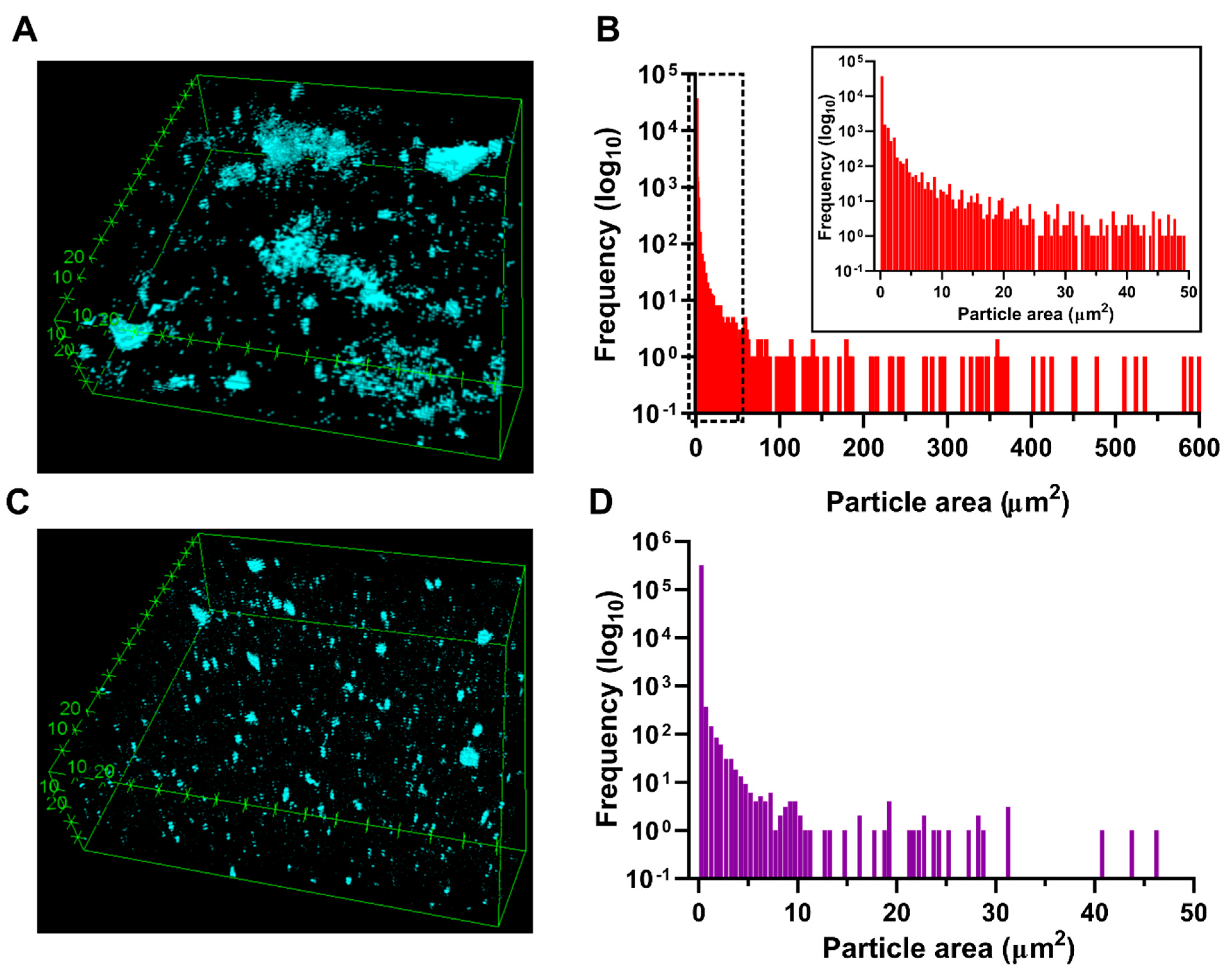
3.4. GO Dispersion and Morphological Structure of the Hydrogels
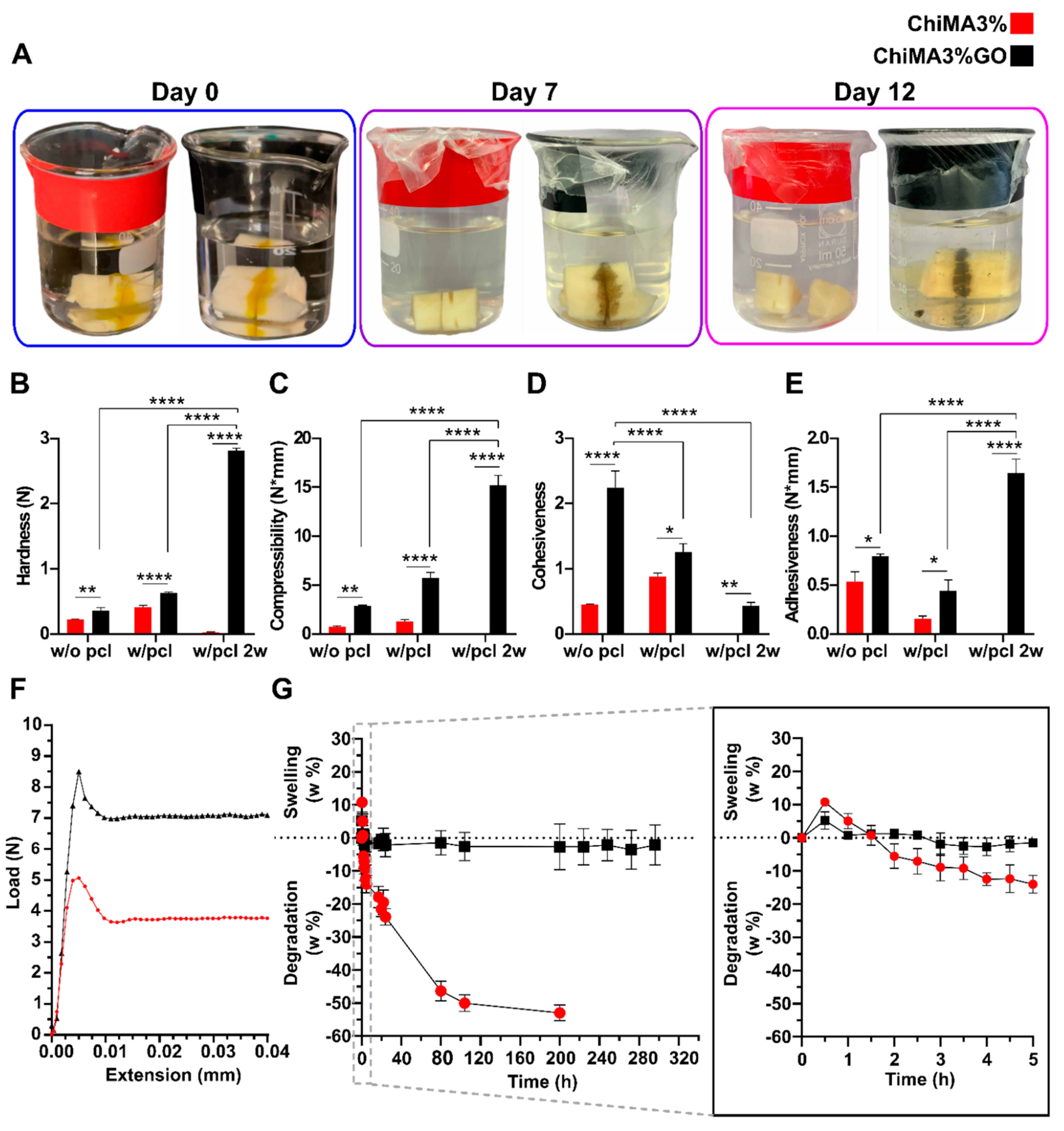
3.5. Mechanical and Adhesion Evaluation
3.6. Swelling and Degradation
3.7. Hemolysis and Platelet Aggregation
3.8. Cytotoxicity
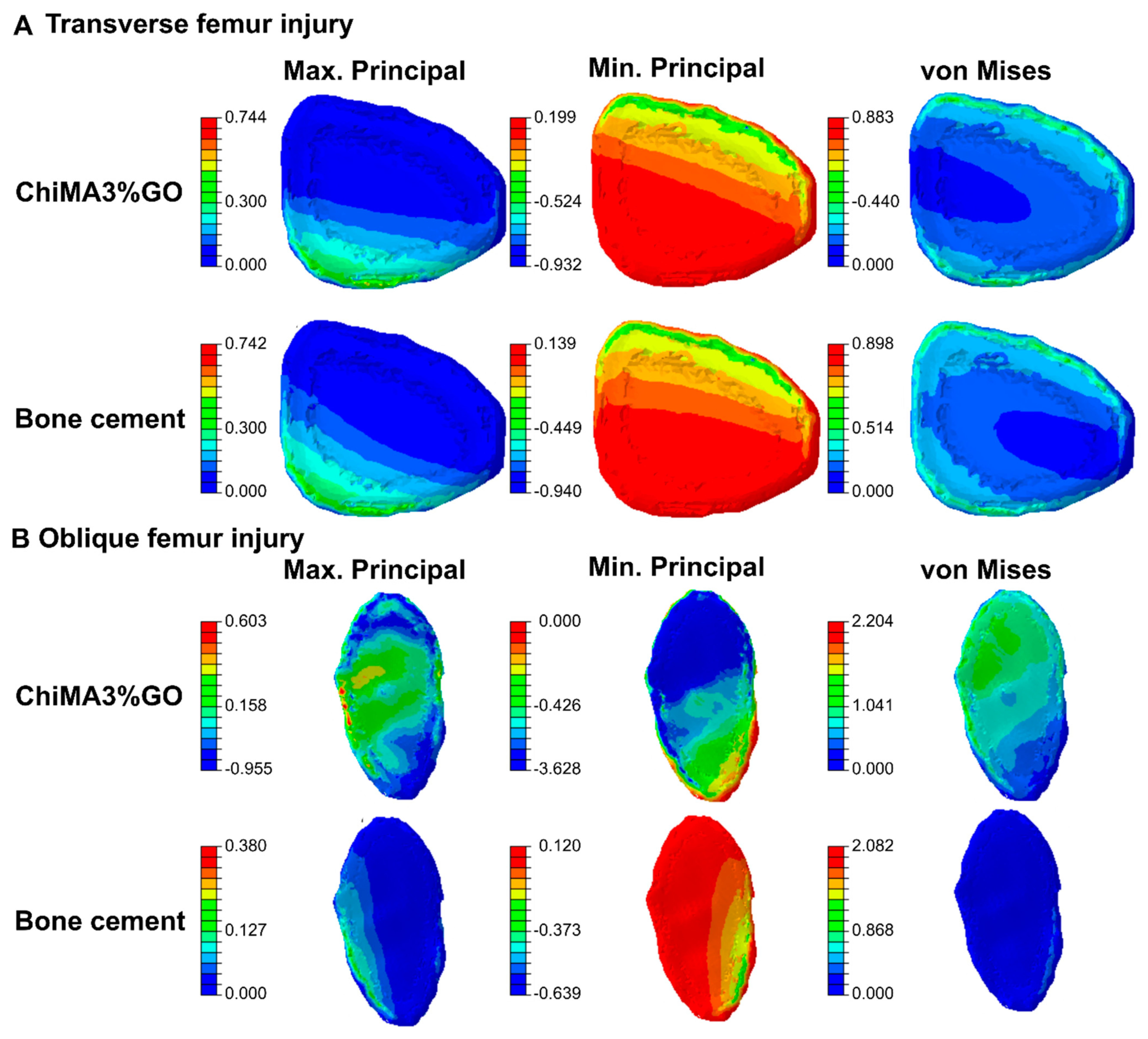
3.9. Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Filardi, V. Healing of tibial comminuted fractures by the meaning of an innovative intramedullary nail. J. Orthop. 2019, 16, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Thakeb, M.F.; Mahran, M.A.; El-Motassem, H.M. Bone transport for the management of severely comminuted fractures without bone loss. Strateg. Trauma Limb. Reconstr. 2016, 11, 19–24. [Google Scholar]
- Massoud, E.I.E. Repair of comminuted fracture of the lower patellar pole. Ulus Travma Acil Cerrahi Derg 2017, 23, 150–155. [Google Scholar] [PubMed]
- Zwingmann, J.; Neumann, M.V.; Hammer, T.O.; Reising, K.; Südkamp, N.P. Comminuted Fracture of Elbow—Ostheosynthesis vs. Total Joint Replacement. Acta Chir. Orthop. Traumatol. Cech. 2016, 83, 231–237. [Google Scholar]
- Kakazu, R.; Archdeacon, M.T. Surgical Management of Patellar Fractures. Orthop. Clin. N. Am. 2016, 47, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Farrar, M.T. Bone adhesives for trauma surgery: A review of challenges and developments. Int. J. Adhes. Adhes. 2012, 33, 89–97. [Google Scholar] [CrossRef]
- Onche, I.I.; Osagie, O.E.; INuhu, S. Removal of orthopaedic implants: Indications, outcome and economic implications. J. West Afr. Coll. Surg. 2011, 1, 101–112. [Google Scholar]
- Haseeb, M.; Butt, M.F.; Altaf, T.; Muzaffar, K.; Gupta, A.; Jallu, A. Indications of implant removal: A study of 83 cases. Int. J. Health Sci. 2017, 11, 1–7. [Google Scholar]
- Iwata, T.; Nozawa, S.; Maeda, M.; Akiyama, H. New Technique for Removal of Screws with Damaged Heads. Orthopedics 2017, 40, e911–e914. [Google Scholar] [CrossRef] [PubMed]
- Endres, K.; Marx, R.; Tinschert, J.; Wirtz, D.C.; Stoll, C.; Riediger, D.; Smeets, R. A new adhesive technique for internal fixation in midfacial surgery. Biomed. Eng. Online 2008, 7, 16. [Google Scholar] [CrossRef]
- Sánchez-Fernández, M.J.; Hammoudeh, H.; Félix Lanao, R.P.; van Erk, M.; van Hest, J.C.M.; Leeuwenburgh, S.C.G. Bone-adhesive materials: Clinical requirements, mechanisms of action, and future perspective. Adv. Mater. Interfaces 2019, 6, 1802021. [Google Scholar] [CrossRef]
- Böker, K.O.; Richter, K.; Jäckle, K.; Taheri, S.; Grunwald, I.; Borcherding, K.; von Byern, J.; Hartwig, A.; Wildemann, B.; Schilling, A.F.; et al. Current State of Bone Adhesives-Necessities and Hurdles. Materials 2019, 12, 3975. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Chauhan, M.; Vaish, A. Bone cement. J. Clin. Orthop. Trauma 2013, 4, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Frazer, R.Q.; Byron, R.T.; Osborne, P.B.; West, K.P. PMMA: An essential material in medicine and dentistry. J. Long. Term. Eff. Med. Implants 2005, 15, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Chan, E.K.; Gupta, S.; Diwan, A.D. Polymethylmethacrylate bone cements and additives: A review of the literature. World J. Orthop. 2013, 4, 67–74. [Google Scholar] [CrossRef]
- Tsukeoka, T.; Suzuki, M.; Ohtsuki, C.; Sugino, A.; Tsuneizumi, Y.; Miyagi, J.; Kuramoto, K.; Moriya, H. Mechanical and histological evaluation of a PMMA-based bone cement modified with gamma-methacryloxypropyltrimethoxysilane and calcium acetate. Biomaterials 2006, 27, 3897–3903. [Google Scholar] [CrossRef] [PubMed]
- Guerra, N.B.; Hernandez, M.L.; Santos, R.G. Acrylic bone cement modified with hydroxiapatyte/vinyl acetate: Mechanical, thermoanalytical characterization and in vitro bioactivity. Polímeros 2010, 20, 98–106. [Google Scholar] [CrossRef]
- Montaño, C.J.; Campos, T.P.R.; Lemos, B.R.S.; Yoshida, M.I.; Almeida, N.G.S.; Aguilar, M.T.P.; Lima, C.V. Effects of hydroxyapatite on PMMA-HAp cement for biomedical applications. Biomed. Mater. Eng. 2020, 31, 191–201. [Google Scholar] [CrossRef] [PubMed]
- de Souza Leão, R.; de Moraes, S.L.D.; de Luna Gomes, J.M.; Lemos, C.A.A.; da Silva Casado, B.G.; do Egito Vasconcelos, B.C.; Pellizzer, E.P. Influence of addition of zirconia on PMMA: A systematic review. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110292. [Google Scholar] [CrossRef]
- Holmes, D. Closing the gap. Nature 2017, 550, S194–S195. [Google Scholar] [CrossRef]
- Majee, S.B. Emerging Concepts in Analysis and Applications of Hydrogels; Books on Demand; IntechOpen: London, UK, 2016. [Google Scholar]
- Hanafy, N.A.N.; Leporatti, S.; El-Kemary, M.A. Mucoadhesive hydrogel nanoparticles as smart biomedical drug delivery system. Appl. Sci. 2019, 9, 825. [Google Scholar] [CrossRef]
- Norouzi, M.; Nazari, B.; Miller, D.W. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov. Today 2016, 21, 1835–1849. [Google Scholar] [CrossRef] [PubMed]
- Serna, J.A.; Florez, S.L.; Talero, V.A.; Briceño, J.C.; Muñoz-Camargo, C.; Cruz, J.C. Formulation and Characterization of a SIS-Based Photocrosslinkable Bioink. Polymers 2019, 11, 569. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, M.H.; Song, J.H.; Kang, C.; Park, W.H. Dual crosslinked alginate hydrogels by riboflavin as photoinitiator. Int. J. Biol. Macromol. 2020, 154, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Francesko, A.; Petkova, P.; Tzanov, T. Hydrogel Dressings for Advanced Wound Management. Curr. Med. Chem. 2018, 25, 5782–5797. [Google Scholar] [CrossRef]
- Chen, T.; Chen, Y.; Rehman, H.U.; Chen, Z.; Yang, Z.; Wang, M.; Li, H.; Liu, H. Ultratough, Self-Healing, and Tissue-Adhesive Hydrogel for Wound Dressing. ACS Appl. Mater. Interfaces 2018, 10, 33523–33531. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef]
- Chen, J.; Wang, D.; Wang, L.H.; Liu, W.; Chiu, A.; Shariati, K.; Liu, Q.; Wang, X.; Zhong, Z.; Webb, J.; et al. An Adhesive Hydrogel with Load-Sharing Effect as Tissue Bandages for Drug and Cell Delivery. Adv. Mater. 2020, 32, e2001628. [Google Scholar] [CrossRef] [PubMed]
- Hasani-Sadrabadi, M.M.; Sarrion, P.; Pouraghaei, S.; Chau, Y.; Ansari, S.; Li, S.; Aghaloo, T.; Moshaverinia, A. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci. Transl. Med. 2020, 12, 534. [Google Scholar] [CrossRef]
- Qiao, Y.; Liu, X.; Zhou, X.; Zhang, H.; Zhang, W.; Xiao, W.; Pan, G.; Cui, W.; Santos, H.A.; Shi, Q. Gelatin Templated Polypeptide Co-Cross-Linked Hydrogel for Bone Regeneration. Adv. Health Mater. 2020, 9, e1901239. [Google Scholar] [CrossRef]
- Ding, J.; He, R.; Zhou, G.; Tang, C.; Yin, C. Multilayered mucoadhesive hydrogel films based on thiolated hyaluronic acid and polyvinylalcohol for insulin delivery. Acta Biomater. 2012, 8, 3643–3651. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Tran, T.T.D. Mucoadhesive Formulation Designs for Oral Controlled Drug Release at the Colon. Curr. Pharm. Des. 2021, 27, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef]
- Husain, S.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Qasim, S.B. Chitosan Biomaterials for Current and Potential Dental Applications. Materials 2017, 10, 602. [Google Scholar] [CrossRef]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; El-Zairy, E.M.R. Chitosan as a biomaterial—Structure, properties, and electrospun nanofibers. Concepts Compd. Altern. Antibact. 2015, 1, 81–101. [Google Scholar]
- Gonzalez-Melo, C.; Garcia-Brand, A.J.; Quezada, V.; Reyes, L.H.; Muñoz-Camargo, C.; Cruz, J.C. Highly Efficient Synthesis of Type B Gelatin and Low Molecular Weight Chitosan Nanoparticles: Potential Applications as Bioactive Molecule Carriers and Cell-Penetrating Agents. Polymers 2021, 13, 4078. [Google Scholar] [CrossRef] [PubMed]
- Diolosà, M.; Donati, I.; Turco, G.; Cadenaro, M.; Di Lenarda, R.; Breschi, L.; Paoletti, S. Use of methacrylate-modified chitosan to increase the durability of dentine bonding systems. Biomacromolecules 2014, 15, 4606–4613. [Google Scholar] [CrossRef] [PubMed]
- Zapata, M.E.V.; Tovar, C.D.G.; Hernandez, J.H.M. The Role of Chitosan and Graphene Oxide in Bioactive and Antibacterial Properties of Acrylic Bone Cements. Biomolecules 2020, 10, 1616. [Google Scholar] [CrossRef]
- Shamekhi, M.A.; Mirzadeh, H.; Mahdavi, H.; Rabiee, A.; Mohebbi-Kalhori, D.; Baghaban, E.M. Graphene oxide containing chitosan scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2019, 127, 396–405. [Google Scholar] [CrossRef]
- Prakash, J.; Prema, D.; Venkataprasanna, K.S.; Balagangadharan, K.; Selvamurugan, N.; Venkatasubbu, G.D. Nanocomposite chitosan film containing graphene oxide/hydroxyapatite/gold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 154, 62–71. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Qin, J.; Siraleartmukul, K.; Siriwongrungson, V. Graphene Oxide/Silver Nanoparticle Coating Produced by Electrophoretic Deposition Improved the Mechanical and Tribological Properties of NiTi Alloy for Biomedical Applications. J. Nanosci. Nanotechnol. 2019, 19, 3804–3810. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Thunyakitpisal, P.; Qin, J.; Rosa, V.; Sapkota, J. Potential Applications of Graphene-Based Nanomaterials in Biomedical, Dental, and Implant Applications. Adv. Dent. Implantol. Nanomater. Allied Technol. Appl. 2021, 1, 77–105. [Google Scholar]
- Srimaneepong, V.; Rokaya, D.; Thunyakitpisal, P.; Qin, J.; Saengkiettiyut, K. Corrosion Resistance of Graphene oxide/Silver Coatings on Ni-Ti alloy and Expression of IL-6 and IL-8 in Human Oral Fibroblasts. Sci. Rep. 2020, 10, 3247. [Google Scholar] [CrossRef] [PubMed]
- Pipattanachat, S.; Qin, J.; Rokaya, D.; Thanyasrisung, P.; Srimaneepong, V. Biofilm inhibition and bactericidal activity of NiTi alloy coated with graphene oxide/silver nanoparticles via electrophoretic deposition. Sci Rep. 2021, 11, 14008. [Google Scholar] [CrossRef]
- Patarroyo, J.L.; Fonseca, E.; Cifuentes, J.; Salcedo, F.; Cruz, J.C.; Reyes, L.H. Gelatin-Graphene Oxide Nanocomposite Hydrogels for Kluyveromyces lactis Encapsulation: Potential Applications in Probiotics and Bioreactor Packings. Biomolecules 2021, 11, 922. [Google Scholar] [CrossRef] [PubMed]
- Raslan, A.; Saenz, D.B.L.; Ciriza, J.; Pedraz, J.L. Graphene oxide and reduced graphene oxide-based scaffolds in regenerative medicine. Int. J. Pharm. 2020, 580, 119226. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, H.; Huang, X.; Hang, R.; Zhang, X.; Wang, Y.; Yao, X. DLP printing photocurable chitosan to build bio-constructs for tissue engineering. Carbohydr. Polym. 2020, 235, 115970. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Rueda-Gensini, L.; Serna, J.A.; Cifuentes, J.; Cruz, J.C.; Muñoz-Camargo, C. Graphene Oxide-Embedded Extracellular Matrix- Derived Hydrogel as a Multiresponsive Platform for 3D Bioprinting Applications. Int. J. Bioprinting 2021, 7, 353. [Google Scholar] [CrossRef]
- Cedano, F.J.; Pinzón, L.M.; Narváez, D.M.; Castro, C.I.; Moreno-Serrano, C.L.; Tabima, D.M.; Salcedo, F.; Briceno, J.C.; Casas-Rodriguez, J.P. Evaluation of a water-resistant and biocompatible adhesive with potential use in bone fractures. J. Adhes. Sci. Technol. 2016, 31, 1480–1495. [Google Scholar] [CrossRef]
- Lopez-Barbosa, N.; Suárez-Arnedo, A.; Cifuentes, J.; Gonzalez Barrios, A.F.; Silvera Batista, C.A.; Osma, J.F.; Muñoz-Camargo, C.; Cruz, J.C. Magnetite OmpA Nanobioconjugates as Cell-Penetrating Vehicles with Endosomal Escape Abilities. ACS Biomater. Sci. Eng. 2019, 6, 415–424. [Google Scholar] [CrossRef]
- Li, S.; Sun, G.X.; Chang, S.M.; Yang, C.S.; Li, Y.; Niu, W.; Zhang, C. Simulated postoperative weight-bearing after fixation of a severe osteoporotic intertrochanteric fracture. Int. J. Clin. Exp. Med. 2017, 10, 8438–8448. [Google Scholar]
- Harper, E.J.; Bonfield, W. Tensile characteristics of ten commercial acrylic bone cements. J. Biomed. Mater. Res. 2002, 53, 605–616. [Google Scholar] [CrossRef]
- Sharma, K.; Kaith, B.S.; Kumar, V.; Kalia, S.; Kapur, B.K.; Swart, H.C. Corrigendum to: A comparative study of the effect of Ni9+ and Au8+ ion beams on the properties of poly(methacrylic acid) grafted gum ghatti films. Radiat. Phys. Chem. 2014, 99, 97. [Google Scholar] [CrossRef]
- Krylova, V.; Dukštienė, N. The structure of PA-Se-S-Cd composite materials probed with FTIR spectroscopy. Appl. Surf. Sci. 2019, 470, 462–471. [Google Scholar] [CrossRef]
- Coussot, P. Introduction to rheology and fluid mechanics. In Mudflow Rheology and Dynamics; Taylor & Francis, Routledge: Abington, UK, 2017; pp. 25–44. [Google Scholar]
- Banerjee, C.; Ghosh, S.; Sen, G.; Mishra, S.; Shukla, P.; Bandopadhyay, R. Study of algal biomass harvesting using cationic guar gum from the natural plant source as flocculant. Carbohydr. Polym. 2013, 92, 675–681. [Google Scholar] [CrossRef]
- Yoo, H.J.; Mahapatra, S.S.; Cho, J.W. High-Speed Actuation and Mechanical Properties of Graphene-Incorporated Shape Memory Polyurethane Nanofibers. J. Phys. Chem. C 2014, 118, 10408–10415. [Google Scholar] [CrossRef]
- Strankowski, M.; Włodarczyk, D.; Piszczyk, L.; Strankowska, J. Polyurethane Nanocomposites Containing Reduced Graphene Oxide FTIR Raman, and XRD Studies. J. Spectrosc. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.-J. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Mohan, R.; Kim, S.-J. Investigation of Raman and photoluminescence studies of reduced graphene oxide sheets. Appl. Phys. A 2011, 106, 501–506. [Google Scholar] [CrossRef]
- Eda, G.; Chhowalla, M. Chemically Derived Graphene Oxide: Towards Large-Area Thin-Film Electronics and Optoelectronics. Adv. Mater. 2010, 22, 2392–2415. [Google Scholar] [CrossRef]
- Muzyka, R.; Drewniak, S.; Pustelny, T.; Chrubasik, M.; Gryglewicz, G. Characterization of Graphite Oxide and Reduced Graphene Oxide Obtained from Different Graphite Precursors and Oxidized by Different Methods Using Raman Spectroscopy. Materials 2018, 11, 1050. [Google Scholar] [CrossRef] [PubMed]
- Arul, R.; Osterbeek, R.N.; Robertson, J.; Xu, G.; Jin, J.; Simpson, M.C. The mechanism of direct laser writing of graphene features into graphene oxide films involves photoreduction and thermally assisted structural rearrangement. Carbon 2016, 99, 423–431. [Google Scholar] [CrossRef]
- Feng, H.; Cheng, R.; Zhao, X.; Duan, X.; Li, J. A low-temperature method to produce highly reduced graphene oxide. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.L.; Wang, X.F.; Qian, Q.Y.; Wang, F.B.; Xia, X.H. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar]
- Song, M.; Yu, L.; Wu, Y. Simple Synthesis and Enhanced Performance of Graphene Oxide-Gold Composites. J. Nanomater. 2012, 2012, 37. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD TEM and electron spectroscopy methods. J. Electron. Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Aziz, M.; Halim, F.S.A.; Jaafar, J. Preparation and Characterization of Graphene Membrane Electrode Assembly. J. Technol. 2014, 146–152. [Google Scholar] [CrossRef]
- Paxton, N.; Smolan, W.; Böck, T.; Melchels, F.; Groll, J.; Jungst, T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 2017, 9, 044107. [Google Scholar] [CrossRef]
- Sandolo, C.; Matricardi, P.; Alhaique, F.; Coviello, T. Effect of temperature and cross-linking density on rheology of chemical cross-linked guar gum at the gel point. Food Hydrocoll. 2009, 23, 210–220. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, L.; Wei, S.; Zhai, M.; Li, J. Stimuli responsive deswelling of radiation synthesized collagen hydrogel in simulated physiological environment. J. Biomed. Mater. Res. A 2012, 101, 2191–2201. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Alonso-González, M.; Romero, A.; Perez-Puyana, V. Applied Rheology as Tool for the Assessment of Chitosan Hydrogels for Regenerative Medicine. Polymers 2021, 13, 2189. [Google Scholar] [CrossRef] [PubMed]
- Jafarigol, E.; Salehi, M.B.; Mortaheb, H.R. Preparation and assessment of electro-conductive poly(acrylamide-co-acrylic acid) carboxymethyl cellulose/reduced graphene oxide hydrogel with high viscoelasticity. Chem. Eng. Res. Des. 2020, 162, 74–84. [Google Scholar] [CrossRef]
- Sahraei, R.; Ghaemy, M. Synthesis of modified gum tragacanth/graphene oxide composite hydrogel for heavy metal ions removal and preparation of silver nanocomposite for antibacterial activity. Carbohydr. Polym. 2017, 157, 823–833. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S.; El-Sakhawy, M.; Kamel, S. Carboxymethyl Cellulose-Grafted Graphene Oxide/Polyethylene Glycol for Efficient Ni(II) Adsorption. J. Polym. Environ. 2020, 29, 859–870. [Google Scholar] [CrossRef]
- Tuğcu-Demiröz, F. Development of in situ poloxamer-chitosan hydrogels for vaginal drug delivery of benzydamine hydrochloride: Textural mucoadhesive and in vitro release properties. Marmara Pharm. J. 2017, 21, 762–770. [Google Scholar] [CrossRef]
- Kakkar, P.; Madhan, B. Fabrication of keratin-silica hydrogel for biomedical applications. Mater. Sci. Eng. 2016, 66, 178–184. [Google Scholar] [CrossRef]
- Karavana, S.Y.H.; Güneri, P.; Ertan, G. Benzydamine hydrochloride buccal bioadhesive gels designed for oral ulcers: Preparation rheological, textural, mucoadhesive and release properties. Pharm. Dev. Technol. 2009, 14, 623–631. [Google Scholar] [CrossRef]
- Da Silva, J.B.; Ferreira, S.; Reis, A.; Cook, M.; Bruschi, M. Assessing Mucoadhesion in Polymer Gels: The Effect of Method Type and Instrument Variables. Polymers 2018, 10, 254. [Google Scholar] [CrossRef]
- Sezer, A.D.; Cevher, E.; Hatipoğlu, F.; Oğurtan, Z.; Baş, A.L.; Akbuğa, J. Preparation of Fucoidan-Chitosan Hydrogel and Its Application as Burn Healing Accelerator on Rabbits. Biol. Pharm. Bull. 2008, 31, 2326–2333. [Google Scholar] [CrossRef]
- Figueroa, T.; Aguayo, C.; Fernandez, K. Design and Characterization of Chitosan-Graphene Oxide Nanocomposites for the Delivery of Proanthocyanidins. Int. J. Nanomed. 2020, 15, 1229–1238. [Google Scholar] [CrossRef]
- Vallabani, N.V.; Mittal, S.; Shukla, R.K.; Pandey, A.K.; Dhakate, S.R.; Pasricha, R.; Dhawan, A. Toxicity of Graphene in Normal Human Lung Cells (BEAS-2B). J. Biomed. Nanotechnol. 2011, 7, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Sharif, P.S.; Abdollahi, M. The Role of Platelets in Bone Remodeling. Inflamm. Allergy Drug Targets 2010, 9, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Nurden, A.T. Platelets and wound healing. Front. Biosci. 2008, 13, 3525. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C.; Fu, E.; Wu, C.-J.; Yeh, J.-H. Chitosan enhances platelet adhesion and aggregation. Biochem. Biophys. Res. Commun. 2003, 302, 480–483. [Google Scholar] [CrossRef]
- Blinc, A.; Božič, M.; Vengust, R.; Stegnar, M. Methyl-methacrylate bone cement surface does not promote platelet aggregation or plasma coagulation in vitro. Thromb. Res. 2004, 114, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Flieger, M.; Bandouchova, H.; Cerny, J.; Chudíčková, M.; Kolarik, M.; Kovacova, V.; Martínková, N.; Novák, P.; Šebesta, O.; Stodůlková, E. Vitamin B2 as a virulence factor in Pseudogymnoascus destructans skin infection. Sci. Rep. 2016, 6, 33200. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Riancho, J.A. Mecanobiología celular y molecular del tejido óseo. Rev. Osteoporos. Metab. Miner. 2013, 5, 51–56. [Google Scholar] [CrossRef]
- Yan, L.; Lim, J.L.; Lee, J.W.; Tia, C.S.H.; O’Neill, G.K.; Chong, D.Y.R. Finite element analysis of bone and implant stresses for customized 3D-printed orthopaedic implants in fracture fixation. Med. Biol. Eng. Comput. 2020, 58, 921–931. [Google Scholar] [CrossRef] [PubMed]






| Sample | Peak | BE (eV) | FWHM | Corrected Area RSF | Ratio (%) |
|---|---|---|---|---|---|
| ChiMA | N1s | 398.83 399.55 400.98 | 1.08 1.04 1.84 | 842 | 6.10 |
| C1s | 284.57 286.07 287.78 | 1.16 1.43 1.31 | 8594 | 62.28 | |
| O1s | 531.58 532.44 | 1.36 1.4 | 4835 | 31.62 | |
| ChiMA 1:1 | N1s | 398.78 399.42 401.09 | 1.25 1.34 2.19 | 698 | 6.61 |
| C1s | 284.56 285.97 287.57 | 1.28 1.41 1.29 | 5884 | 55.71 | |
| O1s | 530.93 532.16 | 1.89 1.51 | 3980 | 37.68 | |
| ChiMA 1:2 | N1s | 398.91 399.53 401.45 | 1.46 1.63 1.43 | 728 | 7.59 |
| C1s | 284.67 286.21 287.82 | 1.42 1.42 1.49 | 5390 | 56.17 | |
| O1s | 531.62 532.53 | 1.85 1.41 | 3478 | 36.24 | |
| ChiMA 1:4 | N1s | 398.69 399.41 401.22 | 1.43 1.36 1.69 | 864 | 7.36 |
| C1s | 284.54 286.10 287.68 | 1.43 1.41 1.33 | 6827 | 58.16 | |
| O1s | 531.35 532.39 | 1.78 1.41 | 4048 | 34.48 |
| ChiMA 1:1 | ChiMA 1:2 | ChiMA 1:4 | |
|---|---|---|---|
| Carbonyl sub-peak (D%) | 24 | 30 | 16 |
| Amide sub-peak (D%) | 17 | 21 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Céspedes-Valenzuela, D.N.; Sánchez-Rentería, S.; Cifuentes, J.; Gantiva-Diaz, M.; Serna, J.A.; Reyes, L.H.; Ostos, C.; Cifuentes-De la Portilla, C.; Muñoz-Camargo, C.; Cruz, J.C. Preparation and Characterization of an Injectable and Photo-Responsive Chitosan Methacrylate/Graphene Oxide Hydrogel: Potential Applications in Bone Tissue Adhesion and Repair. Polymers 2022, 14, 126. https://doi.org/10.3390/polym14010126
Céspedes-Valenzuela DN, Sánchez-Rentería S, Cifuentes J, Gantiva-Diaz M, Serna JA, Reyes LH, Ostos C, Cifuentes-De la Portilla C, Muñoz-Camargo C, Cruz JC. Preparation and Characterization of an Injectable and Photo-Responsive Chitosan Methacrylate/Graphene Oxide Hydrogel: Potential Applications in Bone Tissue Adhesion and Repair. Polymers. 2022; 14(1):126. https://doi.org/10.3390/polym14010126
Chicago/Turabian StyleCéspedes-Valenzuela, Daniela N., Santiago Sánchez-Rentería, Javier Cifuentes, Mónica Gantiva-Diaz, Julian A. Serna, Luis H. Reyes, Carlos Ostos, Christian Cifuentes-De la Portilla, Carolina Muñoz-Camargo, and Juan C. Cruz. 2022. "Preparation and Characterization of an Injectable and Photo-Responsive Chitosan Methacrylate/Graphene Oxide Hydrogel: Potential Applications in Bone Tissue Adhesion and Repair" Polymers 14, no. 1: 126. https://doi.org/10.3390/polym14010126
APA StyleCéspedes-Valenzuela, D. N., Sánchez-Rentería, S., Cifuentes, J., Gantiva-Diaz, M., Serna, J. A., Reyes, L. H., Ostos, C., Cifuentes-De la Portilla, C., Muñoz-Camargo, C., & Cruz, J. C. (2022). Preparation and Characterization of an Injectable and Photo-Responsive Chitosan Methacrylate/Graphene Oxide Hydrogel: Potential Applications in Bone Tissue Adhesion and Repair. Polymers, 14(1), 126. https://doi.org/10.3390/polym14010126












