Populus alba L., an Autochthonous Species of Spain: A Source for Cellulose Nanofibers by Chemical Pretreatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Bleached Pulp Production
2.3. CNF Production
2.4. Pulp and CNF Characterization
2.4.1. Chemical Composition
2.4.2. Fibrillation Yield
2.4.3. Optical Transmittance
2.4.4. Polymerization Degree
2.4.5. Carboxylate Content
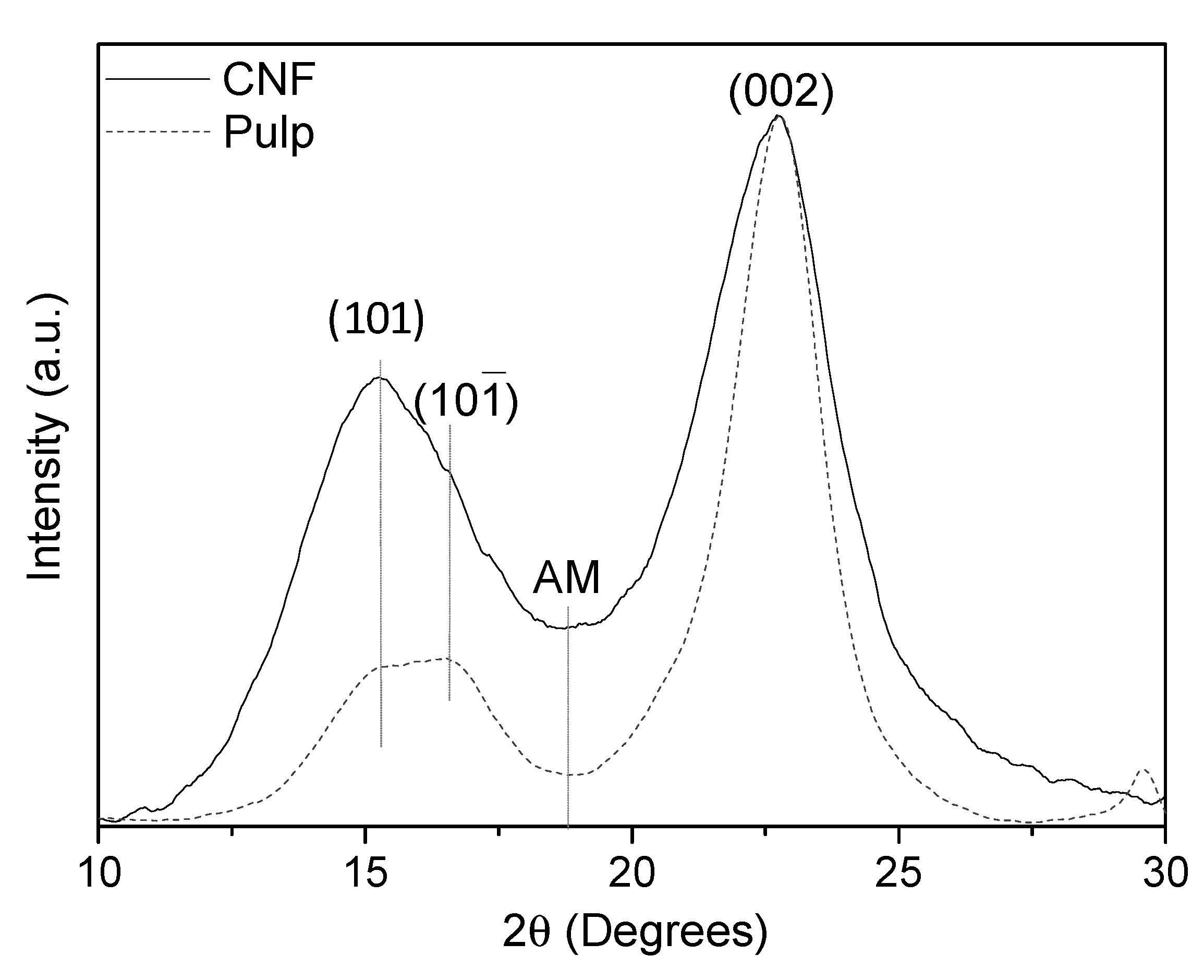
2.4.6. X-ray Diffraction (XRD)
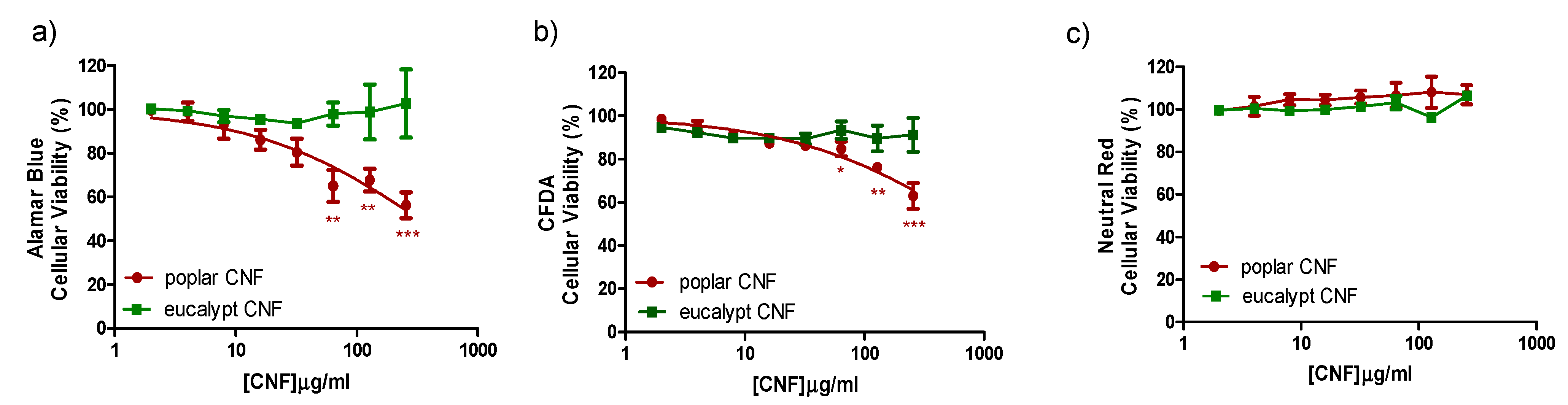
2.4.7. Cytotoxicity Analysis
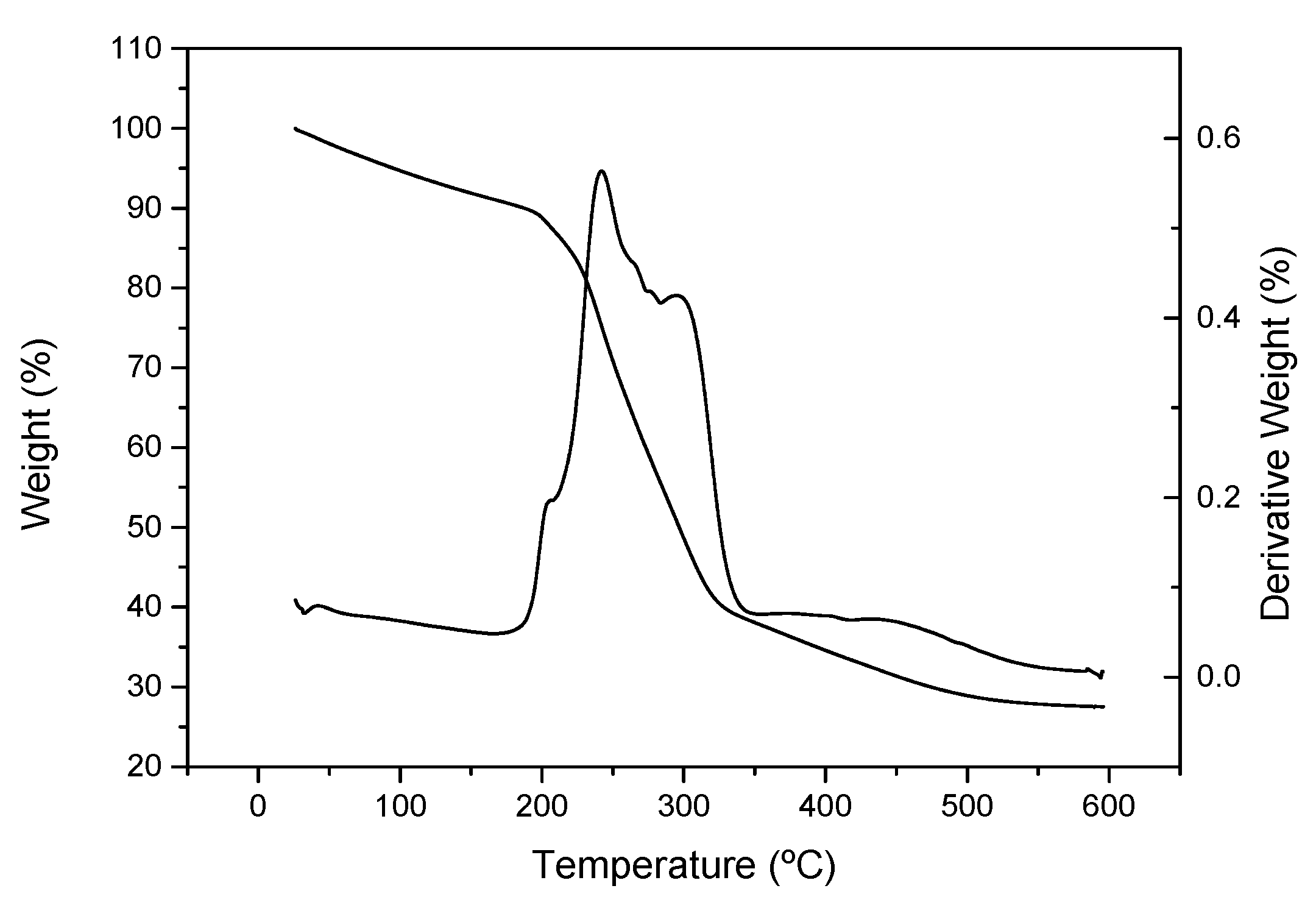
2.4.8. Thermogravimetric Analysis (TGA)
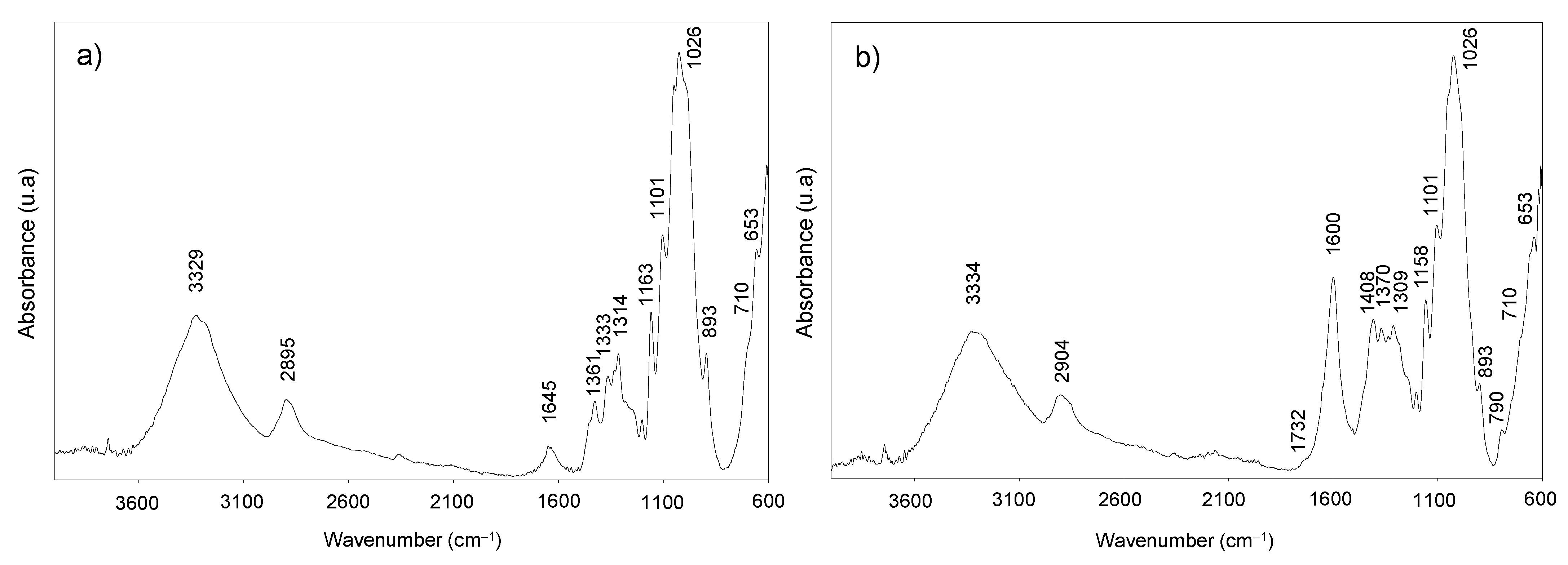
2.4.9. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4.10. Zeta Potential and Particle Size
2.4.11. Rheological Characterization
3. Results and Discussion
3.1. Poplar CNF Composition
3.2. Poplar CNF Characterization
3.3. Cytotoxicity Assays of Poplar CNF
3.4. CNF Zeta Potential, Particle Size and Rheological Properties of Poplar CNF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arevalo-Gallegos, A.; Ahmad, Z.; Asgher, M.; Parra-Saldivar, R.; Iqbal, H. Lignocellulose: A sustainable material to produce value-added products with a zero waste approach. A review. Int. J. Biol. Macromol. 2017, 99, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Sixto, H.; Cañellas, I.; van Arendonk, J.; Ciria, P.; Camps, F.; Sánchez, M.; Sánchez-González, M. Growth potential of different species and genotypes for biomass production in short rotation in Mediterranean environments. For. Ecol. Manag. 2015, 354, 291–299. [Google Scholar] [CrossRef]
- Oliveira, N.; Pérez-Cruzado, C.; Cañellas, I.; Rodríguez-Soalleiro, R.; Sixto, H. Poplar Short Rotation Coppice Plantations under Mediterranean Conditions: The Case of Spain. Forests 2020, 11, 1352. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Ragauskas, A.J. Poplar as a feedstock for biofuels: A review of compositional characteristics. Biofuels Bioprod. Biorefining 2010, 4, 209–226. [Google Scholar] [CrossRef]
- Ibarra, D.; Eugenio, M.E.; Cañellas, I.; Sixto, H.; Martín-Sampedro, R. Potential of different poplar clones for sugar production. Wood Sci. Technol. 2017, 51, 669–684. [Google Scholar] [CrossRef]
- Jiménez-López, L.; Martín-Sampedro, R.; Eugenio, M.E.; Santos, J.I.; Sixto, H.; Cañellas, I.; Ibarra, D. Co-production of soluble sugars and lignin from short rotation white poplar and black locust crops. Wood Sci. Technol. 2020, 54, 1617–1643. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, L.; Gleisner, R.; Zhu, J.Y. Co-production of bioethanol and furfural from poplar wood via low temperature (≤90 °C) acid hydrotropic fractionation (AHF). Fuel 2019, 254, 115572. [Google Scholar] [CrossRef]
- Dalli, S.S.; da Silva, S.S.; Uprety, B.K.; Rakshit, S.K. Enhanced Production of Xylitol from Poplar Wood Hydrolysates Through a Sustainable Process Using Immobilized New Strain Candida tropicalis UFMG BX 12-a. Appl. Biochem. Biotechnol. 2017, 182, 1053–1064. [Google Scholar] [CrossRef]
- Bonyadi, Z.; Kumar, P.S.; Foroutan, R.; Kafaei, R.; Arfaeinia, H.; Farjadfard, S.; Ramavandi, B. Ultrasonic-assisted synthesis of Populus alba activated carbon for water defluorination: Application for real wastewater. Korean J. Chem. Eng. 2019, 36, 1595–1603. [Google Scholar] [CrossRef]
- Wang, S.; Shuai, L.; Saha, B.; Vlachos, D.G.; Epps, T.H. From Tree to Tape: Direct Synthesis of Pressure Sensitive Adhesives from Depolymerized Raw Lignocellulosic Biomass. ACS Cent. Sci. 2018, 4, 701–708. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L.; et al. Developing fibrillated cellulose as a sustainable technological material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef]
- Thakur, V.; Guleria, A.; Kumar, S.; Sharma, S.; Singh, K. Recent advances in nanocellulose processing, functionalization and applications: A review. Mater. Adv. 2021, 2, 1872–1895. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Khiari, R.; Rol, F.; Brochier Salon, M.-C.; Bras, J.; Belgacem, M.N. Efficiency of Cellulose Carbonates to Produce Cellulose Nanofibers. ACS Sustain. Chem. Eng. 2019, 7, 8155–8167. [Google Scholar] [CrossRef]
- Rivadeneyra, A.; Marín-Sánchez, A.; Wicklein, B.; Salmerón, J.F.; Castillo, E.; Bobinger, M.; Salinas-Castillo, A. Cellulose nanofibers as substrate for flexible and biodegradable moisture sensors. Compos. Sci. Technol. 2021, 208, 108738. [Google Scholar] [CrossRef]
- Wicklein, B.; Diem, A.M.; Knöller, A.; Cavalcante, M.S.; Bergström, L.; Bill, J.; Burghard, Z. Dual-Fiber Approach toward Flexible Multifunctional Hybrid Materials. Adv. Funct. Mater. 2018, 28, 1704274. [Google Scholar] [CrossRef]
- Jiang, F.; Kondo, T.; Hsieh, Y.-L. Rice Straw Cellulose Nanofibrils via Aqueous Counter Collision and Differential Centrifugation and Their Self-Assembled Structures. ACS Sustain. Chem. Eng. 2016, 4, 1697–1706. [Google Scholar] [CrossRef]
- Shinoda, R.; Saito, T.; Okita, Y.; Isogai, A. Relationship between Length and Degree of Polymerization of TEMPO-Oxidized Cellulose Nanofibrils. Biomacromolecules 2012, 13, 842–849. [Google Scholar] [CrossRef]
- Isogai, A. Wood nanocelluloses: Fundamentals and applications as new bio-based nanomaterials. J. Wood Sci. 2013, 59, 449–459. [Google Scholar] [CrossRef]
- Saito, T.; Nishiyama, Y.; Putaux, J.-L.; Vignon, M.; Isogai, A. Homogeneous Suspensions of Individualized Microfibrils from TEMPO-Catalyzed Oxidation of Native Cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef]
- Fillat, Ú.; Wicklein, B.; Martín-Sampedro, R.; Ibarra, D.; Ruiz-Hitzky, E.; Valencia, C.; Sarrión, A.; Castro, E.; Eugenio, M.E. Assessing cellulose nanofiber production from olive tree pruning residue. Carbohydr. Polym. 2018, 179, 252–261. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, H.; Xu, D.; He, Z.; Pan, X.; Gui, S.; Dai, X.; Fan, J.; Dong, X.; Li, Y. Screening of Nanocellulose from Different Biomass Resources and Its Integration for Hydrophobic Transparent Nanopaper. Molecules 2020, 25, 227. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Du, J.; Chen, W.; Pan, M.; Chen, D. Preparation and thermostability of cellulose nanocrystals and nanofibrils from two sources of biomass: Rice straw and poplar wood. Cellulose 2019, 26, 8625–8643. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, M.; Wu, X.; Shi, T.; Chen, H.; Wang, H. Preparation of Nanocellulose Aerogel from the Poplar (Populus tomentosa) Catkin Fiber. Forests 2019, 10, 749. [Google Scholar] [CrossRef] [Green Version]
- Ibarra, D.; Martín-Sampedro, R.; Wicklein, B.; Fillat, Ú.; Eugenio, M.E. Production of Microfibrillated Cellulose from Fast-Growing Poplar and Olive Tree Pruning by Physical Pretreatment. Appl. Sci. 2021, 11, 6445. [Google Scholar] [CrossRef]
- Sixto, H.; Hernández, M.J.; Ciria, P.; Carrasco, J.E.; Cañellas, I. Manual de Cultivo de Populus spp. Para la Producción de Biomasa con Fines Energéticos; Monografias INIA: Madrid, Spain, 2010. [Google Scholar]
- Kang, K.Y.; Jo, B.M.; Oh, J.S.; Mansfield, S.D. The effects of biopulping on chemical and energy consumptionduring Kraft pulping of hybrid poplar. Wood Fiber Sci. 2003, 35, 594–600. [Google Scholar]
- Ibarra, D.; Camarero, S.; Romero, J.; Martínez, M.J.; Martínez, A.T. Integrating laccase–mediator treatment into an industrial-type sequence for totally chlorine-free bleaching of eucalypt kraft pulp. J. Chem. Technol. Biotechnol. 2006, 81, 1159–1165. [Google Scholar] [CrossRef]
- NREL P-510-42618-Determination of Structural Carbohydrates and Lignin in Biomass; Laboratory Analytical Procedure; National Renewable Energy Laboratory: Golden, CO, USA, 2011.
- Besbes, I.; Alila, S.; Boufi, S. Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: Effect of the carboxyl content. Carbohydr. Polym. 2011, 84, 975–983. [Google Scholar] [CrossRef]
- Kasaai, M.R. Comparison of various solvents for determination of intrinsic viscosity and viscometric constants for cellulose. J. Appl. Polym. Sci. 2002, 86, 2189–2193. [Google Scholar] [CrossRef]
- Fras, L.; Stana-Kleinschek, K.; Ribitsch, V.; Sfiligoj-Smole, M.; Kreze, T. Quantitative Determination Of Carboxyl Groups In Cellulose Polymers Utilizing Their Ion Exchange Capacity And Using A Complexometric Titration. Mater. Res. Innov. 2004, 8, 145–146. [Google Scholar] [CrossRef]
- Jensen, K.A.; Kembouche, Y.; Christiansen, E.; Jacobsen, N.; Wallin, H.; Guiot, C.; Spalla, O. Final Protocol for Producing Suitable Manufactured Nanomaterial Exposure Media—Report The generic NANOGENOTOX Dispersion Protocol—Standard Operation Procedure (SOP) and Background Documentation. July 2011. Available online: https://www.anses.fr/en/system/files/nanogenotox_deliverable_5.pdf (accessed on 1 November 2021).
- Lammel, T.; Boisseaux, P.; Fernández-Cruz, M.-L.; Navas, J.M. Internalization and cytotoxicity of graphene oxide and carboxyl graphene nanoplatelets in the human hepatocellular carcinoma cell line Hep G2. Part. Fibre Toxicol. 2013, 10, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lammel, T.; Navas, J.M. Graphene nanoplatelets spontaneously translocate into the cytosol and physically interact with cellular organelles in the fish cell line PLHC-1. Aquat. Toxicol. 2014, 150, 55–65. [Google Scholar] [CrossRef]
- Dayeh, V.R.; Bols, N.C.; Tanneberger, K.; Schirmer, K.; Lee, L.E.J. The Use of Fish-Derived Cell Lines for Investigation of Environmental Contaminants: An Update Following OECD’s Fish Toxicity Testing Framework No. 171. Curr. Protoc. Toxicol. 2013, 56, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Martín-Davison, J.S.; Ballesteros, M.; Manzanares, P.; Sepúlveda, X.P.-B.; Vergara-Fernández, A. Effects of Temperature on Steam Explosion Pretreatment of Poplar Hybrids with Different Lignin Contents in Bioethanol Production. Int. J. Green Energy 2015, 12, 832–842. [Google Scholar] [CrossRef]
- Chaker, A.; Alila, S.; Mutjé, P.; Vilar, M.R.; Boufi, S. Key role of the hemicellulose content and the cell morphology on the nanofibrillation effectiveness of cellulose pulps. Cellulose 2013, 20, 2863–2875. [Google Scholar] [CrossRef]
- Rojo, E.; Peresin, M.S.; Sampson, W.W.; Hoeger, I.C.; Vartiainen, J.; Laine, J.; Rojas, O.J. Comprehensive elucidation of the effect of residual lignin on the physical, barrier, mechanical and surface properties of nanocellulose films. Green Chem. 2015, 17, 1853–1866. [Google Scholar] [CrossRef] [Green Version]
- Fall, A.B.; Burman, A.; Wågberg, L. Cellulosic nanofibrils from eucalyptus, acacia and pine fibers. Nord. Pulp Pap. Res. J. 2014, 29, 176–184. [Google Scholar] [CrossRef]
- Solala, I.; Iglesias, M.C.; Peresin, M.S. On the potential of lignin-containing cellulose nanofibrils (LCNFs): A review on properties and applications. Cellulose 2020, 27, 1853–1877. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, M.; Espinosa, E.; Bascón-Villegas, I.; Pérez-Rodríguez, F.; Carrasco, E.; Rodríguez, A. Production of Cellulose Nanofibers from Olive Tree Harvest—A Residue with Wide Applications. Agronomy 2020, 10, 696. [Google Scholar] [CrossRef]
- Rajan, K.; Djioleu, A.; Kandhola, G.; Labbé, N.; Sakon, J.; Carrier, D.J.; Kim, J.-W. Investigating the effects of hemicellulose pre-extraction on the production and characterization of loblolly pine nanocellulose. Cellulose 2020, 27, 3693–3706. [Google Scholar] [CrossRef]
- Jiménez-López, L.; Eugenio, M.E.; Ibarra, D.; Darder, M.; Martín, J.A.; Martín-Sampedro, R. Cellulose nanofibers from a dutch elm disease-resistant ulmus minor clone. Polymers 2020, 12, 2450. [Google Scholar] [CrossRef]
- Balea, A.; Merayo, N.; Fuente, E.; Delgado-Aguilar, M.; Mutje, P.; Blanco, A.; Negro, C. Valorization of corn stalk by the production of cellulose nanofibers to improve recycled paper properties. BioResources 2016, 11, 3416–3431. [Google Scholar] [CrossRef] [Green Version]
- Okita, Y.; Saito, T.; Isogai, A. TEMPO-mediated oxidation of softwood thermomechanical pulp. Holzforschung 2009, 63, 529–535. [Google Scholar] [CrossRef]
- Espinosa, E.; Arrebola, R.I.; Bascón-Villegas, I.; Sánchez-Gutiérrez, M.; Domínguez-Robles, J.; Rodríguez, A. Industrial application of orange tree nanocellulose as papermaking reinforcement agent. Cellulose 2020, 27, 10781–10797. [Google Scholar] [CrossRef]
- Delgado-Aguilar, M.; González Tovar, I.; Tarrés, Q.; Alcalá, M.; Pèlach, M.À.; Mutjé, P. Approaching a Low-Cost Production of Cellulose Nanofibers for Papermaking Applications. BioResources 2015, 10, 5345–5355. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr. Polym. 2013, 95, 32–40. [Google Scholar] [CrossRef]
- Liu, C.; Li, B.; Du, H.; Lv, D.; Zhang, Y.; Yu, G.; Mu, X.; Peng, H. Properties of nanocellulose isolated from corncob residue using sulfuric acid, formic acid, oxidative and mechanical methods. Carbohydr. Polym. 2016, 151, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Montanari, S.; Roumani, M.; Heux, L.; Vignon, M.R. Topochemistry of Carboxylated Cellulose Nanocrystals Resulting from TEMPO-Mediated Oxidation. Macromolecules 2005, 38, 1665–1671. [Google Scholar] [CrossRef]
- Tanaka, A.; Seppänen, V.; Houni, J.; Sneck, A.; Pirkonen, P. BIOREFINERY. Nanocellulose characterization with mechanical fractionation. Nord. Pulp Pap. Res. J. 2012, 27, 689–694. [Google Scholar] [CrossRef]
- Rantuch, P.; Chrebet, T. Thermal decomposition of cellulose insulation. Cellul. Chem. Technol. 2014, 48, 461–467. [Google Scholar]
- Gaspar, D.; Fernandes, S.N.; de Oliveira, A.G.; Fernandes, J.G.; Grey, P.; Pontes, R.V.; Pereira, L.; Martins, R.; Godinho, M.H.; Fortunato, E. Nanocrystalline cellulose applied simultaneously as the gate dielectric and the substrate in flexible field effect transistors. Nanotechnology 2014, 25, 94008. [Google Scholar] [CrossRef] [Green Version]
- Lichtenstein, K.; Lavoine, N. Toward a deeper understanding of the thermal degradation mechanism of nanocellulose. Polym. Degrad. Stab. 2017, 146, 53–60. [Google Scholar] [CrossRef]
- Tsuboi, M. Infrared spectrum and crystal structure of cellulose. J. Polym. Sci. 1957, 25, 159–171. [Google Scholar] [CrossRef]
- Indarti, E.; Marwan; Rohaizu, R.; Wanrosli, W.D. Silylation of TEMPO oxidized nanocellulose from oil palm empty fruit bunch by 3-aminopropyltriethoxysilane. Int. J. Biol. Macromol. 2019, 135, 106–112. [Google Scholar] [CrossRef]
- Sugiyama, J.; Persson, J.; Chanzy, H. Combined infrared and electron diffraction study of the polymorphism of native celluloses. Macromolecules 1991, 24, 2461–2466. [Google Scholar] [CrossRef]
- Fujisawa, S.; Okita, Y.; Fukuzumi, H.; Saito, T.; Isogai, A. Preparation and characterization of TEMPO-oxidized cellulose nanofibril films with free carboxyl groups. Carbohydr. Polym. 2011, 84, 579–583. [Google Scholar] [CrossRef]
- Xue, Y.; Mou, Z.; Xiao, H. Nanocellulose as a sustainable biomass material: Structure, properties, present status and future prospects in biomedical applications. Nanoscale 2017, 9, 14758–14781. [Google Scholar] [CrossRef] [PubMed]
- Vartiainen, J.; Pöhler, T.; Sirola, K.; Pylkkänen, L.; Alenius, H.; Hokkinen, J.; Tapper, U.; Lahtinen, P.; Kapanen, A.; Putkisto, K.; et al. Health and environmental safety aspects of friction grinding and spray drying of microfibrillated cellulose. Cellulose 2011, 18, 775–786. [Google Scholar] [CrossRef]
- Lopes, V.R.; Sanchez-Martinez, C.; Strømme, M.; Ferraz, N. In vitro biological responses to nanofibrillated cellulose by human dermal, lung and immune cells: Surface chemistry aspect. Part. Fibre Toxicol. 2017, 14, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandrescu, L.; Syverud, K.; Gatti, A.; Chinga-Carrasco, G. Cytotoxicity tests of cellulose nanofibril-based structures. Cellulose 2013, 20, 1765–1775. [Google Scholar] [CrossRef]
- Carneiro-Da-Cunha, M.G.; Cerqueira, M.A.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Influence of concentration, ionic strength and pH on zeta potential and mean hydrodynamic diameter of edible polysaccharide solutions envisaged for multinanolayered films production. Carbohydr. Polym. 2011, 85, 522–528. [Google Scholar] [CrossRef] [Green Version]
- Valo, H.; Arola, S.; Laaksonen, P.; Torkkeli, M.; Peltonen, L.; Linder, M.B.; Serimaa, R.; Kuga, S.; Hirvonen, J.; Laaksonen, T. Drug release from nanoparticles embedded in four different nanofibrillar cellulose aerogels. Eur. J. Pharm. Sci. 2013, 50, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Fall, A.B.; Lindström, S.B.; Sundman, O.; Ödberg, L.; Wågberg, L. Colloidal stability of aqueous nanofibrillated cellulose dispersions. Langmuir 2011, 27, 11332–11338. [Google Scholar] [CrossRef] [PubMed]
- Qua, E.H.; Hornsby, P.R.; Sharma, H.S.S.; Lyons, G. Preparation and characterisation of cellulose nanofibres. J. Mater. Sci. 2011, 46, 6029–6045. [Google Scholar] [CrossRef]
- Hujaya, S.D.; Lorite, G.S.; Vainio, S.J.; Liimatainen, H. Polyion complex hydrogels from chemically modified cellulose nanofibrils: Structure-function relationship and potential for controlled and pH-responsive release of doxorubicin. Acta Biomater. 2018, 75, 346–357. [Google Scholar] [CrossRef]
- Aaen, R.; Simon, S.; Wernersson Brodin, F.; Syverud, K. The potential of TEMPO-oxidized cellulose nanofibrils as rheology modifiers in food systems. Cellulose 2019, 26, 5483–5496. [Google Scholar] [CrossRef]
- Costa, V.L.D.; Costa, A.P.; Simões, R.M.S. Nanofibrillated cellulose rheology: Effects of morphology, ethanol/acetone addition, and high NaCl concentration. BioResources 2019, 14, 7636–7654. [Google Scholar] [CrossRef]
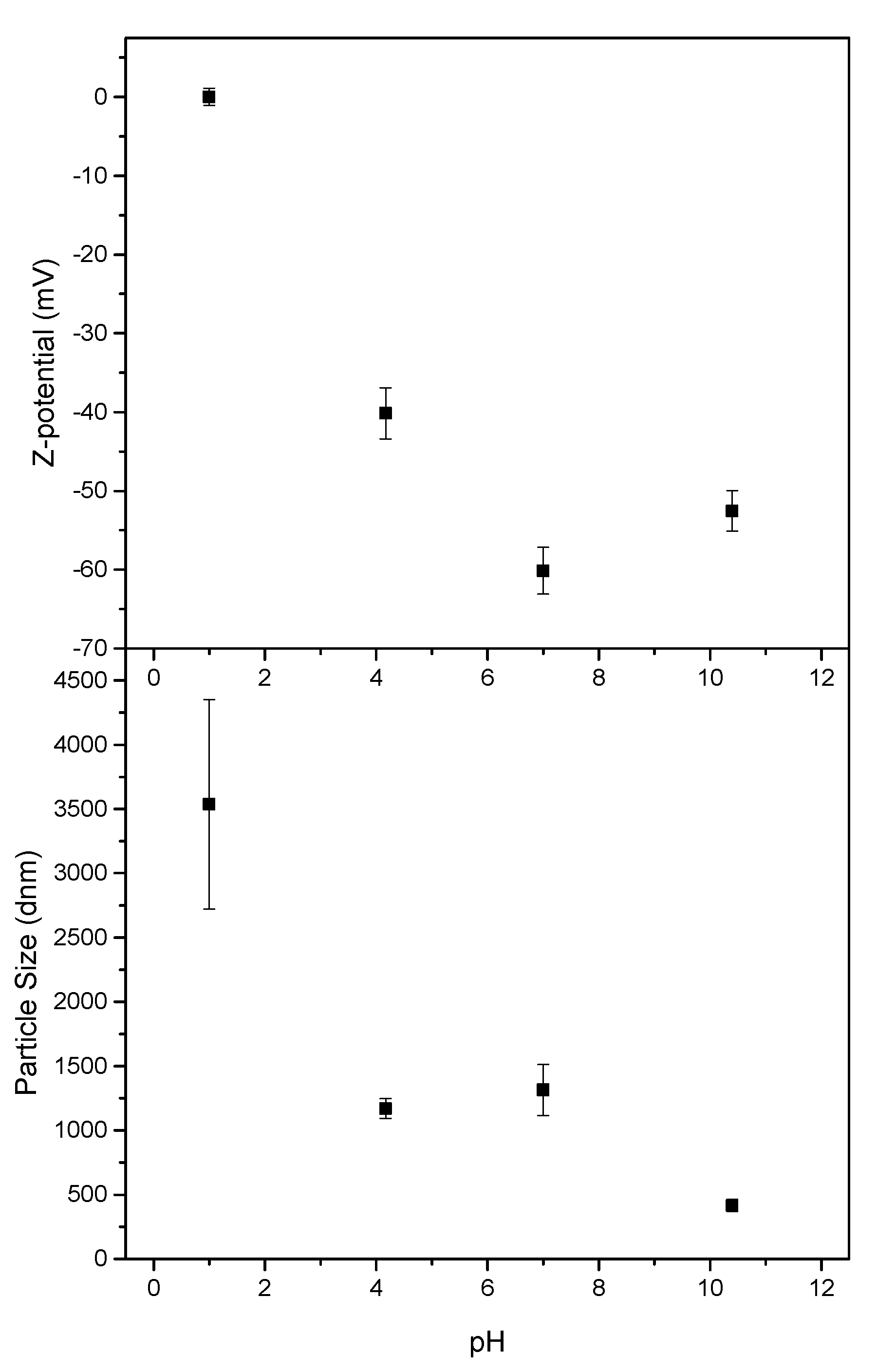
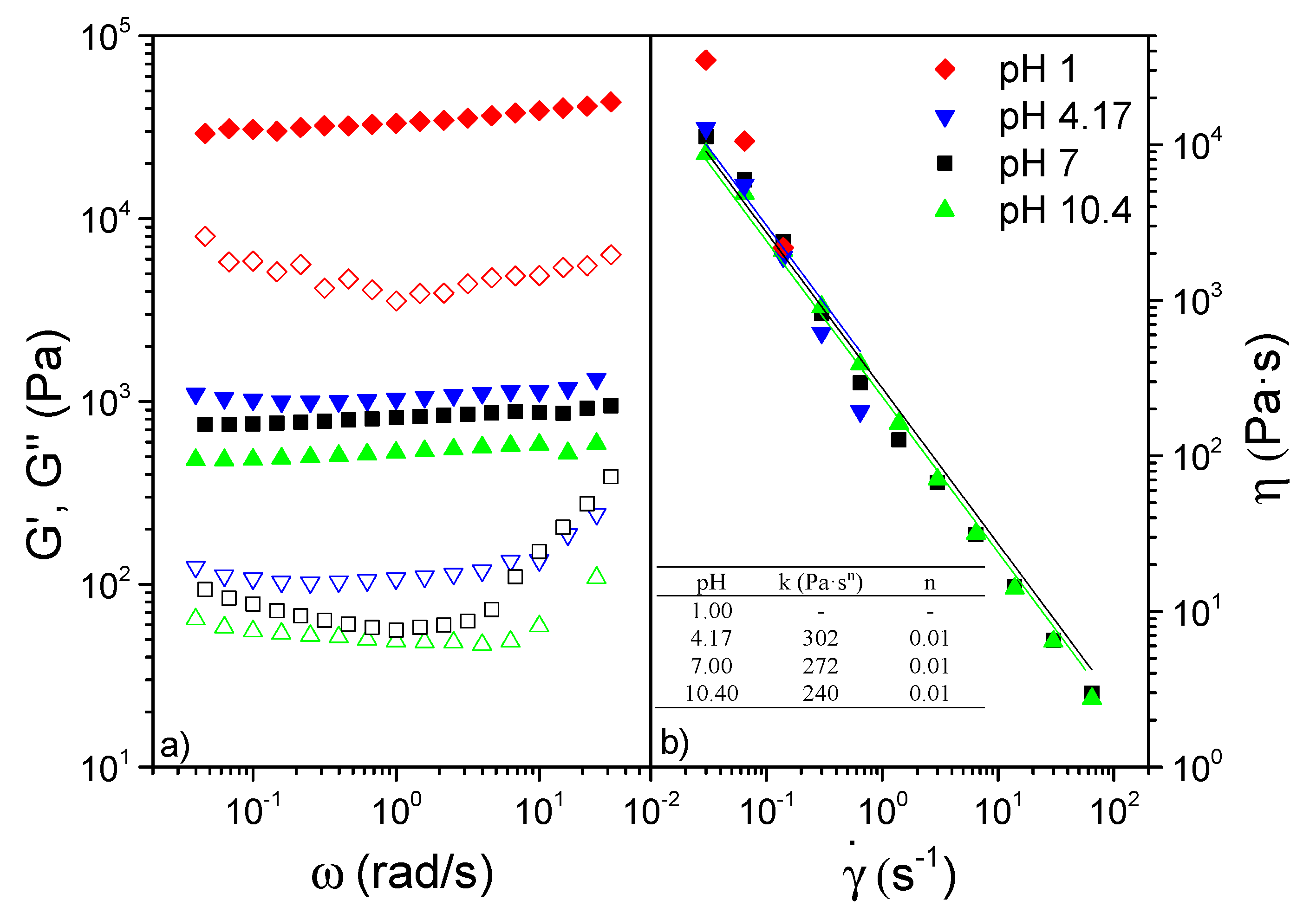
| Extractives (% o.d.p.) | Klason Lignin (% o.d.p.) | Soluble Lignin (% o.d.p.) | Glucans (% o.d.p.) | Xylans (% o.d.p.) | |
|---|---|---|---|---|---|
| P. alba L. “PO-10-10-20” | 6.3 ± 0.5 | 18.7 ± 0.1 | 4.0 ± 0.2 | 39.7 ± 0.4 | 22.7 ± 0.2 |
| Bleached pulp | - | 0.8 ± 0.6 | 0.4 ± 0.3 | 74.0 ± 2.1 | 24.0 ± 1.9 |
| CNF | - | 0.4 ± 0.3 | 0.2 ± 0.1 | 76.2 ± 1.7 | 16.0 ± 1.9 |
| Tonset (°C) | Tmax (°C) | Tfinal (°C) | ∆W (%) | Residue (%) | |
|---|---|---|---|---|---|
| Poplar CNF | 218/270 | 242/295 | 256/320 | 32/40 | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibarra, D.; Martín-Sampedro, R.; Wicklein, B.; Borrero-López, A.M.; Valencia, C.; Valdehíta, A.; Navas, J.M.; Eugenio, M.E. Populus alba L., an Autochthonous Species of Spain: A Source for Cellulose Nanofibers by Chemical Pretreatment. Polymers 2022, 14, 68. https://doi.org/10.3390/polym14010068
Ibarra D, Martín-Sampedro R, Wicklein B, Borrero-López AM, Valencia C, Valdehíta A, Navas JM, Eugenio ME. Populus alba L., an Autochthonous Species of Spain: A Source for Cellulose Nanofibers by Chemical Pretreatment. Polymers. 2022; 14(1):68. https://doi.org/10.3390/polym14010068
Chicago/Turabian StyleIbarra, David, Raquel Martín-Sampedro, Bernd Wicklein, Antonio M. Borrero-López, Concepción Valencia, Ana Valdehíta, José M. Navas, and María E. Eugenio. 2022. "Populus alba L., an Autochthonous Species of Spain: A Source for Cellulose Nanofibers by Chemical Pretreatment" Polymers 14, no. 1: 68. https://doi.org/10.3390/polym14010068
APA StyleIbarra, D., Martín-Sampedro, R., Wicklein, B., Borrero-López, A. M., Valencia, C., Valdehíta, A., Navas, J. M., & Eugenio, M. E. (2022). Populus alba L., an Autochthonous Species of Spain: A Source for Cellulose Nanofibers by Chemical Pretreatment. Polymers, 14(1), 68. https://doi.org/10.3390/polym14010068








