Biodegradable Hydrogel Scaffolds Based on 2-Hydroxyethyl Methacrylate, Gelatin, Poly(β-amino esters), and Hydroxyapatite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Multidoped Hydroxyapatite
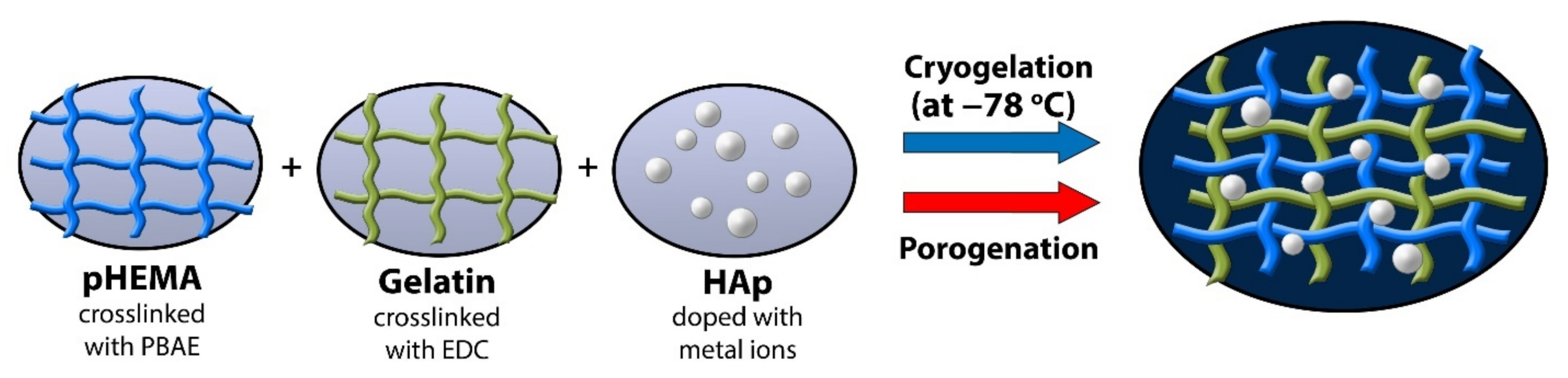
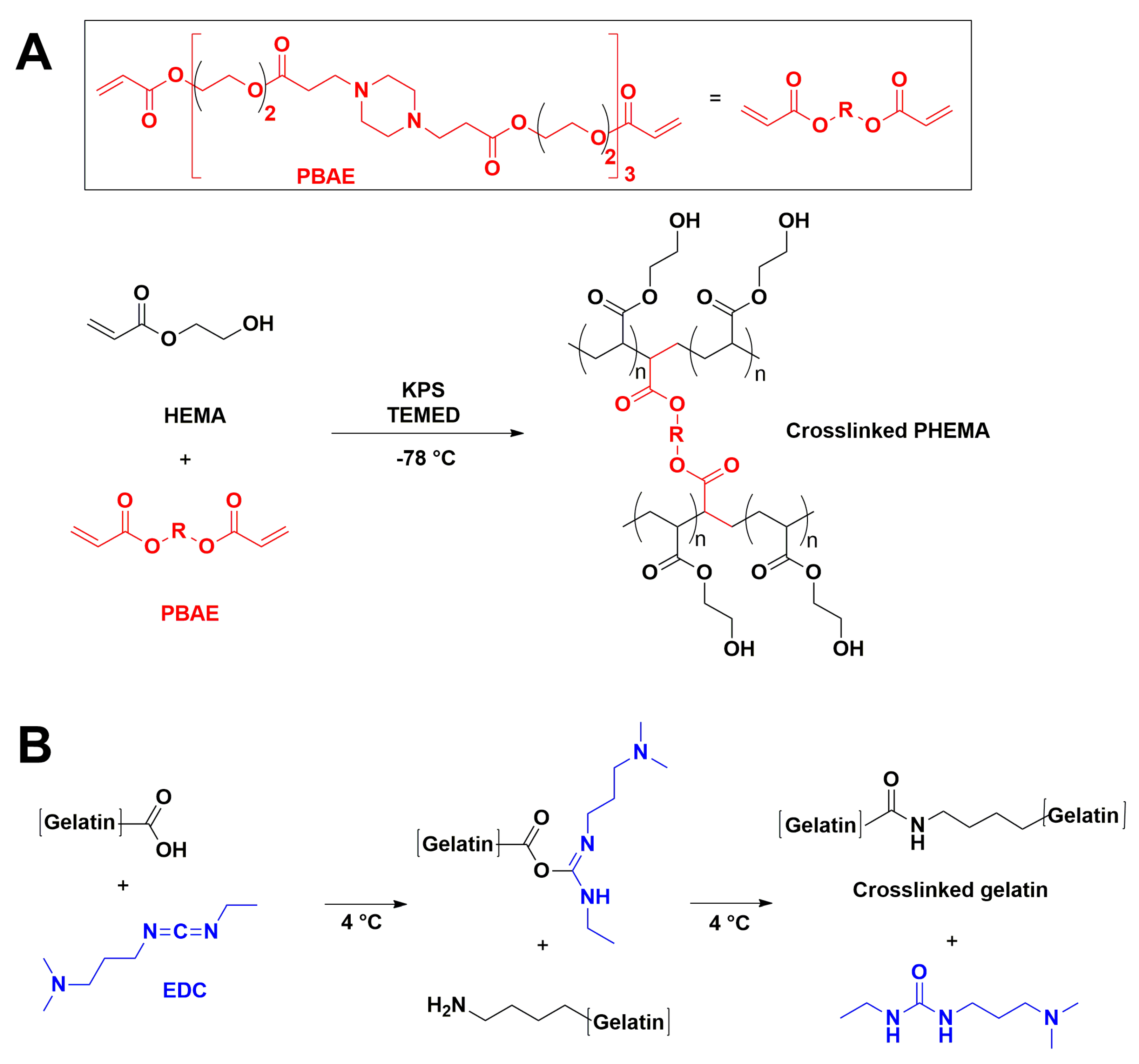
2.2.1. PHEMA/Gelatin Hydrogels Synthesis
2.2.2. Hydrogel Synthesis by Cryogelation
2.2.3. Hydrogel Synthesis by Porogenation
2.2.4. Hydroxyapatite (HAp) Incorporation in Scaffolds
2.3. Scaffold Characterization
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Porosity Measurements
2.3.4. Mechanical Characterization
2.3.5. Swelling Study
2.3.6. Water Contact Angle Measurement
2.3.7. In Vitro Degradation Study
2.4. Biological Activity Studies
2.4.1. Cell Expansion
2.4.2. DNA Quantification
2.4.3. Lactate Dehydrogenase (LDH) Activity
2.4.4. AlamarBlue Assay
2.4.5. RNA Isolation and Real-Time PCR Analysis
2.4.6. Statistics
3. Results and Discussion
3.1. Structural Characteristics of Scaffolds
3.2. Scaffold Morphology
3.3. Scaffold Porosity
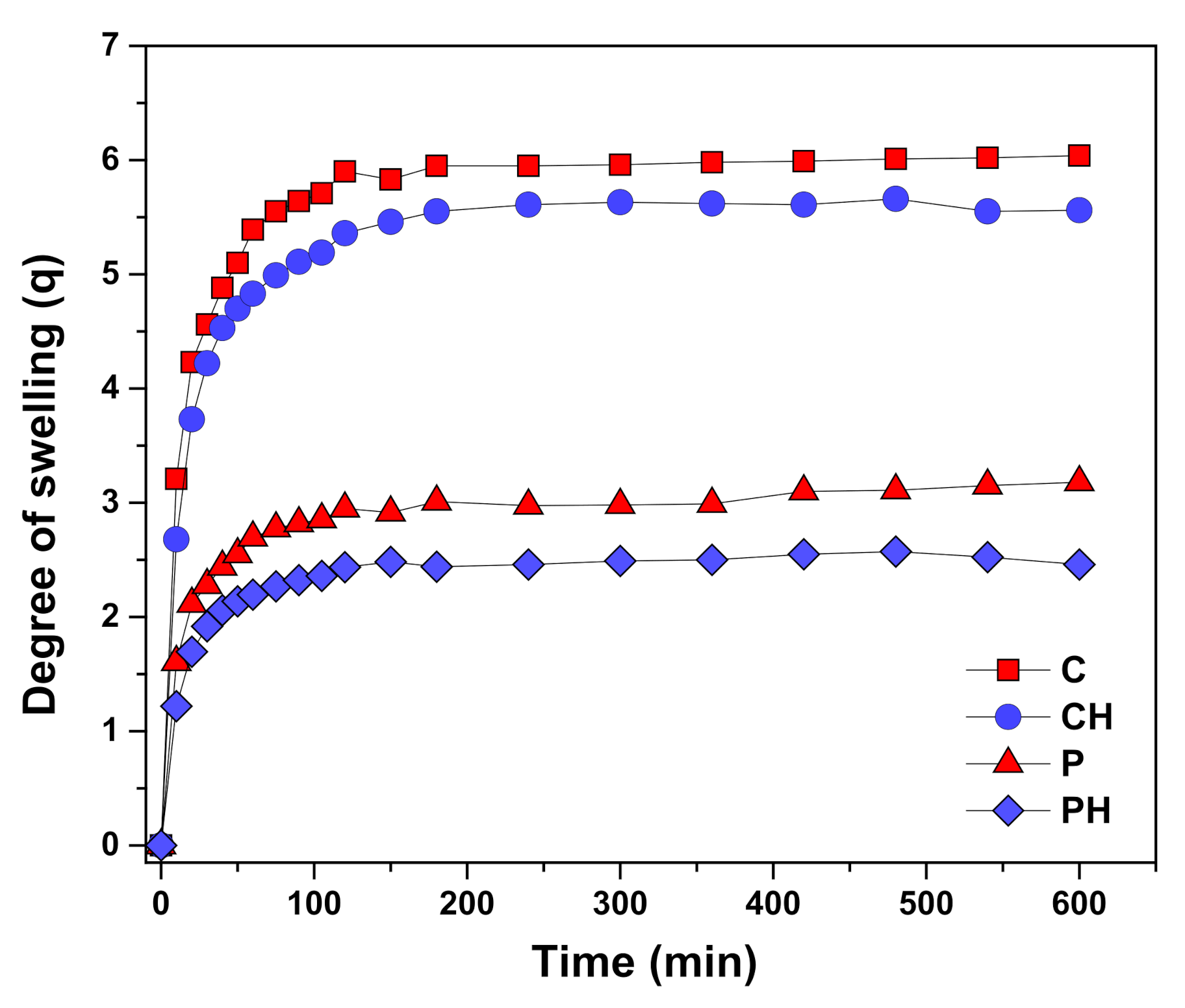
3.4. Scaffold Swelling Properties
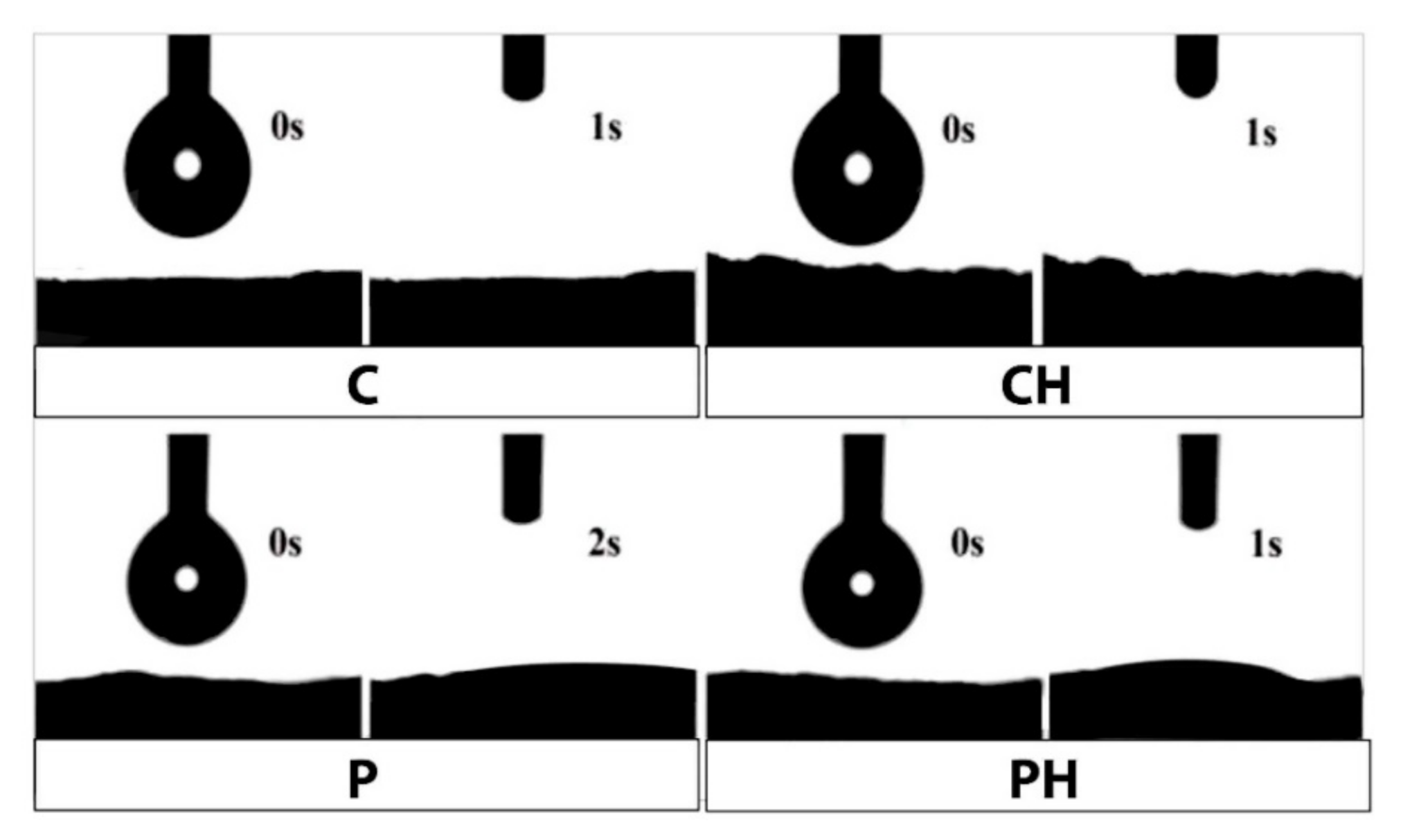
3.5. Hydrophilicity of Scaffolds
3.6. In Vitro Degradation Behavior
3.7. Scaffold Mechanical Properties
3.8. Scaffold Biological Properties
3.9. Cell Adhesion
3.10. Influence of Scaffolds on Cell Viability
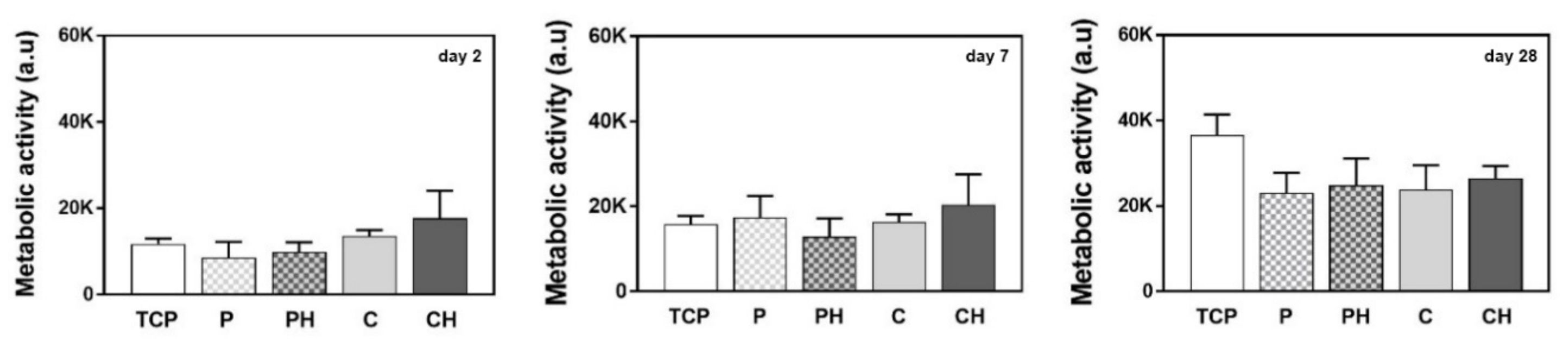
3.11. Cell Metabolic Activity
3.12. Upregulation of Osteogenesis-Related mRNA Levels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffith, L.G.; Naughton, G. Tissue Engineering—Current Challenges and Expanding Opportunities. Science 2002, 295, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.A. Biomaterials in tissue engineering. Biotechnology 1995, 13, 565–576. [Google Scholar] [CrossRef]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [Green Version]
- Buwalda, S.J.; Vermonden, T.; Hennink, W.E. Hydrogels for Therapeutic Delivery: Current Developments and Future Directions. Biomacromolecules 2017, 18, 316–330. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [Green Version]
- Deiber, J.A.; Ottone, M.L.; Piaggio, M.V.; Peirotti, M.B. Characterization of cross-linked polyampholytic gelatin hydrogels through the rubber elasticity and thermodynamic swelling theories. Polymer 2009, 50, 6065–6075. [Google Scholar] [CrossRef]
- Freudenberg, U.; Hermann, A.; Welzel, P.B.; Stirl, K.; Schwarz, S.C.; Grimmer, M.; Zieris, A.; Panyanuwat, W.; Zschoche, S.; Meinhold, D.; et al. A star-PEG–heparin hydrogel platform to aid cell replacement therapies for neurodegenerative diseases. Biomaterials 2009, 30, 5049–5060. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef] [Green Version]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact. Mater. 2021, 6, 490–502. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Myoui, A. Bone tissue engineering with porous hydroxyapatite ceramics. J. Artif. Organs 2005, 8, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Xie, P.; Zhang, K.; Tang, Z.; Chen, X.; Zhu, X.; Fan, Y.; Yang, X.; Zhang, X. Selective effect of hydroxyapatite nanoparticles on osteoporotic and healthy bone formation correlates with intracellular calcium homeostasis regulation. Acta Biomater. 2017, 59, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhao, D.; Dangaria, S.J.; Luan, X.; Diekwisch, T.G.; Jiang, G.; Saiz, E.; Liu, G.; Tomsia, A.P. Combinatorial design of hydrolytically degradable, bone-like biocomposites based on PHEMA and hydroxyapatite. Polymer 2013, 54, 909–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Xu, J.; Filion, T.; Saiz, E.; Tomsia, A.P.; Lian, J.B.; Stein, G.S.; Ayers, D.C.; Bertozzi, C.R. Elastomeric high-mineral content hydrogel-hydroxyapatite composites for orthopedic applications. J. Biomed. Mater. Res. Part A 2009, 89A, 1098–1107. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.S.; Um, S.-H.; Yoo, D.S.; Chung, Y.-C.; Chung, S.H.; Lee, J.-C.; Rhee, S.-H. Enhanced osteoconductivity of sodium-substituted hydroxyapatite by system instability. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1046–1062. [Google Scholar] [CrossRef]
- Filipović, V.V.; Nedeljković, B.B.; Vukomanović, M.; Tomić, S.Lj. Biocompatible and degradable scaffolds based on 2-hydroxyethyl methacrylate, gelatin and poly(beta amino ester) crosslinkers. Polym. Test. 2018, 68, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Kurtjak, M.; Vukomanović, M.; Krajnc, A.; Kramer, L.; Turk, B.; Suvorov, D. Designing Ga(iii)-containing hydroxyapatite with antibacterial activity. RSC Adv. 2016, 6, 112839–112852. [Google Scholar] [CrossRef]
- Hofmann, S.; Hagenmüller, H.; Koch, A.M.; Müller, R.; Vunjak-Novakovic, G.; Kaplan, D.L.; Merkle, H.P.; Meinel, L. Control of in vitro tissue-engineered bone-like structures using human mesenchymal stem cells and porous silk scaffolds. Biomaterials 2007, 28, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Kaniewska, K.; Karbarz, M.; Katz, E. Nanocomposite hydrogel films and coatings—Features and applications. Appl. Mater. Today 2020, 20, 100776. [Google Scholar] [CrossRef]
- Saveleva, M.; Prikhozhdenko, E.; Gorin, D.; Skirtach, A.G.; Yashchenok, A.; Parakhonskiy, B. Polycaprolactone-Based, Porous CaCO3 and Ag Nanoparticle Modified Scaffolds as a SERS Platform With Molecule-Specific Adsorption. Front. Chem. 2020, 7, 888. [Google Scholar] [CrossRef] [Green Version]
- Lishchynskyi, O.; Stetsyshyn, Y.; Raczkowska, J.; Awsiuk, K.; Orzechowska, B.; Abalymov, A.; Skirtach, A.; Bernasik, A.; Nastyshyn, S.; Budkowski, A. Fabrication and Impact of Fouling-Reducing Temperature-Responsive POEGMA Coatings with Embedded CaCO3 Nanoparticles on Different Cell Lines. Materials 2021, 14, 1417. [Google Scholar] [CrossRef] [PubMed]
- Nastyshyn, S.; Raczkowska, J.; Stetsyshyn, Y.; Orzechowska, B.; Bernasik, A.; Shymborska, Y.; Brzychczy-Włoch, M.; Gosiewski, T.; Lishchynskyi, O.; Ohar, H.; et al. Non-cytotoxic, temperature-responsive and antibacterial POEGMA based nanocomposite coatings with silver nanoparticles. RSC Adv. 2020, 10, 10155–10166. [Google Scholar] [CrossRef]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef] [Green Version]
- Russell, S.W.; Luptak, K.A.; Suchicital, C.T.A.; Alford, T.L.; Pizziconi, V.B. Chemical and Structural Evolution of Sol-Gel-Derived Hydroxyapatite Thin Films under Rapid Thermal Processing. J. Am. Ceram. Soc. 1996, 79, 837–842. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Park, K. Swelling and mechanical properties of superporous hydrogels of poly(acrylamide-co-acrylic acid)/polyethylenimine interpenetrating polymer networks. Polymer 2004, 45, 189–196. [Google Scholar] [CrossRef]
- Idaszek, J.; Kijeńska, E.; Łojkowski, M.; Swieszkowski, W. How important are scaffolds and their surface properties in regenerative medicine. Appl. Surf. Sci. 2016, 388, 762–774. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, X.; Li, M.; Shi, L.; Ong, J.L.T.; Jańczewski, D.; Neoh, K.G. Parallel Control over Surface Charge and Wettability Using Polyelectrolyte Architecture: Effect on Protein Adsorption and Cell Adhesion. ACS Appl. Mater. Interfaces 2016, 8, 30552–30563. [Google Scholar] [CrossRef] [PubMed]
- Ayala, R.; Zhang, C.; Yang, D.; Hwang, Y.; Aung, A.; Shroff, S.S.; Arce, F.T.; Lal, R.; Arya, G.; Varghese, S. Engineering the cell–material interface for controlling stem cell adhesion, migration, and differentiation. Biomaterials 2011, 32, 3700–3711. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Mishra, R.K.; Asiri, A.M. (Eds.) Sustainable Polymer Composites and Nanocomposites; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Nakasaki, M.; Shih, Y.-R.V.; Varghese, S. Effect of age on biomaterial-mediated in situ bone tissue regeneration. Acta Biomater. 2018, 78, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioeng. Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef]
- Şelaru, A.; Drăgușin, D.-M.; Olăreț, E.; Serafim, A.; Steinmüller-Nethl, D.; Vasile, E.; Iovu, H.; Stancu, I.-C.; Costache, M.; Dinescu, S. Fabrication and Biocompatibility Evaluation of Nanodiamonds-Gelatin Electrospun Materials Designed for Prospective Tissue Regeneration Applications. Materials 2019, 12, 2933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mpoyi, E.N.; Cantini, M.; Reynolds, P.M.; Gadegaard, N.; Dalby, M.; Salmerón-Sánchez, M. Protein Adsorption as a Key Mediator in the Nanotopographical Control of Cell Behavior. ACS Nano 2016, 10, 6638–6647. [Google Scholar] [CrossRef] [PubMed]
- Penningroth, S. Essentials of Toxic Chemical Risk: Science and Society; CRC Press: Boca Raton, FL, USA, 2016; pp. 1–212. [Google Scholar]
- Fields, R.D.; Lancaster, M.V. Dual-attribute continuous monitoring of cell proliferation/cytotoxicity. Am. Biotechnol. Lab. 1993, 11, 48–50. [Google Scholar] [PubMed]
- Ahmed, S.A.; Gogal, R.M.; Walsh, J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H] thymidine incorporation assay. J. Immunol. Methods 1994, 170, 211–224. [Google Scholar] [CrossRef]





| Sample | Component 1 | Component 2 | Cross-Linker for pHEMA | Cross-Linker for Gelatin | Initiator/Initiation Catalyst | Pore Formation Method (Foaming Agent/Stabilizer) | nHAp Doped |
|---|---|---|---|---|---|---|---|
| C | HEMA | Gelatin | PBEA | EDC | APS/TEMED | Cryogelation | - |
| CH | HEMA | Gelatin | PBEA | EDC | APS/TEMED | Cryogelation | yes |
| P | HEMA | Gelatin | PBEA | EDC | APS/TEMED | Porogenation (NaHCO3/TWEEN) | - |
| PH | HEMA | Gelatin | PBEA | EDC | APS/TEMED | Porogenation (NaHCO3/TWEEN) | yes |
| Sample | Equilibrium Degree of Swelling | Young’s Modulus (MPa) | Porosity (%) | Percentage of Mass Loss (after 16 Weeks) | Elongation at Break (%) |
|---|---|---|---|---|---|
| C | 5.12 ± 0.05 | 4.25 ± 0.22 | 83.92 | 23.17 ± 0.85 | 26.96 ± 1.12 |
| CH | 4.87 ± 0.04 | 5.87 ± 0.24 | 78.96 | 21.93 ± 1.12 | 24.37 ± 1.18 |
| P | 1.93 ± 0.03 | 8.63 ± 0.28 | 71.17 | 9.24 ± 0.67 | 20.45 ± 1.36 |
| PH | 1.74 ± 0.03 | 10.14 ± 0.31 | 65.66 | 7.55 ± 0.69 | 17.58 ± 1.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipović, V.V.; Babić Radić, M.M.; Vuković, J.S.; Vukomanović, M.; Rubert, M.; Hofmann, S.; Müller, R.; Tomić, S.L. Biodegradable Hydrogel Scaffolds Based on 2-Hydroxyethyl Methacrylate, Gelatin, Poly(β-amino esters), and Hydroxyapatite. Polymers 2022, 14, 18. https://doi.org/10.3390/polym14010018
Filipović VV, Babić Radić MM, Vuković JS, Vukomanović M, Rubert M, Hofmann S, Müller R, Tomić SL. Biodegradable Hydrogel Scaffolds Based on 2-Hydroxyethyl Methacrylate, Gelatin, Poly(β-amino esters), and Hydroxyapatite. Polymers. 2022; 14(1):18. https://doi.org/10.3390/polym14010018
Chicago/Turabian StyleFilipović, Vuk V., Marija M. Babić Radić, Jovana S. Vuković, Marija Vukomanović, Marina Rubert, Sandra Hofmann, Ralph Müller, and Simonida Lj. Tomić. 2022. "Biodegradable Hydrogel Scaffolds Based on 2-Hydroxyethyl Methacrylate, Gelatin, Poly(β-amino esters), and Hydroxyapatite" Polymers 14, no. 1: 18. https://doi.org/10.3390/polym14010018
APA StyleFilipović, V. V., Babić Radić, M. M., Vuković, J. S., Vukomanović, M., Rubert, M., Hofmann, S., Müller, R., & Tomić, S. L. (2022). Biodegradable Hydrogel Scaffolds Based on 2-Hydroxyethyl Methacrylate, Gelatin, Poly(β-amino esters), and Hydroxyapatite. Polymers, 14(1), 18. https://doi.org/10.3390/polym14010018











