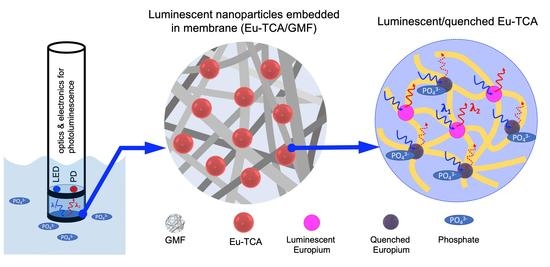
Reusable and pH-Stable Luminescent Sensors for Highly Selective Detection of Phosphate
Abstract
:1. Introduction
2. Materials and Methods
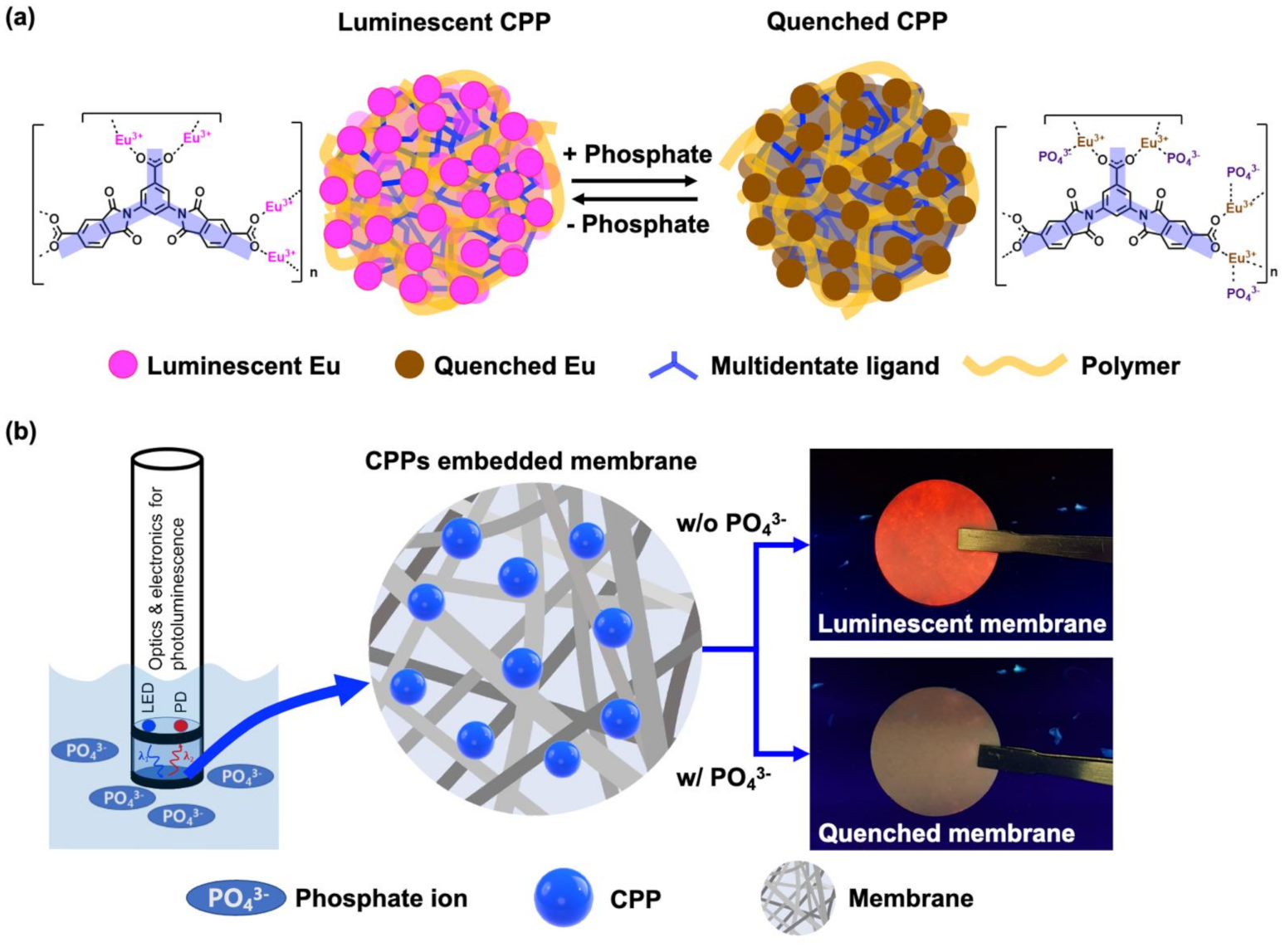
2.1. Preparation of Luminescent CPP
2.2. Preparation of Eu-TCA Embedded in Glass Microfiber Filters (Eu-TCA/GMF)
2.3. Phosphate Detection of Eu-TCA Dispersion
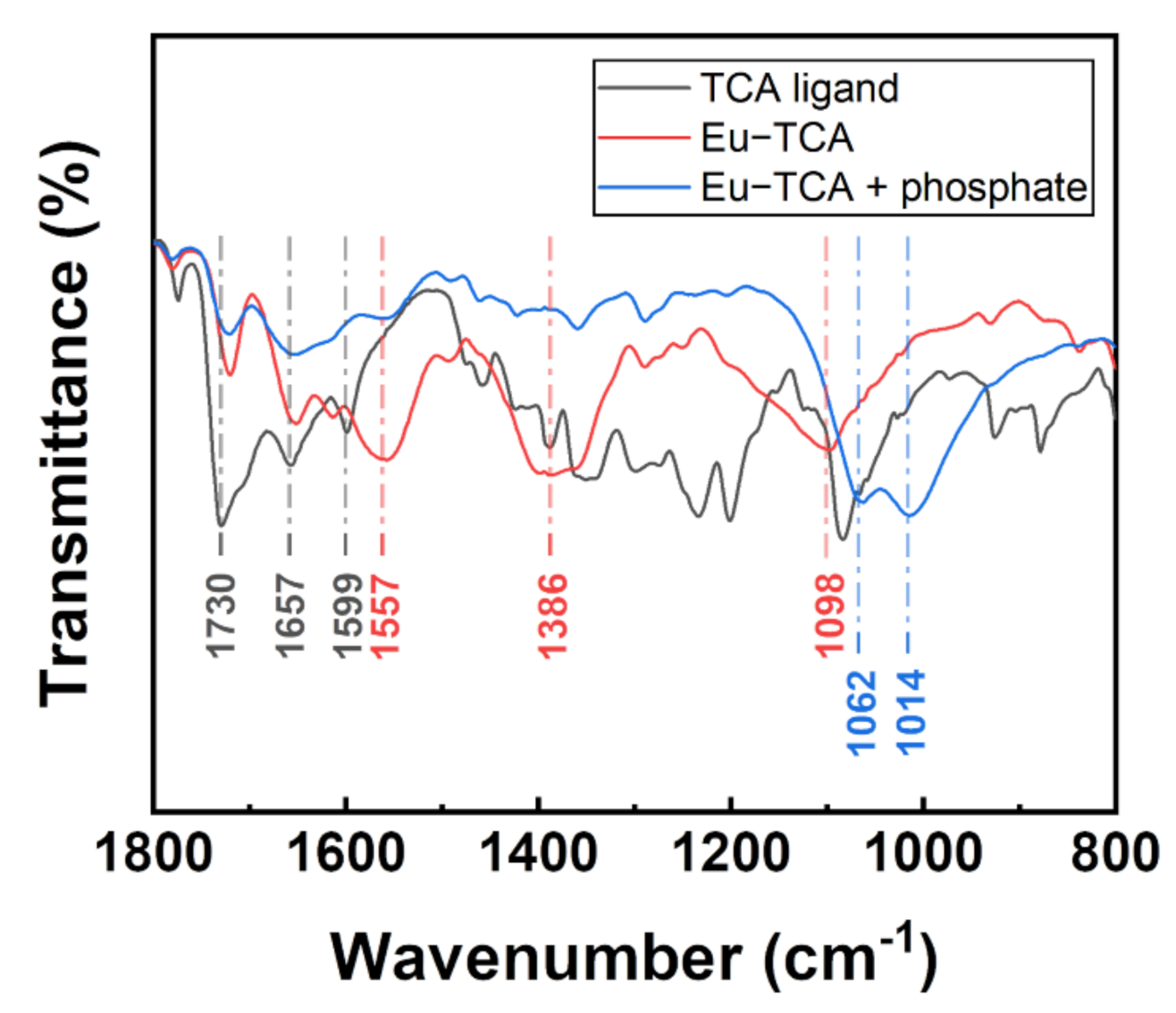
2.4. Characterization
3. Results and Discussion
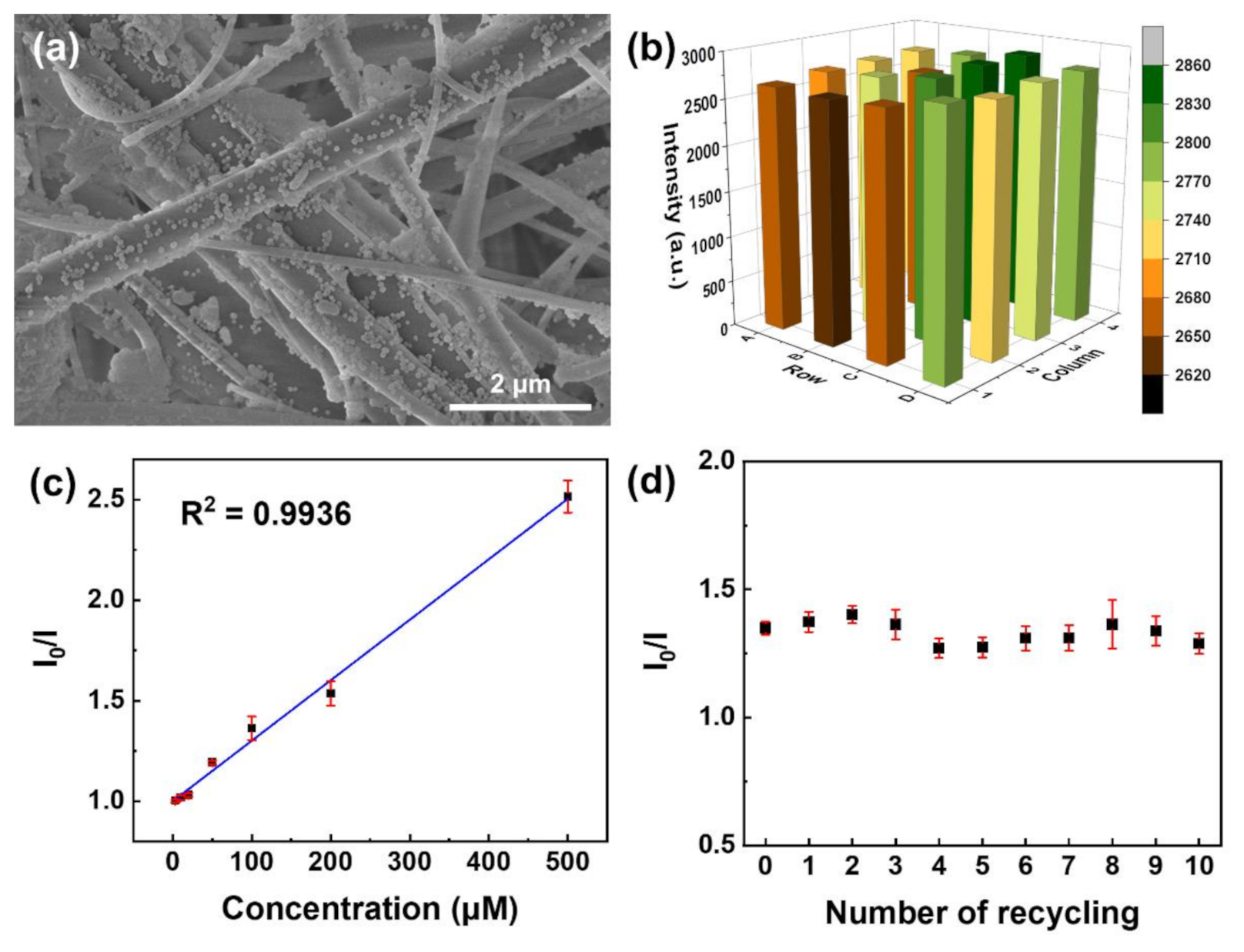
3.1. Morphology and Structure Analysis
3.2. Evaluation of Phosphate Sensing Materials Based on Luminescence Quenching
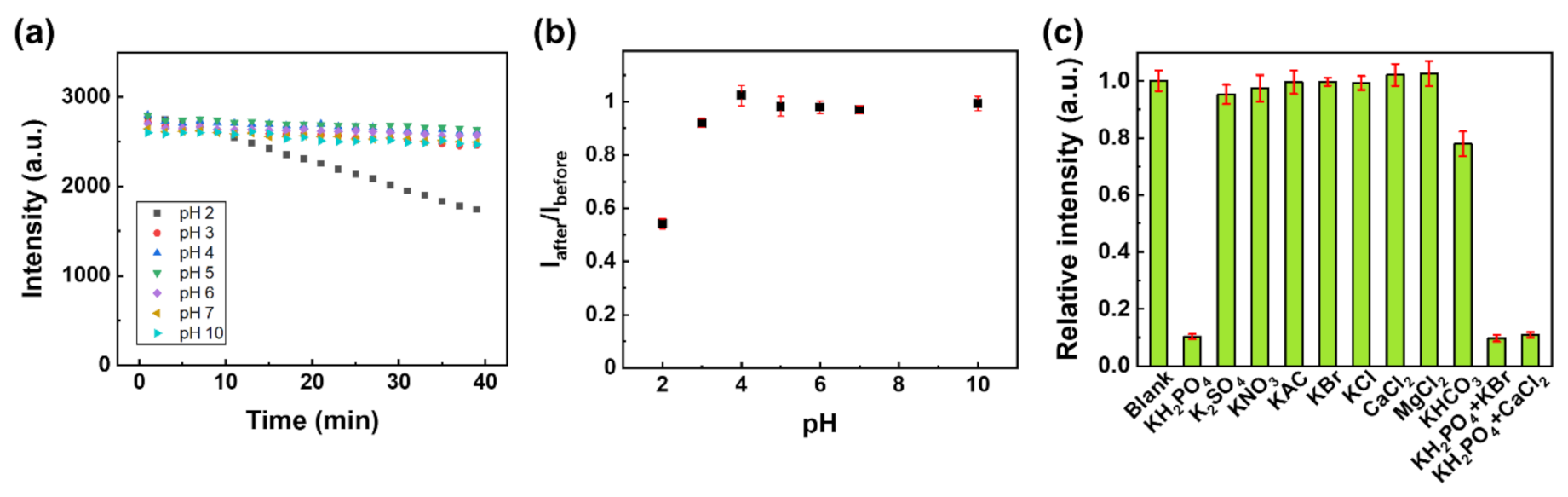
3.3. Phosphate Sensing Properties of Eu-TCA/GMF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Worsfold, P.; McKelvie, I.; Monbet, P. Determination of Phosphorus in Natural Waters: A Historical Review. Anal. Chim. Acta 2016, 918, 8–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The Rise of Harmful Cyanobacteria Blooms: The Potential Roles of Eutrophication and Climate Change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate Change: Links to Global Expansion of Harmful Cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef]
- Bertone, E.; Burford, M.A.; Hamilton, D.P. Fluorescence Probes for Real-Time Remote Cyanobacteria Monitoring: A Review of Challenges and Opportunities. Water Res. 2018, 141, 152–162. [Google Scholar] [CrossRef]
- Patel, V.; Selvaganapathy, P.R. Enhancing the Sensitivity of Cobalt Based Solid-State Phosphate Sensor Using Electrical Pretreatment. Sens. Actuators B Chem. 2021, 349, 130789. [Google Scholar] [CrossRef]
- Matveichuk, Y.; Rakhman’ko, E.; Akayeu, Y.; Stanishevskii, D. Ion-Selective Electrodes Based on Long-Chain Quaternary Ammonium Salts with Enhanced Steric Accessibility, and Their Application for Determination of Hydrophilic Double-Charged Inorganic Anion. Chem. Pap. 2018, 72, 731–739. [Google Scholar] [CrossRef]
- Xu, K.; Kitazumi, Y.; Kano, K.; Shirai, O. Phosphate Ion Sensor Using a Cobalt Phosphate Coated Cobalt Electrode. Electrochim. Acta 2018, 282, 242–246. [Google Scholar] [CrossRef]
- Huang, Y.; Ye, Y.; Zhao, G.; Wu, X.; Kan, Y.; Mur, L.; Han, J.; Qin, H. An All-Solid-State Phosphate Electrode with H3PO4 Doped Polyaniline as the Sensitive Layer. Int. J. Electrochem. Sci. 2017, 12, 4677–4691. [Google Scholar] [CrossRef]
- Chandra Rao, P.; Mandal, S. Europium-Based Metal–Organic Framework as a Dual Luminescence Sensor for the Selective Detection of the Phosphate Anion and Fe3+ Ion in Aqueous Media. Inorg. Chem. 2018, 57, 11855–11858. [Google Scholar] [CrossRef]
- Gao, M.; Tang, B.Z. Fluorescent Sensors Based on Aggregation-Induced Emission: Recent Advances and Perspectives. ACS Sens. 2017, 2, 1382–1399. [Google Scholar] [CrossRef]
- Wu, H.; Tong, C. A Specific Turn-On Fluorescent Sensing for Ultrasensitive and Selective Detection of Phosphate in Environmental Samples Based on Antenna Effect-Improved FRET by Surfactant. ACS Sens. 2018, 3, 1539–1545. [Google Scholar] [CrossRef]
- Tafesse, F. Detection Mechanisms of Phosphate Sensitive Electrodes. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2015, 45, 1687–1692. [Google Scholar] [CrossRef]
- Lrving, H.M.N.H.; Zettler, H.; Baudin, G.; Freiser, H.; Guilbault, G.G.; Menis, O.; Rice, N.M.; Robertson, A.J.B.; Docherty, A.C.; Fischer, W.; et al. Recommendations for Nomenclature of Ion-Selective Electrodes (Recommendations 1975). Pure Appl. Chem. 1976, 48, 127–132. [Google Scholar] [CrossRef]
- Morais, I.P.A.; Tóth, I.V.; Rangel, A.O.S.S. An Overview on Flow Methods for the Chemiluminescence Determination of Phosphorus. Anal. Phosphorus Environ. Agric. Samples 2005, 66, 341–347. [Google Scholar] [CrossRef]
- Clinton-Bailey, G.S.; Grand, M.M.; Beaton, A.D.; Nightingale, A.M.; Owsianka, D.R.; Slavik, G.J.; Connelly, D.P.; Cardwell, C.L.; Mowlem, M.C. A Lab-on-Chip Analyzer for in Situ Measurement of Soluble Reactive Phosphate: Improved Phosphate Blue Assay and Application to Fluvial Monitoring. Environ. Sci. Technol. 2017, 51, 9989–9995. [Google Scholar] [CrossRef]
- Einkauf, J.D.; Clark, J.M.; Paulive, A.; Tanner, G.P.; de Lill, D.T. A General Model of Sensitized Luminescence in Lanthanide-Based Coordination Polymers and Metal–Organic Framework Materials. Inorg. Chem. 2017, 56, 5544–5552. [Google Scholar] [CrossRef]
- Sakata, Y.; Furukawa, S.; Kondo, M.; Hirai, K.; Horike, N.; Takashima, Y.; Uehara, H.; Louvain, N.; Meilikhov, M.; Tsuruoka, T.; et al. Shape-Memory Nanopores Induced in Coordination Frameworks by Crystal Downsizing. Science 2013, 339, 193. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.-M.; Heo, J.; Mirkin, C.A. Dynamic Interconversion of Amorphous Microparticles and Crystalline Rods in Salen-Based Homochiral Infinite Coordination Polymers. J. Am. Chem. Soc. 2007, 129, 7480–7481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, Y.-M.; Armatas, G.S.; Heo, J.; Kanatzidis, M.G.; Mirkin, C.A. Amorphous Infinite Coordination Polymer Microparticles: A New Class of Selective Hydrogen Storage Materials. Adv. Mater. 2008, 20, 2105–2110. [Google Scholar] [CrossRef]
- Wang, S.; McGuirk, C.M.; d’Aquino, A.; Mason, J.A.; Mirkin, C.A. Metal–Organic Framework Nanoparticles. Adv. Mater. 2018, 30, 1800202. [Google Scholar] [CrossRef]
- Das, A.; Das, S.; Trivedi, V.; Biswas, S. A Dual Functional MOF-Based Fluorescent Sensor for Intracellular Phosphate and Extracellular 4-Nitrobenzaldehyde. Dalton Trans. 2019, 48, 1332–1343. [Google Scholar] [CrossRef]
- Asha, K.S.; Bhattacharjee, R.; Mandal, S. Complete Transmetalation in a Metal–Organic Framework by Metal Ion Metathesis in a Single Crystal for Selective Sensing of Phosphate Ions in Aqueous Media. Angew. Chem. Int. Ed. 2016, 55, 11528–11532. [Google Scholar] [CrossRef]
- Fan, C.; Lv, X.; Tian, M.; Yu, Q.; Mao, Y.; Qiu, W.; Wang, H.; Liu, G. A Terbium(III)-Functionalized Zinc(II)-Organic Framework for Fluorometric Determination of Phosphate. Microchim. Acta 2020, 187, 84. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Ma, Y.; Ge, X.; Zhou, H.; Wang, G.; Zhang, H.; Tang, X.; Zhang, Y. Europium-Based Infinite Coordination Polymer Nanospheres as an Effective Fluorescence Probe for Phosphate Sensing. RSC Adv. 2017, 7, 8661–8669. [Google Scholar] [CrossRef] [Green Version]
- Spokoyny, A.M.; Kim, D.; Sumrein, A.; Mirkin, C.A. Infinite Coordination Polymer Nano- and Microparticle Structures. Chem. Soc. Rev. 2009, 38, 1218–1227. [Google Scholar] [CrossRef]
- Vegas, V.G.; Villar-Alonso, M.; Gómez-García, C.J.; Zamora, F.; Amo-Ochoa, P. Direct Formation of Sub-Micron and Nanoparticles of a Bioinspired Coordination Polymer Based on Copper with Adenine. Polymers 2017, 9, 565. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Jiao, Y.; An, D.; Li, D.; Li, W.; Wei, Q. Review of Dissolved Oxygen Detection Technology: From Laboratory Analysis to Online Intelligent Detection. Sensors 2019, 19, 3995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukubayashi, Y.; Nakai, M.; Yanaka. A Method for Producing Organic Compound. WO 2020/009016 A1, 9 January 2020. [Google Scholar]
- Heravi, M.M.; Ghavidel, M.; Mohammadkhani, L. Beyond a Solvent: Triple Roles of Dimethylformamide in Organic Chemistry. RSC Adv. 2018, 8, 27832–27862. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, R.D.L.; Mazali, I.O.; Sigoli, F.A. Particle Size Tailoring and Luminescence of Europium(III)-Doped Gadolinium Oxide Obtained by the Modified Homogeneous Precipitation Method: Dielectric Constant and Counter Anion Effects. Colloids Surf. Physicochem. Eng. Asp. 2010, 367, 155–160. [Google Scholar] [CrossRef]
- Zhou, X.; Li, H.; Xiao, H.; Li, L.; Zhao, Q.; Yang, T.; Zuo, J.; Huang, W. A Microporous Luminescent Europium Metal–Organic Framework for Nitro Explosive Sensing. Dalton Trans. 2013, 42, 5718–5723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Yan, B. A Ratiometric Fluorescent PH Sensor Based on Nanoscale Metal–Organic Frameworks (MOFs) Modified by Europium(Iii) Complexes. Chem. Commun. 2014, 50, 13323–13326. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, A.F.Y.; Tapia, M.J.; Pais, A.A.C.C.; Valente, A.J.M. Luminescent Properties of Lanthanoid-Poly(Sodium Acrylate) Composites: Insights on the Interaction Mechanism. Polymers 2020, 12, 1314. [Google Scholar] [CrossRef]
- Zhou, X.-H.; Li, L.; Li, H.-H.; Li, A.; Yang, T.; Huang, W. A Flexible Eu(Iii)-Based Metal–Organic Framework: Turn-off Luminescent Sensor for the Detection of Fe(Iii) and Picric Acid. Dalton Trans. 2013, 42, 12403–12409. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Gypser, S.; Leinweber, P.; Freese, D.; Kühn, O. Infrared Spectroscopic Characterization of Phosphate Binding at the Goethite–Water Interface. Phys. Chem. Chem. Phys. 2019, 21, 4421–4434. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Dai, Y.; Zhu, X.; Wang, Z.; Li, Y.; Zhuang, Q.; Shi, J.; Gu, J. Metal–Organic Frameworks with Inherent Recognition Sites for Selective Phosphate Sensing through Their Coordination-Induced Fluorescence Enhancement Effect. J. Mater. Chem. A 2015, 3, 7445–7452. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Hu, K.; Li, Y.; Feng, J.; Shi, J.; Gu, J. Rapid and Specific Aqueous-Phase Detection of Nitroaromatic Explosives with Inherent Porphyrin Recognition Sites in Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2015, 7, 11956–11964. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, H.; Pan, S.; Liu, H.; Hu, X. Fluorescence-Scattering Dual-Signal Response of Carbon Dots@ZIF-90 for Phosphate Ratiometric Detection. ACS Sens. 2020, 5, 2211–2220. [Google Scholar] [CrossRef] [PubMed]



| Samples | Spiked (μM) | Measured (μM) (Mean ± Standard Deviation, n = 3) | Recovery (%) (Mean ± Standard Deviation, n = 3) |
|---|---|---|---|
| Tap water | 0 | not detected 1 | |
| 20 | 20.82 ± 0.58 | 104.10 ± 2.90 | |
| 50 | 50.30 ± 1.22 | 100.60 ± 2.44 | |
| Lake water (Daecheong Lake) | 0 | not detected 1 | |
| 20 | 19.77 ± 0.46 | 98.85 ± 2.30 | |
| 50 | 47.45 ± 2.06 | 94.90 ± 4.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.Y.; Kim, D.G.; Jeong, B.; Kim, Y.I.; Heo, J.; Lee, H.-K. Reusable and pH-Stable Luminescent Sensors for Highly Selective Detection of Phosphate. Polymers 2022, 14, 190. https://doi.org/10.3390/polym14010190
Kim DY, Kim DG, Jeong B, Kim YI, Heo J, Lee H-K. Reusable and pH-Stable Luminescent Sensors for Highly Selective Detection of Phosphate. Polymers. 2022; 14(1):190. https://doi.org/10.3390/polym14010190
Chicago/Turabian StyleKim, Do Yeob, Dong Gyu Kim, Bongjin Jeong, Young Il Kim, Jungseok Heo, and Hyung-Kun Lee. 2022. "Reusable and pH-Stable Luminescent Sensors for Highly Selective Detection of Phosphate" Polymers 14, no. 1: 190. https://doi.org/10.3390/polym14010190
APA StyleKim, D. Y., Kim, D. G., Jeong, B., Kim, Y. I., Heo, J., & Lee, H.-K. (2022). Reusable and pH-Stable Luminescent Sensors for Highly Selective Detection of Phosphate. Polymers, 14(1), 190. https://doi.org/10.3390/polym14010190