A Three-Dimensional Coordination Framework with a Ferromagnetic Coupled Ni(II)-CrO4 Layer: Synthesis, Structure, and Magnetic Studies
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of [Ni(CrO4)(bpym)(H2O)]n (1)
2.3. X-ray Crystallography
2.4. Physical Measurements
3. Results and Discussion
3.1. Syntheses and Characterization of Compound 1
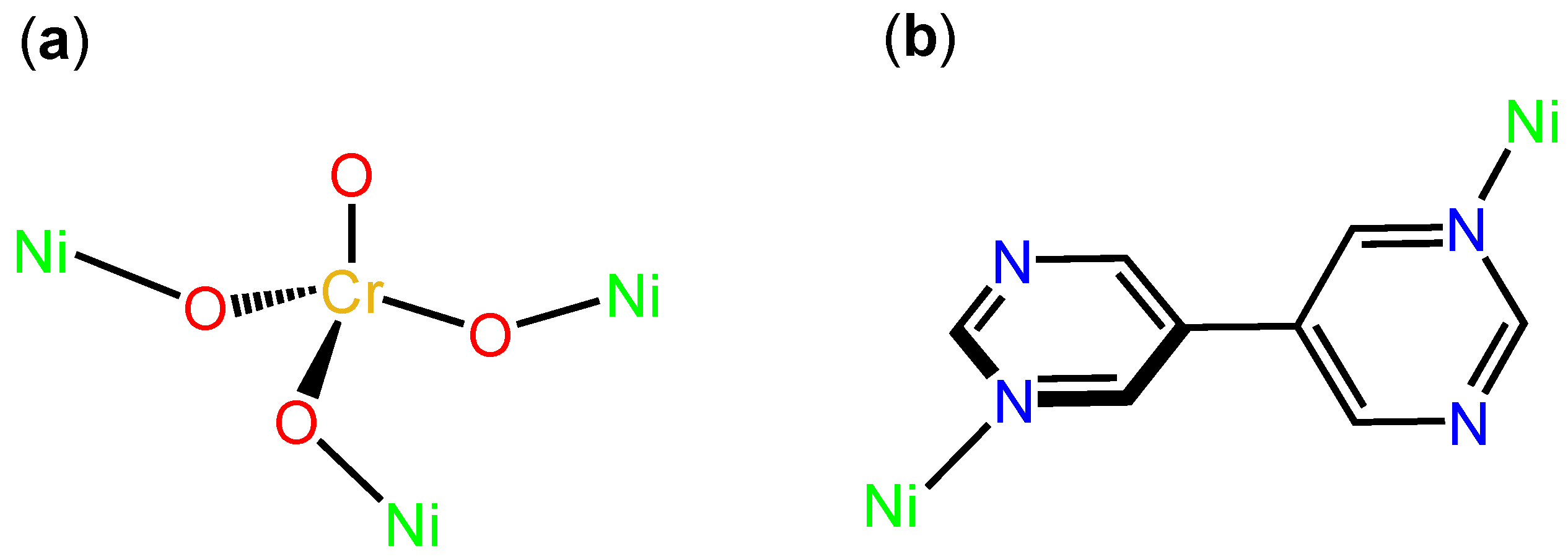
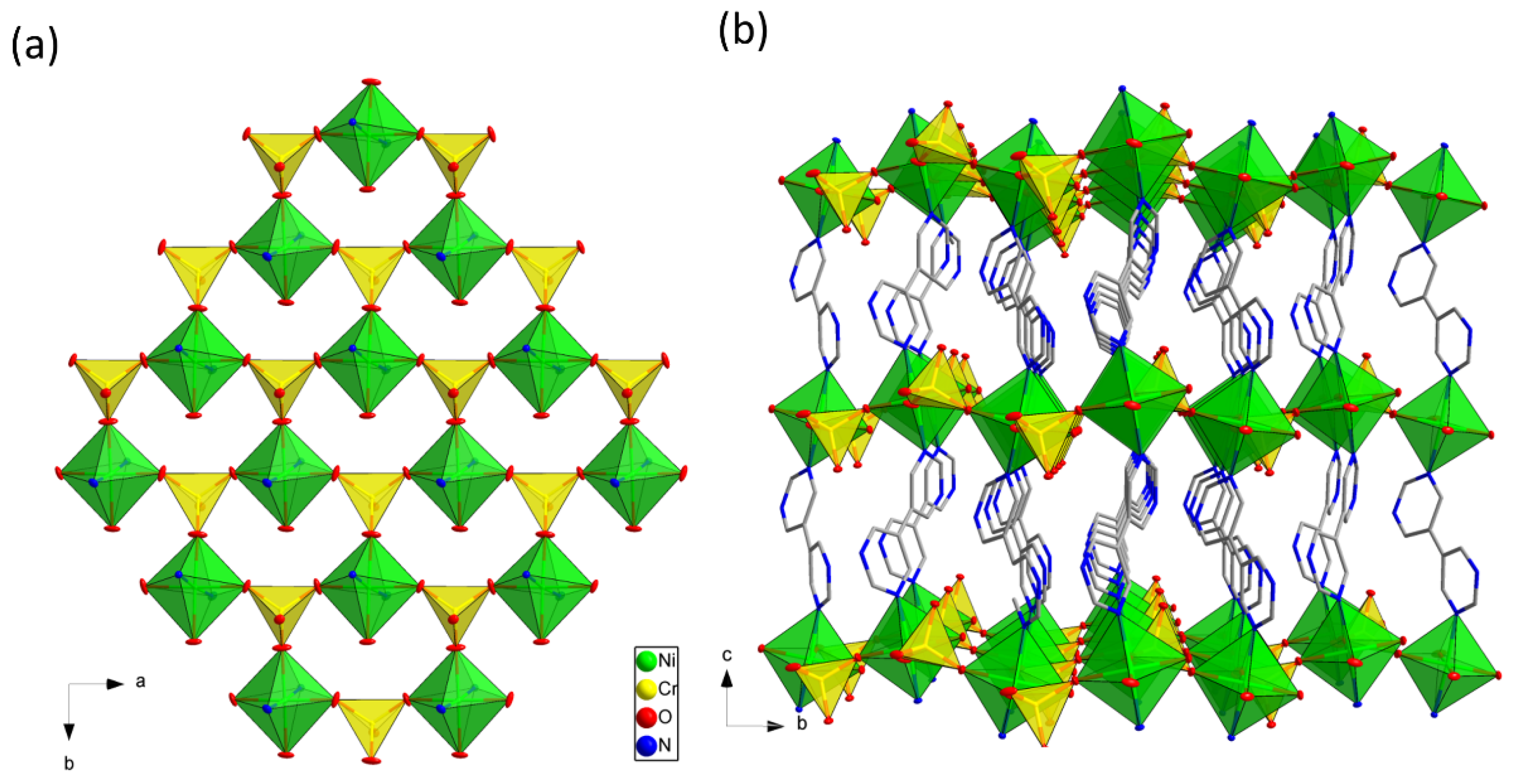
3.2. Description of Structure
Crystal Structures Description of [Ni(CrO4)(bpym)(H2O)]n (1)
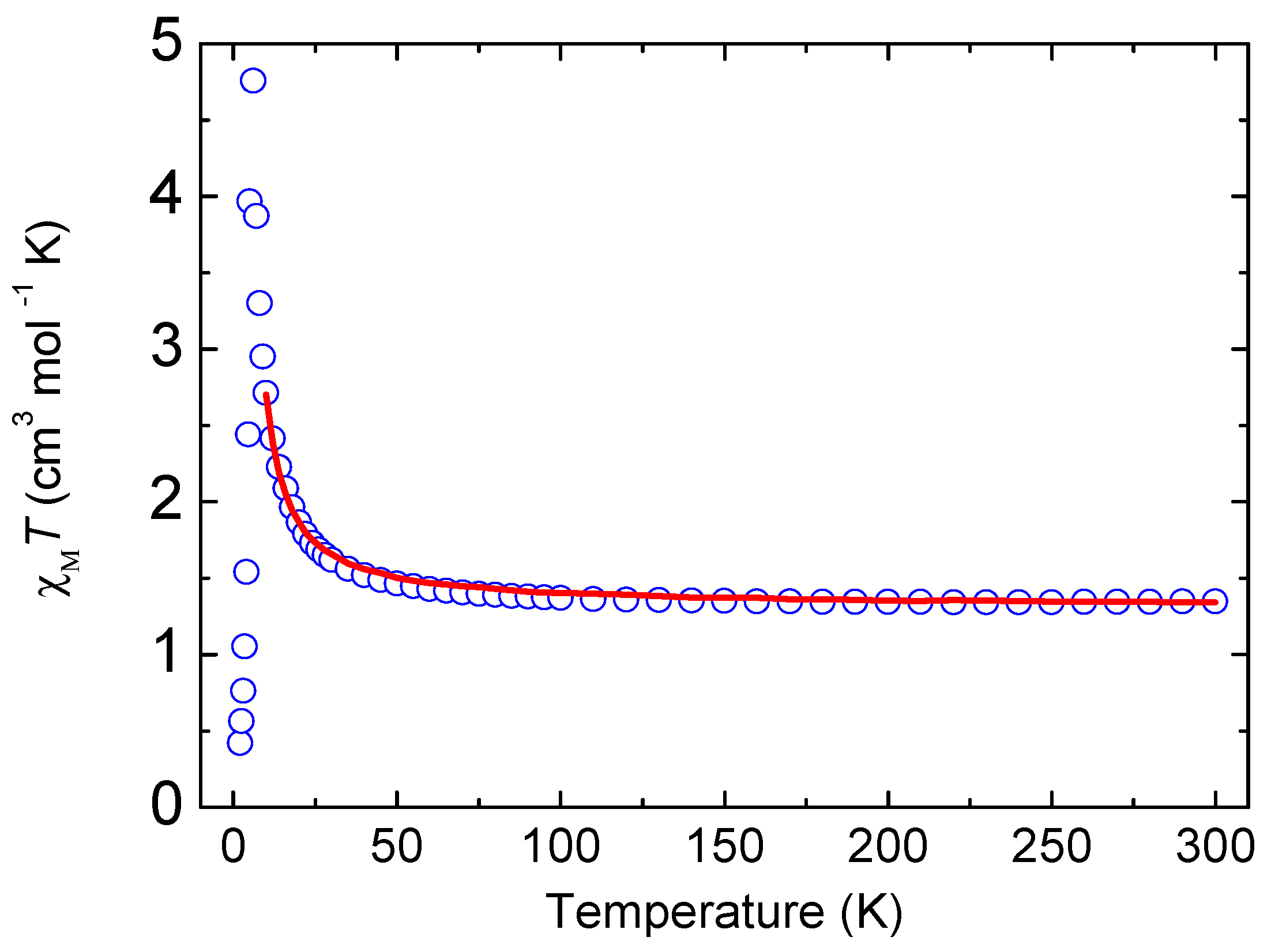
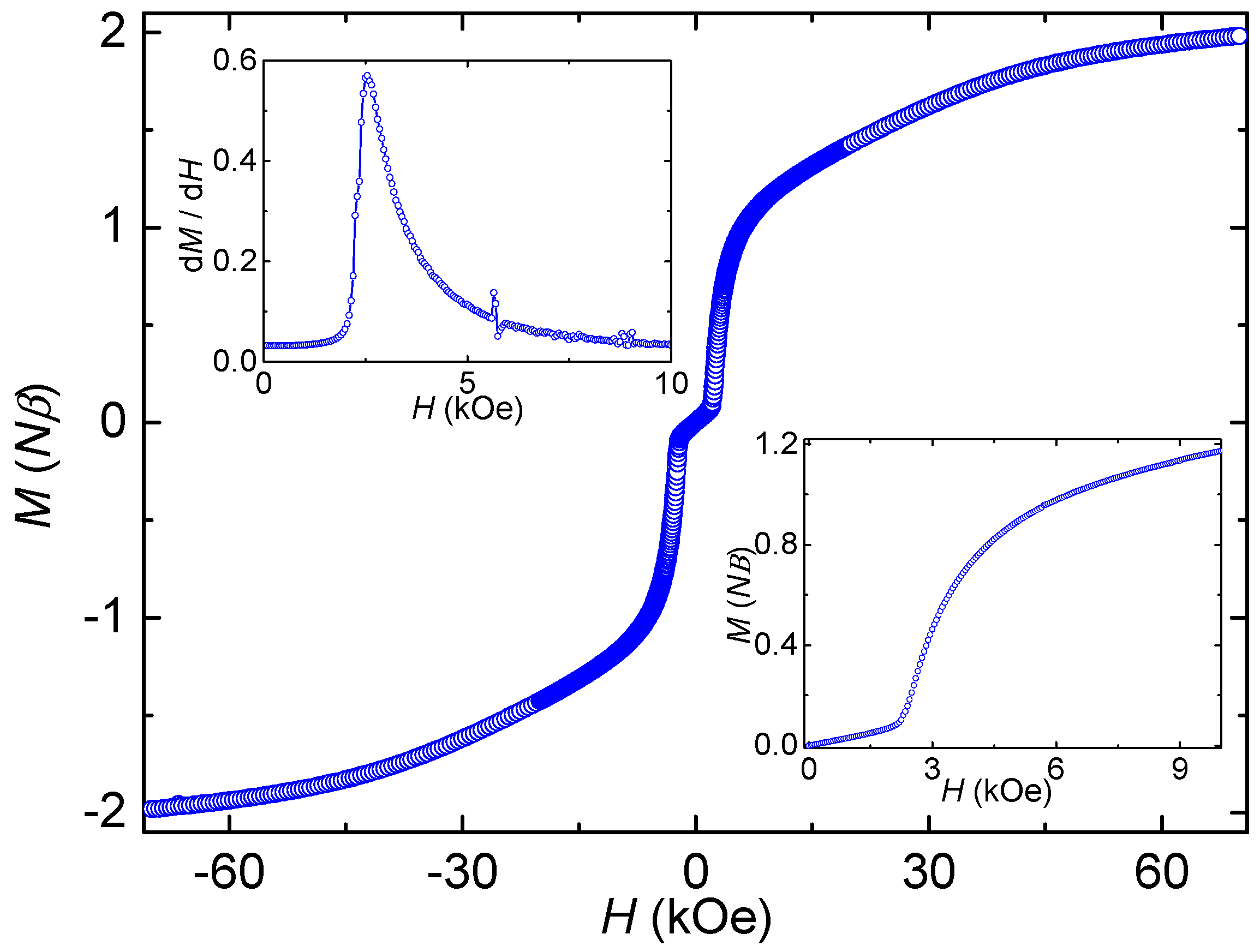
3.3. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Ferey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F. Crystallized frameworks with giant pores: Are there limits to the possible? Acc. Chem. Res. 2005, 38, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Wu, X.; Zhang, J.; Fu, R.; Hu, S.; Zhang, X. 3D Canted Antiferromagnetic Porous Metal—Organic Framework with Anatase Topology through Assembly of an Analogue of Polyoxometalate. J. Am. Chem. Soc. 2005, 127, 16352–16353. [Google Scholar] [CrossRef] [PubMed]
- Ockwig, N.W.; Delgado-Friedrichs, O.; O’Keeffe, M.; Yaghi, O.M. Reticular chemistry: Occurrence and taxonomy of nets and grammar for the design of frameworks. Acc. Chem. Res. 2005, 38, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Ferey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Moulton, B.; Zaworotko, M.J. From Molecules to Crystal Engineering: Supramolecular Isomerism and Polymorphism in Network Solids. Chem. Rev. 2001, 101, 1629–1658. [Google Scholar] [CrossRef]
- Welte, L.; Calzolari, A.; Di Felice, R.; Zamora, F.; Gomez-Herrero, J. Highly conductive self-assembled nanoribbons of coordination polymers. Nat. Nanotechnol. 2010, 5, 110–115. [Google Scholar] [CrossRef]
- Barth, J.V.; Costantini, G.; Kern, K. Engineering atomic and molecular nanostructures at surfaces. Nature 2005, 437, 671–679. [Google Scholar] [CrossRef]
- Guijarro, A.; Castillo, O.; Welte, L.; Calzolari, A.; Miguel, P.J.S.; Gómez-García, C.J.; Olea, D.; di Felice, R.; Gómez-Herrero, J.; Zamora, F. Conductive Nanostructures of MMX Chains. Adv. Funct. Mater. 2010, 20, 1451–1457. [Google Scholar] [CrossRef]
- Noro, S.-I.; Kitaura, R.; Kondo, M.; Kitagawa, S.; Ishii, T.; Matsuzaka, H.; Yamashita, M. Framework Engineering by Anions and Porous Functionalities of Cu(II)/4,4′-bpy Coordination Polymers. J. Am. Chem. Soc. 2002, 124, 2568–2583. [Google Scholar] [CrossRef] [PubMed]
- Masciocchi, N.; Cairati, P.; Carlucci, L.; Mezza, G.; Ciani, G.; Sironi, A. Ab-initio X-ray powder diffraction structural characterization of coordination compounds: Polymeric [{MX,(bipy)},] complexes (M = Ni or Cu; X = C1 or Br; bipy = 4,4′-bipyridyl). J. Chem. Soc. Dalton Trans. 1996, 2739–2746. [Google Scholar] [CrossRef]
- Rancan, M.; Armelao, L. Exploiting dimensional variability in coordination polymers: Solvent promotes reversible conversion between 3D and chiral 1D architectures. ChemComm 2015, 51, 12947–12949. [Google Scholar] [CrossRef]
- Rancan, M.; Carlotto, A.; Bottaro, G.; Armelao, L. Effect of Coordinating Solvents on the Structure of Cu(II)-4,4′-bipyridine Coordination Polymers. Inorganics 2019, 7, 103. [Google Scholar] [CrossRef] [Green Version]
- Coronado, E.; Minguez Espallargas, G. Dynamic magnetic MOFs. Chem. Soc. Rev. 2013, 42, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Chaemchuen, S.; Kabir, N.A.; Zhou, K.; Verpoort, F. Metal–organic frameworks for upgrading biogas via CO2 adsorption to biogas green energy. Chem. Soc. Rev. 2013, 42, 9304. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, E.; Ali, L.; El Sayah, A.; Alkhatib, S.A.; Abdulsalam, H.; Juma, M.; Al-Muhtaseb, A.a.H. Implementing Metal-Organic Frameworks for Natural Gas Storage. Crystals 2019, 9, 406. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liang, C.; Zou, X.; Gu, J.; Kirillova, M.V.; Kirillov, A.M. Metal(II) Coordination Polymers from Tetracarboxylate Linkers: Synthesis, Structures, and Catalytic Cyanosilylation of Benzaldehydes. Catalysts 2021, 11, 204. [Google Scholar] [CrossRef]
- Connolly, B.M.; Madden, D.G.; Wheatley, A.E.H.; Fairen-Jimenez, D. Shaping the Future of Fuel: Monolithic Metal-Organic Frameworks for High-Density Gas Storage. J. Am. Chem. Soc. 2020, 142, 8541–8549. [Google Scholar] [CrossRef]
- Yoon, J.; Solomon, E.I. Electronic structures of exchange coupled trigonal trimeric Cu(II) complexes: Spin frustration, antisymmetric exchange, pseudo-A terms, and their relation to O2 activation in the multicopper oxidases. Coordin. Chem. Rev. 2007, 251, 379–400. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, B.; Chen, L. First-Principles Study of Microporous Magnets M-MOF-74 (M = Ni, Co, Fe, Mn): The Role of Metal Centers. Inorg. Chem. 2013, 52, 9356–9362. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, A.; Miyasaka, H.; Yamashita, M.; Clérac, R. Direct evidence of exchange interaction dependence of magnetization relaxation in a family of ferromagnetic-type single-chain magnets. J. Mater. Chem. 2007, 17, 2002–2012. [Google Scholar] [CrossRef]
- Kurmoo, M. Magnetic metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1353–1379. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.F.; Hu, X.; Liu, F.C.; Bu, X.H. Azido-mediated systems showing different magnetic behaviors. Chem. Soc. Rev. 2009, 38, 469–480. [Google Scholar] [CrossRef]
- Gatteschi, D.; Kahn, O.; Miller, J.S.; Palacio, F. Magnetic Molecular Materials; Kluwer Academic: Dordrecht, The Netherlands, 1991. [Google Scholar]
- Willet, R.D.; Gatteschi, D.; Kahn, O. Magneto-Structural Correlations in Exchange Coupled Systems; Reidel Publishing: Dordrecht, The Netherlands, 1985. [Google Scholar]
- Salah, M.B.; Vilminot, S.; Andre, G.; Richard-Plouet, M.; Mhiri, T.; Takagi, S.; Kurmoo, M. Nuclear and Magnetic Structures and Magnetic Properties of the Layered Cobalt Hydroxysulfate Co5(OH)6(SO4)2(H2O)4 and Its Deuterated Analogue, Co5(OD)6(SO4)2(D2O)4. J. Am. Chem. Soc. 2006, 128, 7972–7981. [Google Scholar] [CrossRef]
- Maalej, W.; Vilminot, S.; Andre, G.; Kurmoo, M. Synthesis, magnetic structure, and properties of a layered cobalt-hydroxide ferromagnet, Co5(OH)6(SeO4)2(H2O)4. Inorg. Chem. 2010, 49, 3019–3024. [Google Scholar] [CrossRef]
- Palii, A.V.; Reu, O.S.; Ostrovsky, S.M.; Klokishner, S.I.; Tsukerblat, B.S.; Sun, Z.-M.; Mao, J.-G.; Prosvirin, A.V.; Zhao, H.-H.; Dunbar, K.R. A Highly Anisotropic Cobalt(II)-Based Single-Chain Magnet: Exploration of Spin Canting in an Antiferromagnetic Array. J. Am. Chem. Soc. 2008, 130, 14729–14738. [Google Scholar] [CrossRef]
- Leclercq, B.; Kabbour, H.; Damay, F.; Colin, C.V.; Pautrat, A.; Arevalo-Lopez, A.M.; Mentre, O. Metamagnetic Transitions versus Magnetocrystalline Anisotropy in Two Cobalt Arsenates with 1D Co(2+) Chains. Inorg. Chem. 2019, 58, 12609–12617. [Google Scholar] [CrossRef]
- Oshio, H.; Okamoto, H.; Kikuchi, T.; Ito, T. The Ferromagnetic Chain System catena-(µ-CrO4-O,O′)[NiII(cyclam)]·2H2O. Inorg. Chem. 1997, 36, 3201–3203. [Google Scholar] [CrossRef]
- Choi, K.-Y.; Suh, I.-H.; Kim, D.W. Structure and magnetic properties of catena-(μ-CrO4-O,O′)[MII(L)]·5H2O (M=NiII, CuII.; L=3,14-dimethyl-2,6,13,17-tetraazatricyclo[14,4,01.18,07.12]docosane). Inorg. Chim. Acta 1999, 293, 100–105. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Winter, M.; Wieghardt, K.; Gehring, S.; Haase, W.; Nuber, B.; Weisss, J. Syntheses and Magnetic Properties of a Heteropolyoxotungsten(VI)iron(III) Cation and [LFeII(μ-MO4)3FeIIIL] Complexes (M = Chromium(VI), Molybdenum(VI)). Crystal Structures of L2Fe2(CrO4)3·H2O and [L3Fe3W4O14(OCH3)3](C1O4)2·0.5H2O (L = 1,4,7-Trimethyl-1,4,7-triazacyclononane). Inorg. Chem. 1988, 27, 1562–1569. [Google Scholar] [CrossRef]
- Daszkiewicz, M.; Bronowska, W.; Pietraszko, A.; Staszak, Z.; Cieślak-Golonka, M. Coordination geometry of chromium(VI) species. The first example of cis-bridging chromate ions in helically structured catena(μ-CrO4-O,O′)[Ni(HIm)3H2O]. J. Mol. Struct. 2005, 754, 124–132. [Google Scholar] [CrossRef]
- Lee, K.; Lee, P.H. Efficient homo-coupling reactions of heterocyclic aromatic bromides catalyzed by Pd(OAc)2 using indium. Tetrahedron Lett. 2008, 49, 4302–4305. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1993. [Google Scholar]
- Sheldrick, G.M. SHELXL2014; University of Goëttingen: Goëttingen, Germany, 2014. [Google Scholar]
- Mulay, L.N.; Boudreaux, E.A. Theory and Applications of Molecular Diamagnetism; Wiley-VCH: New York, NY, USA, 1976; pp. 491–494, ISBN-10 047162358X. [Google Scholar]
- O’Keeffe, M.; Peskov, M.A.; Ramsden, S.J.; Yaghi, O.M. The Reticular Chemistry Structure Resource (RCSR) Database of, and Symbols for, Crystal Nets. Acc. Chem. Res. 2008, 41, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.-D.; Yang, C.-I. Utilization of ligand containing 2,2′-bipyridyl and tetrazolate groups to construct of a 2D Co(II) coordination polymer: Spin canting and metamagnetism. Dalton Trans. 2014, 43, 15576–15582. [Google Scholar] [CrossRef] [PubMed]
- Metropolis, M.; Rosenbluth, A.W.; Rosenbluth, M.N.; Teller, A.H. Equation of State Calculations by Fast Computing Machines. J. Chem. Phys. 1953, 21, 1087–1092. [Google Scholar] [CrossRef] [Green Version]
- Li, R.-Y.; Wang, X.-Y.; Liu, T.; Xu, H.-B.; Zhao, F.; Wang, Z.-M.; Gao, S. Synthesis, structure, and magnetism of three azido-bridged Co2+ compounds with a flexible coligand 1,2-(tetrazole-1-yl)ethane. Inorg. Chem. 2008, 47, 8134–8142. [Google Scholar] [CrossRef]
- Jiang, Y.-C.; Wang, S.-L.; Lee, S.-F.; Lii, K.-H. Metamagnetism in cobalt phosphates with pillared layer structures: [Co3(pyz)(HPO4)2F2] and [Co3(4,4′-bpy)(HPO4)2F2]·xH2O. Inorg. Chem. 2004, 43, 2564–2571. [Google Scholar] [CrossRef]
- Shao, D.; Zhou, Y.; Pi, Q.; Shen, F.-X.; Yang, S.-R.; Zhang, S.-L.; Wang, X.-Y. Two-dimensional frameworks formed by pentagonal bipyramidal cobalt(II) ions and hexacyanometallates: Antiferromagnetic ordering, metamagnetism and slow magnetic relaxation. Dalton Trans. 2017, 46, 9088–9096. [Google Scholar] [CrossRef]
- Zou, J.-Y.; Shi, W.; Gao, H.-L.; Cui, J.-Z.; Cheng, P. Spin canting and metamagnetism in 3D pillared-layer homospin cobalt(II) molecular magnetic materials constructed via a mixed ligands approach. Inorg. Chem. Front. 2014, 1, 242–248. [Google Scholar] [CrossRef]
- Yang, B.-P.; Prosvirin, A.V.; Guo, Y.-Q.; Mao, J.-G. Co[HO2C(CH2)3NH(CH2PO3H)2]2: A New Canted Antiferromagnet. Inorg. Chem. 2008, 47, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Balanda, M.; Falk, K.; Griesar, K.; Tomkowicz, Z.; Haase, W. Characterization of magnetic ordering in porphyrin-based molecular magnets [Mn(R)4TPP][TCNE] (R = OC12H25, F, CN). J. Magn. Magn. Mater. 1999, 205, 14–26. [Google Scholar] [CrossRef]
- Lazari, G.; Stamatatos, T.C.; Raptopoulou, P.C.; Psycharis, V.; Pissas, M.; Perlepes, S.P.; Boudalis, A.K. A metamagnetic 2D copper(II)-azide complex with 1D ferromagnetism and a hysteretic spin-flop transition. Dalton Trans. 2009, 3215–3221. [Google Scholar] [CrossRef] [PubMed]




| Formula | C8H8CrN4NiO5 |
|---|---|
| Fw | 350.89 |
| Crystal system | Monoclinic |
| Space group | P21 |
| a/Å | 6.6931(2) |
| b/Å | 8.4975(2) |
| c/Å | 9.8328(2) |
| α/° | 90 |
| β/° | 102.7114(9) |
| γ/° | 90 |
| V/Å3 | 545.53(2) |
| Z | 2 |
| T/K | 150(2) |
| Dc/g cm−3 | 2.136 |
| μ/mm−1 | 2.745 |
| Rint | 0.0255 |
| θmax/° | 34.983 |
| Goodness-of-fits on F2 | 1.030 |
| R11, wR22 (all data) | 0.0274, 0.0611 |
| R11, wR22 (I > 2σ (I)) | 0.0255, 0.0598 |
| Flack | 0.026(13) |
| Compound 1 | |||
|---|---|---|---|
| Ni(1)-O(2) 1 | 2.0233(19) | Ni(1)-O(5) | 2.087(2) |
| Ni(1)-O(1) | 2.0242(17) | Ni(1)-N(1) | 2.0998(19) |
| Ni(1)-O(3) 2 | 2.0505(17) | Ni(1)-N(3) 3 | 2.122(2) |
| Cr(1)-O(4) | 1.6132(18) | Cr(1)-O(2) | 1.647(2) |
| Cr(1)-O(3) | 1.6594(18) | Cr(1)-O(1) | 1.6649(19) |
| O(3)2-Ni(1)-N(1) | 88.17(7) | O(1)-Ni(1)-N(3) 3 | 89.57(8) |
| O(5)-Ni(1)-N(1) | 92.04(8) | O(3)2-Ni(1)-N(3) 3 | 90.88(7) |
| O(2)1-Ni(1)-N(3) 3 | 87.18(8) | O(5)-Ni(1)-N(3) 3 | 92.32(8) |
| O(2)1-Ni(1)-O(1) | 88.20(11) | N(1)-Ni(1)-N(3) 3 | 175.51(8) |
| O(2)1-Ni(1)-O(3) 2 | 89.96(10) | Cr(1)-O(1)-Ni(1) | 134.85(13) |
| O(1)-Ni(1)-O(3) 2 | 178.08(11) | Cr(1)-O(2)-Ni(1) 4 | 159.65(14) |
| O(2)1-Ni(1)-O(5) | 178.50(11) | Cr(1)-O(3)-Ni(1) 5 | 145.98(14) |
| O(1)-Ni(1)-O(5) | 93.21(10) | C(2)-N(1)-Ni(1) | 122.84(15) |
| O(3)2-Ni(1)-O(5) | 88.64(9) | C(1)-N(1)-Ni(1) | 120.28(16) |
| O(2)1-Ni(1)-N(1) | 88.44(8) | C(5)-N(3)-Ni(1) 6 | 119.9(5) |
| O(1)-Ni(1)-N(1) | 91.25(8) | C(6)-N(3)-Ni(1) 6 | 121.83(15) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.-Y.; Lee, G.-H.; Ng, K.-K.; Yang, C.-I. A Three-Dimensional Coordination Framework with a Ferromagnetic Coupled Ni(II)-CrO4 Layer: Synthesis, Structure, and Magnetic Studies. Polymers 2022, 14, 1735. https://doi.org/10.3390/polym14091735
Tang H-Y, Lee G-H, Ng K-K, Yang C-I. A Three-Dimensional Coordination Framework with a Ferromagnetic Coupled Ni(II)-CrO4 Layer: Synthesis, Structure, and Magnetic Studies. Polymers. 2022; 14(9):1735. https://doi.org/10.3390/polym14091735
Chicago/Turabian StyleTang, Hsu-Yen, Gene-Hsiang Lee, Kwai-Kong Ng, and Chen-I Yang. 2022. "A Three-Dimensional Coordination Framework with a Ferromagnetic Coupled Ni(II)-CrO4 Layer: Synthesis, Structure, and Magnetic Studies" Polymers 14, no. 9: 1735. https://doi.org/10.3390/polym14091735






