Release of Bisphenol A from Pit and Fissure Sealants According to Different pH Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Pre-Investigation on the Amount of Sealant
2.2.2. Sealant Specimens for BPA Release Test
2.2.3. Procedure of BPA Release Test
2.2.4. Conditions and Calibration of GC/MS
2.3. Statistical Analysis
3. Results
3.1. The Difference in BPA Concentration (ppm) According to pH Levels and Time
3.2. Comparison of BPA Concentration According to pH Level and Time of Each Sealant
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferracane, J.L. Current trends in dental composites. Crit. Rev. Oral. Biol. Med. 1995, 6, 302–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahovuo-Saloranta, A.; Forss, H.; Walsh, T.; Nordblad, A.; Mäkelä, M.; Worthington, H.V. Pit and fissure sealants for preventing dental decay in permanent teeth. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef] [Green Version]
- Azarpazhooh, A.; Main, P.A. Pit and fissure sealants in the prevention of dental caries in children and adolescents: A systematic review. J. Can. Dent. Assoc. 2008, 74, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Wiener, R.C.; Findley, P.A.; Shen, C.; Dwibedi, N.; Sambamoorthi, U. Acculturation and dental sealant use among US children. Community Dent. Oral Epidemiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Olea, N.; Pulgar, R.; Pérez, P.; Olea-Serrano, F.; Rivas, A.; Novillo-Fertrell, A.; Pedraza, V.; Soto, A.M.; Sonnenschein, C. Estrogenicity of resin-based composites and sealants used in dentistry. Environ. Health Perspect. 1996, 104, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Schafer, T.E.; Lapp, C.A.; Hanes, C.M.; Lewis, J.B.; Wataha, J.C.; Schuster, G.S. Estrogenicity of bisphenol A and bisphenol A dimethacrylate in vitro. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. 1999, 45, 192–197. [Google Scholar] [CrossRef]
- Fung, E.Y.; Ewoldsen, N.O.; Germain, H.A.S., Jr.; Marx, D.B.; Miaw, C.-L.; Siew, C.; Chou, H.-N.; Gruninger, S.E.; Meyer, D.M. Pharmacokinetics of bisphenol A released from a dental sealant. J. Am. Dent. Assoc. 2000, 131, 51–58. [Google Scholar] [CrossRef]
- Vogel, S.A. The politics of plastics: The making and unmaking of bisphenol a “safety”. Am. J. Public Health 2009, 99, S559–S566. [Google Scholar] [CrossRef]
- Hamid, A.; Hume, W.R. A study of component release from resin pit and fissure sealants in vitro. Dent. Mater. 1997, 13, 98–102. [Google Scholar] [CrossRef]
- Moriyama, K.; Tagami, T.; Akamizu, T.; Usui, T.; Saijo, M.; Kanamoto, N.; Hataya, Y.; Shimatsu, A.; Kuzuya, H.; Nakao, K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J. Clin. Endocrinol. Metab. 2002, 87, 5185–5190. [Google Scholar] [CrossRef] [PubMed]
- Maffini, M.V.; Rubin, B.S.; Sonnenschein, C.; Soto, A.M. Endocrine disruptors and reproductive health: The case of bisphenol-A. Mol. Cell. Endocrinol. 2006, 254, 179–186. [Google Scholar] [CrossRef]
- Chapin, R.E.; Adams, J.; Boekelheide, K.; Gray, L.E., Jr.; Hayward, S.W.; Lees, P.S.; McIntyre, B.S.; Portier, K.M.; Schnorr, T.M.; Selevan, S.G. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2008, 83, 157–395. [Google Scholar] [CrossRef] [PubMed]
- Nah, W.H.; Park, M.J.; Gye, M.C. Effects of early prepubertal exposure to bisphenol A on the onset of puberty, ovarian weights, and estrous cycle in female mice. Clin. Exp. Reprod. Med. 2011, 38, 75. [Google Scholar] [CrossRef] [Green Version]
- Hunt, P.A.; Susiarjo, M.; Rubio, C.; Hassold, T.J. The bisphenol A experience: A primer for the analysis of environmental effects on mammalian reproduction. Biol. Reprod. 2009, 81, 807–813. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.A.; Welshons, W.V.; Vom Saal, F.S. No effect of route of exposure (oral; subcutaneous injection) on plasma bisphenol A throughout 24 h after administration in neonatal female mice. Reprod. Toxicol. 2008, 25, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Braun, J.M.; Kalkbrenner, A.E.; Calafat, A.M.; Yolton, K.; Ye, X.; Dietrich, K.N.; Lanphear, B.P. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics 2011, 128, 873–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes-Rocha, L.; Ribeiro-Goncalves, L.; Henriques, B.; Ozcan, M.; Tiritan, M.E.; Souza, J.C.M. An integrative review on the toxicity of Bisphenol A (BPA) released from resin composites used in dentistry. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1942–1952. [Google Scholar] [CrossRef] [PubMed]
- Pachori, A.; Kambalimath, H.; Maran, S.; Niranjan, B.; Bhambhani, G.; Malhotra, G. Evaluation of changes in salivary pH after intake of different eatables and beverages in children at different time intervals. Int. J. Clin. Pediatric Dent. 2018, 11, 177. [Google Scholar] [CrossRef]
- Nielsen, S.J.; Popkin, B.M. Changes in beverage intake between 1977 and 2001. Am. J. Prev. Med. 2004, 27, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Storey, M.L.; Forshee, R.A.; Anderson, P.A. Beverage consumption in the US population. J. Am. Diet. Assoc. 2006, 106, 1992–2000. [Google Scholar] [CrossRef]
- Reddy, A.; Norris, D.F.; Momeni, S.S.; Waldo, B.; Ruby, J.D. The pH of beverages in the United States. J. Am. Dent. Assoc. 2016, 147, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Okamoto, A.; Fukushima, M.; Okiji, T. Evaluation of flowable resin composite surfaces eroded by acidic and alcoholic drinks. Dent. Mater. J. 2008, 27, 455–465. [Google Scholar] [CrossRef] [Green Version]
- Jeon, C.-M.; Yoo, H.-M.; Kwon, H.-C. Effect of pH and storage time on the elution of residual monomers from polymerized composite resins. J. Korean Acad. Conserv. Dent. 2004, 29, 249–266. [Google Scholar] [CrossRef] [Green Version]
- Arenholt-Bindslev, D.; Breinholt, V.; Preiss, A.; Schmalz, G. Time-related bisphenol-A content and estrogenic activity in saliva samples collected in relation to placement of fissure sealants. Clin. Oral Investig. 1999, 3, 120–125. [Google Scholar] [CrossRef]
- Pulgar, R.; Olea-Serrano, M.F.; Novillo-Fertrell, A.; Rivas, A.; Pazos, P.; Pedraza, V.; Navajas, J.-M.; Olea, N. Determination of bisphenol A and related aromatic compounds released from bis-GMA-based composites and sealants by high performance liquid chromatography. Environ. Health Perspect. 2000, 108, 21–27. [Google Scholar] [CrossRef]
- Gruninger, S.; Tiba, A.; Vinh, R. Bisphenol A released from resin based dental sealants. ADA Prof. Prod. Rev. 2015, 10, 8–16. [Google Scholar]
- ABU-BAKR, N.H.; HAN, L.; OKAMOTO, A.; IWAKU, M. Effect of Alcoholic and Low-pH Soft Drinks on Fluoride Release from Compomer. J. Esthet. Restor. Dent. 2000, 12, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Øysæd, H.; Ruyter, I.E. Water Sorption and Filler Characteristics of Composites for Use in Posterior Teeth. J. Dent. Res. 1986, 65, 1315–1318. [Google Scholar] [CrossRef]
- Finer, Y.; Santerre, J.P. Biodegradation of a dental composite by esterases: Dependence on enzyme concentration and specificity. J. Biomater. Sci. Polym. Ed. 2003, 14, 837–849. [Google Scholar] [CrossRef]
- Göpferich, A. Mechanisms of polymer degradation and erosion. Biomaterials 1996, 17, 103–114. [Google Scholar] [CrossRef]
- Finer, Y.; Santerre, J.P. The influence of resin chemistry on a dental composite’s biodegradation. J. Biomed. Mater. Res. 2004, 69A, 233–246. [Google Scholar] [CrossRef]
- Atkinson, J.C.; Diamond, F.; Eichmiller, F.; Selwitz, R.; Jones, G. Stability of bisphenol A, triethylene-glycol dimethacrylate, and bisphenol A dimethacrylate in whole saliva. Dent. Mater. 2002, 18, 128–135. [Google Scholar] [CrossRef]
- Hoekman, D. Exploring QSAR fundamentals and applications in chemistry and biology, volume 1. hydrophobic, electronic and steric constants, Volume 2. J. Am. Chem. Soc. 1996, 39, 1189–1190. [Google Scholar] [CrossRef]
- Manabe, A.; Kaneko, S.; Numazawa, S.; Itoh, K.; Inoue, M.; Hisamitsu, H.; Sasa, R.; Yoshida, T. Detection of Bisphenol-A in Dental Materials by Gas Chromatography-Mass Spectrometry. Dent. Mater. J. 2000, 19, 75–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarja Laaninen. New Rules on Bisphenol A in Food Contact Materials. Available online: https://www.europarl.europa.eu/thinktank/en/document.html?reference=EPRS_ATA(2018)614705 (accessed on 1 November 2021).
- Ministry of Food and Drug Safety. Standards and Specifications for Appliances, Containers and Packaging [Announcement No. 2021-234]. Available online: https://www.mfds.go.kr/brd/m_209/view.do?seq=43478&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1 (accessed on 1 November 2021).
- Chang Yeob, S.; Woong, K.; Myung Chan, G. Current State of Use and the Risks of Bisphenols: A Minireview. Korean J. Environ. Biol. 2017, 35, 581–594. [Google Scholar] [CrossRef]
- Örtengren, U.; Andersson, F.; Elgh, U.; Terselius, B.; Karlsson, S. Influence of pH and storage time on the sorption and solubility behaviour of three composite resin materials. J. Dent. 2001, 29, 35–41. [Google Scholar] [CrossRef]



| Sealant | Composition (% by Wt) | Manufacturer | |
|---|---|---|---|
| A | ClinproTM | bis-GMA * (40~50), TEGDMA (40~50) | 3M ESPE, Seefeld, Germany |
| B | Eco-s® | bis-GMA * (50~55), TEGDMA (35~40) | Vericom, Gyeonggi, Korea |
| C | UltraSeal XT® plus | bis-GMA* (not revealed), TEGDMA (10~25), DUDMA (2.5~10) | Ultradent Products, South Jordan, UT, USA |
| D | Charmseal® | bis-GMA * (not revealed), TEGDMA, UDMA | DenKist, Gyeonggi, Korea |
| E | Seal-it® | bis-EMA * (30~50), TEGDMA (20~30) | Spident, Incheon, Korea |
| F | FORTIFY® | bis-DMA * (5~10), UDMA (30~50) | Bisco, Schaumburg, IL, USA |
| No. | N | Amount of Sealant (mg) | F/p | |
|---|---|---|---|---|
| M ± SD | Min–Max | |||
| 1 | 5 | 4.47 ± 0.30 | 4.11–4.85 | 4.812/0.007 |
| 2 | 5 | 5.92 ± 0.70 | 4.69–6.47 | |
| 3 | 5 | 4.71 ± 0.71 | 4.06–6.01 | |
| 4 | 5 | 4.38 ± 0.39 | 4.00–4.98 | |
| 5 | 5 | 5.03 ± 0.70 | 4.07–5.77 | |
| Total | 25 | 4.90 ± 0.79 | 4.00–6.47 | |
| Conditions | N | Sealant Specimens (ea) | ||
|---|---|---|---|---|
| 10 min | 1 h | 24 h | ||
| pH 3.0 | 90 | 30 (6 S × 5 each) | 30 (6 S × 5 each) | 30 (6 S × 5 each) |
| pH 6.5 | 90 | 30 (6 S × 5 each) | 30 (6 S × 5 each) | 30 (6 S × 5 each) |
| pH 10.0 | 90 | 30 (6 S × 5 each) | 30 (6 S × 5 each) | 30 (6 S × 5 each) |
| Total | 270 | 90 | 90 | 90 |
| Conditions | ||||
|---|---|---|---|---|
| Column | HP-5ms Ultra Inert (30 m 250 μm 0.25 μm) | |||
| Oven Temp. | Unit | Rate (℃/min) | Temp. (℃) | Hold (min) |
| Initial | - | 40 | 0 | |
| Ramp1 | 5 | 50 | 0 | |
| Ramp2 | 5 | 80 | 2 | |
| Ramp3 | 10 | 120 | 5 | |
| Ramp4 | 10 | 280 | 1 | |
| Ramp5 | 10 | 320 | 0 | |
| Inlet Temp. | 250 ℃ | |||
| Injection Mode | splitless | |||
| Injection Vol. | 1 μL | |||
| Carrier Gas | Helium | |||
| Carrier Flow | 0.7 mL/min | |||
| Scan Parameters | 40~615 | |||
| Sim Parameters | bisphenol A: 213.3, 119, 228, 214 | |||
| Group | BPA (ppm) | ||||
| 10 min M (SD) | 1 h M (SD) | 24 h M (SD) | (Min–Max) | F/p | |
| pH 3.0 | 0.35 (0.30) b,A | 0.72 (0.73) b,A | 2.14 (2.55) a,A | (0.07–7.74) | 11.196 * |
| pH 6.5 | 0.09 (0.14) b,B | 0.18 (0.23) ab,B | 0.28 (0.28) a,B | (<0.01–1.06) | 5.303 * |
| pH 10.0 | 0.09 (0.13) b,B | 0.25 (0.31) b,B | 0.52 (0.60) a,B | (<0.01–2.81) | 9.189 * |
| F/p | 15.492 * | 11.518 * | 13.158 * | ||
| Source | F/p | ||||
| pH | 24.440 * | ||||
| Time | 18.153 * | ||||
| pH * Time | 7.361 * | ||||
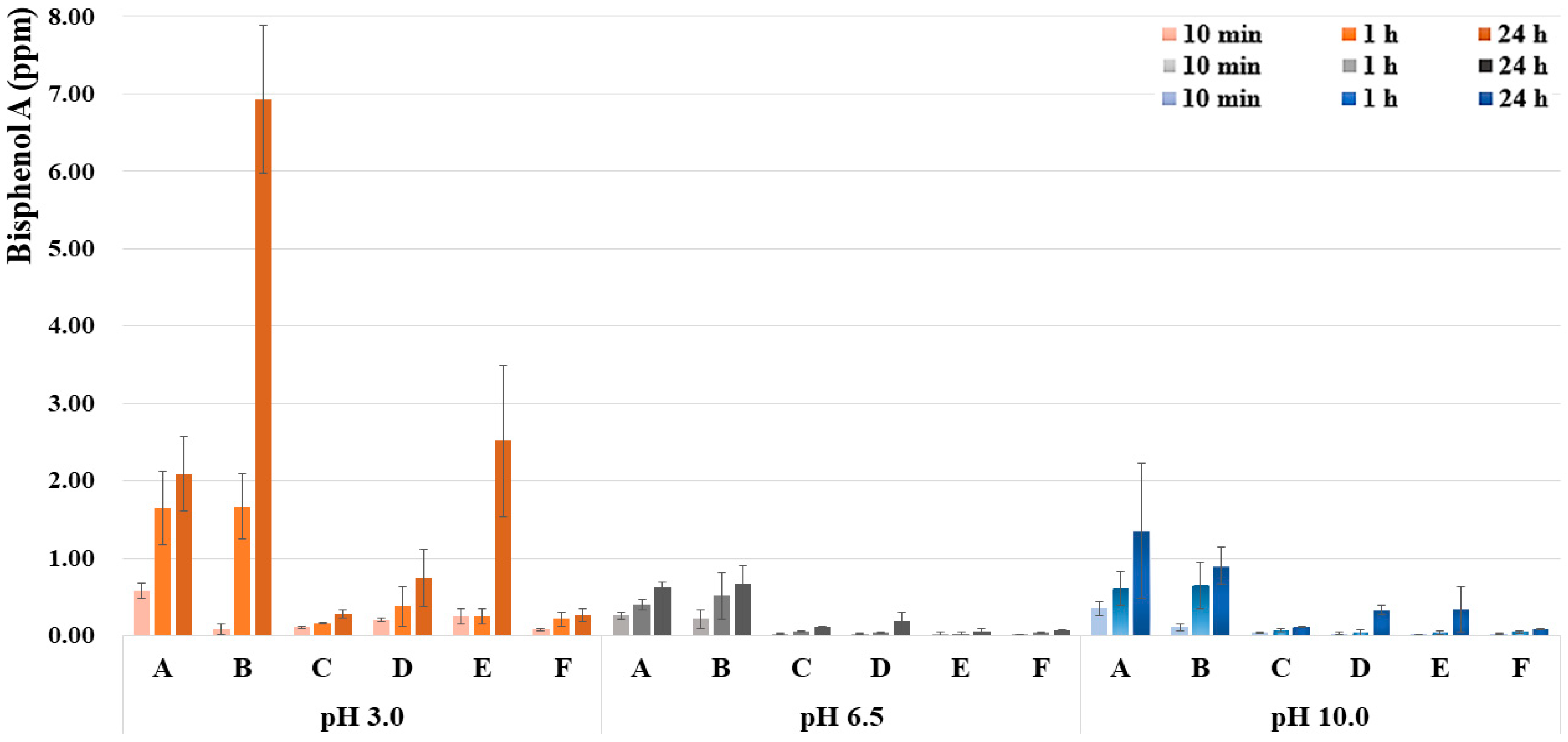
| Sealants/Group | Bisphenol A (ppm) | ||||
|---|---|---|---|---|---|
| 10 min M (SD) | 1 h M (SD) | 24 h M (SD) | F/p | ||
| A | pH 3.0 | 0.58 (0.10) b,A | 1.65 (0.47) a,A | 2.09 (0.48) a,A | 19.638 * |
| pH 6.5 | 0.26 (0.05) c,B | 0.40 (0.07) b,B | 0.62 (0.08) a,AB | 35.813 * | |
| pH 10.0 | 0.35 (0.09) b,B | 0.61 (0.22) ab,B | 1.36 (0.87) a,B | 5.049 * | |
| F/p | 19.457 * | 24.396 * | 8.192 * | ||
| B | pH 3.0 | 0.09 (0.07) b,A | 1.67 (0.42) b,A | 6.93 (0.95) a,A | 145.406 * |
| pH 6.5 | 0.22 (0.12) b,B | 0.52 (0.30) ab,B | 0.67 (0.24) a,B | 4.019 * | |
| pH 10.0 | 0.11 (0.05) b,B | 0.65 (0.30) a,B | 0.90 (0.24) a,B | 16.704 * | |
| F/p | 33.311 * | 16.905 * | 186.455 * | ||
| C | pH 3.0 | 0.11 (0.01) b,A | 0.16 (0.01) b,A | 0.28 (0.05) a,A | 42.419 * |
| pH 6.5 | 0.03 (<01) c,B | 0.06 (<01) b,B | 0.11 (0.01) a,B | 201.652 * | |
| pH 10.0 | 0.04 (0.01) c,B | 0.07 (0.02) b,B | 0.12 (0.01) a,B | 64.039 * | |
| F/p | 121.907 * | 97.903 * | 51.062 * | ||
| D | pH 3.0 | 0.21 (0.02) b,A | 0.38 (0.25) ab,A | 0.75 (0.37) a,A | 5.780 * |
| pH 6.5 | 0.03 (0.04) b,B | 0.04 (<01) b,B | 0.19 (0.12) a,B | 8.322 * | |
| pH 10.0 | 0.03 (0.01) b,B | 0.04 (0.04) b,B | 0.33 (0.06) a,B | 61.164 * | |
| F/p | 374.387 * | 8.902 * | 8.138 * | ||
| E | pH 3.0 | 0.25 (0.10) b,A | 0.25 (0.10) b,A | 2.52 (0.98) a,A | 4.188 * |
| pH 6.5 | 0.02 (0.02) B | 0.02 (0.02) B | 0.05 (0.04) AB | 0.851 | |
| pH 10.0 | 0.02 (<01) B | 0.04 (0.02) B | 0.34 (0.30) B | 2.462 | |
| F/p | 26.069 * | 23.278 * | 4.292 * | ||
| F | pH 3.0 | 0.08 (0.01) b,A | 0.22 (0.09) a,A | 0.27 (0.08) a,A | 10.108 * |
| pH 6.5 | 0.02 (<01) c,B | 0.04 (<01) b,B | 0.07 (<01) a,B | 781.970 * | |
| pH 10.0 | 0.02 (0.01) c,B | 0.05 (0.02) b,B | 0.09 (0.01) a,B | 49.230 * | |
| F/p | 124.536 * | 17.505 * | 32.187 * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, E.-D.; Lee, S.-B.; Kang, C.-M.; Kim, K.-M.; Kwon, J.-S. Release of Bisphenol A from Pit and Fissure Sealants According to Different pH Conditions. Polymers 2022, 14, 37. https://doi.org/10.3390/polym14010037
Jo E-D, Lee S-B, Kang C-M, Kim K-M, Kwon J-S. Release of Bisphenol A from Pit and Fissure Sealants According to Different pH Conditions. Polymers. 2022; 14(1):37. https://doi.org/10.3390/polym14010037
Chicago/Turabian StyleJo, Eun-Deok, Sang-Bae Lee, Chung-Min Kang, Kwang-Mahn Kim, and Jae-Sung Kwon. 2022. "Release of Bisphenol A from Pit and Fissure Sealants According to Different pH Conditions" Polymers 14, no. 1: 37. https://doi.org/10.3390/polym14010037








