Effects and Impacts of Different Oxidative Digestion Treatments on Virgin and Aged Microplastic Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
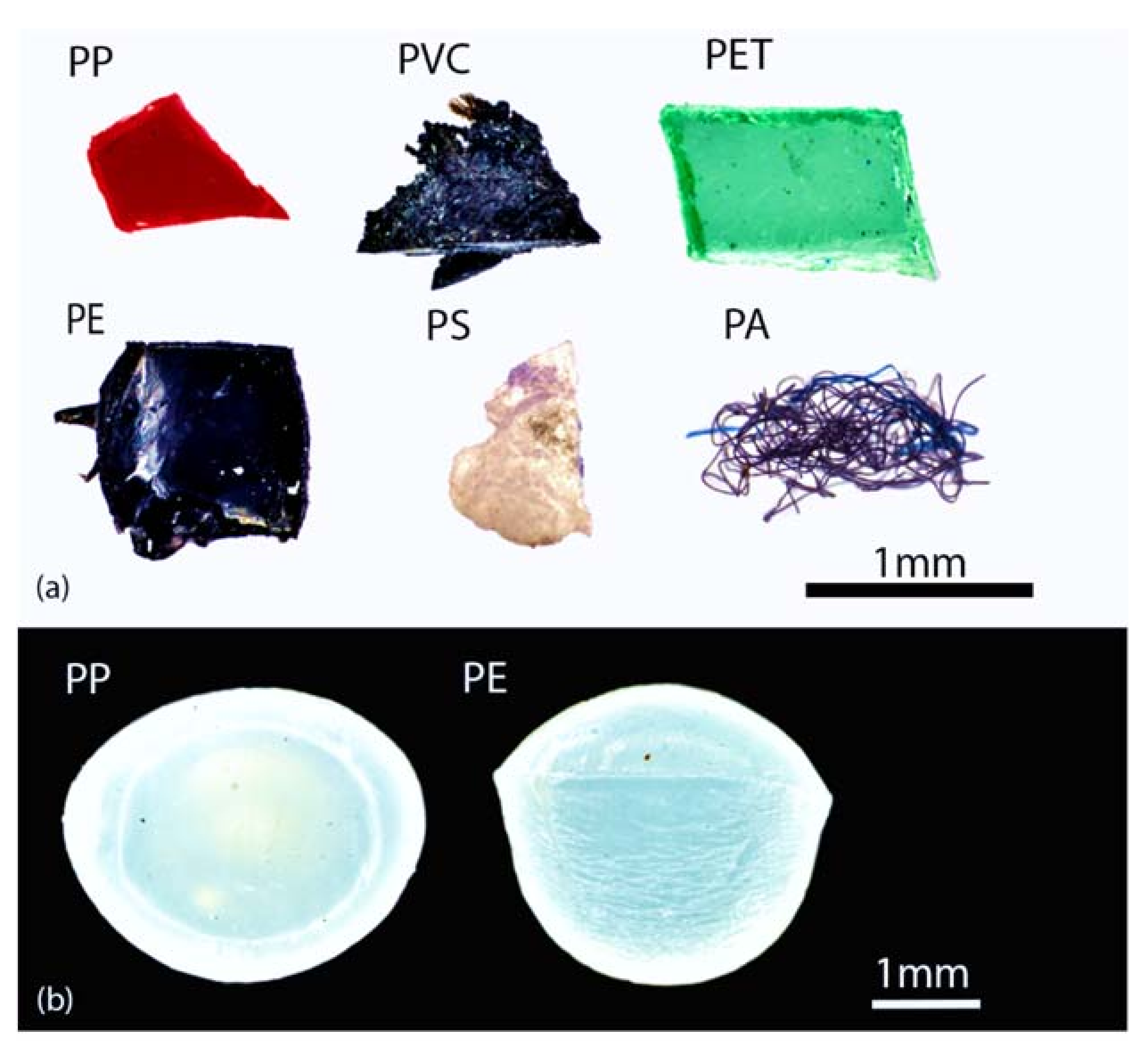
2.2. Microplastic Standards Selection
2.3. Experiment One: Evaluating the Efficiency of MPs Digestion Treatment through Recovery Tests
Digestion Treatment Conditions
2.4. Experiment Two: Evaluating the Impact of Digestion on Virgin vs. Aged MPs Integrity
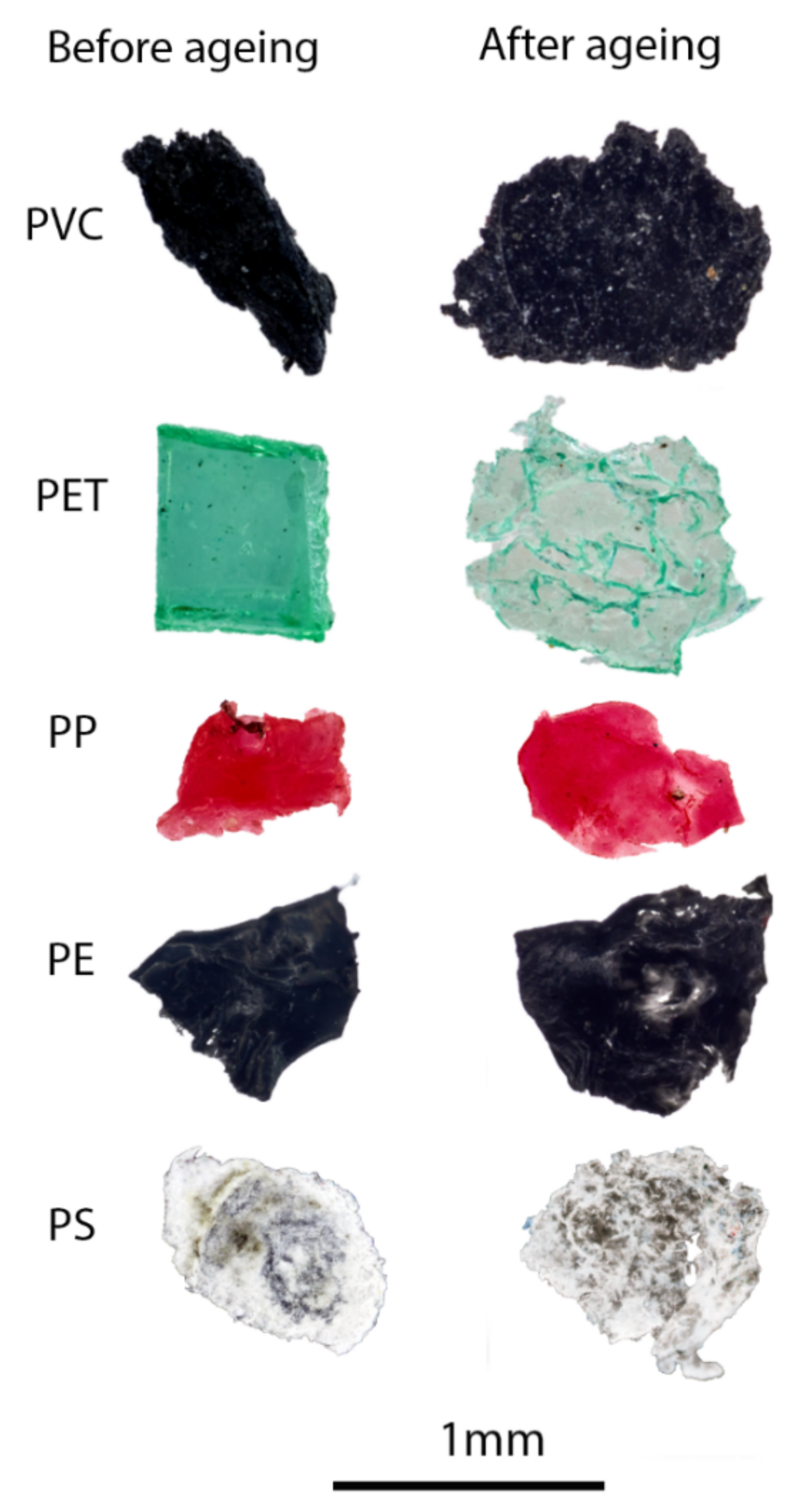
2.4.1. Ageing of Microplastics
2.4.2. Digestion Treatment Conditions
2.5. Fourier Transform Infrared Spectroscopy (FTIR) Acquisition
2.6. Scanning Electron Microscopy (SEM) Acquisition
2.7. Quality Control
3. Results
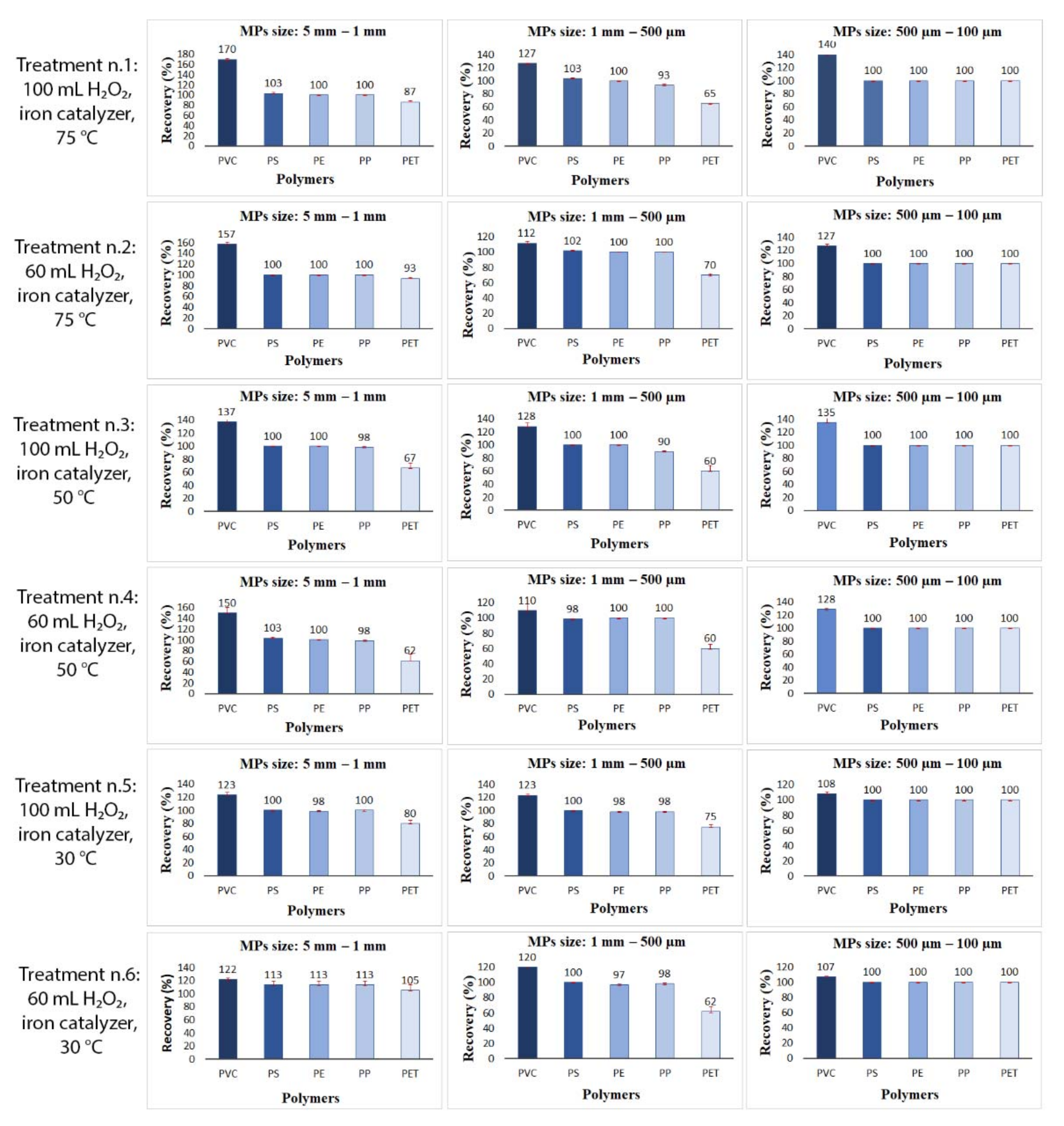
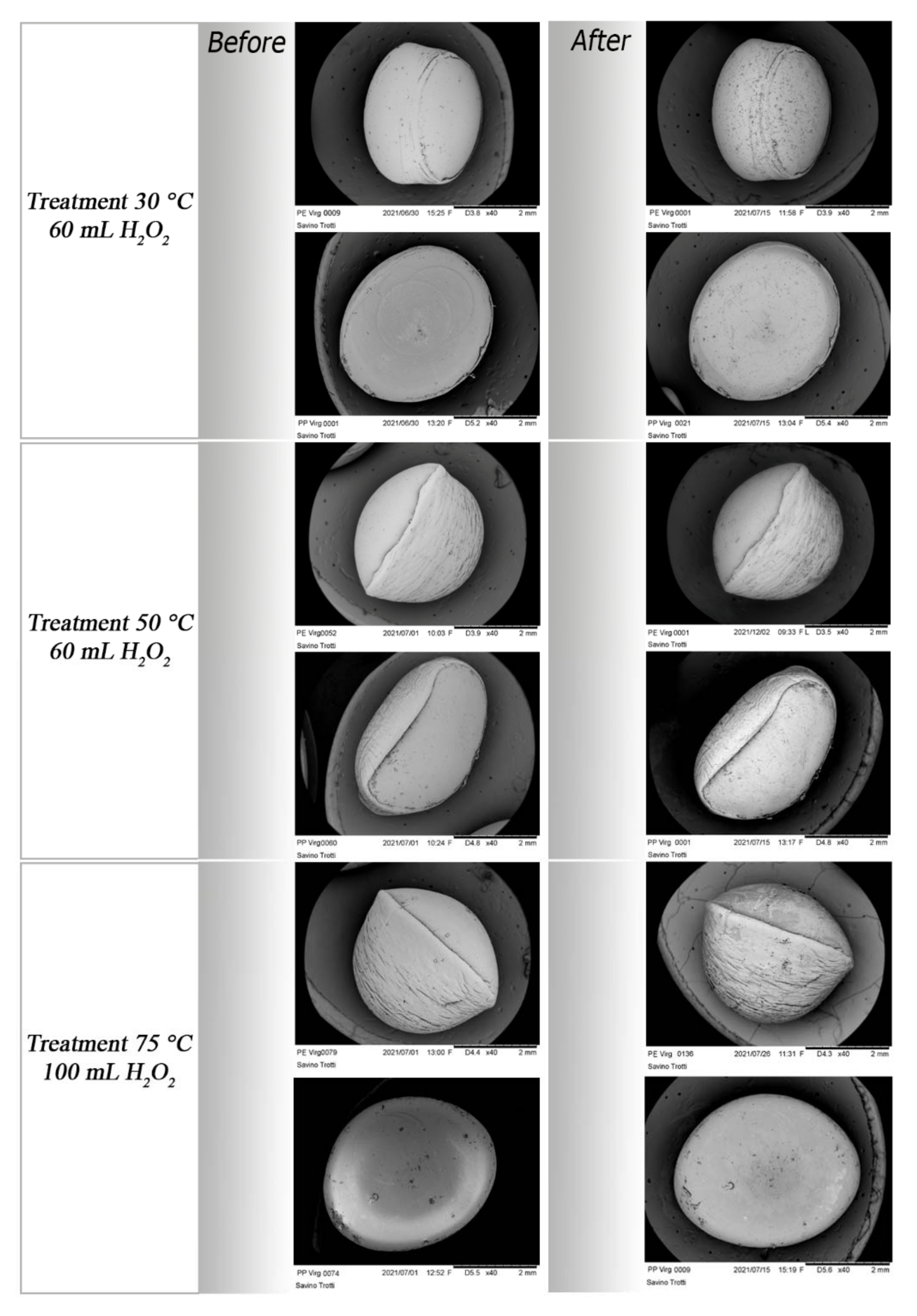
3.1. Results of Experiment One: Evaluating the Efficiency of MPs Digestion Treatment through Recovery Tests
3.2. Results of Experiment Two: Evaluating the Impact of Digestion Treatment on Virgin and Aged MPs through Qualitative Evaluations
3.2.1. Ageing of Microplastics: FTIR Acquisition
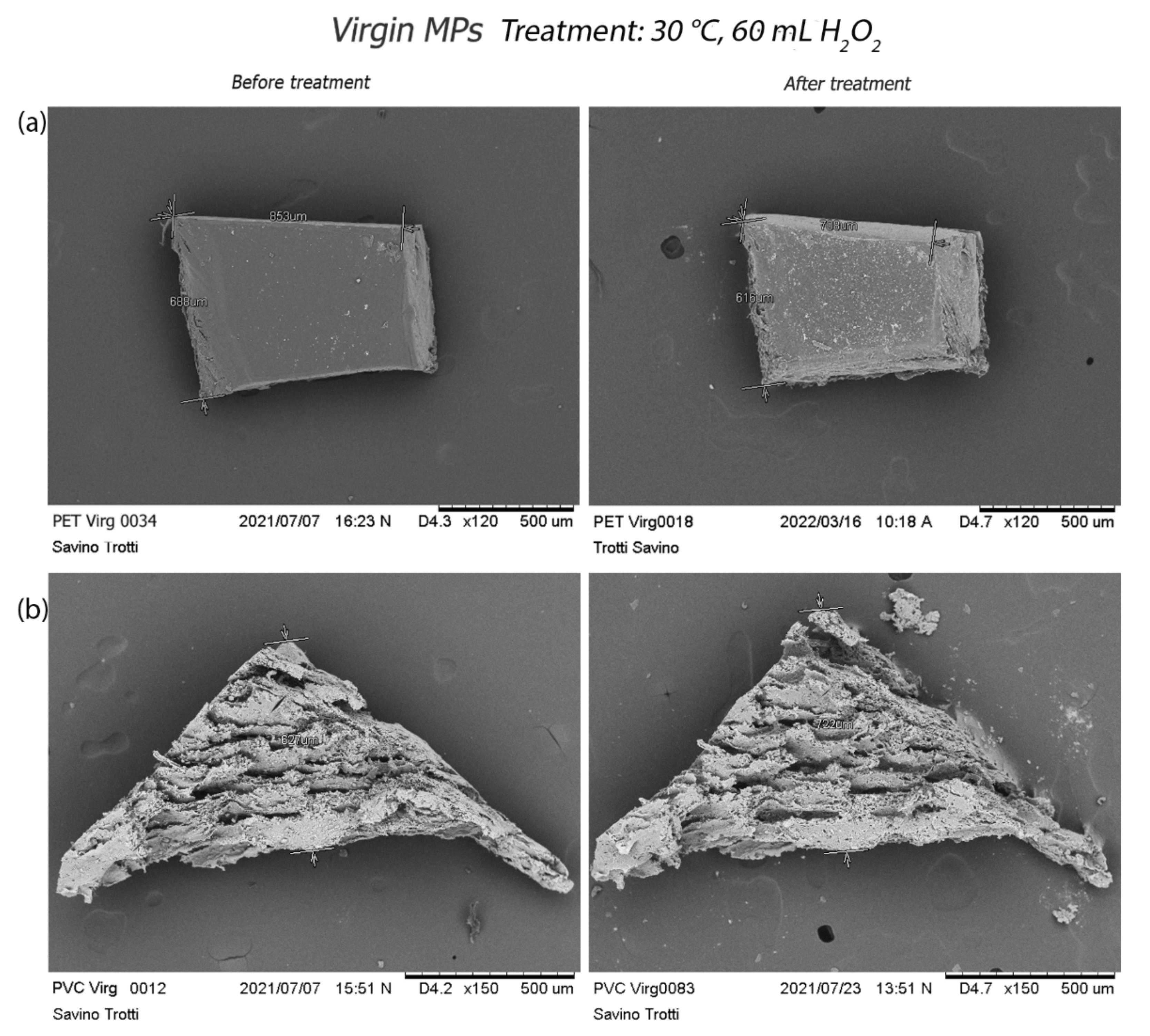
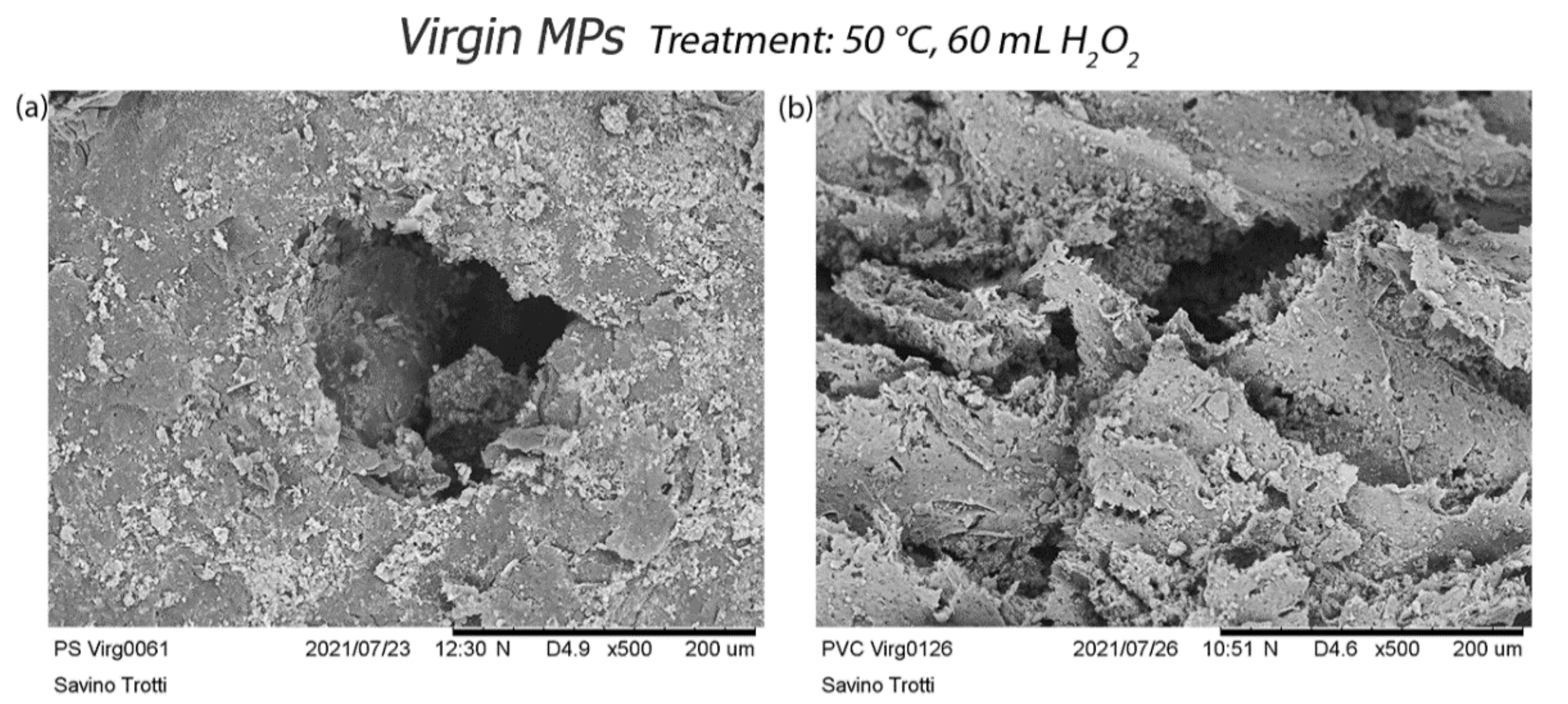
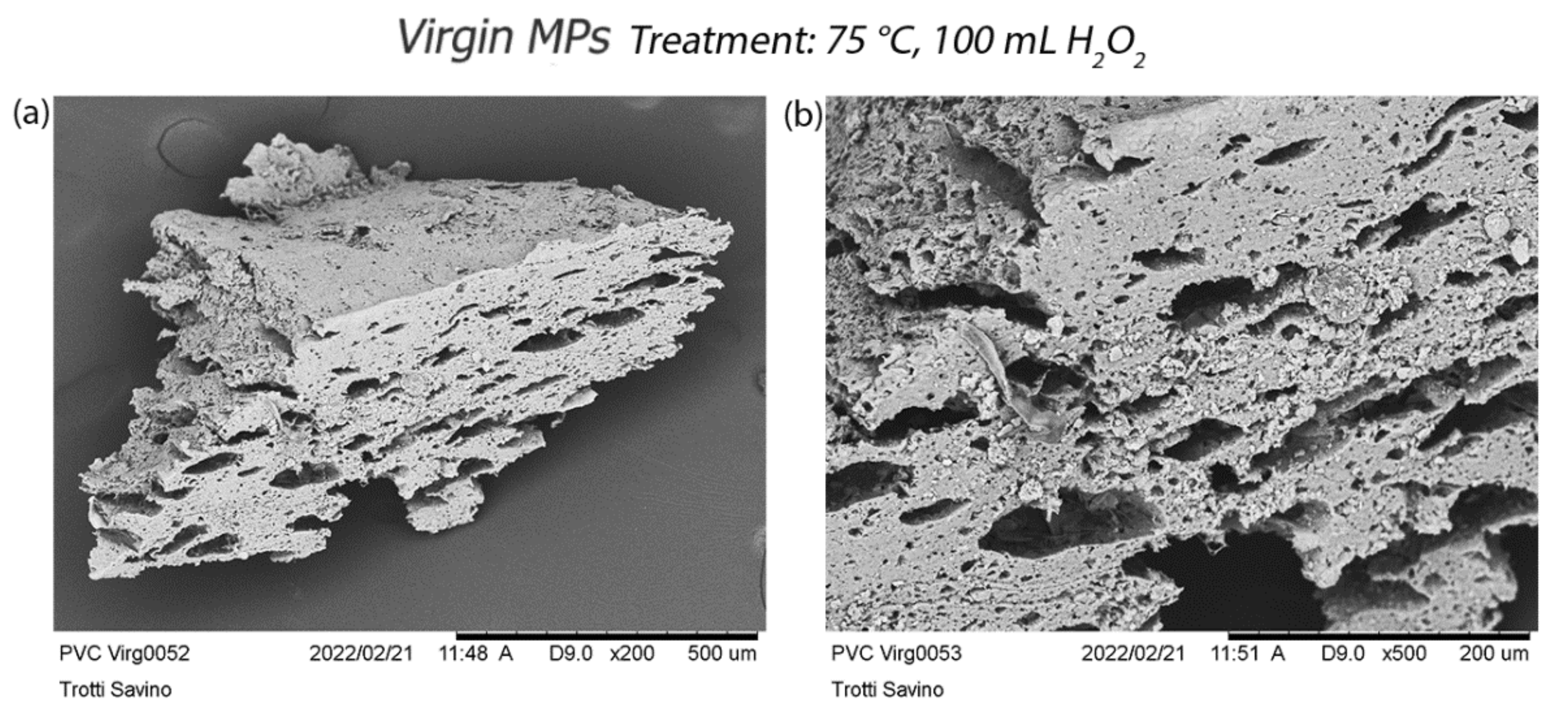
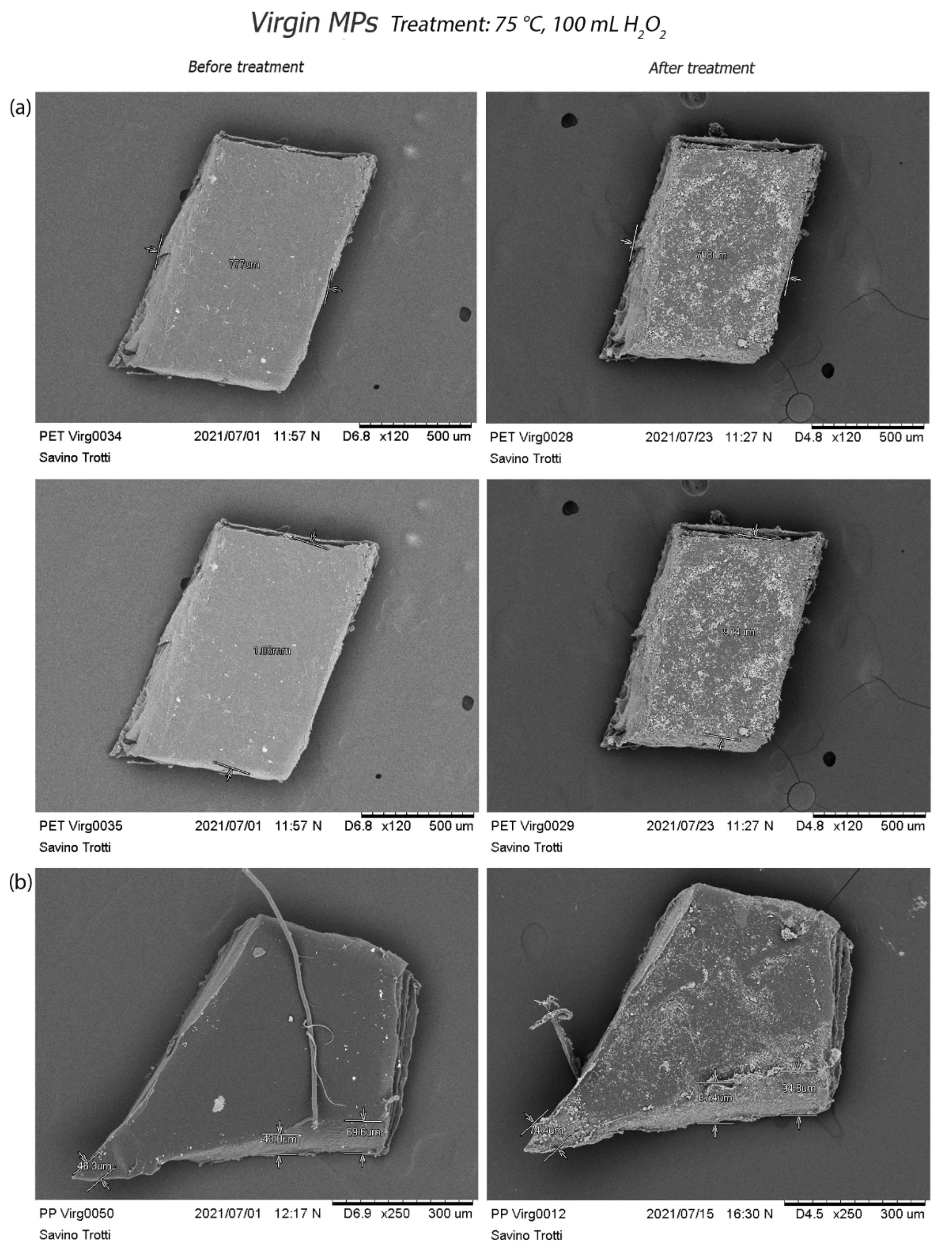
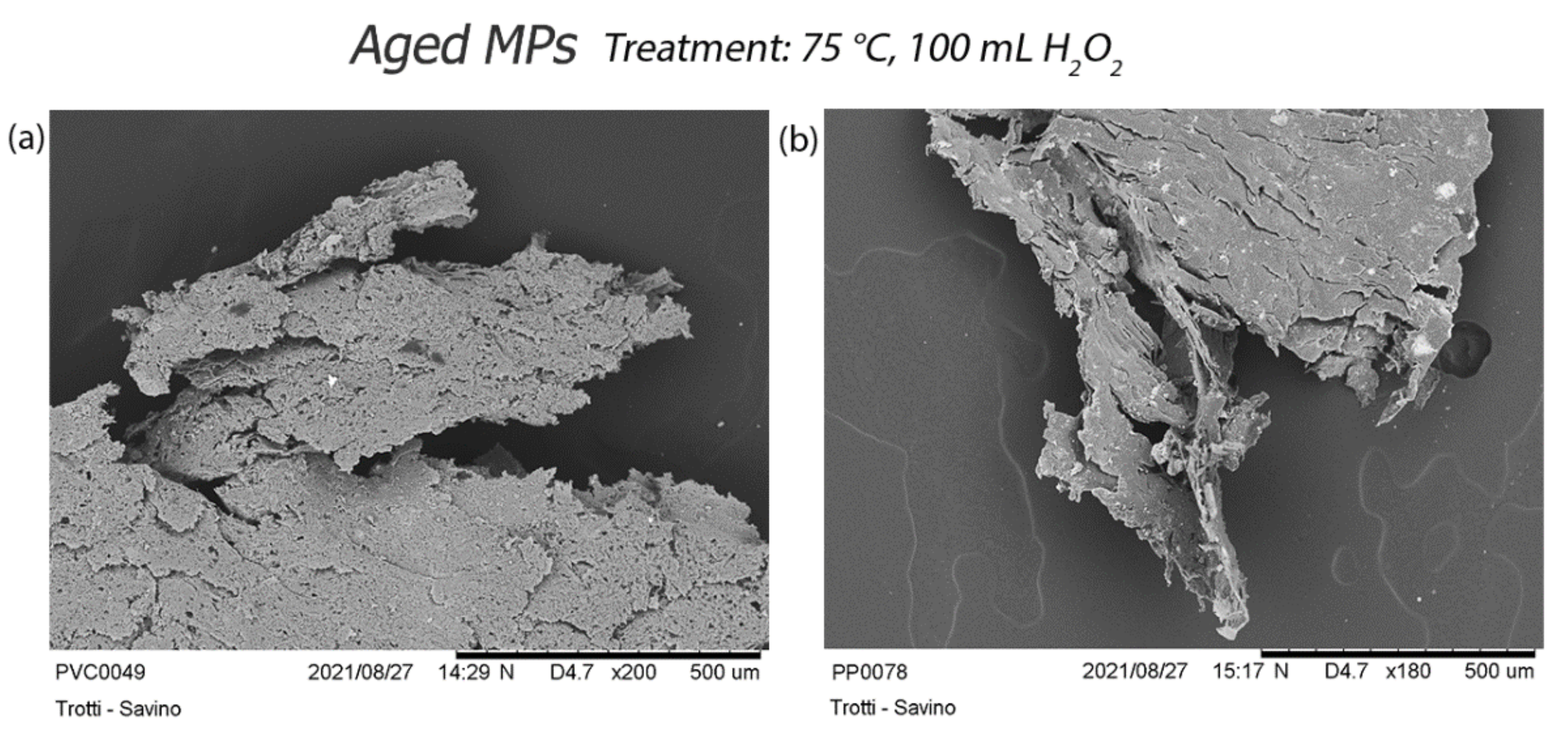
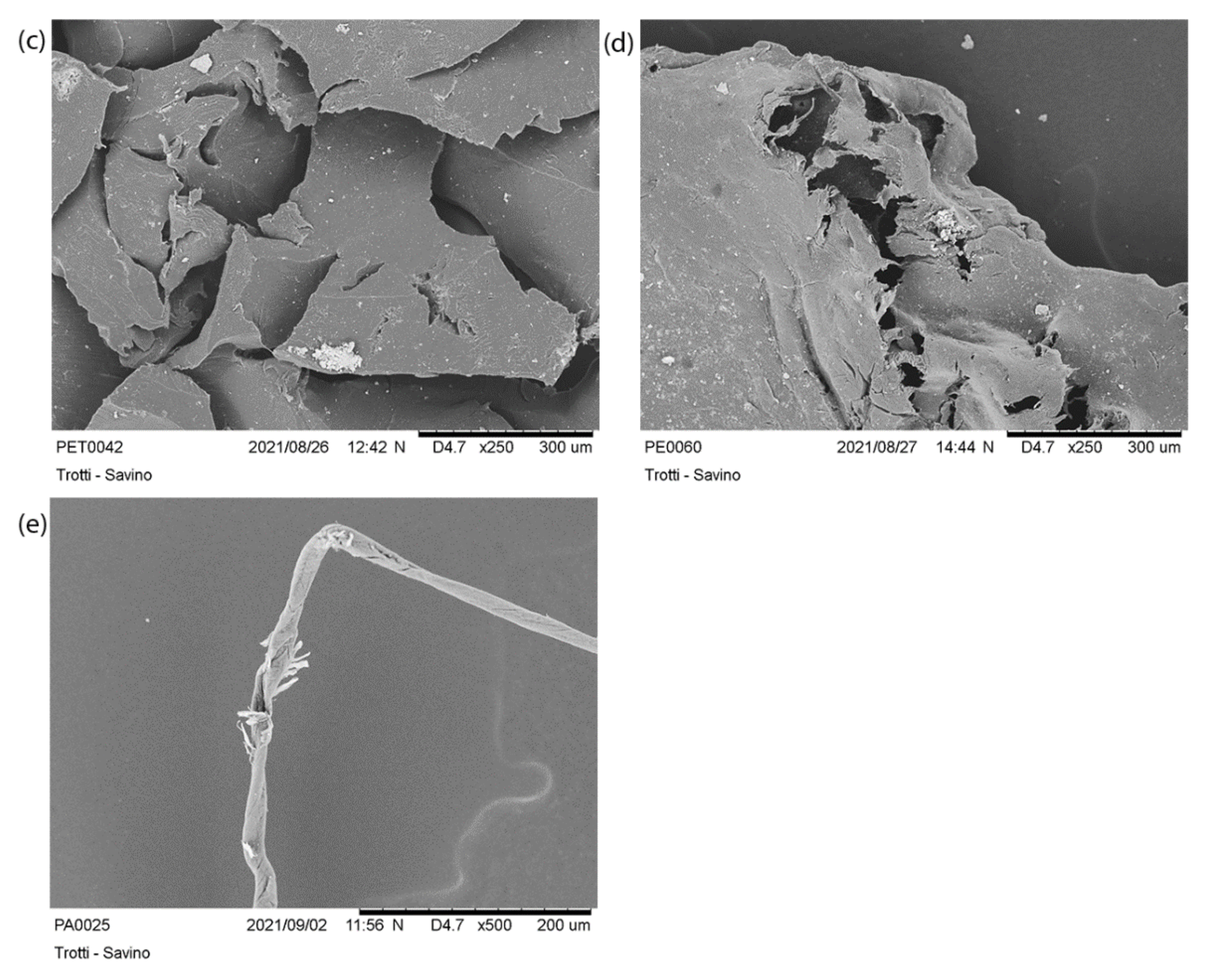
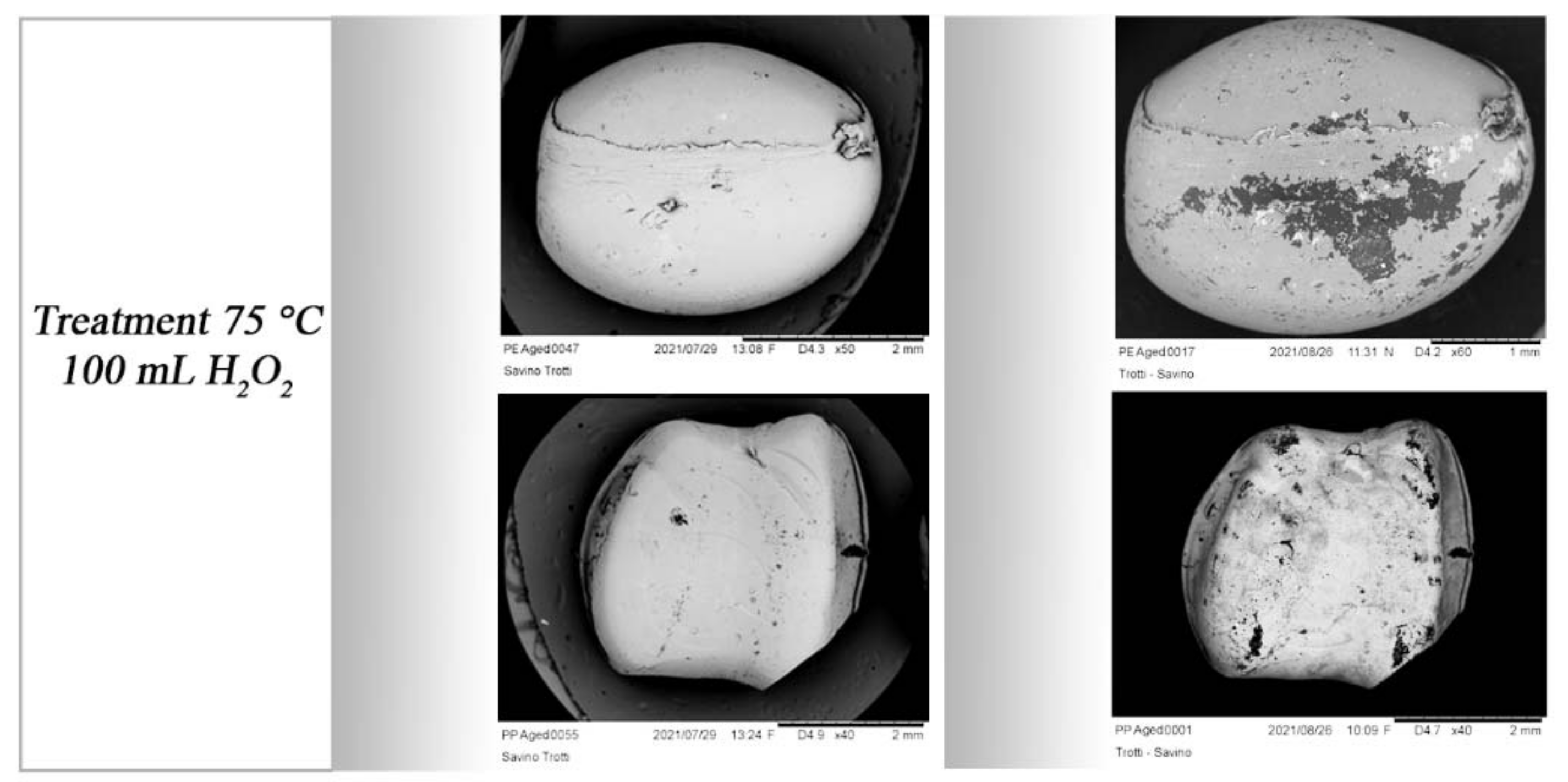
3.2.2. Scanning Electron Microscopy (SEM) Acquisition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Kanhai, L.D.K.; Gårdfeldt, K.; Lyashevska, O.; Hassellöv, M.; Thompson, R.C.; O’Connor, I. Microplastics in sub-surface waters of the Arctic Central Basin. Mar. Pollut. Bull. 2018, 130, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Galafassi, S.; Savino, I.; Massarelli, C.; Ancona, V.; Volta, P.; Uricchio, V.F. Microplastics pollution in the terrestrial environments: Poorly known diffuse sources and implications for plants. Sci. Total Environ. 2022, 805, 150431. [Google Scholar] [CrossRef]
- Campanale, C.; Dierkes, G.; Massarelli, C.; Bagnuolo, G.; Uricchio, V.F. A Relevant Screening of Organic Contaminants Present on Freshwater and Pre-Production Microplastics. Toxics 2020, 8, 100. [Google Scholar] [CrossRef]
- Batel, A.; Borchert, F.; Reinwald, H.; Erdinger, L.; Braunbeck, T. Microplastic accumulation patterns and transfer of benzo[a]pyrene to adult zebrafish (Danio rerio) gills and zebrafish embryos. Environ. Pollut. 2018, 235, 918–930. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [Green Version]
- Cormier, B.; Gambardella, C.; Tato, T.; Perdriat, Q.; Costa, E.; Veclin, C.; Le Bihanic, F.; Grassl, B.; Dubocq, F.; Kärrman, A.; et al. Chemicals sorbed to environmental microplastics are toxic to early life stages of aquatic organisms. Ecotoxicol. Environ. Saf. 2021, 208, 111665. [Google Scholar] [CrossRef]
- Campanale, C.; Savino, I.; Pojar, I.; Massarelli, C.; Uricchio, V.F. A Practical Overview of Methodologies for Sampling and Analysis of Microplastics in Riverine Environments. Sustainability 2020, 12, 6755. [Google Scholar] [CrossRef]
- Massarelli, C.; Campanale, C.; Uricchio, V.F. A handy open-source application based on computer vision and machine learning algorithms to count and classify microplastics. Water 2021, 13, 2104. [Google Scholar] [CrossRef]
- Lusher, A.L.; Munno, K.; Hermabessiere, L.; Carr, S. Isolation and Extraction of Microplastics from Environmental Samples: An Evaluation of Practical Approaches and Recommendations for Further Harmonization. Appl. Spectrosc. 2020, 74, 1049–1065. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, J.; Zhao, X.; Gu, X.; Ji, R. Separation and identification of microplastics from soil and sewage sludge. Environ. Pollut. 2019, 254, 113076. [Google Scholar] [CrossRef]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C.; Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments. NOAA Technical Memorandum NOS-OR&R-48. 2015. Available online: https://repository.library.noaa.gov/view/noaa/10296 (accessed on 1 January 2022).
- von Friesen, L.W.; Granberg, M.E.; Hassellöv, M.; Gabrielsen, G.W.; Magnusson, K. An efficient and gentle enzymatic digestion protocol for the extraction of microplastics from bivalve tissue. Mar. Pollut. Bull. 2019, 142, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Bretas Alvim, C.; Bes-Piá, M.A.; Mendoza-Roca, J.A. Separation and identification of microplastics from primary and secondary effluents and activated sludge from wastewater treatment plants. Chem. Eng. J. 2020, 402, 126293. [Google Scholar] [CrossRef]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E. Evaluation of existing methods to extract microplastics from bivalve tissue: Adapted KOH digestion protocol improves filtration at single-digit pore size. Mar. Pollut. Bull. 2019, 142, 384–393. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Girão, A.V.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Identifying a quick and efficient method of removing organic matter without damaging microplastic samples. Sci. Total Environ. 2019, 686, 131–139. [Google Scholar] [CrossRef]
- Pfohl, P.; Roth, C.; Meyer, L.; Heinemeyer, U.; Gruendling, T.; Lang, C.; Nestle, N.; Hofmann, T.; Wohlleben, W.; Jessl, S. Microplastic extraction protocols can impact the polymer structure. Microplast. Nanoplast. 2021, 1, 8. [Google Scholar] [CrossRef]
- Naidoo, T.; Goordiyal, K.; Glassom, D. Are Nitric Acid (HNO3) Digestions Efficient in Isolating Microplastics from Juvenile Fish? Water Air Soil Pollut. 2017, 228, 470. [Google Scholar] [CrossRef]
- Stock, F.; Kochleus, C.; Bänsch-Baltruschat, B.; Brennholt, N.; Reifferscheid, G. Sampling techniques and preparation methods for microplastic analyses in the aquatic environment—A review. TrAC Trends Anal. Chem. 2019, 113, 84–92. [Google Scholar] [CrossRef]
- Löder, M.G.J.; Imhof, H.K.; Ladehoff, M.; Löschel, L.A.; Lorenz, C.; Mintenig, S.; Piehl, S.; Primpke, S.; Schrank, I.; Laforsch, C.; et al. Enzymatic Purification of Microplastics in Environmental Samples. Environ. Sci. Technol. 2017, 51, 14283–14292. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; van Werven, B.; van Oyen, A.; Meijboom, A.; Bravo Rebolledo, E.L.; van Franeker, J.A. The use of potassium hydroxide (KOH) solution as a suitable approach to isolate plastics ingested by marine organisms. Mar. Pollut. Bull. 2017, 115, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Mbachu, O.; Jenkins, G.; Pratt, C.; Kaparaju, P. Enzymatic purification of microplastics in soil. MethodsX 2021, 8, 101254. [Google Scholar] [CrossRef] [PubMed]
- Tagg, A.S.; Harrison, J.P.; Ju-Nam, Y.; Sapp, M.; Bradley, E.L.; Sinclair, C.J.; Ojeda, J.J. Fenton’s reagent for the rapid and efficient isolation of microplastics from wastewater. Chem. Commun. 2017, 53, 372–375. [Google Scholar] [CrossRef] [Green Version]
- Zobkov, M.; Belkina, N.; Kovalevski, V.; Zobkova, M.; Efremova, T.; Galakhina, N. Microplastic abundance and accumulation behavior in Lake Onego sediments: A journey from the river mouth to pelagic waters of the large boreal lake. J. Environ. Chem. Eng. 2020, 8, 104367. [Google Scholar] [CrossRef]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a Method for Extracting Microplastics from Complex, Organic-Rich, Environmental Matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef] [Green Version]
- Munno, K.; Helm, P.A.; Jackson, D.A.; Rochman, C.; Sims, A. Impacts of temperature and selected chemical digestion methods on microplastic particles. Environ. Toxicol. Chem. 2018, 37, 91–98. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Fischer, E.K. Various Digestion Protocols Within Microplastic Sample Processing—Evaluating the Resistance of Different Synthetic Polymers and the Efficiency of Biogenic Organic Matter Destruction. Front. Environ. Sci. 2020, 8, 263. [Google Scholar] [CrossRef]
- Enders, K.; Lenz, R.; Beer, S.; Stedmon, C.A. Extraction of microplastic from biota: Recommended acidic digestion destroys common plastic polymers. ICES J. Mar. Sci. 2017, 74, 326–331. [Google Scholar] [CrossRef] [Green Version]
- Gulizia, A.M.; Brodie, E.; Daumuller, R.; Bloom, S.B.; Corbett, T.; Santana, M.M.F.; Motti, C.A.; Vamvounis, G. Evaluating the Effect of Chemical Digestion Treatments on Polystyrene Microplastics: Recommended Updates to Chemical Digestion Protocols. Macromol. Chem. Phys. 2022, 2100485. [Google Scholar] [CrossRef]
- Alfonso, M.B.; Takashima, K.; Yamaguchi, S.; Tanaka, M.; Isobe, A. Microplastics on plankton samples: Multiple digestion techniques assessment based on weight, size, and FTIR spectroscopy analyses. Mar. Pollut. Bull. 2021, 173, 113027. [Google Scholar] [CrossRef] [PubMed]
- Kallenbach, E.M.F.; Hurley, R.R.; Lusher, A.; Friberg, N. Chitinase digestion for the analysis of microplastics in chitinaceous organisms using the terrestrial isopod Oniscus asellus L. as a model organism. Sci. Total Environ. 2021, 786, 147455. [Google Scholar] [CrossRef] [PubMed]
- Dordevic, D.; Necasova, L.; Antonic, B.; Jancikova, S.; Tremlová, B. Plastic cutlery alternative: Case study with biodegradable spoons. Foods 2021, 10, 1612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Hamidian, A.H.; Tubić, A.; Zhang, Y.; Fang, J.K.H.; Wu, C.; Lam, P.K.S. Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef]
- Lang, K.; Bhattacharya, S.; Ning, Z.; Sánchez-Leija, R.J.; Bramson, M.T.K.; Centore, R.; Corr, D.T.; Linhardt, R.J.; Gross, R.A. Enzymatic Polymerization of Poly(glycerol-1,8-octanediol-sebacate): Versatile Poly(glycerol sebacate) Analogues that Form Monocomponent Biodegradable Fiber Scaffolds. Biomacromolecules 2020, 21, 3197–3206. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Lear, G.; Maday, S.D.M.; Gambarini, V.; Northcott, G.; Abbel, R.; Kingsbury, J.M.; Weaver, L.; Wallbank, J.A.; Pantos, O. Microbial abilities to degrade global environmental plastic polymer waste are overstated. Environ. Res. Lett. 2022, 17, 043002. [Google Scholar] [CrossRef]
- Glaser, J.A. Biological Degradation of Polymers in the Environment. In Plastics in the Environment; IntechOpen: London, UK, 2019. [Google Scholar]
- Fairbrother, A.; Hsueh, H.-C.; Kim, J.H.; Jacobs, D.; Perry, L.; Goodwin, D.; White, C.; Watson, S.; Sung, L.-P. Temperature and light intensity effects on photodegradation of high-density polyethylene. Polym. Degrad. Stab. 2019, 165, 153–160. [Google Scholar] [CrossRef]
- Lyngsie, G.; Krumina, L.; Tunlid, A.; Persson, P. Generation of hydroxyl radicals from reactions between a dimethoxyhydroquinone and iron oxide nanoparticles. Sci. Rep. 2018, 8, 10834. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, S.S.; Rocha-Santos, T.; Prata, J.C.; Duarte, A.C.; Girão, A.V.; Lopes, P.; Cristovão, T.; da Costa, J.P. A straightforward method for microplastic extraction from organic-rich freshwater samples. Sci. Total Environ. 2022, 815, 152941. [Google Scholar] [CrossRef]
- Grause, G.; Kuniyasu, Y.; Chien, M.-F.; Inoue, C. Separation of microplastic from soil by centrifugation and its application to agricultural soil. Chemosphere 2022, 288, 132654. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.T.; Le, P.T.; Nguyen, T.N.H.; Hoang, T.Q.; Ngo, H.M.; Doan, T.O.; Le, T.P.Q.; Bui, H.T.; Bui, M.H.; Trinh, V.T.; et al. Selection of a density separation solution to study microplastics in tropical riverine sediment. Environ. Monit. Assess. 2022, 194, 65. [Google Scholar] [CrossRef] [PubMed]
- Pongstabodee, S.; Kunachitpimol, N.; Damronglerd, S. Combination of three-stage sink–float method and selective flotation technique for separation of mixed post-consumer plastic waste. Waste Manag. 2008, 28, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Cashman, M.A.; Ho, K.T.; Boving, T.B.; Russo, S.; Robinson, S.; Burgess, R.M. Comparison of microplastic isolation and extraction procedures from marine sediments. Mar. Pollut. Bull. 2020, 159, 111507. [Google Scholar] [CrossRef]
- Nguyen, B.; Claveau-Mallet, D.; Hernandez, L.M.; Xu, E.G.; Farner, J.M.; Tufenkji, N. Separation and Analysis of Microplastics and Nanoplastics in Complex Environmental Samples. Acc. Chem. Res. 2019, 52, 858–866. [Google Scholar] [CrossRef] [Green Version]
- Brewer, A.; Dror, I.; Berkowitz, B. The Mobility of Plastic Nanoparticles in Aqueous and Soil Environments: A Critical Review. ACS ES&T Water 2021, 1, 48–57. [Google Scholar] [CrossRef]
- He, J.; Liu, W.; Huang, Y.-X. Simultaneous Determination of Glass Transition Temperatures of Several Polymers. PLoS ONE 2016, 11, e0151454. [Google Scholar] [CrossRef]
- Catarino, A.I.; Thompson, R.; Sanderson, W.; Henry, T.B. Development and optimization of a standard method for extraction of microplastics in mussels by enzyme digestion of soft tissues. Environ. Toxicol. Chem. 2017, 36, 947–951. [Google Scholar] [CrossRef]
- Treilles, R.; Cayla, A.; Gaspéri, J.; Strich, B.; Ausset, P.; Tassin, B. Impacts of organic matter digestion protocols on synthetic, artificial and natural raw fibers. Sci. Total Environ. 2020, 748, 141230. [Google Scholar] [CrossRef]
- Hu, K.; Zhou, P.; Yang, Y.; Hall, T.; Nie, G.; Yao, Y.; Duan, X.; Wang, S. Degradation of Microplastics by a Thermal Fenton Reaction. ACS ES&T Eng. 2021, 2, 110–120. [Google Scholar] [CrossRef]
- Nuelle, M.-T.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Han, J.; Zhou, H.; Lau, Y.L.; An, W.; Wei, P.; Cheung, S.G.; Yang, Y.; Tam, N.F. Development of a digestion method for determining microplastic pollution in vegetal-rich clayey mangrove sediments. Sci. Total Environ. 2020, 707, 136030. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, K.N.; Karapanagioti, H.K. Degradation of Various Plastics in the Environment. In Hazardous Chemicals Associated with Plastics in the Marine Environment; Barcelò, D., Kostianoy, A.G., Eds.; The Handbook of Environmental Chemistry; Springer: Cham, Switzerland; New York, NY, USA, 2017; Volume 78, pp. 71–92. [Google Scholar] [CrossRef]
- McGivney, E.; Cederholm, L.; Barth, A.; Hakkarainen, M.; Hamacher-Barth, E.; Ogonowski, M.; Gorokhova, E. Rapid Physicochemical Changes in Microplastic Induced by Biofilm Formation. Front. Bioeng. Biotechnol. 2020, 8, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-González, V.; Andrade-Garda, J.M.; López-Mahía, P.; Muniategui-Lorenzo, S. Impact of weathering on the chemical identification of microplastics from usual packaging polymers in the marine environment. Anal. Chim. Acta 2021, 1142, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Reis, V.; Paço, A.; Martins, P.; Cruz, A.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Effects of spatial and seasonal factors on the characteristics and carbonyl index of (micro)plastics in a sandy beach in Aveiro, Portugal. Sci. Total Environ. 2020, 709, 135892. [Google Scholar] [CrossRef]
- Rodrigues, M.O.; Abrantes, N.; Gonçalves, F.J.M.; Nogueira, H.; Marques, J.C.; Gonçalves, A.M.M. Spatial and temporal distribution of microplastics in water and sediments of a freshwater system (Antuã River, Portugal). Sci. Total Environ. 2018, 633, 1549–1559. [Google Scholar] [CrossRef]
- Brandon, J.; Goldstein, M.; Ohman, M.D. Long-term aging and degradation of microplastic particles: Comparing in situ oceanic and experimental weathering patterns. Mar. Pollut. Bull. 2016, 110, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Miranda, M.N.; Sampaio, M.J.; Tavares, P.B.; Silva, A.M.T.; Pereira, M.F.R. Aging assessment of microplastics (LDPE, PET and uPVC) under urban environment stressors. Sci. Total Environ. 2021, 796, 148914. [Google Scholar] [CrossRef]
- Xu, J.L.; Thomas, K.V.; Luo, Z.; Gowen, A.A. FTIR and Raman imaging for microplastics analysis: State of the art, challenges and prospects. TrAC Trends Anal. Chem. 2019, 119, 115629. [Google Scholar] [CrossRef]
- Liu, P.; Qian, L.; Wang, H.; Zhan, X.; Lu, K.; Gu, C.; Gao, S. New Insights into the Aging Behavior of Microplastics Accelerated by Advanced Oxidation Processes. Environ. Sci. Technol. 2019, 53, 3579–3588. [Google Scholar] [CrossRef]
- Gulmine, J.V.; Akcelrud, L. FTIR characterization of aged XLPE. Polym. Test. 2006, 25, 932–942. [Google Scholar] [CrossRef]
- Lacoste, J.; Carlsson, D.J. Gamma-, photo-, and thermally-initiated oxidation of linear low density polyethylene: A quantitative comparison of oxidation products. J. Polym. Sci. Part A Polym. Chem. 1992, 30, 493–500. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts—George Socrates—Google Libri, 3rd ed.; Chichester, E., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Veerasingam, S.; Ranjani, M.; Venkatachalapathy, R.; Bagaev, A.; Mukhanov, V.; Litvinyuk, D.; Mugilarasan, M.; Gurumoorthi, K.; Guganathan, L.; Aboobacker, V.M.; et al. Contributions of Fourier transform infrared spectroscopy in microplastic pollution research: A review. Crit. Rev. Environ. Sci. Technol. 2020, 51, 2681–2743. [Google Scholar] [CrossRef]




| Experiment One | |
|---|---|
| AIM | Evaluate the efficiency of extraction of the most commonly used chemical digestion protocol (based on Wet Peroxide Oxidation [14]) on the recovery of virgin MPs standards from a complex matrix |
| Particle selection | Virgin MPs |
| Matrix selected | Soil |
| Starting digestion condition | Reagents volume: 20 mL of 30% H2O2 solution add to 20 mL of 0.05 M iron sulphate heptahydrate (FeSO4·7H2O) every 30′ until complete sample digestion. The temperature of reaction: 75 °C. |
| Density separation | NaI (1.8 g cm3) |
| Qualitative analysis | Stereomicroscope |
| Experiment Two | |
| AIM | Evaluate the impact of the most commonly used chemical digestion protocol on the integrity of virgin and aged MPs standards |
| Particle selection | Virgin and aged MPs |
| Matrix selected | Soil |
| Starting digestion condition | Reagents volume: 20 mL of 30% H2O2 solution add to 20 mL of 0.05 M iron sulphate heptahydrate (FeSO4·7H2O) every 30′ until complete sample digestion. The temperature of reaction: 75 °C. |
| Density separation | NaI (1.8 g cm3) |
| Qualitative analysis | FTIR—SEM |
| Polymers | Density (g cm 3) (*) | Source | Colour | Shape |
|---|---|---|---|---|
| Polystyrene (PS) | 0.01–1.06 | Food box | White | Fragment |
| Polypropylene (PP) | 0.85–0.92 | Disposable glass | Red | Fragment |
| Polyethylene (PE) | 0.89–0.98 | Mulching films | Black | Fragment |
| Polyamide (PA) | 1.12–1.15 | Textile | Black | Fibre |
| Polyvinyl chloride (PVC) | 1.38–1.41 | Building material | Black | Fragment |
| Polyethylene terephthalate (PET) | 1.38–1.41 | Plastics bottle | Green | Fragment |
| Treatment | Reagent Volumes | Temperature (°C) | Polymers | Size | Soil Matrix (g) |
|---|---|---|---|---|---|
| 1 | 100 mL H2O2 + 20 mL FeSO4·7H2O | 75 °C | PE, PP, PET, PVC, PS | 5–1 mm 1 mm–500 µm | 50 |
| 500–100 µm | - | ||||
| 2 | 60 mL H2O2 + 20 mL FeSO4·7H2O | 75 °C | PE, PP, PET, PVC, PS | 5–1 mm 1 mm–500 µm | 50 |
| 500–100 µm | - | ||||
| 3 | 100 mL H2O2 + 20 mL FeSO4·7H2O | 50 °C | PE, PP, PET, PVC, PS | 5–1 mm 1 mm–500 µm | 50 |
| 500–100 µm | - | ||||
| 4 | 60 mL H2O2 + 20 mL FeSO4·7H2O | 50 °C | PE, PP, PET, PVC, PS | 5–1 mm 1 mm–500 µm | 50 |
| 500–100 µm | - | ||||
| 5 | 100 mL H2O2 + 20 mL FeSO4·7H2O | 30 °C | PE, PP, PET, PVC; PS | 5–1 mm 1 mm–500 µm | 50 |
| 500–100 µm | - | ||||
| 6 | 60 mL H2O2 + 20 mL FeSO4·7H2O | 30 °C | PE, PP, PET, PVC, PS | 5–1 mm 1 mm–500 µm | 50 |
| 500–100 µm | - |
| Treatment | Reagent Volumes | Temperature (°C) | Polymers | Size | Soil Matrix (g) |
|---|---|---|---|---|---|
| a | 100 mL H2O2 + 20 mL FeSO4·7H2O | 75 °C | PE, PP, PET, PVC, PS | 5–1 mm 1 mm–500 µm | 13 |
| PA | 5–1 mm | - | |||
| b | 60 mL H2O2 + 20 mL FeSO4·7H2O | 50 °C | PE, PP, PET, PVC, PS | 5–1 mm 1 mm–500 µm | 13 |
| PA | 5–1 mm | - | |||
| c | 60 mL H2O2 + 20 mL FeSO4·7H2O | 30 °C | PE, PP, PET, PVC, PS | 5–1 mm 1 mm–500 µm | 13 |
| PA | 5–1 mm | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savino, I.; Campanale, C.; Trotti, P.; Massarelli, C.; Corriero, G.; Uricchio, V.F. Effects and Impacts of Different Oxidative Digestion Treatments on Virgin and Aged Microplastic Particles. Polymers 2022, 14, 1958. https://doi.org/10.3390/polym14101958
Savino I, Campanale C, Trotti P, Massarelli C, Corriero G, Uricchio VF. Effects and Impacts of Different Oxidative Digestion Treatments on Virgin and Aged Microplastic Particles. Polymers. 2022; 14(10):1958. https://doi.org/10.3390/polym14101958
Chicago/Turabian StyleSavino, Ilaria, Claudia Campanale, Pasquale Trotti, Carmine Massarelli, Giuseppe Corriero, and Vito Felice Uricchio. 2022. "Effects and Impacts of Different Oxidative Digestion Treatments on Virgin and Aged Microplastic Particles" Polymers 14, no. 10: 1958. https://doi.org/10.3390/polym14101958








