Removal of Methylene Blue from Aqueous Solutions Using a New Natural Lignocellulosic Adsorbent—Raspberry (Rubus idaeus) Leaves Powder
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Adsorbent Material Characterization
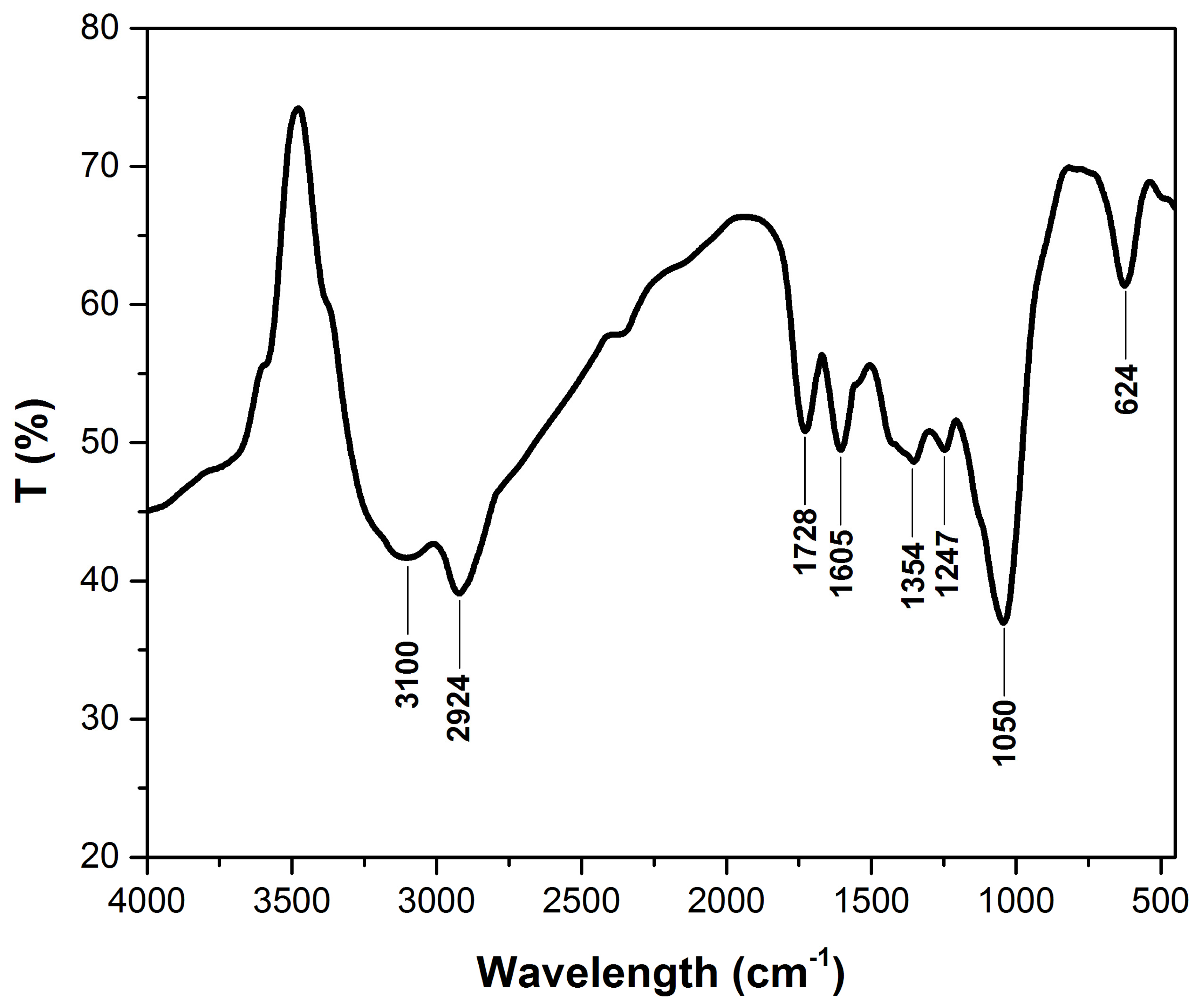
3.1.1. FTIR Analysis
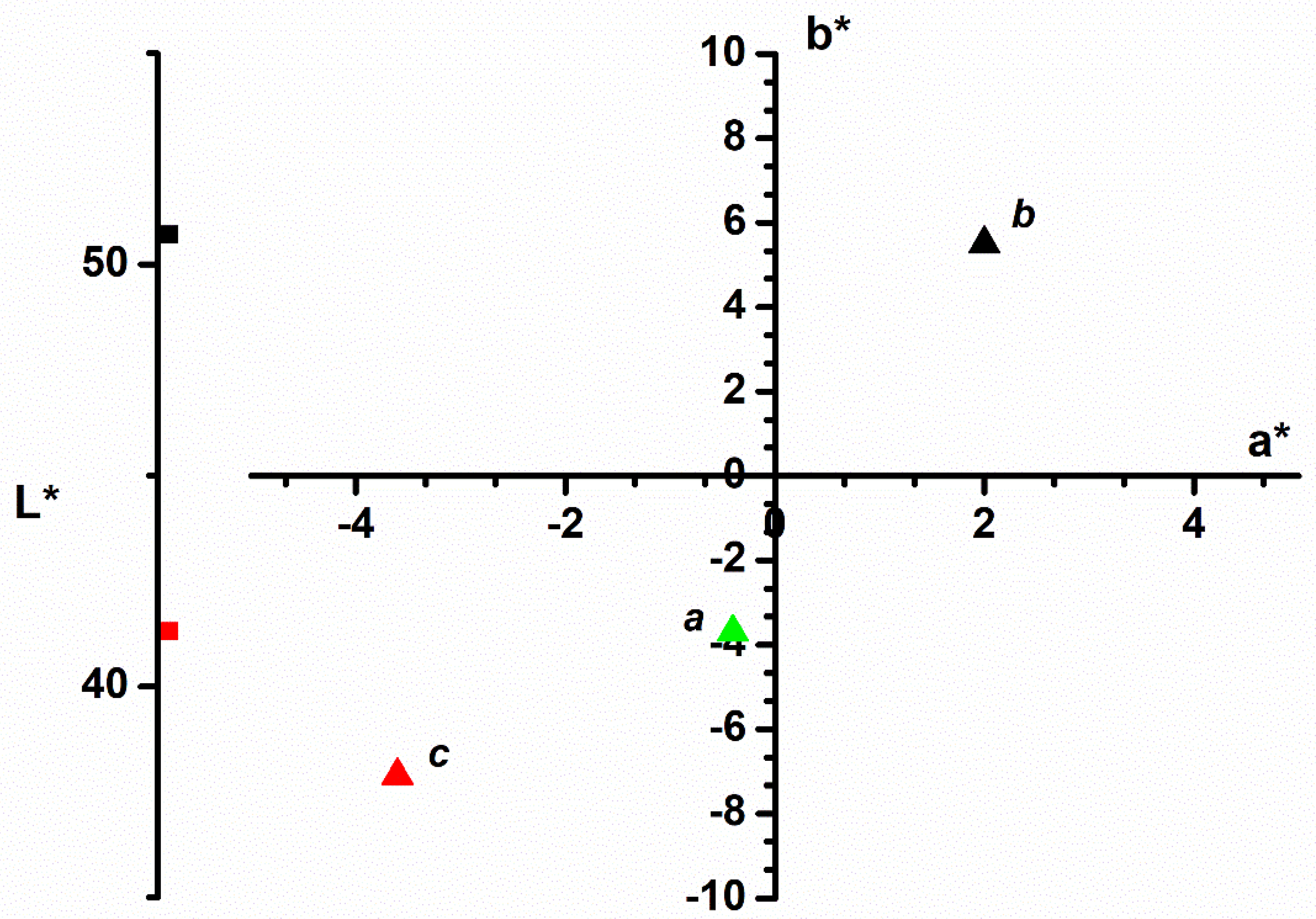
3.1.2. Color Analysis
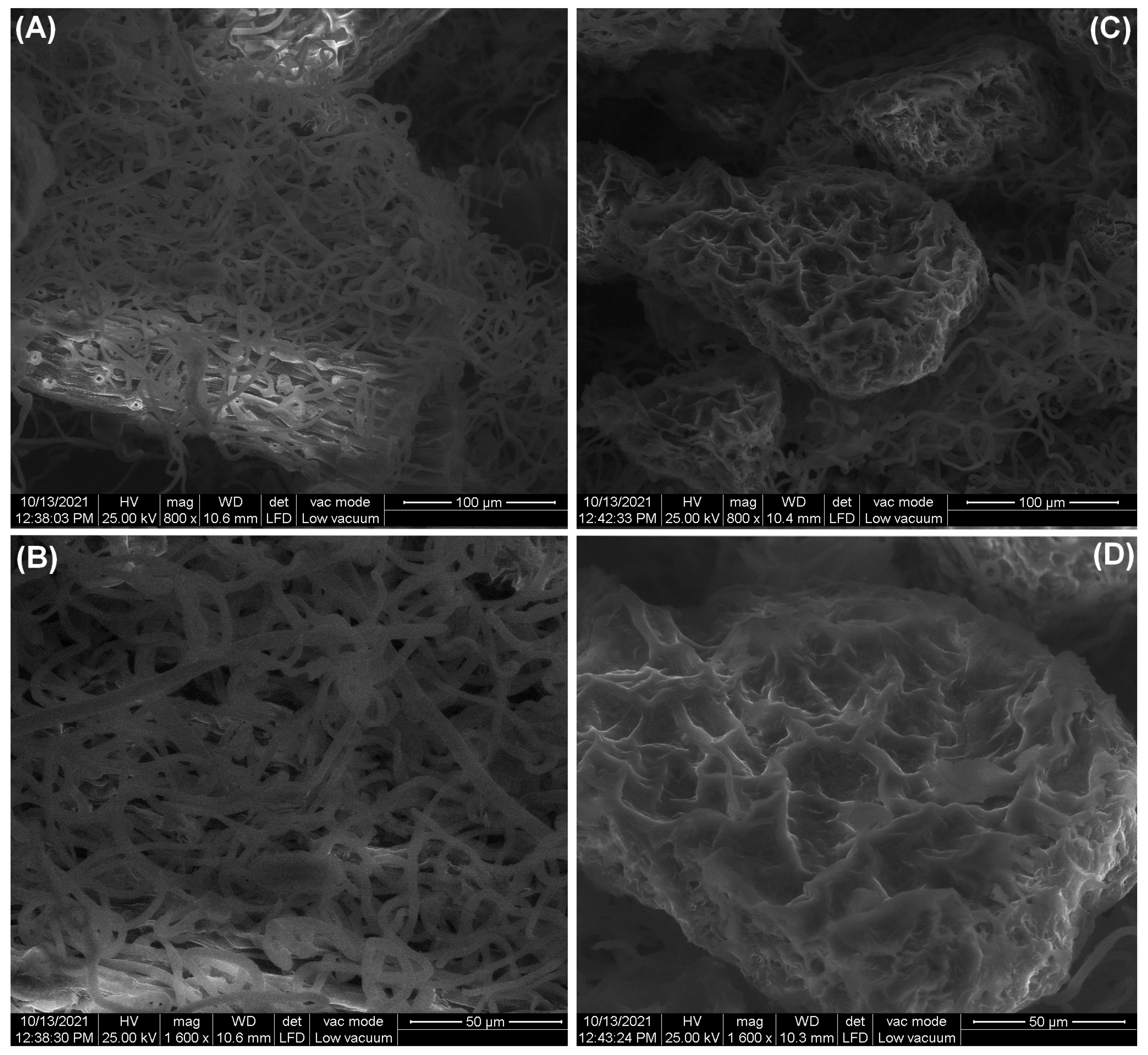
3.1.3. SEM Analysis
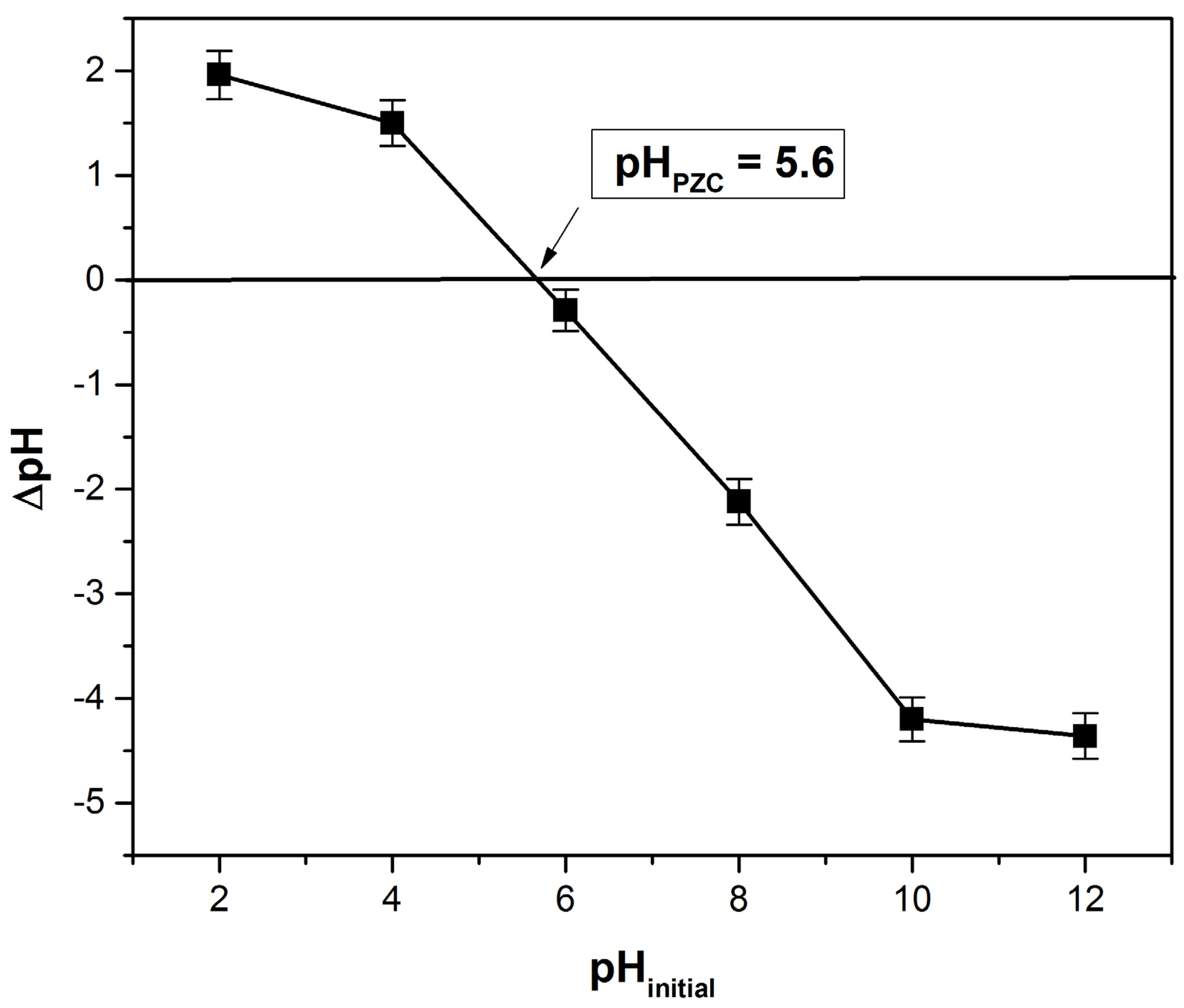
3.1.4. Point of Zero Charge Determination
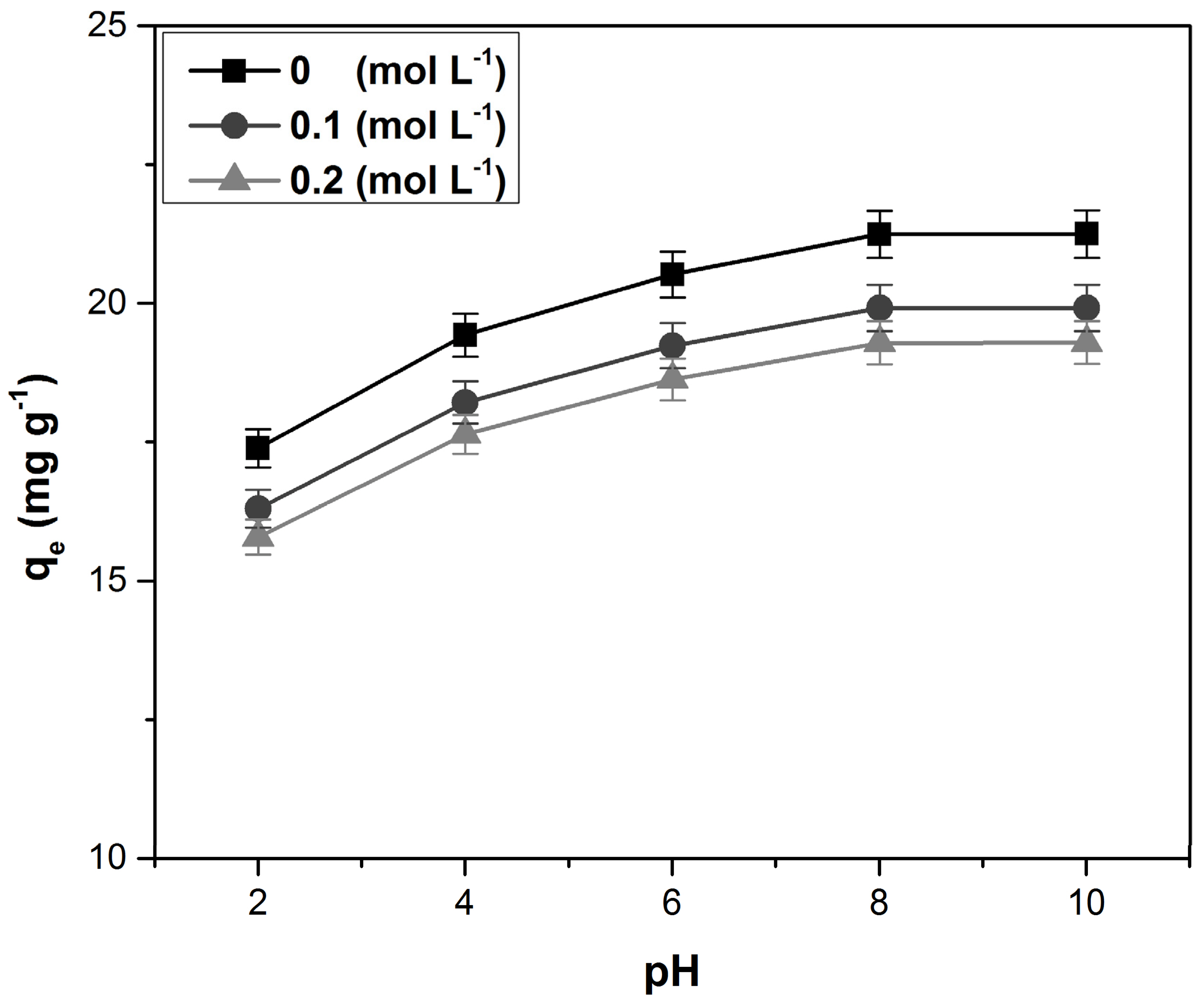
3.2. Influence of pH and Ionic Strength
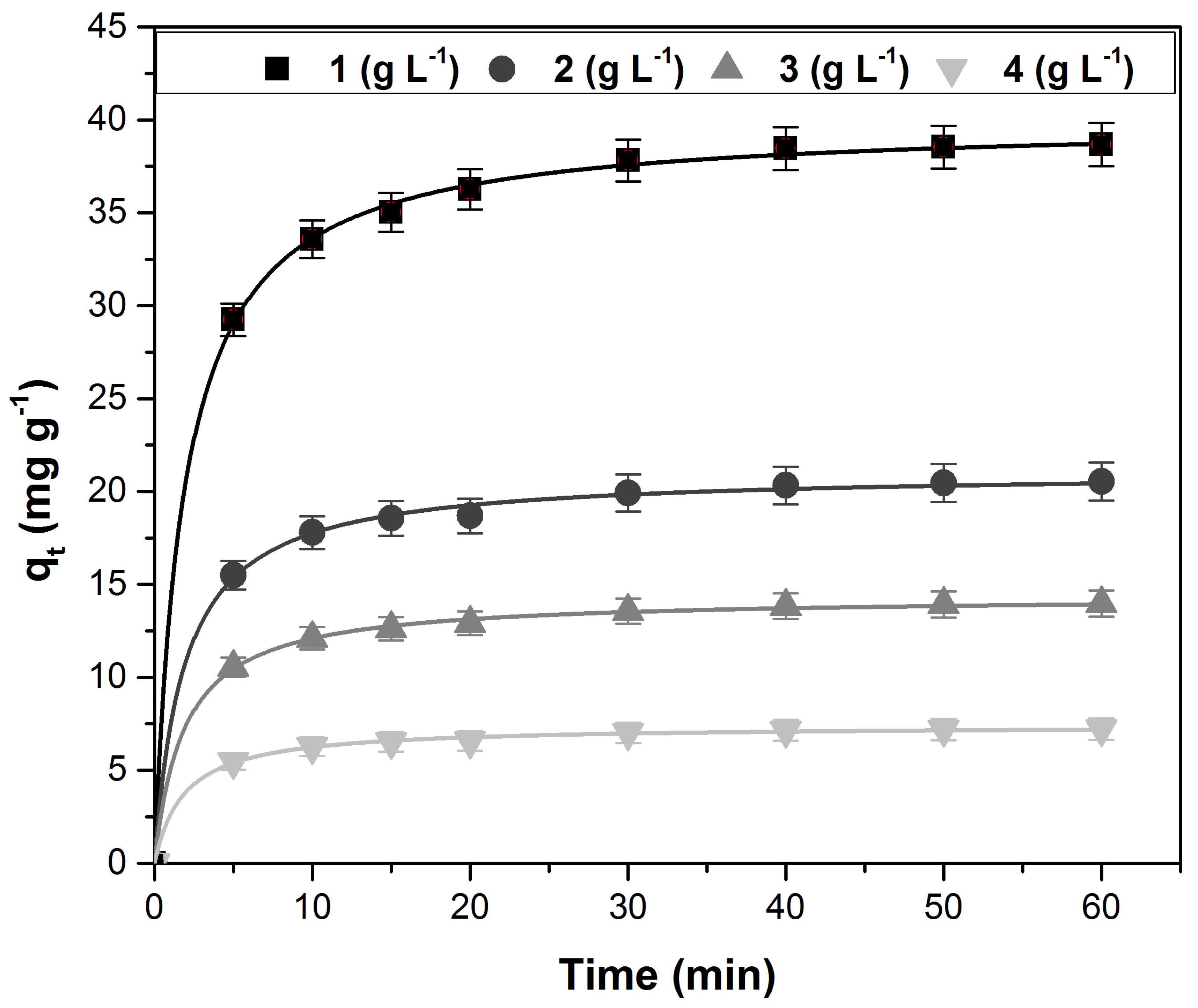
3.3. Kinetic Study
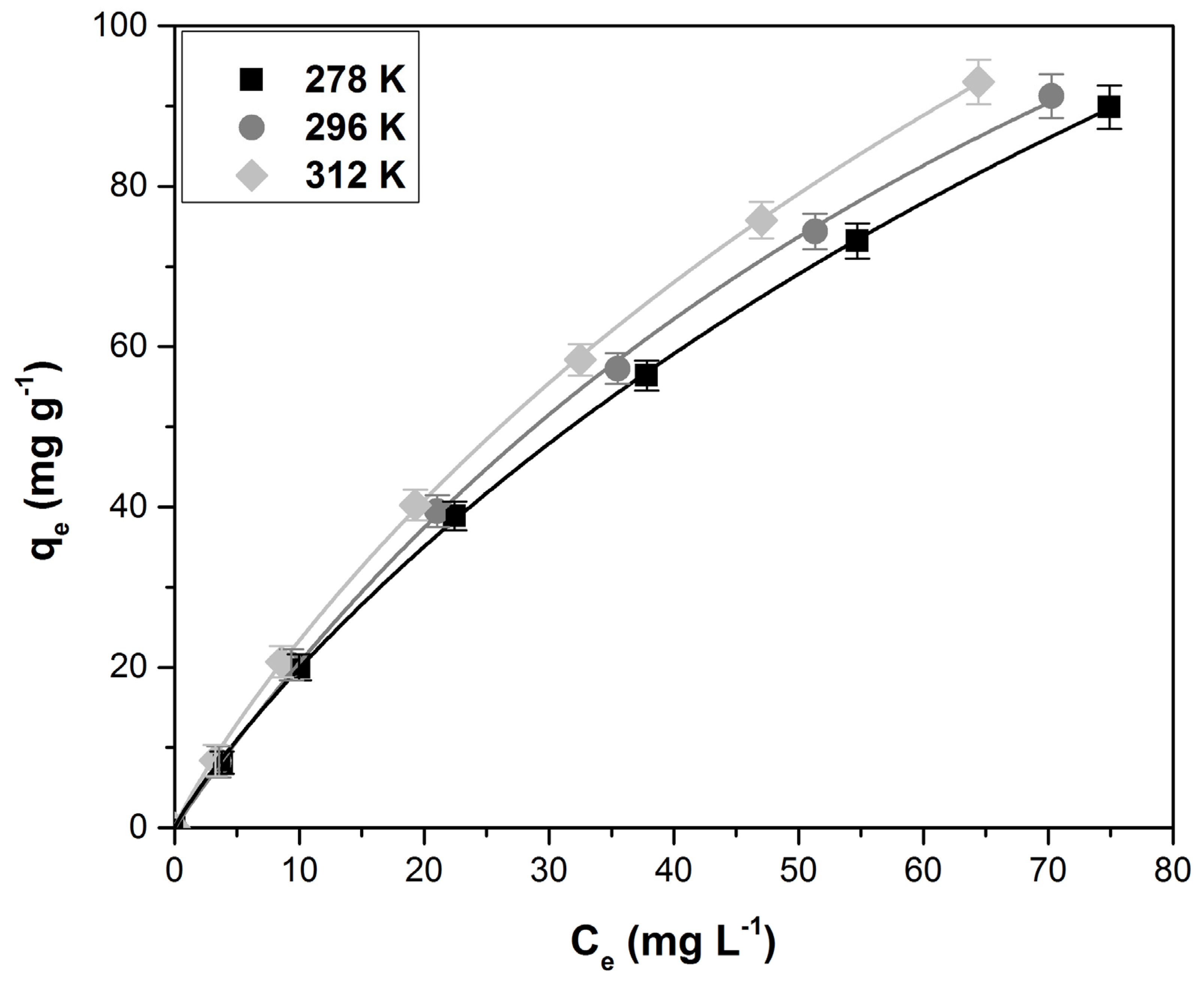
3.4. Equilibrum Study
3.5. Thermodynamic Parameters
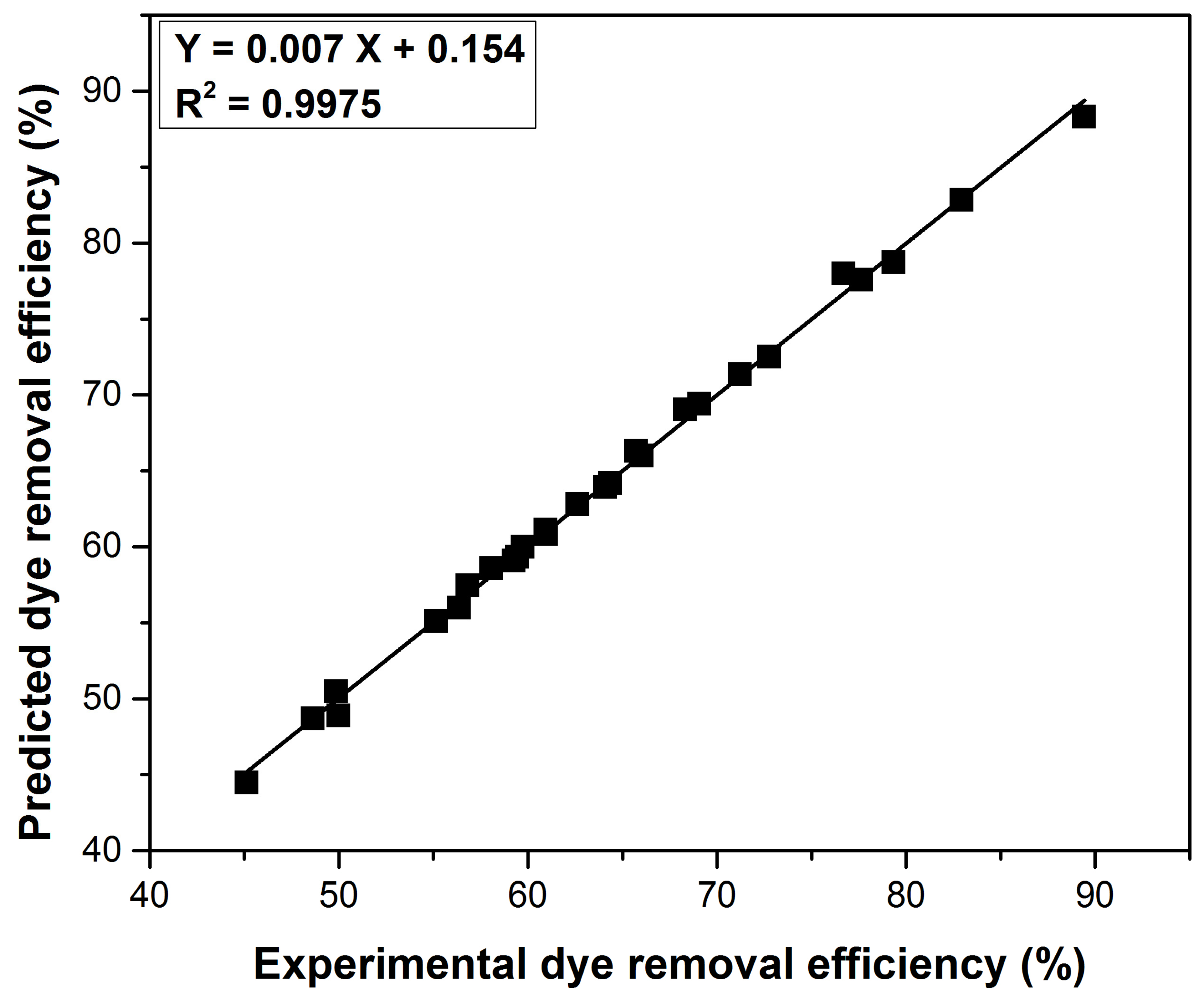
3.6. Adsorption Parameters Optimization
3.7. Desorption Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Gorair, A.S.; Sayed, A.; Mahmoud, G.A. Engineered Superabsorbent Nanocomposite Reinforced with Cellulose Nanocrystals for Remediation of Basic Dyes: Isotherm, Kinetic, and Thermodynamic Studies. Polymers 2022, 14, 567. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Yadav, V.K.; Yadav, K.K.; Almohana, A.I.; Almojil, S.F.; Gnanamoorthy, G.; Kim, D.-H.; Islam, S.; Kumar, P.; Jeon, B.-H. Application of Green Synthesized MMT/Ag Nanocomposite for Removal of Methylene Blue from Aqueous Solution. Water 2021, 13, 3206. [Google Scholar] [CrossRef]
- Elbedwehy, A.M.; Atta, A.M. Novel Superadsorbent Highly Porous Hydrogel Based on Arabic Gum and Acrylamide Grafts for Fast and Efficient Methylene Blue Removal. Polymers 2020, 12, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamad, H.N.; Idrus, S. Recent Developments in the Application of Bio-Waste-Derived Adsorbents for the Removal of Methylene Blue from Wastewater: A Review. Polymers 2022, 14, 783. [Google Scholar] [CrossRef] [PubMed]
- Maleš, L.; Fakin, D.; Bračič, M.; Gorgieva, S. Efficiency of Differently Processed Membranes Based on Cellulose as Cationic Dye Adsorbents. Nanomaterials 2020, 10, 642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aljar, M.A.A.; Rashdan, S.; Abd El-Fattah, A. Environmentally Friendly Polyvinyl Alcohol−Alginate/Bentonite Semi-Interpenetrating Polymer Network Nanocomposite Hydrogel Beads as an Efficient Adsorbent for the Removal of Methylene Blue from Aqueous Solution. Polymers 2021, 13, 4000. [Google Scholar] [CrossRef]
- Mokhtar, N.; Aziz, E.A.; Aris, A.; Ishak, W.F.W.; Ali, N.S.M. Biosorption of azo-dye using marine macro-alga of Euchema spinosum. J. Environ. Chem. Eng. 2017, 5, 5721–5731. [Google Scholar] [CrossRef]
- Shakoor, S.; Nasar, A. Removal of methylene blue dye from artificially contaminated water using citrus limetta peel waste as a very low cost adsorbent. J. Taiwan Inst. Chem. Eng. 2016, 66, 154–163. [Google Scholar] [CrossRef]
- Reddy, P.M.K.; Verma, P.; Subrahmanyam, C. Bio-waste derived adsorbent material for methylene blue adsorption. J. Taiwan Inst. Chem. Eng. 2016, 58, 500–508. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, S.; Sharma, V.; Mishra, P.K.; Ekielski, A.; Sharma, V.; Kumar, V. Methylene blue dye adsorption from wastewater using hydroxyapatite/gold nanocomposite: Kinetic and thermodynamics studies. Nanomaterials 2021, 11, 1403. [Google Scholar] [CrossRef]
- Aysu, T.; Küçük, M.M. Removal of crystal violet and methylene blue from aqueous solutions by activated carbon prepared from Ferula orientalis. Int. J. Environ. Sci. Technol. 2014, 12, 2273–2284. [Google Scholar] [CrossRef] [Green Version]
- Alghamdi, W.M.; El Mannoubi, I. Investigation of Seeds and Peels of Citrullus colocynthis as Efficient Natural Adsorbent for Methylene Blue Dye. Processes 2021, 9, 1279. [Google Scholar] [CrossRef]
- Alvarez-Torrellas, S.; Boutahala, M.; Boukhalfa, N.; Munoz, M. Effective Adsorption of Methylene Blue dye onto Magnetic Nanocomposites. Modeling and Reuse Studies. Appl. Sci. 2019, 9, 4563. [Google Scholar] [CrossRef] [Green Version]
- Giovannetti, R.; Rommozzi, E.; Zannotti, M.; D’Amato, C.A. Recent advances in graphene based TiO2 nanocomposites (GTiO2Ns) for photocatalytic degradation of synthetic dyes. Catalysts 2017, 7, 305. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Ighalo, J.O.; Ogunfowora, L.A.; Adeniyi, A.G.; Igwegbe, C.A. An empirical literature analysis of adsorbent performance for methylene blue uptake from aqueous media. J. Environ. Chem. Eng. 2021, 9, 105658. [Google Scholar] [CrossRef]
- Kadhom, M.; Albayati, N.; Alalwan, H.; Al-Furaiji, M. Removal of dyes by agricultural waste. Sustain. Chem. Pharm. 2020, 16, 100259. [Google Scholar] [CrossRef]
- Khodabandehloo, A.; Rahbar-Kelishami, A.; Shayesteh, H. Methylene blue removal using Salix babylonica (Weeping willow) leaves powder as a low-cost biosorbent in batch mode: Kinetic, equilibrium, and thermodynamic studies. J. Mol. Liq. 2017, 244, 540–548. [Google Scholar] [CrossRef]
- Miraboutalebi, S.M.; Nikouzad, S.K.; Peydayesh, M.; Allahgholi, N.; Vafajoo, L.; McKay, G. Methylene blue adsorption via maize silk powder: Kinetic, equilibrium, thermodynamic studies and residual error analysis. Process Saf. Environ. Prot. 2017, 106, 191–202. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Abdelrahman, E.A.; Aly, A.A.; Mohamed, T.Y. A facile synthesis of mordenite zeolite nanostructures for efficient bleaching of crude soybean oil and removal of methylene blue dye from aqueous media. J. Mol. Liq. 2017, 248, 302–313. [Google Scholar] [CrossRef]
- Setiabudi, H.D.; Jusoh, R.; Suhaimi, S.F.R.M.; Masrur, S.F. Adsorption of methylene blue onto oil palm (Elaeis guineensis) leaves: Process optimization, isotherm, kinetics and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2016, 63, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Dalal, C.; Garg, A.K.; Sonkar, S.K. Carboxylic Acid-Terminated Carbon Nanoflakes for Selective Adsorption of Water-Soluble Cationic Dyes. ACS Appl. Nano Mater. 2021, 4, 5611–5620. [Google Scholar] [CrossRef]
- Cseri, L.; Topuz, F.; Abdulhamid, M.A.; Alammar, A.; Budd, P.M.; Szekely, G. Electrospun Adsorptive Nanofibrous Membranes from Ion Exchange Polymers to Snare Textile Dyes from Wastewater. Adv. Mater. Technol. 2021, 6, 2000955. [Google Scholar] [CrossRef]
- Topuz, F.; Holtzl, T.; Szekely, G. Scavenging organic micropollutants from water with nanofibrous hypercrosslinked cyclodextrin membranes derived from green resources. Chem. Eng. J. 2021, 419, 129443. [Google Scholar] [CrossRef]
- Chevallier, A. Encyclopedia of Herbal Medicine: 550 Herbs and Remedies for Common Ailments, 3rd ed.; DK Publishing: New York, NY, USA, 2016; p. 264. [Google Scholar]
- Chwil, M.; Kostryco, M. Histochemical assays of secretory trichomes and the structure and content of mineral nutrients in Rubus idaeus L. leaves. Protoplasma 2020, 257, 119–139. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yin, X.; Zhang, D.; Lu, J.; Wang, X. Isolation, Structural Characterization and Macrophage Activation Activity of an Acidic Polysaccharide from Raspberry Pulp. Molecules 2022, 27, 1674. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z.; Li, X.; Abubaker, M.A.; Liu, X.; Li, Z.; Wang, X.; Zhu, X.; Zhang, J.; Chen, X. Comparative Study of Three Raspberry Cultivar (Rubus idaeus L.) Leaves Metabolites: Metabolome Profiling and Antioxidant Activities. Appl. Sci. 2022, 12, 990. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; Gupta, N.; Chattopadhyaya, M.C. Removal of cationic methylene blue and malachite green dyes from aqueous solution by waste materials of Daucus carota. J. Saudi Chem. Soc. 2014, 18, 200–207. [Google Scholar] [CrossRef]
- Dotto, G.L.; Salau, N.P.G.; Piccin, J.S.; Cadaval, T.R.S.; de Pinto, L.A.A. Adsorption Kinetics in Liquid Phase: Modeling for Discontinuous and Continuous Systems. In Adsorption Processes for Water Treatment and Purification; Bonilla-Petriciolet, A., Mendoza-Castillo, D., Reynel-Avila, H., Eds.; Springer: Cham, Switzerland, 2017; pp. 53–76. [Google Scholar]
- Esquerdo, V.M.; Quintana, T.M.; Dotto, G.L.; Pinto, L.A.A. Kinetics and mass transfer aspects about the adsorption of tartrazine by a porous chitosan sponge. React. Kinet. Mech. Catal. 2015, 116, 105–117. [Google Scholar] [CrossRef]
- Netto, M.S.; Georgin, J.; Franco, D.S.P.; Mallmann, E.S.; Foletto, E.L.; Godinho, M.; Pinto, D.; Dotto, G.L. Effective adsorptive removal of atrazine herbicide in river waters by a novel hydrochar derived from Prunus serrulata bark. Environ. Sci. Pollut. Res. 2022, 29, 3672–3685. [Google Scholar] [CrossRef]
- Piccin, J.S.; Cadaval, T.R.S.; de Pinto, L.A.A.; Dotto, G.L. Adsorption Isotherms in Liquid Phase: Experimental, Modeling, and Interpretations. In Adsorption Processes for Water Treatment and Purification; Bonilla-Petriciolet, A., Mendoza-Castillo, D., Reynel-Avila, H., Eds.; Springer: Cham, Switzerland, 2017; pp. 19–51. [Google Scholar]
- Zaidi, N.A.H.M.; Lim, L.B.L.; Usman, A. Artocarpus odoratissimus leaf-based cellulose as adsorbent for removal of methyl violet and crystal violet dyes from aqueous solution. Cellulose 2018, 25, 3037–3049. [Google Scholar] [CrossRef]
- Filho, A.C.D.; Mazzocato, A.C.; Dotto, G.L.; Thue, P.S.; Pavan, F.A. Eragrostis plana Nees as a novel eco-friendly adsorbent for removal of crystal violet from aqueous solutions. Environ. Sci. Pollut. Res. 2017, 24, 19909–19919. [Google Scholar] [CrossRef] [PubMed]
- Zaltariov, M.F.; Filip, D.; Varganici, C.D.; Macocinschi, D. ATR-FTIR and thermal behavior studies of new hydrogel formulations based on hydroxypropyl methylcellulose/poly(acrilic acid) polymeric blends. Cell. Chem. Technol. 2018, 52, 619–631. [Google Scholar]
- Pekel Bayramgil, N. Preparation of graft copolymers of cellulose derivatives and their use in recovery processes. In Cellulose-Based Graft Copolymers: Structure and Chemistry, 1st ed.; Thakur, V.K., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 335–364. [Google Scholar]
- Plermjai, K.; Phoohinkong, W.; Pavasupree, S.; Mekprasart, W.; Pecharapa, W.; Boonyarattanakalin, K. UV shielding properties of cellulose/TiO2 composite film. Curr. J. Appl. Sci. Technol. 2018, 18, 111–118. [Google Scholar]
- Özgenç, Ö.; Durmaz, S.; Kuştaş, S. Chemical Analysis of Tree Barks using ATR-FTIR Spectroscopy and Conventional Techniques. Bioresources 2017, 12, 9143–9151. [Google Scholar]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Asric, S.E.A.M.; Ismailab, A.F. Physicochemical properties of “green” nanocrystalline cellulose isolated from recycled newspaper. RSC Adv. 2015, 5, 29842–29849. [Google Scholar] [CrossRef]
- Gupta, N.; Kushwaha, A.K.; Chattopadhyaya, M.C. Application of potato (Solanum tuberosum) plant wastes for the removal of methylene blue and malachite green dye from aqueous solution. Arab. J. Chem. 2016, 9, S707–S716. [Google Scholar] [CrossRef] [Green Version]
- Meisam, T.M. Biosorption of lanthanum and cerium from aqueous solutions using tangerine (Citrus reticulata) peel: Equilibrium, kinetic, and thermodynamic studies. Chem. Ind. Chem. Eng. Q. 2013, 19, 79–88. [Google Scholar]
- Peydayesh, M.; Rahbar-Kelishami, A. Adsorption of methylene blue onto Platanus orientalis leaf powder: Kinetic, equi-librium and thermodynamic studies. J. Ind. Eng. Chem. 2015, 21, 1014–1019. [Google Scholar] [CrossRef]
- Han, R.; Zou, W.; Yu, W.; Cheng, S.; Wang, Y.; Shi, J. Biosorption of methylene blue from aqueous solution by fallen phoenix tree’s leaves. J. Hazard. Mater. 2007, 141, 156–162. [Google Scholar] [CrossRef]
- Karley, A.J.; Mitchell, C.; Brookes, C.; McNicol, J.; O’Neill, T.; Roberts, H.; Graham, J.; Johnson, S.N. Exploiting physical defence traits for crop protection: Leaf trichomes of Rubus idaeus have deterrent effects on spider mites but not aphids. Ann. Appl. Biol. 2016, 168, 159–172. [Google Scholar] [CrossRef]
- Han, X.; Niu, X.; Ma, X. Adsorption characteristics of methylene blue on poplar leaf in batch mode: Equilibrium, kinetics and thermodynamics. Korean J. Chem. Eng. 2012, 29, 494–502. [Google Scholar] [CrossRef]
- Guo, D.; Li, Y.; Cui, B.; Hu, M.; Luo, S.; Ji, B.; Liu, Y. Natural adsorption of methylene blue by waste fallen leaves of Magnoli-aceae and its repeated thermal regeneration for reuse. J. Clean. Prod. 2020, 267, 121903. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Gheju, M.; Boran, S. Syringa vulgaris leaves powder a novel low-cost adsorbent for meth-ylene blue removal: Isotherms, kinetics, thermodynamic and optimization by Taguchi method. Sci. Rep. 2020, 10, 17676. [Google Scholar] [CrossRef] [PubMed]
- Mukhlish, M.Z.B.; Khan, M.R.; Bhoumick, M.C.; Paul, S. Leaf powder: Novel adsorbent for removal of methylene blue from aqueous solution. Water Air Soil Pollut. 2012, 223, 4949–4958. [Google Scholar] [CrossRef] [Green Version]
- Boumaza, S.; Yenounne, A.; Hachi, W.; Kaouah, F.; Bouhamidi, Y.; Trari, M. Application of Typha angustifolia (L.) dead leaves waste as biomaterial for the removal of cationic dye from aqueous solution. Int. J. Environ. Res. 2018, 12, 561–573. [Google Scholar] [CrossRef]
- Franco, D.S.P.; Georgin, J.; Drumm, F.C.; Netto, M.S.; Allasia, D.; Oliveira, M.L.S.; Dotto, G.L. Araticum (Annona crassiflora) seed powder (ASP) for the treatment of colored effluents by biosorption. Environ. Sci. Pollut. Res. 2020, 27, 11184–11194. [Google Scholar] [CrossRef]
- Aryee, A.A.; Zhang, R.; Liu, H.; Han, R.; Li, Z.; Qu, L. Application of magnetic peanut husk for methylene blue adsorption in batch mode. Desalination Water Treat. 2020, 194, 269–279. [Google Scholar] [CrossRef]
- Weng, C.H.; Lin, Y.T.; Tzeng, T.W. Removal of methylene blue from aqueous solution by adsorption onto pineapple leaf powder. J. Hazard. Mater. 2009, 170, 417–424. [Google Scholar] [CrossRef]
- Han, X.; Wang, W.; Ma, X. Adsorption characteristics of methylene blue onto low cost biomass material lotus leaf. Chem. Eng. J. 2011, 171, 1–8. [Google Scholar] [CrossRef]
- Sadare, O.O.; Ayeni, A.O.; Daramola, M.O. Evaluation of adsorption and kinetics of neem leaf powder (Azadirachta indica) as a bio-sorbent for desulfurization of dibenzothiophene (DBT) from synthetic diesel. J. Saudi Chem. Soc. 2022, 26, 101433. [Google Scholar] [CrossRef]
- Huang, Z.G.; Wang, T.; Yi, H.Y.; Li, X.B. Study on the adsorption of methylene blue from dye wastewater by Humulus japonicas leaves. In E3S Web of Conferences; EDP Sciences: Ulis, France, 2021; Volume 236, p. 03028. [Google Scholar]
- Singh, R.; Singh, T.S.; Odiyo, J.O.; Smith, J.A.; Edokpayi, J.N. Evaluation of methylene blue sorption onto low-cost biosorbents: Equilibrium, kinetics, and thermodynamics. J. Chem. 2020, 2020, 8318049. [Google Scholar] [CrossRef] [Green Version]
- Yagub, M.T.; Sen, T.K.; Ang, H.M. Equilibrium, kinetics, and thermodynamics of methylene blue adsorption by Pine Tree leaves. Water Air Soil Pollut. 2012, 223, 5267–5282. [Google Scholar] [CrossRef]
- Elmorsi, T.M. Equilibrium isotherms and kinetic studies of removal of methylene blue dye by adsorption onto miswak leaves as a natural adsorbent. J. Environ. Prot. 2011, 2, 817–827. [Google Scholar] [CrossRef] [Green Version]
- Sips, R. On the structure of a catalyst surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Fiaz, R.; Hafeez, M.; Mahmood, R. Ficcus palmata leaves as a low-cost biosorbent for methylene blue: Thermodynamic and kinetic studies. Water Environ. Res. 2019, 91, 689–699. [Google Scholar] [CrossRef]
- Reçber, Z. Adsorption of methylene blue onto spent Alchemilla vulgaris leaves: Characterization, isotherms, kinetic and thermodynamic studies. Int. J. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Zolgharnein, J.; Bagtash, M. Hybrid central composite design optimization for removal of Methylene blue by Acer tree leaves: Characterization of adsorption. Desalin. Water Treat. 2015, 54, 2601–2610. [Google Scholar] [CrossRef]
- Krishni, R.R.; Foo, K.Y.; Hameed, B.H. Adsorptive removal of methylene blue using the natural adsorbent-banana leaves. Desalin. Water Treat. 2014, 52, 6104–6112. [Google Scholar] [CrossRef]
- Jawad, A.H.; Rashid, R.A.; Mahmuod, R.M.A.; Ishak, M.A.M.; Kasim, N.N.; Ismail, K. Adsorption of methylene blue onto coconut (Cocos nucifera) leaf: Optimization, isotherm and kinetic studies. Desalin. Water Treat. 2015, 57, 8839–8853. [Google Scholar] [CrossRef]
- Ponnusami, V.; Gunasekar, V.; Srivastava, S.N. Kinetics of methylene blue removal from aqueous solution using gulmohar (Delonix regia) plant leaf powder: Multivariate regression analysis. J. Hazard. Mater. 2009, 169, 119–127. [Google Scholar] [CrossRef]
- Ponnusami, V.; Vikram, S.; Srivastava, S.N. Guava (Psidium guajava) leaf powder: Novel adsorbent for removal of methylene blue from aqueous solutions. J. Hazard. Mater. 2008, 152, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Rahman-Setayesh, M.R.; Kelishami, A.R.; Shayesteh, H. Equilibrium, kinetic, and thermodynamic applications for methylene blue removal using Buxus sempervirens leaf powder as a powerful low-cost adsorbent. J. Part. Sci. Technol. 2019, 5, 161–170. [Google Scholar]
- Zhai, Q.-Z. Studies of adsorption of crystal violet from aqueous solution by nano mesocellular foam silica: Process equilibrium, kinetic, isotherm, and thermodynamic studies. Water Sci. Technol. 2020, 81, 2092–2108. [Google Scholar] [CrossRef] [PubMed]
- Gerçel, O.; Özcan, A.; Özcan, A.S.; Gerçel, H.F. Preparation of activated carbon from a renewable bio-plant of Euphorbia rigida by H2SO4 activation and its adsorption behavior in aqueous solutions. Appl. Surf. Sci. 2007, 253, 4843–4852. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K.; Phan, C. Adsorption removal of Methylene Blue (MB) dye from aqueous solution by bio-char prepared from Eucalyptus sheathiana bark: Kinetic, equilibrium, mechanism, thermodynamic and process design. Desalin. Water Treat. 2016, 57, 28964–28980. [Google Scholar] [CrossRef]
- Jiang, Z.; Hu, D. Molecular mechanism of anionic dyes adsorption on cationized rice husk cellulose from agricultural wastes. J. Mol. Liq. 2019, 276, 105–114. [Google Scholar] [CrossRef]
- Wakkel, M.; Khiari, B.; Zagrouba, F. Textile wastewater treatment by agro-industrial waste: Equilibrium modelling, thermodynamics and mass transfer mechanisms of cationic dyes adsorption onto low-cost lignocellulosic adsorbent. J. Taiwan Inst. Chem. Eng. 2019, 96, 439–452. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Angosto, J.M.; Roca, M.J.; Miñarro, M.D. Taguchi design-based enhancement of heavy metals bioremoval by agroindustrial waste biomass from artichoke. Sci. Total Environ. 2018, 653, 55–63. [Google Scholar] [CrossRef]
- Zolgharnein, J.; Rastgordani, M. Optimization of simultaneous removal of binary mixture of indigo carmine and methyl orange dyes by cobalt hydroxide nano-particles through Taguchi method. J. Mol. Liq. 2018, 262, 405–414. [Google Scholar] [CrossRef]
| Kinetic Model | Parameters | Adsorbent Dose (g L−1) | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Pseudo-first-order | k1 (min−1) | 0.275 ± 0.035 | 0.279 ± 0.047 | 0.277 ± 0.054 | 0.279 ± 0.051 |
| qe,calc (mg g−1) | 37.48 ± 0.82 | 19.76 ± 0.29 | 13.47 ± 0.31 | 6.95 ± 0.27 | |
| R2 | 0.9901 | 0.9876 | 0.9891 | 0.9876 | |
| χ2 | 0.3429 | 0.2235 | 0.1347 | 0.0786 | |
| SSE | 12.11 | 4.23 | 1.72 | 0.52 | |
| ARE (%) | 13.96 | 14.34 | 14.14 | 14.36 | |
| Pseudo-second-order | k2 (min−1) | 0.013 ± 0.005 | 0.025 ± 0.010 | 0.037 ± 0.011 | 0.071 ± 0.026 |
| qe,calc (g mg−1 min−1) | 39.92 ± 0.89 | 21.07 ± 0.74 | 14.36 ± 0.71 | 7.40 ± 0.32 | |
| R2 | 0.9996 | 0.9986 | 0.9993 | 0.9986 | |
| χ2 | 0.0110 | 0.0251 | 0.0078 | 0.0087 | |
| SSE | 0.38 | 0.47 | 0.10 | 0.05 | |
| ARE (%) | 0.50 | 0.99 | 0.72 | 0.99 | |
| Elovich | a (g mg−1) | 0.267 ± 0.052 | 0.506 ± 0.061 | 0.741 ± 0.057 | 1.447 ± 0.075 |
| b (mg g−1 min−1) | 2508 ± 153 | 1328 ± 125 | 895 ± 96 | 895 ± 96 | |
| R2 | 0.9969 | 0.9973 | 0.9973 | 0.9974 | |
| χ2 | 0.1336 | 0.0637 | 0.0436 | 0.0227 | |
| SSE | 3.72 | 0.90 | 0.42 | 0.11 | |
| ARE (%) | 12.68 | 12.57 | 12.58 | 12.52 | |
| Avrami | kAV (min−1) | 0.633 ± 0.046 | 0.638 ± 0.057 | 0.635 ± 0.062 | 0.638 ± 0.073 |
| qAV (mg g−1) | 37.48 ± 0.51 | 19.76 ± 0.41 | 13.47 ± 0.45 | 6.95 ± 0.26 | |
| nAV | 0.435 | 0.437 | 0.436 | 0.437 | |
| R2 | 0.9901 | 0.9876 | 0.9891 | 0.9876 | |
| χ2 | 0.3419 | 0.2231 | 0.1344 | 0.0784 | |
| SSE | 12.11 | 4.23 | 1.72 | 0.52 | |
| ARE (%) | 13.96 | 14.34 | 14.14 | 14.34 | |
| Isotherm Model | Parameters | Value | ||
|---|---|---|---|---|
| 278 K | 297 K | 311 K | ||
| Langmuir | KL (L mg−1) | 0.011 ± 0.001 | 0.012 ± 0.001 | 0.013 ± 0.002 |
| qmax (mg g−1) | 186.08 ± 7.25 | 188.96 ± 6.12 | 192.58 ± 6.73 | |
| R2 | 0.9997 | 0.9997 | 0.9997 | |
| χ2 | 0.0321 | 0.0326 | 0.0332 | |
| SSE | 1.18 | 1.21 | 1.26 | |
| ARE (%) | 1.43 | 1.44 | 1.43 | |
| Freundlich | Kf (mg g−1) | 3.54 ± 0.61 | 3.78 ± 0.52 | 4.11 ± 0.71 |
| 1/n | 0.76 ± 0.07 | 0.75 ± 0.05 | 0.76 ± 0.04 | |
| R2 | 0.9988 | 0.9988 | 0.9988 | |
| χ2 | 0.3367 | 0.3419 | 0.3483 | |
| SSE | 5.23 | 5.40 | 5.60 | |
| ARE (%) | 4.74 | 4.74 | 4.74 | |
| Temkin | KT (L mg−1) | 0.291 ± 0.043 | 0.311 ± 0.057 | 0.339 ± 0.052 |
| b (kJ g−1) | 102.22 ± 7.49 | 100.67 ± 5.71 | 98.77 ± 6.18 | |
| R2 | 0.9632 | 0.9632 | 0.9633 | |
| χ2 | 16.41 | 16.67 | 17.00 | |
| SSE | 149.75 | 154.42 | 160.39 | |
| ARE (%) | 57.29 | 57.29 | 58.15 | |
| Sips | Qsat (mg g−1) | 235.5 ± 8.45 | 239.1 ± 6.27 | 244.6 ± 7.64 |
| KS (L mg−1) | 0.010 ± 0.001 | 0.011 ± 0.002 | 0.012 ± 0.002 | |
| n | 0.9326 | 0.9326 | 0.9319 | |
| R2 | 0.9998 | 0.9998 | 0.9998 | |
| χ2 | 0.0242 | 0.0246 | 0.0254 | |
| SSE | 0.56 | 0.58 | 0.60 | |
| ARE (%) | 1.42 | 1.42 | 1.43 | |
| Adsorbent | Maximum Adsorption Capacity (mg g−1) | Reference |
|---|---|---|
| Ficcus Palmata leaves | 6.89 | [60] |
| Alchemilla Vulgaris leaves | 45.66 | [61] |
| Ginkgo biloba leaves | 48.07 | [56] |
| Salix babylonica leaves | 60.9 | [17] |
| Daucus carota leaves powder | 66.5 | [28] |
| Phoenix tree’s leaves | 80.90 | [43] |
| Acer tree leaves | 97.07 | [62] |
| Elaeis guineensis leaves | 103.00 | [20] |
| Typha angustifolia leaves | 106.75 | [49] |
| Banana leaves | 109.90 | [63] |
| Cocos nucifera leaf | 112.35 | [64] |
| Platanus orientalis leaf powder | 114.90 | [42] |
| Pine Tree leaves | 126.58 | [57] |
| Poplar leaf | 135.40 | [45] |
| Humulus japonicas leaves | 145.56 | [55] |
| Magnolia grandiflora leaves | 149.25 | [46] |
| Magnolia denudate leaves | 185.19 | [46] |
| Gulmohar leaf | 186.24 | [65] |
| Syringa vulgaris leaves | 188.2 | [47] |
| Miswak leaves | 200 | [58] |
| Lotus leaf | 221.7 | [53] |
| Michelia figo leaves | 238.1 | [46] |
| Raspberry (Rubus idaeus) leaves | 244.6 | This study |
| Guava leaf | 295.04 | [66] |
| Buxus sepmervirens leaves | 384.61 | [67] |
| Papaya leaf | 512.55 | [48] |
| ΔG0 (kJ mol−1) | ΔH0 (kJ mol−1) | ΔS0 (J mol−1 K−1) | ||
|---|---|---|---|---|
| 278 K | 297 K | 311 K | ||
| −19.27 | −20.68 | −22.03 | 0.38 | 9.70 |
| pH | Ionic Strength (mol L−1) | Time (min) | Adsorbent Dose (g L−1) | Initial Dye Concentration (mg g−1) | Temperature (K) | Dye Removal Efficiency (%) | S/N Ratio |
|---|---|---|---|---|---|---|---|
| 2 | 0 | 5 | 1 | 20 | 278 | 49.87 | 33.95 |
| 2 | 0 | 5 | 1 | 100 | 297 | 48.62 | 33.73 |
| 2 | 0 | 5 | 1 | 250 | 311 | 45.12 | 33.08 |
| 2 | 0.1 | 20 | 3 | 20 | 278 | 60.96 | 35.70 |
| 2 | 0.1 | 20 | 3 | 100 | 297 | 59.42 | 35.47 |
| 2 | 0.1 | 20 | 3 | 250 | 311 | 55.15 | 34.83 |
| 2 | 0.2 | 40 | 5 | 20 | 278 | 66.03 | 36.39 |
| 2 | 0.2 | 40 | 5 | 100 | 297 | 64.37 | 36.17 |
| 2 | 0.2 | 40 | 5 | 250 | 311 | 59.74 | 35.52 |
| 6 | 0 | 20 | 5 | 20 | 297 | 79.35 | 37.99 |
| 6 | 0 | 20 | 5 | 100 | 311 | 77.64 | 37.80 |
| 6 | 0 | 20 | 5 | 250 | 278 | 68.30 | 36.68 |
| 6 | 0.1 | 40 | 1 | 20 | 297 | 72.78 | 37.24 |
| 6 | 0.1 | 40 | 1 | 100 | 311 | 71.21 | 37.05 |
| 6 | 0.1 | 40 | 1 | 250 | 278 | 62.65 | 35.93 |
| 6 | 0.2 | 5 | 3 | 20 | 297 | 58.07 | 35.28 |
| 6 | 0.2 | 5 | 3 | 100 | 311 | 56.82 | 35.09 |
| 6 | 0.2 | 5 | 3 | 250 | 278 | 49.99 | 33.97 |
| 10 | 0 | 40 | 3 | 20 | 311 | 89.43 | 39.03 |
| 10 | 0 | 40 | 3 | 100 | 278 | 82.95 | 38.37 |
| 10 | 0 | 40 | 3 | 250 | 297 | 76.70 | 37.69 |
| 10 | 0.1 | 5 | 5 | 20 | 311 | 65.73 | 36.35 |
| 10 | 0.1 | 5 | 5 | 100 | 278 | 60.97 | 35.70 |
| 10 | 0.1 | 5 | 5 | 250 | 297 | 56.37 | 35.02 |
| 10 | 0.2 | 20 | 1 | 20 | 311 | 69.08 | 36.78 |
| 10 | 0.2 | 20 | 1 | 100 | 278 | 64.08 | 36.13 |
| 10 | 0.2 | 20 | 1 | 250 | 297 | 59.25 | 35.45 |
| Level | pH | Ionic Strength | Time | Adsorbent Dose | Initial Concentration | Temperature |
|---|---|---|---|---|---|---|
| 1 | 34.99 | 36.49 | 34.69 | 35.49 | 36.53 | 35.87 |
| 2 | 36.34 | 35.92 | 36.32 | 36.16 | 36.17 | 36.01 |
| 3 | 36.73 | 35.65 | 37.05 | 36.41 | 35.36 | 36.17 |
| Delta | 1.74 | 0.84 | 2.36 | 0.92 | 1.17 | 0.30 |
| Rank | 2 | 5 | 1 | 4 | 3 | 6 |
| Contribution (%) | 27.10 | 5.92 | 47.26 | 7.35 | 11.65 | 0.73 |
| Desorbing Agent | Desorption Efficiencies (%) |
|---|---|
| HCl | 77.63 ± 2.51 |
| Distilled water | 8.08 ± 0.25 |
| NaOH | 36.95 ± 1.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosoarca, G.; Popa, S.; Vancea, C.; Dan, M.; Boran, S. Removal of Methylene Blue from Aqueous Solutions Using a New Natural Lignocellulosic Adsorbent—Raspberry (Rubus idaeus) Leaves Powder. Polymers 2022, 14, 1966. https://doi.org/10.3390/polym14101966
Mosoarca G, Popa S, Vancea C, Dan M, Boran S. Removal of Methylene Blue from Aqueous Solutions Using a New Natural Lignocellulosic Adsorbent—Raspberry (Rubus idaeus) Leaves Powder. Polymers. 2022; 14(10):1966. https://doi.org/10.3390/polym14101966
Chicago/Turabian StyleMosoarca, Giannin, Simona Popa, Cosmin Vancea, Mircea Dan, and Sorina Boran. 2022. "Removal of Methylene Blue from Aqueous Solutions Using a New Natural Lignocellulosic Adsorbent—Raspberry (Rubus idaeus) Leaves Powder" Polymers 14, no. 10: 1966. https://doi.org/10.3390/polym14101966






