Synthesis and Optimization of a Free-Radical/Cationic Hybrid Photosensitive UV Curable Resin Using Polyurethane Acrylate and Graphene Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Free Radical/Cationic Hybrid Photosensitive UV Cured Resin (UVR)
2.2.2. Preparation of PUA Modified Photosensitive UV Cured Resin (PUA-UVR)
2.2.3. Preparation of GO Modified Photosensitive UV Cured Resin (GO-UVR)
2.3. Measurement and Characterization
2.3.1. Fourier-Transform Infrared (FTIR) Spectroscopy
2.3.2. Contact Angle Measurement
2.3.3. Apparent Morphology
2.3.4. Thermal Stability
2.3.5. Crystallinity
2.3.6. Mechanical Structure
2.3.7. Volume Shrinkage Determination
2.3.8. Gel Rate Determination
2.3.9. Rheological Properties
3. Results and Discussion
3.1. Orthogonal Reaction Analysis
3.2. Structure Elucidation Analysis
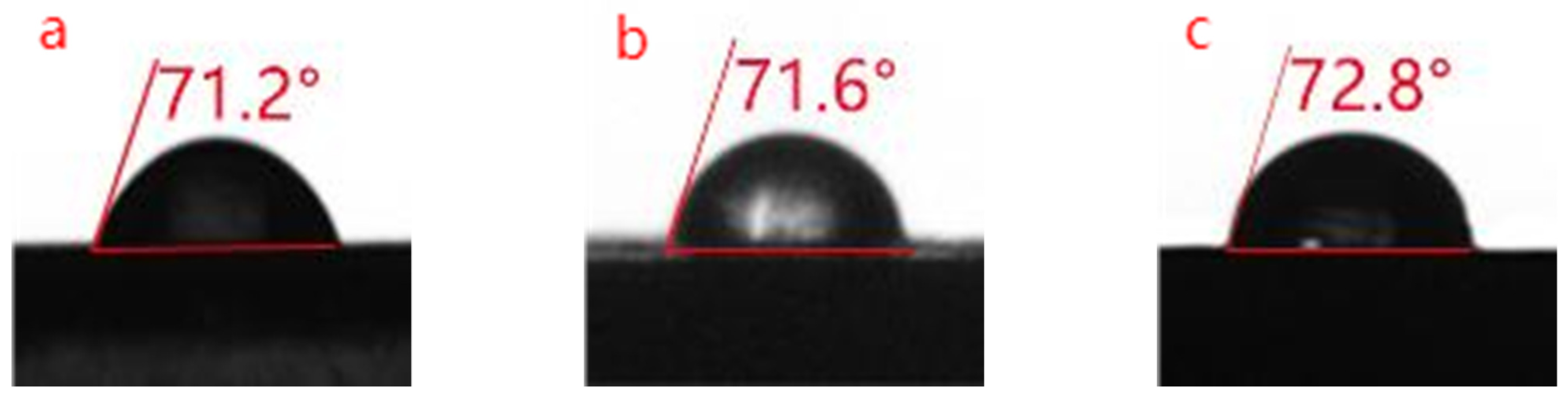
3.3. Hydrophobicity Analysis
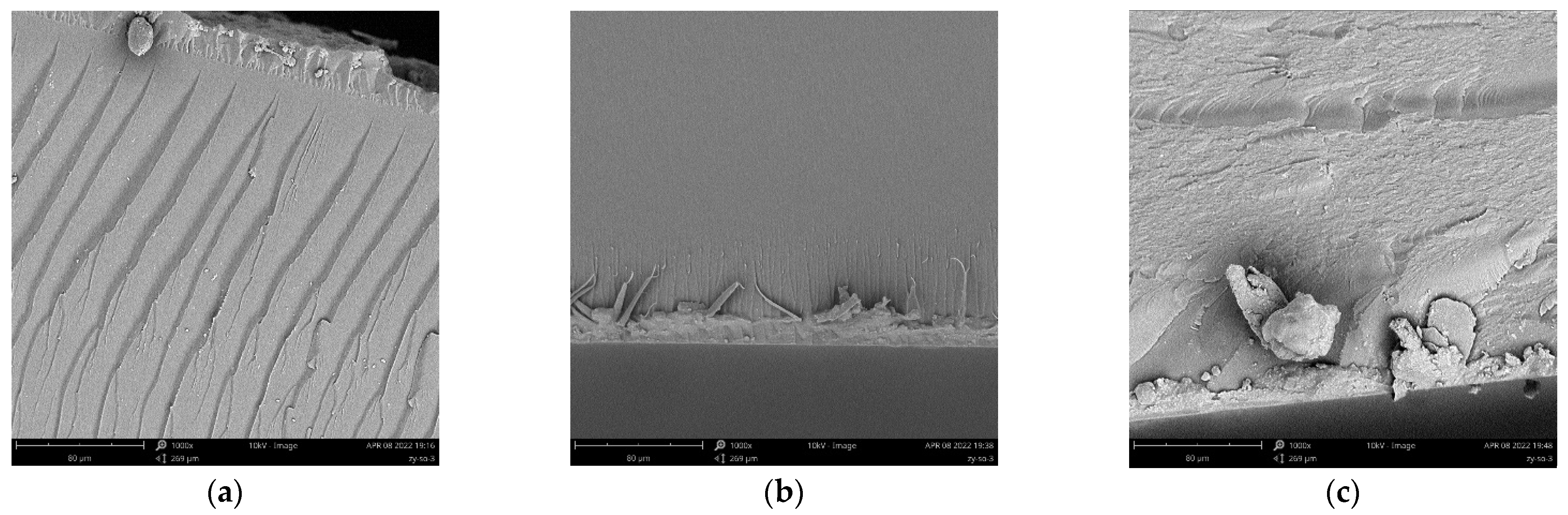
3.4. Morphological Analysis
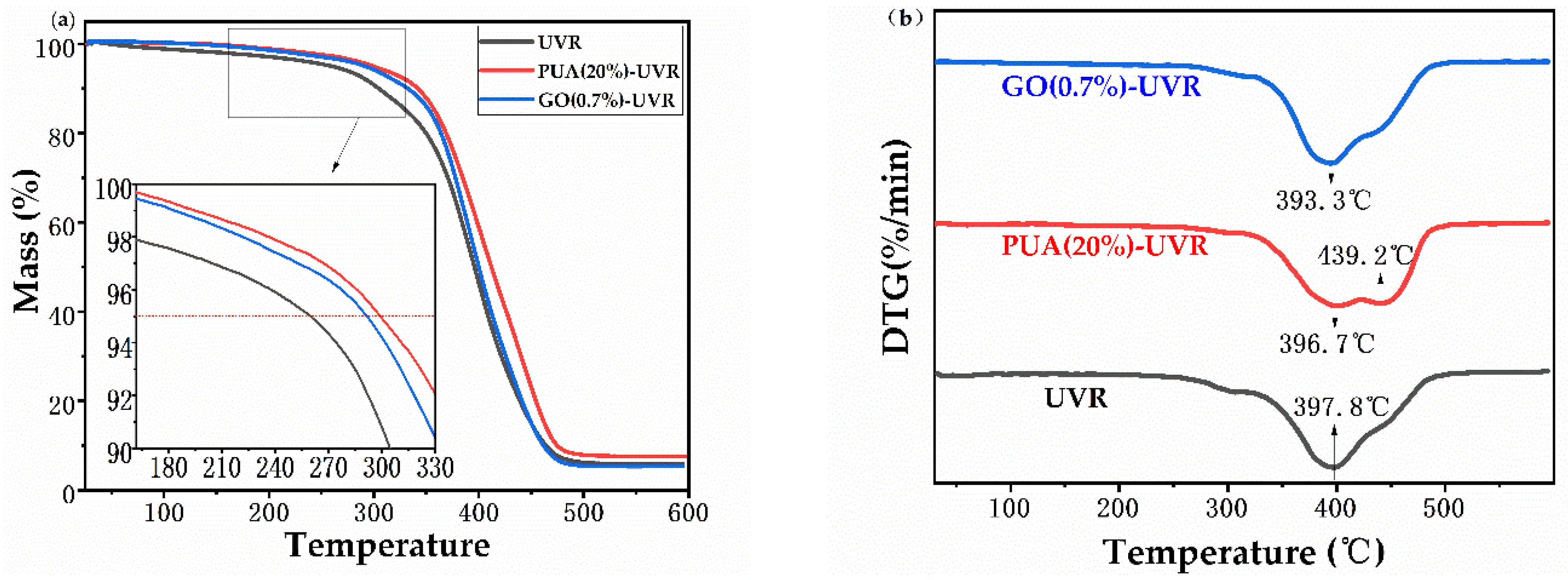
3.5. Thermogravimetric Analysis
3.6. Crystallinity Analysis
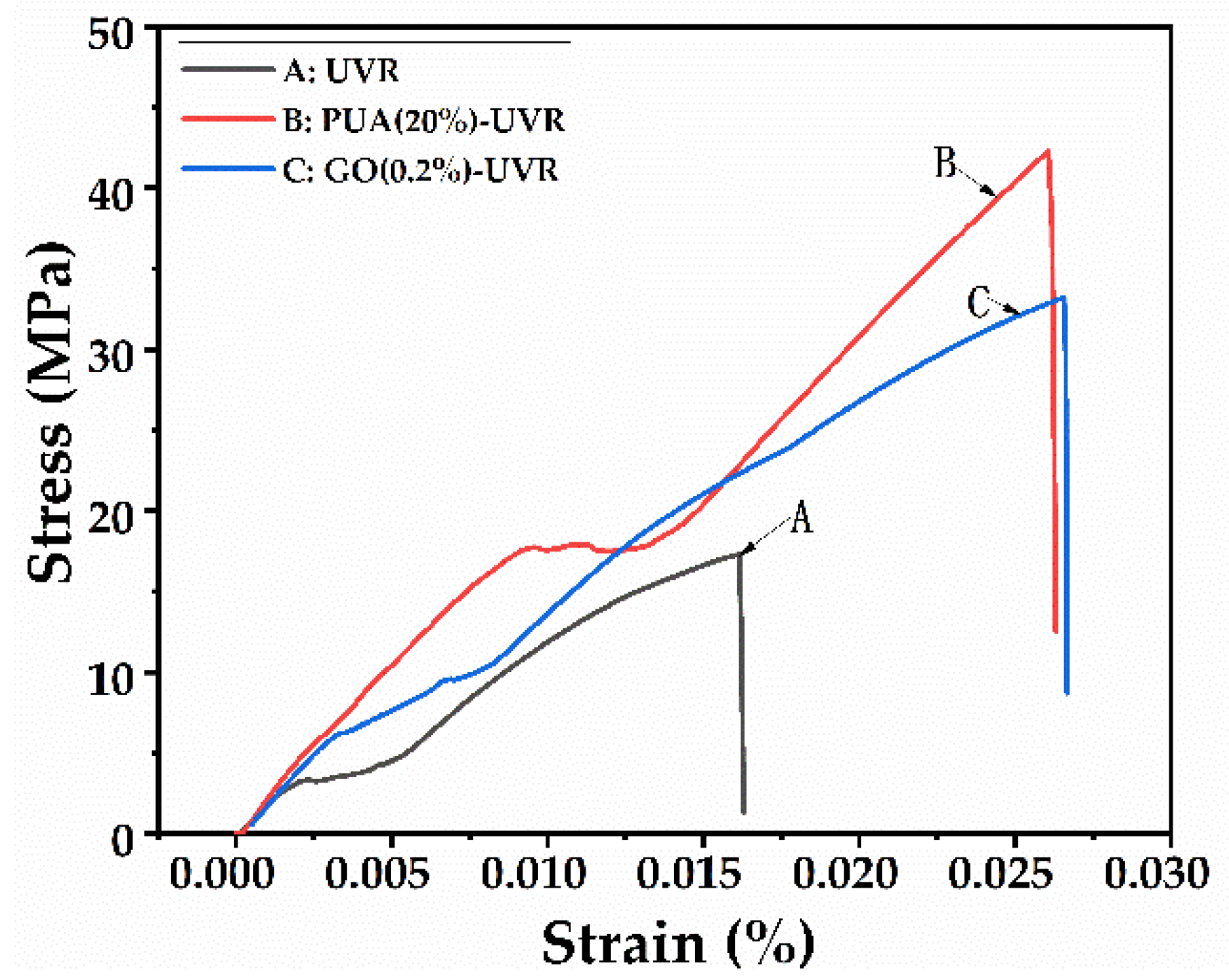
3.7. Mechanical Properties Analysis
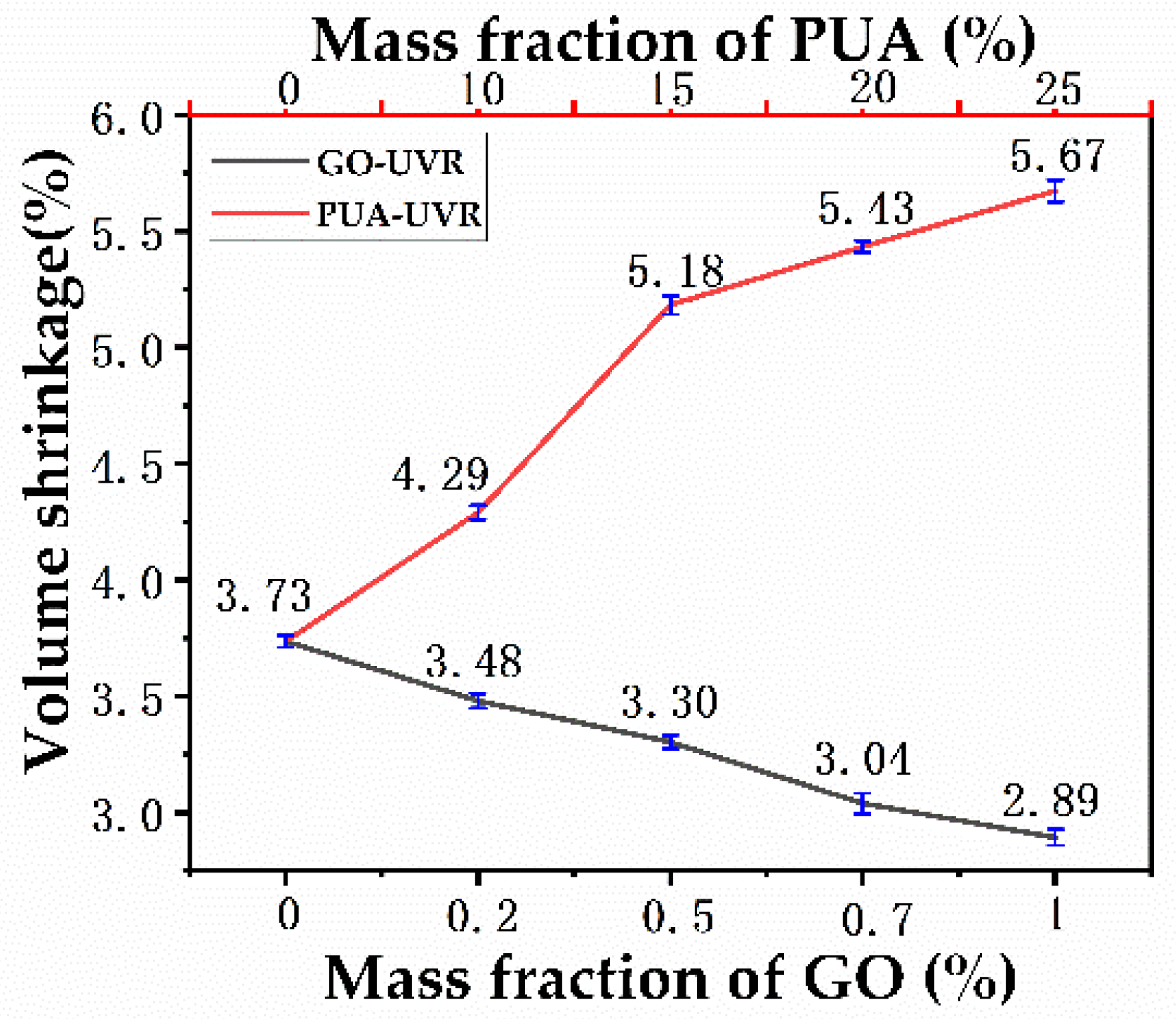
3.8. Volume Shrinkage Rate Analysis
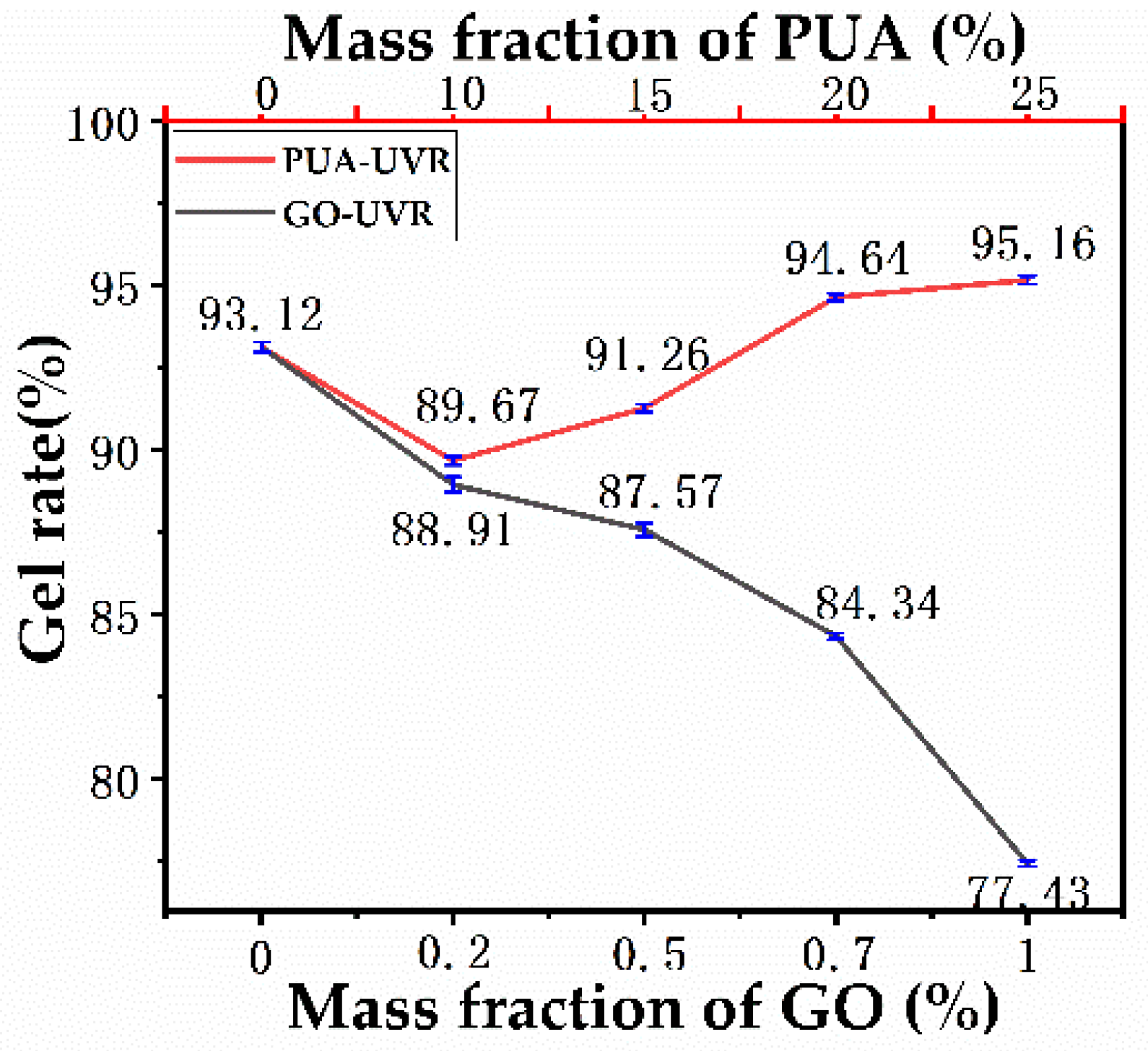
3.9. Gelation Rate Analysis
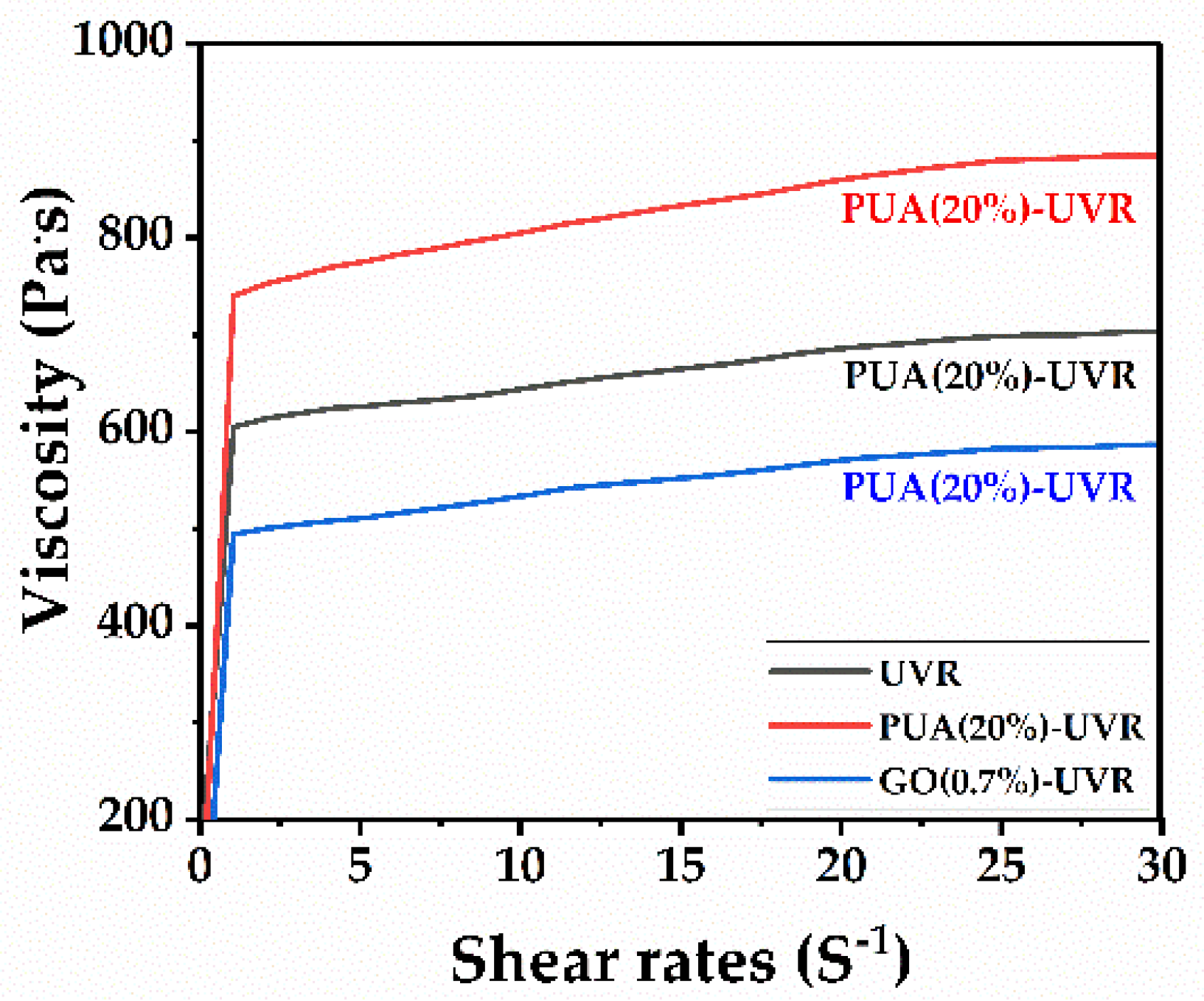
3.10. Rheological Properties Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakhshandeh, E.; Bastani, S.; Saeb, M.R.; Croutxe-Barghorn, C.; Allonas, X. High-performance water-based UV-curable soft systems with variable chain architecture for advanced coating applications. Prog. Org. Coat. 2019, 130, 99–113. [Google Scholar] [CrossRef]
- Rehbein, T.; Johlitz, M.; Lion, A.; Sekmen, K.; Constantinescu, A. Temperature-and degree of cure-dependent viscoelastic properties of photopolymer resins used in digital light processing. Prog. Addit. Manuf. 2021, 6, 743–756. [Google Scholar] [CrossRef]
- Ren, Y.; Dong, Y.; Zhu, Y.; Xu, J.; Yao, Y. Preparation, characterization, and properties of novel ultraviolet-curable and flame-retardant polyurethane acrylate. Prog. Org. Coat. 2019, 129, 309–317. [Google Scholar] [CrossRef]
- Wang, D. Research on Application of Stereolithography 3D Printing Materials. J. New Ind. 2016, 6, 59–63. [Google Scholar]
- Li, X.; Yu, R.; He, Y.; Zhang, Y.; Yang, X.; Zhao, X.; Huang, W. Self-healing polyurethane elastomers based on a disulfide bond by digital light processing 3D printing. ACS Macro Lett. 2019, 8, 1511–1516. [Google Scholar] [CrossRef]
- Wu, X.; Yang, X.; Yu, R.; Zhao, X.; Zhang, Y.; Huang, W. A facile access to stiff epoxy vitrimers with excellent mechanical properties via siloxane equilibration. J. Mater. Chem. A 2018, 6, 10184–10188. [Google Scholar] [CrossRef]
- Rizwan, U.I.H.; Soohwan, J.; Jongwon, S. Fabrication of A Functionally Graded and Magnetically Responsive Shape Memory Polymer Using A 3D Printing Technique and Its Characterization. J. Appl. Polym. Sci. 2018, 135, 45997–46002. [Google Scholar]
- Zhao, T.; Yu, R.; Li, S.; Li, X.; Zhang, Y.; Yang, X.; Zhao, X.; Wang, C.; Liu, Z.; Dou, R.; et al. Superstretchable and processable silicone elastomers by digital light processing 3D printing. ACS Appl. Mater. Interfaces 2019, 11, 14391–14398. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Fan, S.; Feng, X.; Cao, K.; Ge, Q.; Gao, L.; Lu, Y. Three-Dimensional Stretchable Microelectronics by Projection Microstereolithography (PμSL). ACS Appl. Mater. Interfaces 2021, 13, 8901–8908. [Google Scholar] [CrossRef]
- Wang, D.; Li, L.; Zhang, B.; Zhang, Y.; Wu, M.; Gu, G.; Ge, Q. Effect of temperature on the programmable helical deformation of a reconfigurable anisotropic soft actuator. Int. J. Solids Struct. 2020, 199, 169–180. [Google Scholar] [CrossRef]
- Park, S.; Lee, D.H.; Ryoo, H.I.; Lim, T.W.; Yang, D.Y.; Kim, D.P. Fabrication of Three-dimensional SiC Ceramic Microstructures with Near-zero Shrinkage via Dual Crosslinking Induced Stereolithography. Chem. Commun. 2009, 32, 4880–4882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Noirbent, G.; Brunel, D.; Liu, F.; Gigmes, D.; Sun, K. Ketone derivatives as photoinitiators for both radical and cationic photopolymerizations under visible LED and application in 3D printing. Eur. Polym. J. 2020, 132, 109737. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Z.; Hamel, C.M.; Mu, X.; Kuang, X.; Guo, Z.; Qi, H.J. Evolution of material properties during free radical photopolymerization. J. Mech. Phys. Solids 2018, 112, 25–49. [Google Scholar] [CrossRef]
- Kim, Y.; Hong, S.; Nam, J.; Sun, H.; Kim, M.G.; Choi, K.; Cho, J.; Choi, H.R. UV Curing Kinetics and Performance Development of in situ Curable 3D Printing Materi-al. Eur. Polym. J. 2017, 93, 140–147. [Google Scholar] [CrossRef]
- Hossain, M.; Steinmann, P. Degree of cure-dependent modelling for polymer curing processes at small-strain. Part I: Consistent reformulation. Comput. Mech. 2014, 53, 777–787. [Google Scholar] [CrossRef]
- Zhao, T.T.; Li, X.P.; Yu, R.; Zhang, Y.; Yang, X.; Zhao, X.; Wang, L.; Huang, W. Silicone-epoxy-based Hybrid Photo-polymers for 3D Printing. Macromol. Chem. Phys. 2018, 219, 1700530–1700540. [Google Scholar] [CrossRef]
- Winfield, R.J.; O’Brien, S. Two-photon Polymerization of An Epoxy-acrylate Resin Material System. Appl. Surf. Sci. 2011, 257, 5389–5392. [Google Scholar] [CrossRef]
- Min, Y.; Wang, H.; Huang, W.; Hong, J.; Zhang, X.; Du, B. Development of polymeric materials for 3D printing technology. Adhesion 2016, 37, 36–41. [Google Scholar]
- Yang, Z.; Shan, J.; Huang, Y.; Dong, X.; Zheng, W.; Jin, Y.; Zhou, W. Preparation and mechanism of free-radical/cationic hybrid photosensitive resin with high tensile strength for three-dimensional printing application. J. Appl. Polym. Sci. 2021, 138, 49881. [Google Scholar] [CrossRef]
- Shan, J.; Yang, Z.; Chen, G.; Hu, Y.; Luo, Y.; Dong, X.; Zheng, W.; Zhou, W. Design and synthesis of free-radical/cationic photosensitive resin applied for 3D printer with liquid crystal display (LCD) irradiation. Polymers 2020, 12, 1346. [Google Scholar] [CrossRef]
- Noè, C.; Malburet, S.; Bouvet-Marchand, A.; Graillot, A.; Loubat, C.; Sangermano, M. Cationic photopolymerization of bio-renewable epoxidized monomers. Prog. Org. Coat. 2019, 133, 131–138. [Google Scholar] [CrossRef]
- You, S.; Wang, P.; Schimelman, J.; Hwang, H.H.; Chen, S. High-fidelity 3D printing using flashing photopolymerization. Addit. Manuf. 2019, 30, 100834. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xu, Y.; Jia, J.; Chen, P.; Zhang, F.; Zhang, L.; Chen, Y. Improvement on curing performance and morphology of E5I/TPGDA mixture in a free radical-cationic hybrid photopolymerization system. J. Polym. Res. 2020, 27, 166. [Google Scholar] [CrossRef]
- Hola, E.; Ortyl, J.; Jankowska, M.; Pilch, M.; Galek, M.; Morlet-Savary, F.; Graff, B.; Dietlin, C.; Lalevee, J. New bimolecular photoinitiating systems based on terphenyl derivatives as highly efficient photosensitizers for 3D printing application. Polym. Chem. 2020, 11, 922–935. [Google Scholar] [CrossRef]
- Xu, Y.; Noirbent, G.; Brunel, D.; Ding, Z.; Gigmes, D.; Graff, B.; Xiao, P.; Dumur, F.; Lalevee, J. Allyloxy ketones as efficient photoinitiators with high migration stability in free radical polymerization and 3D printing. Dye. Pigment. 2021, 185, 108900. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Liu, Y.; Du, S.; Leng, J. Photosensitive Composite Inks for Digital Light Processing Four-Dimensional Printing of Shape Memory Capture Devices. ACS Appl. Mater. Interfaces 2021, 13, 18110–18119. [Google Scholar] [CrossRef]
- Zhu, Y.; Dong, Y.; Chen, L.; Yao, Y.; Hong, Y.; Tang, G. Synthesis and Properties of Rapid UV Curable Polyurethane with Comb-Like Structure. Fine Chem. 2018, 35, 17–23. [Google Scholar]
- Abadie MJ, M.; Manole, I.; Fetecau, C. Photosensitive Formulation for Additive Manufacturing-3D Printing. Mater. Plast. 2020, 57, 141–152. [Google Scholar] [CrossRef]
- Huang, B.; Wu, B.; Han, L.; Lu, Z.; Zhou, W. Preparation of a novel cationic photosensitive resin (3D-SLR01) for stereolithography 3D printing and determination of its some properties. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2019, 34, 761–768. [Google Scholar] [CrossRef]
- Huang, B.; Du, Z.; Yong, T.; Han, W. Preparation of a novel hybrid type photosensitive resinfor stereolithography in 3D printing and testing on the accuracy of the fabricated parts. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2017, 32, 726–732. [Google Scholar] [CrossRef]
- Lin, G.; Yi, J.; Huang, H.; Huang, W.; Cai, M.; Xiang, H.; Liu, X. Photocuring kinetics properties of hybrid UV-curing resin for 3D printing. J. Mater. Eng. 2019, 47, 143–150. [Google Scholar]
- Jiao, T.; Lin, Y.; Liu, Y.; Liu, J.; Lu, G. Effect of modified calcium sulphate whiskers on free-radical/cationic hybrid photopolymers for 3D printing. Mater. Res. Express 2020, 7, 015334. [Google Scholar] [CrossRef]
- Škola, O.; Jašúrek, B.; Veselý, D.; Nemec, P. Mechanical properties of polymer layers fabricated via hybrid free radical-cationic polymerization of acrylate, epoxide, and oxetane binders. Prog. Org. Coat. 2016, 101, 279–287. [Google Scholar] [CrossRef]
- Sahu, R.; Patra, K.; Szpunar, J. Experimental study and numerical modelling of creep and stress relaxation of dielectric elastomers. Strain 2015, 51, 43–54. [Google Scholar] [CrossRef]
- Wissler, M.; Mazza, E. Mechanical behavior of an acrylic elastomer used in dielectric elastomer actuators. Sens. Actuators A Phys. 2007, 134, 494–504. [Google Scholar] [CrossRef]
- Liao, Z.; Hossain, M.; Yao, X.; Mehnert, M.; Steinmann, P. On thermo-viscoelastic experimental characterization and numerical modelling of VHB polymer. Int. J. Non-Linear Mech. 2020, 118, 103263. [Google Scholar] [CrossRef]
- Pang, Z.; Ling, X. Study Relative Internal Stress and Shrinkage of Cured Volume for UV-curing Coatings. J. Beijing Univ. Chem. Technol. 1995, 4, 22–26. [Google Scholar]


| Level | A | B/% | C/% | |
|---|---|---|---|---|
| Factors | ||||
| 1 | 1:1 | 20 | 3 | |
| 2 | 2:1 | 30 | 4 | |
| 3 | 3:1 | 40 | 5 | |
| A | B/% | C/% | Null Columns | |
|---|---|---|---|---|
| ① | 1:1 | 20 | 3 | 1 |
| ② | 1:1 | 30 | 4 | 2 |
| ③ | 1:1 | 40 | 5 | 3 |
| ④ | 2:1 | 20 | 4 | 3 |
| ⑤ | 2:1 | 30 | 5 | 1 |
| ⑥ | 2:1 | 40 | 3 | 2 |
| ⑦ | 3:1 | 20 | 5 | 2 |
| ⑧ | 3:1 | 30 | 3 | 3 |
| ⑨ | 3:1 | 40 | 4 | 1 |
| A | B/% | C/% | Null Columns | Tensile Strength/MPa | Volume Shrinkage/% | Gel Rate/% |
|---|---|---|---|---|---|---|
| 1:1 | 20 | 3 | 1 | 17.35 | 3.81 | 87.39 |
| 1:1 | 30 | 4 | 2 | 19.44 | 4.87 | 90.11 |
| 1:1 | 40 | 5 | 3 | 15.58 | 6.38 | 88.65 |
| 2:1 | 20 | 4 | 3 | 16.23 | 4.52 | 89.26 |
| 2:1 | 30 | 5 | 1 | 18.50 | 5.30 | 95.28 |
| 2:1 | 40 | 3 | 2 | 14.90 | 6.96 | 84.53 |
| 3:1 | 20 | 5 | 2 | 15.05 | 5.44 | 90.72 |
| 3:1 | 30 | 3 | 3 | 11.80 | 6.53 | 83.70 |
| 3:1 | 40 | 4 | 1 | 10.34 | 7.3 | 85.14 |
| A | B | C | Null Columns | Primary and Secondary Order | Optimum Combination | ||
|---|---|---|---|---|---|---|---|
| Tensile strength | k1 | 17.46 | 16.21 | 14.68 | 15.4 | A > B > C | A1B2C3 |
| k2 | 16.54 | 16.58 | 15.34 | 16.46 | |||
| k3 | 12.4 | 13.61 | 16.38 | 14.54 | |||
| R | 5.06 | 2.97 | 1.69 | 1.93 | |||
| Volume shrinkage | k1 | 5.02 | 4.59 | 5.77 | 5.49 | B > A > C | A1B1C2 |
| k2 | 5.59 | 5.57 | 5.58 | 5.76 | |||
| k3 | 6.44 | 6.9 | 5.71 | 5.81 | |||
| R | 1.42 | 2.31 | 0.18 | 0.32 | |||
| Gel rate | k1 | 88.72 | 89.12 | 85.21 | 89.27 | C > B > A | A2B2C3 |
| k2 | 89.69 | 89.7 | 88.17 | 88.45 | |||
| k3 | 86.52 | 86.11 | 91.55 | 87.2 | |||
| R | 3.17 | 3.59 | 6.34 | 2.07 |
| Sum of Squares of Deviation | Degrees of Freedom | The Variance | F | Fa | Significant Level | ||
|---|---|---|---|---|---|---|---|
| Tensile strength | A | 43.633 | 2 | 21.8165 | 7.806 | 19 | F < Fa |
| B | 15.755 | 2 | 7.8775 | 2.818 | 19 | F < Fa | |
| C | 4.376 | 2 | 2.188 | 0.783 | 19 | F < Fa | |
| error | 5.59 | 2 | 2.795 | ||||
| sum | 69.354 | ||||||
| Volume shrinkage | A | 3.077 | 2 | 1.5385 | 17.094 | 19 | F < Fa |
| B | 8.068 | 2 | 4.034 | 44.822 | 19 | F > Fa, remarkable | |
| C | 0.052 | 2 | 0.026 | 0.289 | 19 | F < Fa | |
| error | 0.18 | 2 | 0.09 | ||||
| sum | 11.377 | ||||||
| Gel rate | A | 15.822 | 2 | 7.911 | 2.434 | 19 | F < Fa |
| B | 22.317 | 2 | 11.1585 | 3.433 | 19 | F < Fa | |
| C | 60.444 | 2 | 30.222 | 9.299 | 19 | F < Fa | |
| error | 6.5 | 2 | 3.25 | ||||
| sum | 105.083 |
| Tensile Strength (MPa) | Young’s Modulus (MPa) | |
|---|---|---|
| UVR | 16.42 | 1820.62 |
| PUA (10%)-UVR | 28.76 | 1893.24 |
| PUA (15%)-UVR | 32.11 | 1726.12 |
| PUA (20%)-UVR | 36.89 | 2296.14 |
| PUA (25%)-UVR | 28.31 | 2128.34 |
| GO (0.2%)-UVR | 29.27 | 1549.99 |
| GO (0.5%)-UVR | 13.16 | 2642.22 |
| GO (0.7%)-UVR | 11.84 | 2597.72 |
| GO (1%)-UVR | 12.07 | 1712.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Wang, Y.; Wei, Z.; Han, X.; Mo, Q.; Wang, X.; Li, Y. Synthesis and Optimization of a Free-Radical/Cationic Hybrid Photosensitive UV Curable Resin Using Polyurethane Acrylate and Graphene Oxide. Polymers 2022, 14, 1959. https://doi.org/10.3390/polym14101959
Huang L, Wang Y, Wei Z, Han X, Mo Q, Wang X, Li Y. Synthesis and Optimization of a Free-Radical/Cationic Hybrid Photosensitive UV Curable Resin Using Polyurethane Acrylate and Graphene Oxide. Polymers. 2022; 14(10):1959. https://doi.org/10.3390/polym14101959
Chicago/Turabian StyleHuang, Lijie, Yanan Wang, Zhehao Wei, Xiaoxue Han, Qi Mo, Xiyue Wang, and Yishan Li. 2022. "Synthesis and Optimization of a Free-Radical/Cationic Hybrid Photosensitive UV Curable Resin Using Polyurethane Acrylate and Graphene Oxide" Polymers 14, no. 10: 1959. https://doi.org/10.3390/polym14101959
APA StyleHuang, L., Wang, Y., Wei, Z., Han, X., Mo, Q., Wang, X., & Li, Y. (2022). Synthesis and Optimization of a Free-Radical/Cationic Hybrid Photosensitive UV Curable Resin Using Polyurethane Acrylate and Graphene Oxide. Polymers, 14(10), 1959. https://doi.org/10.3390/polym14101959






