Species Surface Distribution and Surface Tension of Aqueous Solutions of MIBC and NaCl Using Molecular Dynamics Simulations
Abstract
:1. Introduction
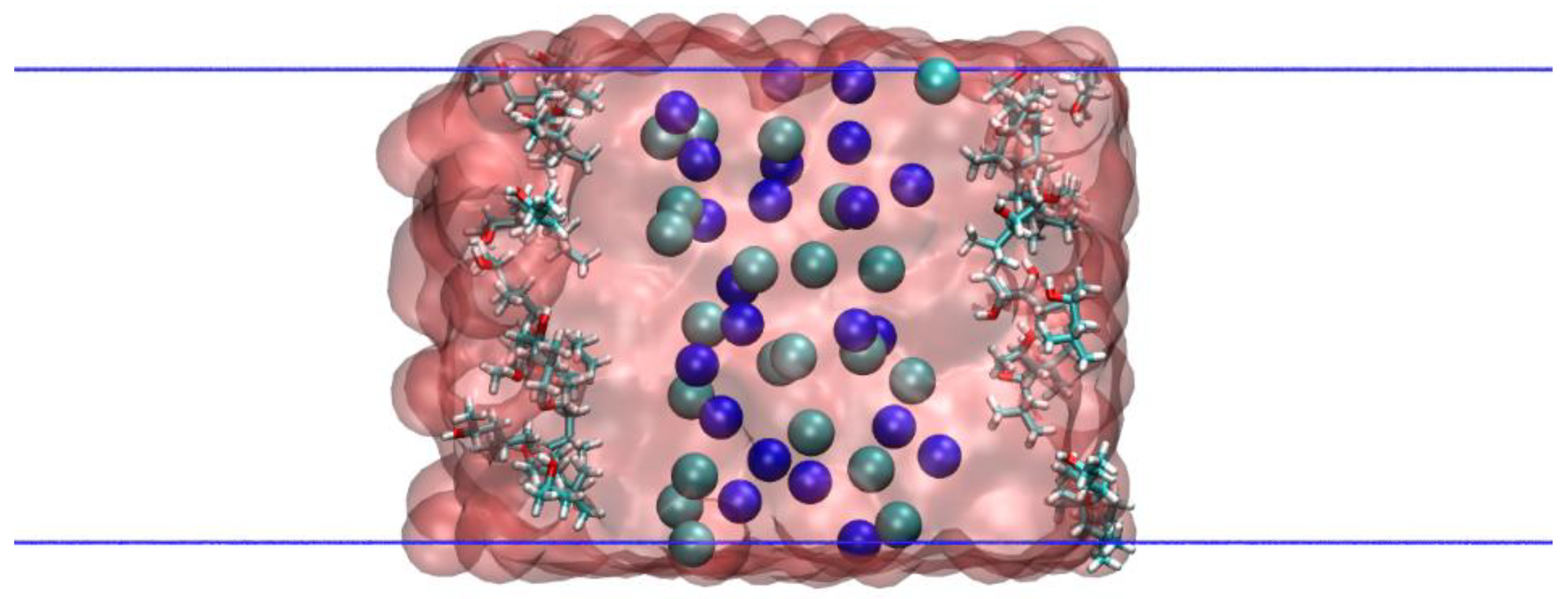
2. Methodology
3. Results
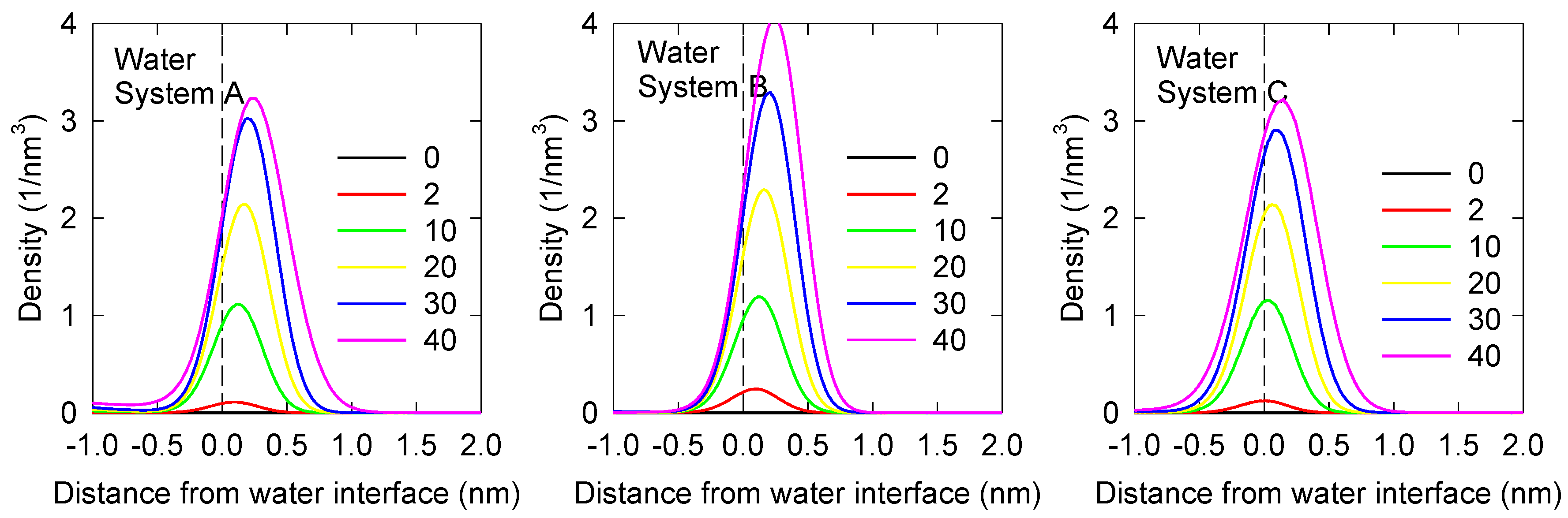
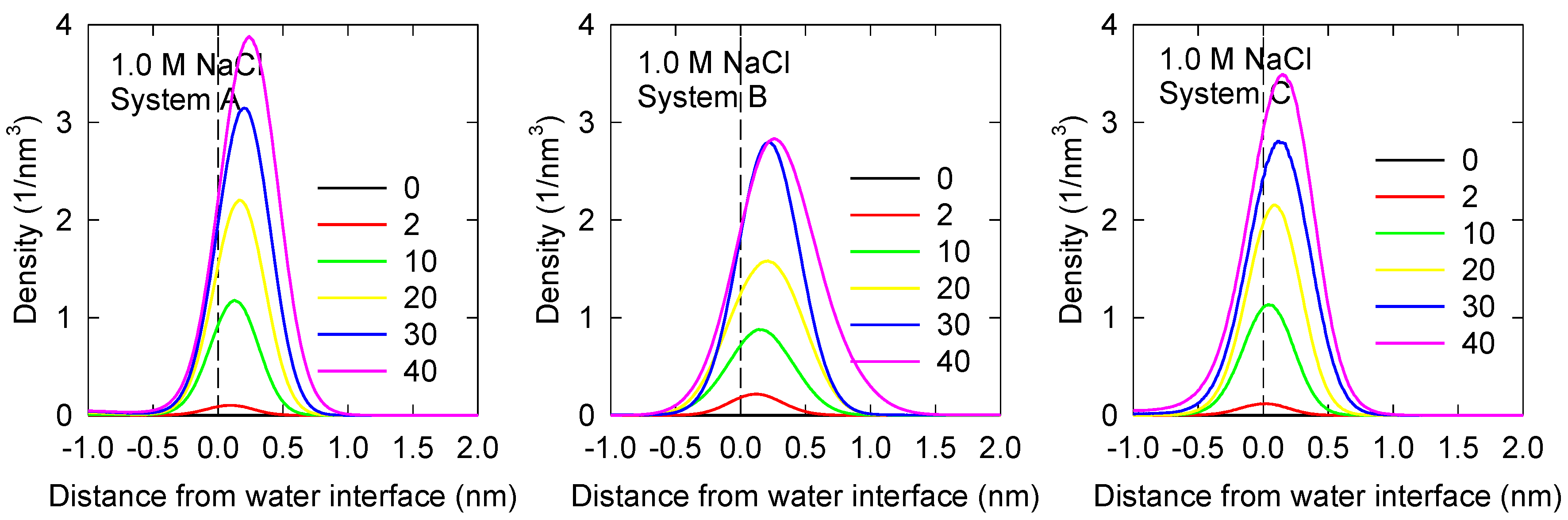
3.1. MIBC Density Profiles and Orientation
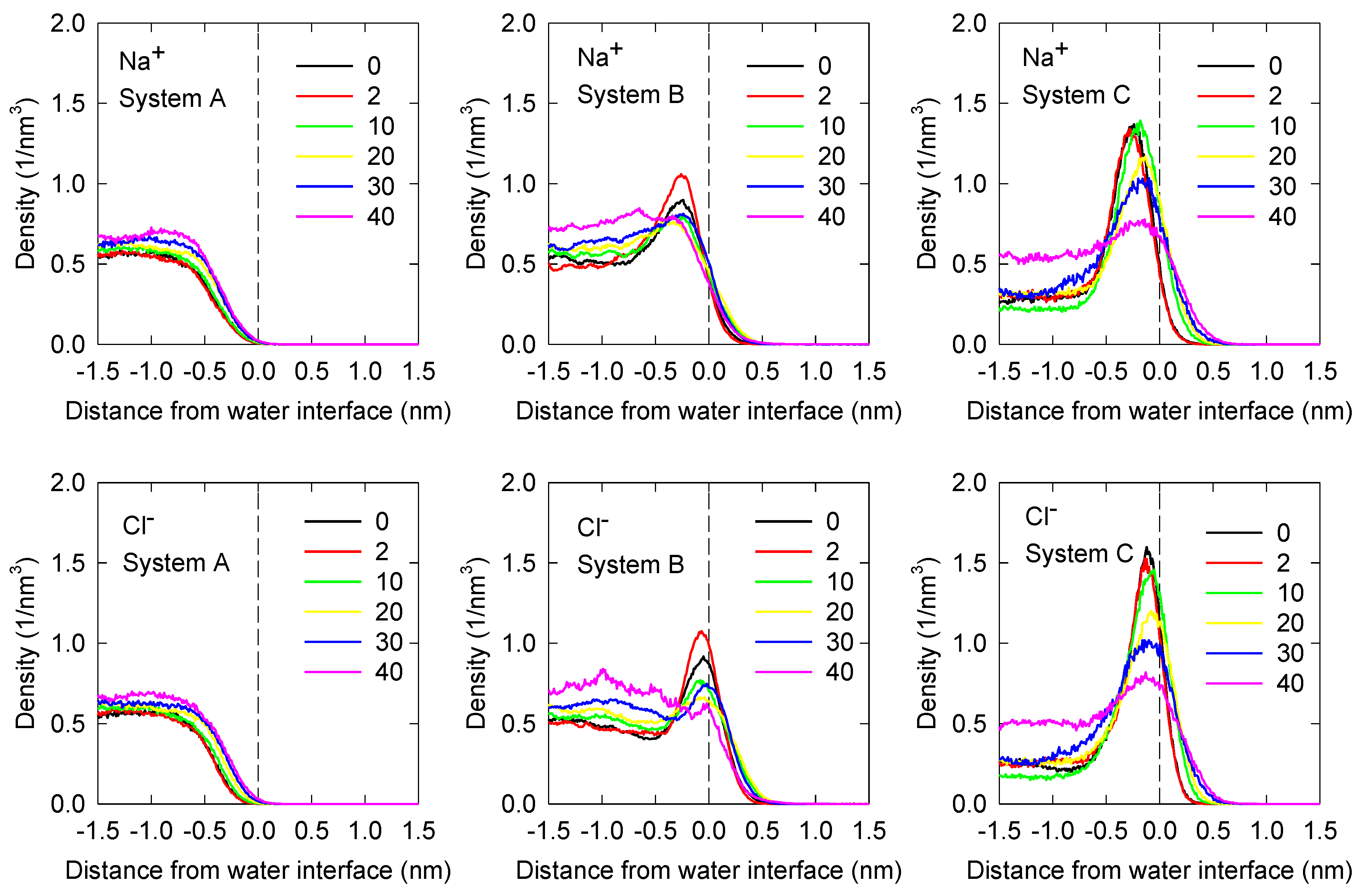
3.2. Ion Density Profiles
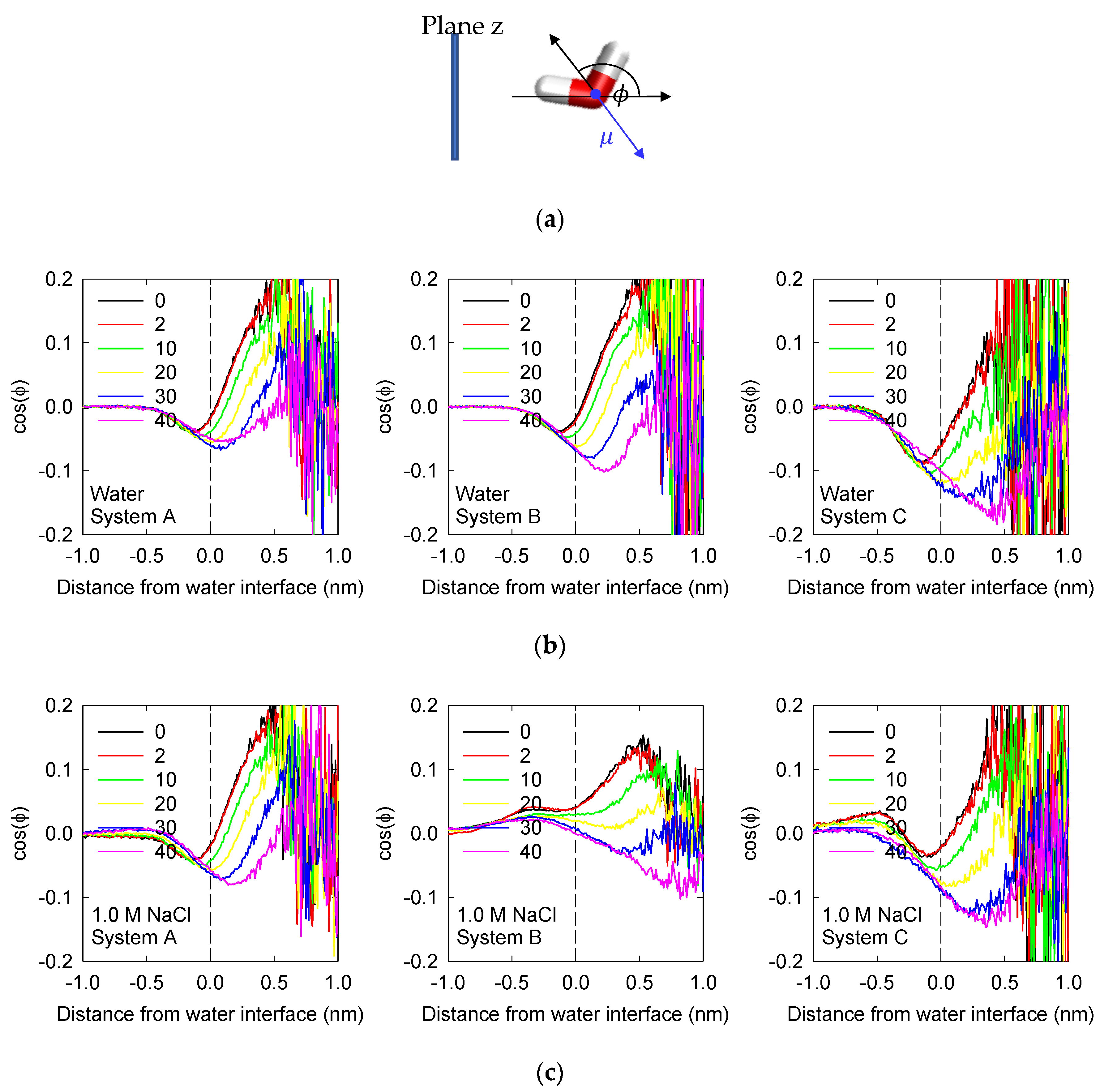
3.3. Water Orientation
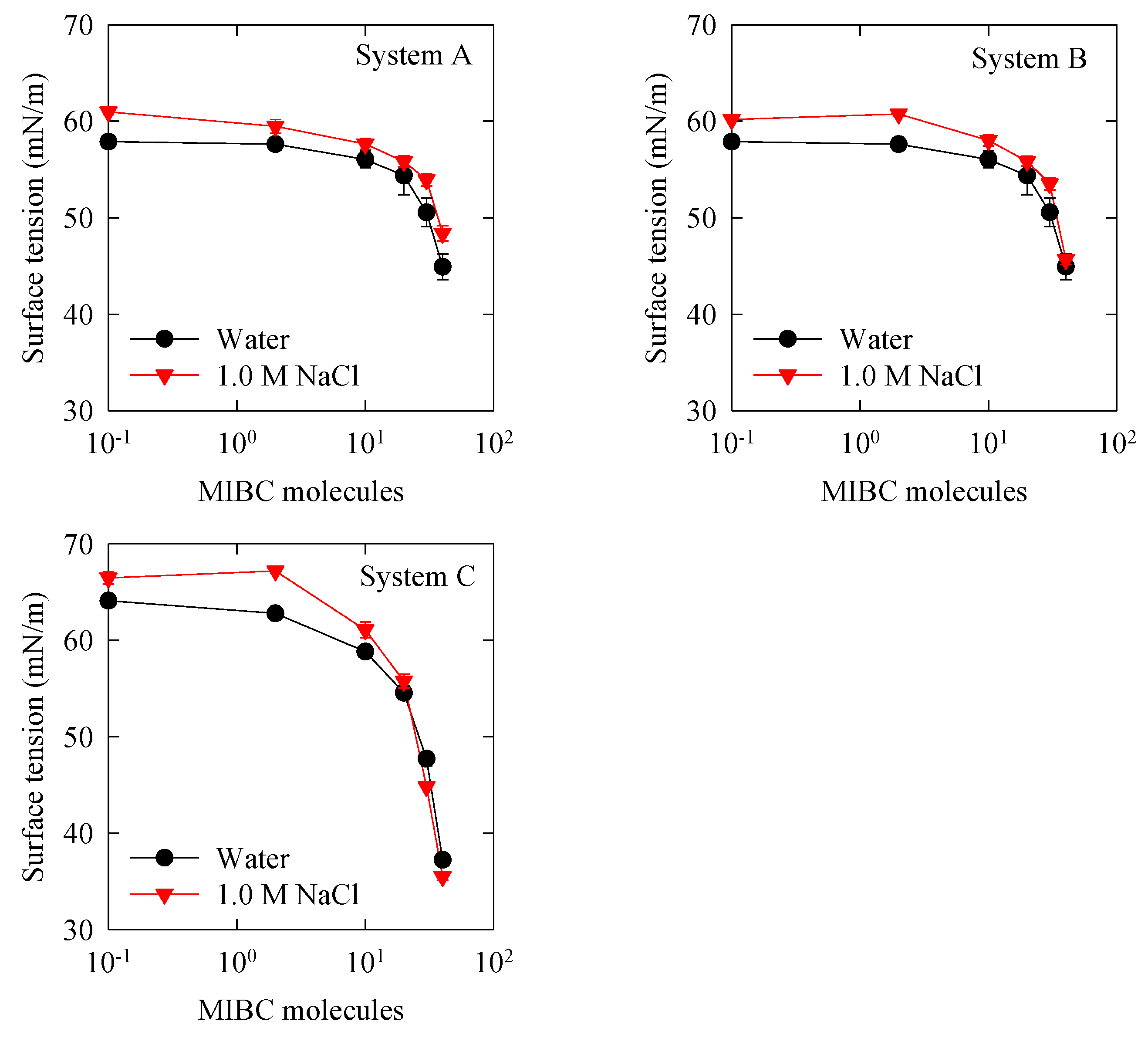
3.4. Surface Tension
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Comley, B.A.; Harris, P.J.; Bradshaw, D.J.; Harris, M.C. Frother Characterisation Using Dynamic Surface Tension Measurements. Int. J. Miner. Process. 2002, 64, 81–100. [Google Scholar] [CrossRef]
- Le, T.N.; Phan, C.M.; Ang, H.M. Influence of Hydrophobic Tail on the Adsorption of Isomeric Alcohols at Air/Water Interface. Asia Pac. J. Chem. Eng. 2012, 7, 250–255. [Google Scholar] [CrossRef]
- Nguyen, C.V.; Chaudhary, D. Surface Potential of MIBC at Air/Water Interface: A Molecular Dynamics Study. e-J. Surf. Sci. Nanotech. 2012, 10, 437–440. [Google Scholar]
- Nguyen, C.V.; Phan, C.M.; Ang, H.M.; Nakahara, H.; Shibata, O.; Moroi, Y. Molecular Dynamics Investigation on Adsorption Layer of Alcohols at the Air/Brine Interface. Langmuir 2015, 31, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Nguyen, C.V.; Phan, C.M. Molecular Arrangement Between Electrolyte and Alcohol at the Air/Water Interface. J. Mol. Liq. 2017, 242, 859–867. [Google Scholar] [CrossRef]
- Jungwirth, P.; Tobias, D.J. Ions at the Air/Water Interface. J. Phys. Chem. B 2002, 106, 6361–6373. [Google Scholar] [CrossRef]
- Jungwirth, P.; Tobias, D.J. Specific Ion Effects at the Air/Water Interface. Chem. Rev. 2006, 106, 1259–1281. [Google Scholar] [CrossRef]
- Liu, D.; Ma, G.; Levering, L.M.; Allen, H.C. Vibrational Spectroscopy of Aqueous Sodium Halide Solutions and Air-Liquid Interfaces: Observation of Increased Interfacial Depth. J. Phys. Chem. B 2004, 108, 2252–2260. [Google Scholar] [CrossRef]
- Mucha, M.; Frigato, T.; Levering, L.M.; Allen, H.C.; Tobias, D.J.; Dang, L.X.; Jungwirth, P. Unified Molecular Picture of the Surfaces of Aqueous Acid, Base, and Salt Solutions. J. Phys. Chem. B 2005, 109, 7617–7623. [Google Scholar] [CrossRef]
- Shen, Y.R.; Ostroverkhov, V. Sum-Frequency Vibrational Spectroscopy on Water Interfaces: Polar Orientation of Water Molecules at Interfaces. Chem. Rev. 2006, 106, 1140–1154. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Liu, D.F.; Allen, H.C.; Kuo, M.; Shultz, M.J. Vibrational Spectroscopic Studies of Aqueous Interfaces: Salts, Acids, Bases, and Nanodrops. Chem. Rev. 2006, 106, 1155–1175. [Google Scholar] [CrossRef] [PubMed]
- Tarbuck, T.L.; Ota, S.T.; Richmond, G.L. Spectroscopic Studies of Solvated Hydrogen and Hydroxide Ions at Aqueous Surfaces. J. Am. Chem. Soc. 2006, 128, 14519–14527. [Google Scholar] [CrossRef] [PubMed]
- Levering, L.M.; Sierra-Hernandez, M.R.; Allen, H.C. Observation of Hydronium Ions at the Air-Aqueous Acid Interface: Vibrational Spectroscopic Studies of Aqueous HCl, HBr, and HI. J. Phys. Chem. C 2007, 111, 8814–8826. [Google Scholar] [CrossRef]
- Tian, C.; Ji, N.; Waychunas, G.A.; Shen, Y.R. Interfacial Structures of Acidic and Basic Aqueous Solutions. J. Am. Chem. Soc. 2008, 130, 13033–13039. [Google Scholar] [CrossRef] [Green Version]
- D’Auria, R.; Tobias, D.J. Relation Between Surface Tension and Ion Adsorption at the Air–Water Interface: A Molecular Dynamics Simulation Study. J. Phys. Chem. A 2009, 113, 7286–7293. [Google Scholar] [CrossRef]
- Chattoraj, D.K. Adsorption and the Gibbs Surface Excess; Chattoraj, D.K., Birdi, K.S., Eds.; Plenum Press: New York, NY, USA, 1984. [Google Scholar]
- Onsager, L.; Samaras, N.N.T. The Surface Tension of Debye-Hückel Electrolytes. J. Chem. Phys. 1934, 2, 528–536. [Google Scholar] [CrossRef]
- Ozdemir, O.; Karakashev, S.I.; Nguyen, A.V.; Miller, J.D. Adsorption and Surface Tension Analysis of Concentrated Alkali Halide Brine Solutions. Miner. Eng. 2009, 22, 263–271. [Google Scholar] [CrossRef]
- Phan, C.M.; Nakahara, H.; Shibata, O.; Moroi, Y.; Le, T.N.; Ang, H.M. Surface Potential of Methyl Isobutyl Carbinol Adsorption Layer at the Air/Water Interface. J. Phys. Chem. B 2012, 116, 980–986. [Google Scholar] [CrossRef]
- Shahir, A.A.; Nguyen, K.T.; Nguyen, A.V. A Sum-Frequency Generation Spectroscopic Study of the Gibbs Analysis Paradox: Monolayer or Sub-Monolayer Adsorption? Phys. Chem. Chem. Phys. 2016, 18, 8794. [Google Scholar] [CrossRef]
- Castro, S.; Miranda, C.; Toledo, P.; Laskowski, J.S. Effect of Frothers on Bubble Coalescence and Foaming in Electrolyte Solutions and Seawater. Int. J. Miner. Processing 2013, 124, 8–14. [Google Scholar] [CrossRef]
- Nguyen, C.V.; Phan, C.M.; Ang, H.M.; Nakahara, H.; Shibata, O.; Moroi, Y. Surface Potential of 1-Hexanol Solution: Comparison with Methyl Isobutyl Carbinol. J. Phys. Chem. B 2013, 117, 7615–7620. [Google Scholar] [CrossRef] [PubMed]
- Sahu, K.; McNeill, V.F.; Eisenthal, K.B. Effect of Salt on the Adsorption Affinity of An Aromatic Carbonyl Molecule to the Air-Aqueous Interface: Insight for Aqueous Environmental Interfaces. J. Phys. Chem. C 2010, 114, 18258–18262. [Google Scholar] [CrossRef] [Green Version]
- Krisch, M.J.; D’Auria, R.; Brown, M.A.; Tobias, D.J.; Hemminger, C.; Ammann, M.; Starr, D.E.; Bluhm, H. The Effect of An Organic Surfactant on the Liquid-Vapor Interface of An Electrolyte Solution. J. Phys. Chem. C 2007, 111, 13497–13509. [Google Scholar] [CrossRef]
- Gungoren, C. An Investigation of Air/Water Interface in Mixed Aqueous Solutions of KCl, NaCl, and DAH. Physicochem. Probl. Miner. Process. 2019, 5, 1259–1270. [Google Scholar]
- Lemkul, J.A.; Roux, B.; van der Spoel, D.; MacKerell, A.D., Jr. Implementation of Extended Lagrangian Dynamics in GROMACS for Polarizable Simulations Using the Classical Drude Oscillator Model. J. Comput. Chem. 2015, 36, 1473–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Song, L.F.; Merz Jr, K.M. Systematic Parameterization of Monovalent Ions Employing the Nonbonded Model. J. Chem. Theory Comput. 2015, 11, 1645–1657. [Google Scholar] [CrossRef]
- Cornell, W.D.; Caldwell, J.W.; Kollman, P.A. A Second Generation Force Field for the Simulation of Proteins, Nucleic Acids, and Organic Molecules. J. Am. Chem. Soc. 1995, 117, 5179–5197. [Google Scholar] [CrossRef] [Green Version]
- Sorin, E.J.; Pande, V.S. Exploring the Helix-Coil Transition Via All-Atom Equilibrium Ensemble Simulations. Biophys. J. 2005, 88, 2472–2493. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Whitfield, T.W.; Harder, E.; Lamoureux, G.; Vorobyov, I.; Anisimov, V.M.; MacKerell, A.D., Jr.; Roux, B. Simulating Monovalent and Divalent Ions in Aqueous Solution Using a Drude Polarizable Force Field. J. Chem. Theory Comput. 2010, 6, 774–786. [Google Scholar] [CrossRef] [Green Version]
- Lamoureux, G.; Harder, E.; Vorobyov, I.V.; Roux, B.; MacKerell, A.D., Jr. A Polarizable Model of Water for Molecular Dynamics Simulations of Biomolecules. Chem. Phys. Lett. 2006, 418, 245–249. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle Mesh Ewald: An N log(N) Method for Ewald Sums in Large Systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef] [Green Version]
- Asmadi, A.; Kirchner, T.; Abdallah, W.; Fedorov, M.V.; Mikhail, R. Stukan, M.R. Influence of the Drude Charge Value on the Performance of Polarisable Water Model: A test for Microscopic and Macroscopic Parameters. J. Mol. Liq. 2013, 188, 245–251. [Google Scholar] [CrossRef]
- Vega, C.; de Miguel, E. Surface Tension of the Most Popular Models of Water by Using the Test-Area Simulation Method. J. Chem. Phys. 2007, 126, 154707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, A.E.; Grest, G.S.; Stevens, M.J. Capillary Waves at the Liquid-Vapor Interface and the Surface Tension of Water. J. Chem. Phys. 2006, 125, 014702. [Google Scholar] [CrossRef]
- Chen, F.; Smith, P.E. Simulated Surface Tensions of Common Water Models. J. Chem. Phys. 2007, 126, 3. [Google Scholar] [CrossRef]


| Atom Type | Charge | Meaning |
|---|---|---|
| H | +0.06000 | Aliplatic hydrogen |
| C1 | −0.06000 | Aliphatic carbon with 1 H |
| C2 | −0.12000 | Aliphatic carbon with 2 H |
| C3 | −0.18000 | Aliphatic carbon with 3 H |
| COH | +0.20500 | Carbon bond with OH |
| OH | −0.68300 | Hydroxide oxygen |
| HO | +0.41800 | Hydroxide hydrogen |
| Components | Systems | ||
|---|---|---|---|
| A | B | C | |
| Water model | SPC/E | SPC/E | SWM4-NDP |
| Ions model | Li-IOD-2015 | Drude-2013 | Drude-2013 |
| MIBC model | Amber99sb | Amber99sb | Amber99sb |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado, O.; Quezada, G.R.; Saavedra, J.H.; Rozas, R.E.; Toledo, P.G. Species Surface Distribution and Surface Tension of Aqueous Solutions of MIBC and NaCl Using Molecular Dynamics Simulations. Polymers 2022, 14, 1967. https://doi.org/10.3390/polym14101967
Alvarado O, Quezada GR, Saavedra JH, Rozas RE, Toledo PG. Species Surface Distribution and Surface Tension of Aqueous Solutions of MIBC and NaCl Using Molecular Dynamics Simulations. Polymers. 2022; 14(10):1967. https://doi.org/10.3390/polym14101967
Chicago/Turabian StyleAlvarado, Omar, Gonzalo R. Quezada, Jorge H. Saavedra, Roberto E. Rozas, and Pedro G. Toledo. 2022. "Species Surface Distribution and Surface Tension of Aqueous Solutions of MIBC and NaCl Using Molecular Dynamics Simulations" Polymers 14, no. 10: 1967. https://doi.org/10.3390/polym14101967









