Stimulus-Responsiveness of Thermo-Sensitive Polymer Hybridized with N-Doped Carbon Quantum Dots and Its Applications in Solvent Recognition and Fe3+ Ion Detection
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of N-CQDs
2.3. Synthesis of the Thermo-Sensitive Poly(NIPAAm-co-AAc) Copolymers
2.4. Preparation of N-CQDs Hybrid Thermo-Sensitive Copolymers (Poly-N-CQDs)
2.5. Characterization
2.5.1. Fourier Transform Infrared Spectroscopy (FT IR)
2.5.2. Gel Permeation Chromatography (GPC)
2.5.3. Particle Size Distribution (PSD)
2.5.4. Transmittance Measurement
2.5.5. Transmission Electron Microscopy (TEM)
2.5.6. Fluorescence Spectrum
3. Results and Discussion
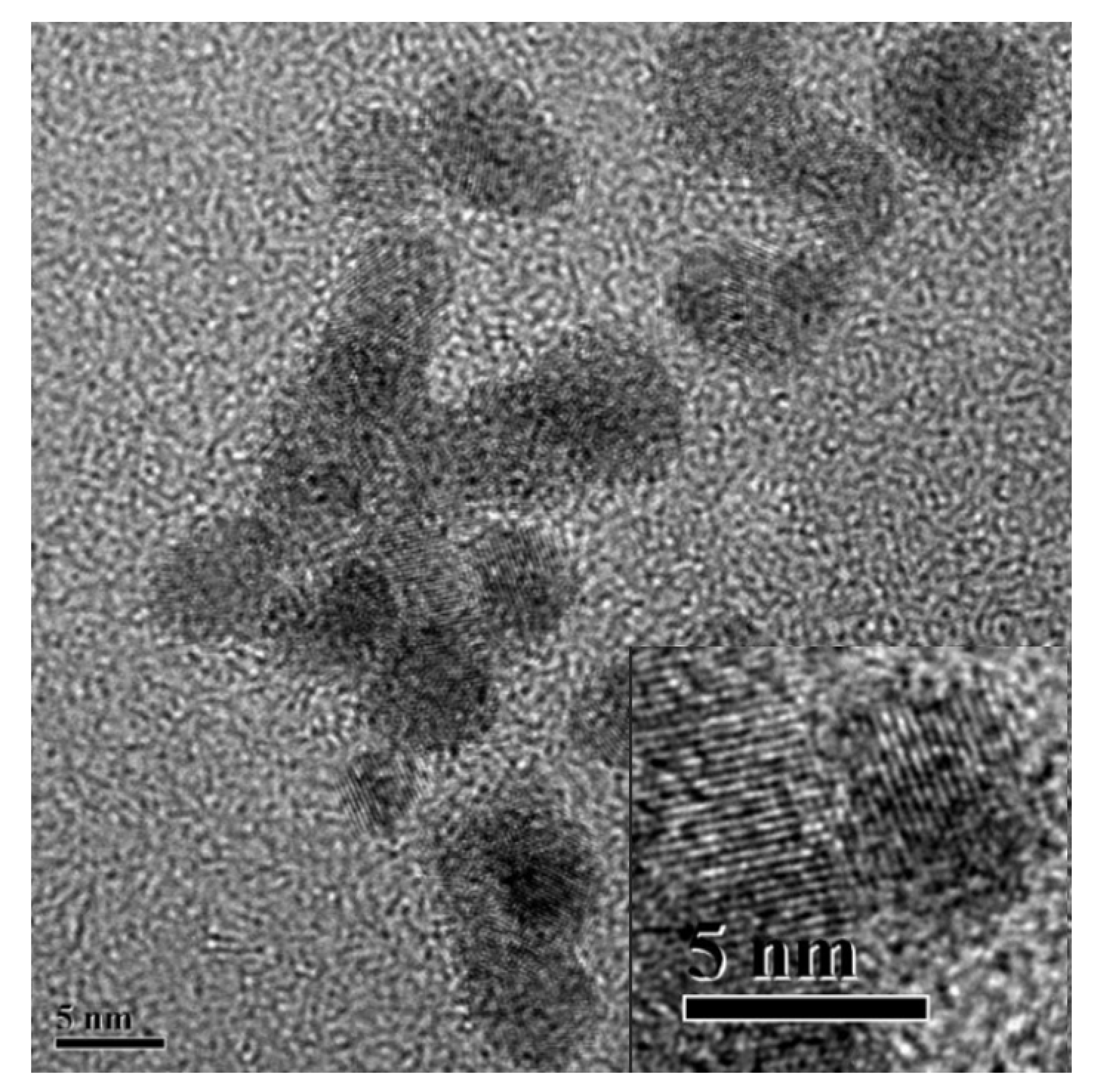
3.1. The Structure and Morphology of N-CQDs, Poly(NIPAAm-co-AAc) and Poly-N-CQDs
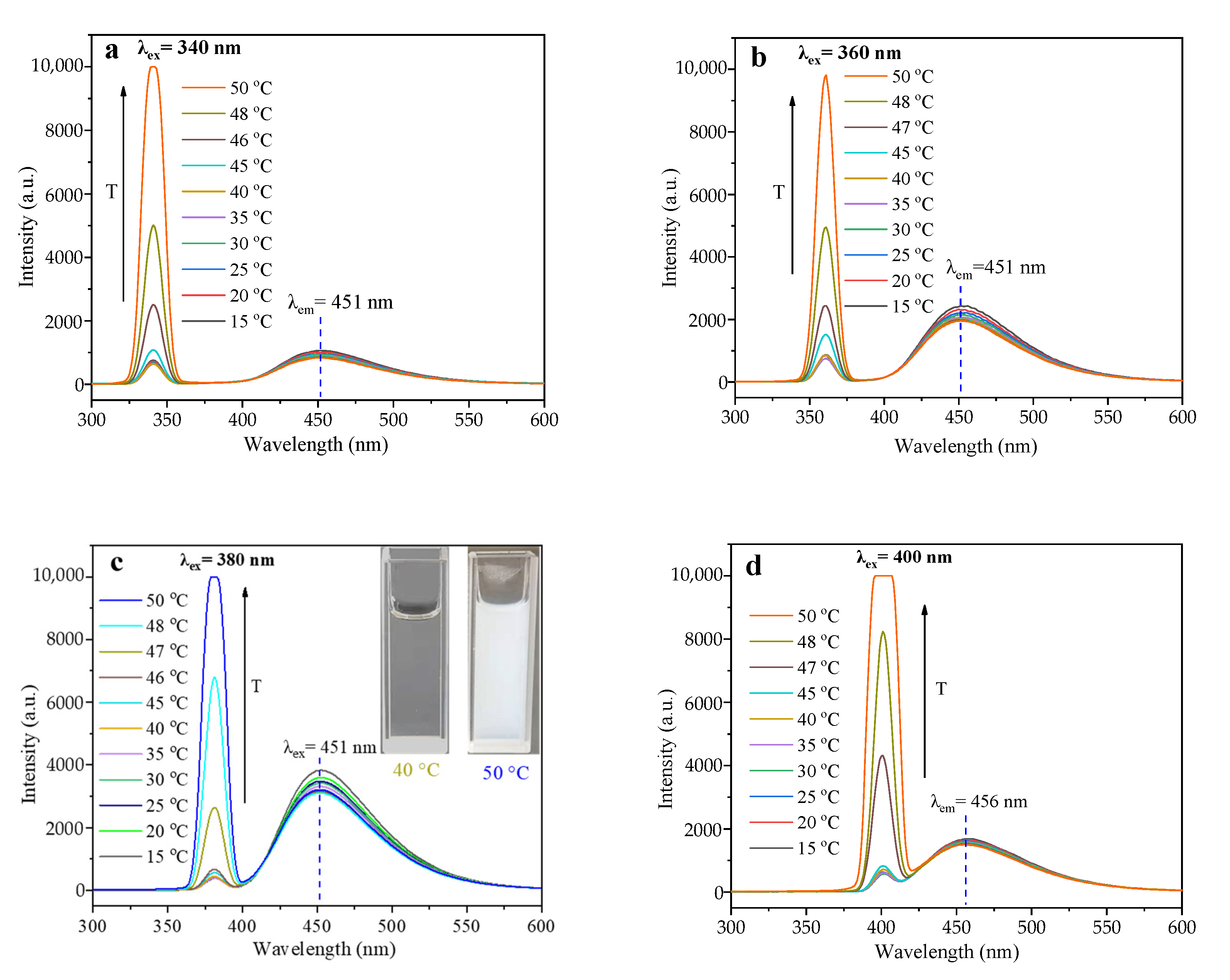
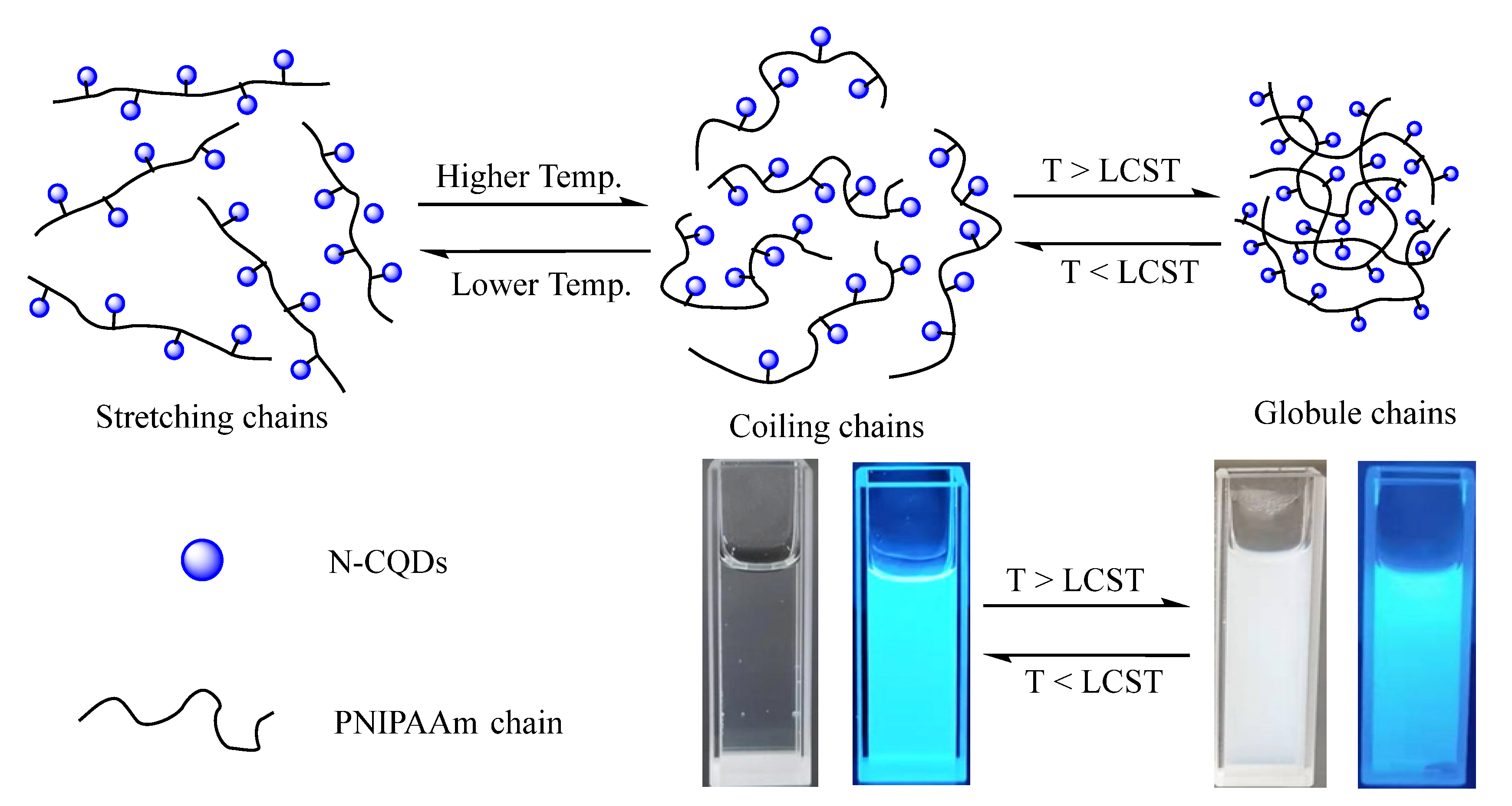
3.2. The Thermo-Sensitivity and Optical Properties of Poly-N-CQDs
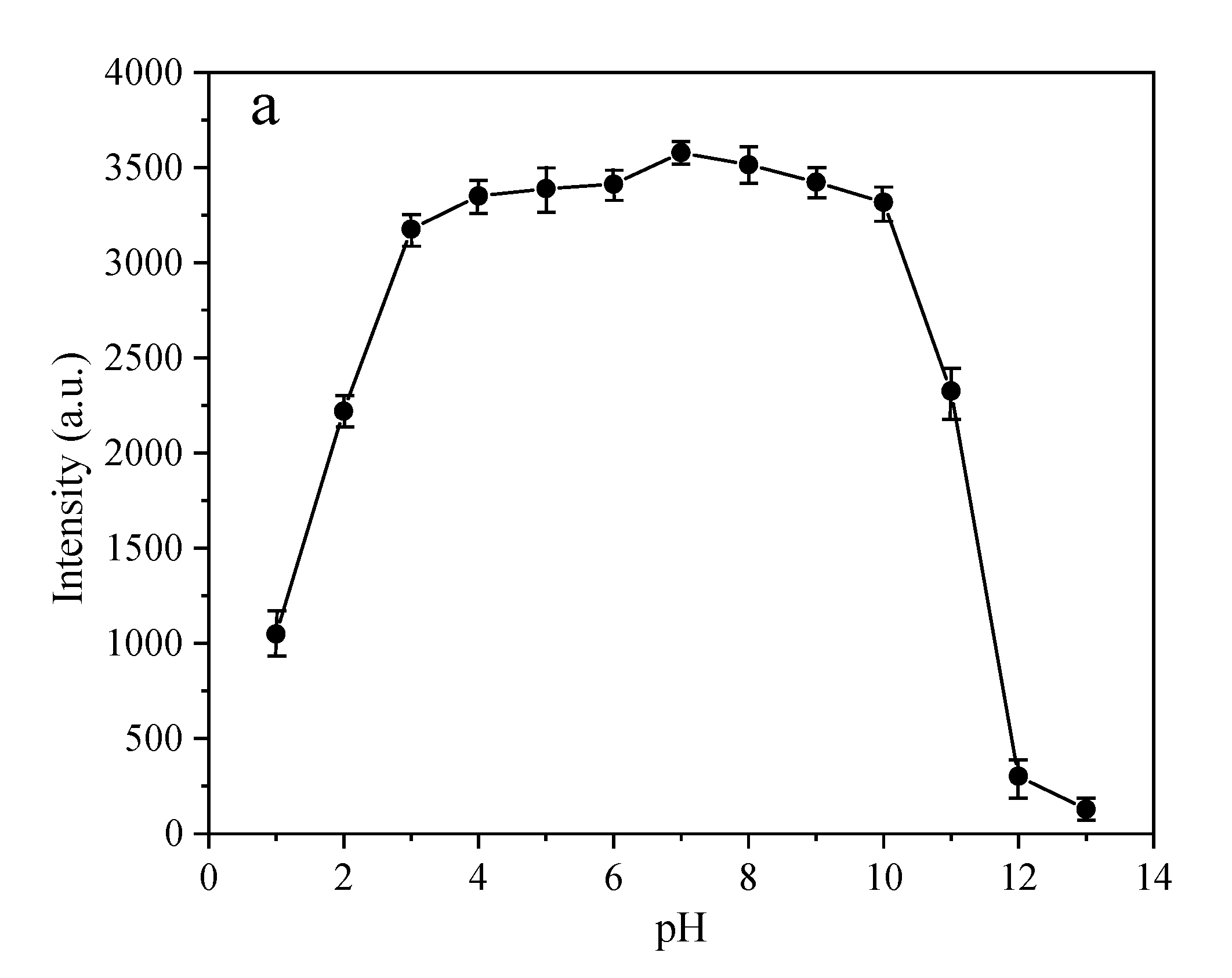
3.3. The Dependency of the Fluorescence Intensity on the pH
3.4. The Poly-N-CQDs in Different Solvents for Solvent Recognition
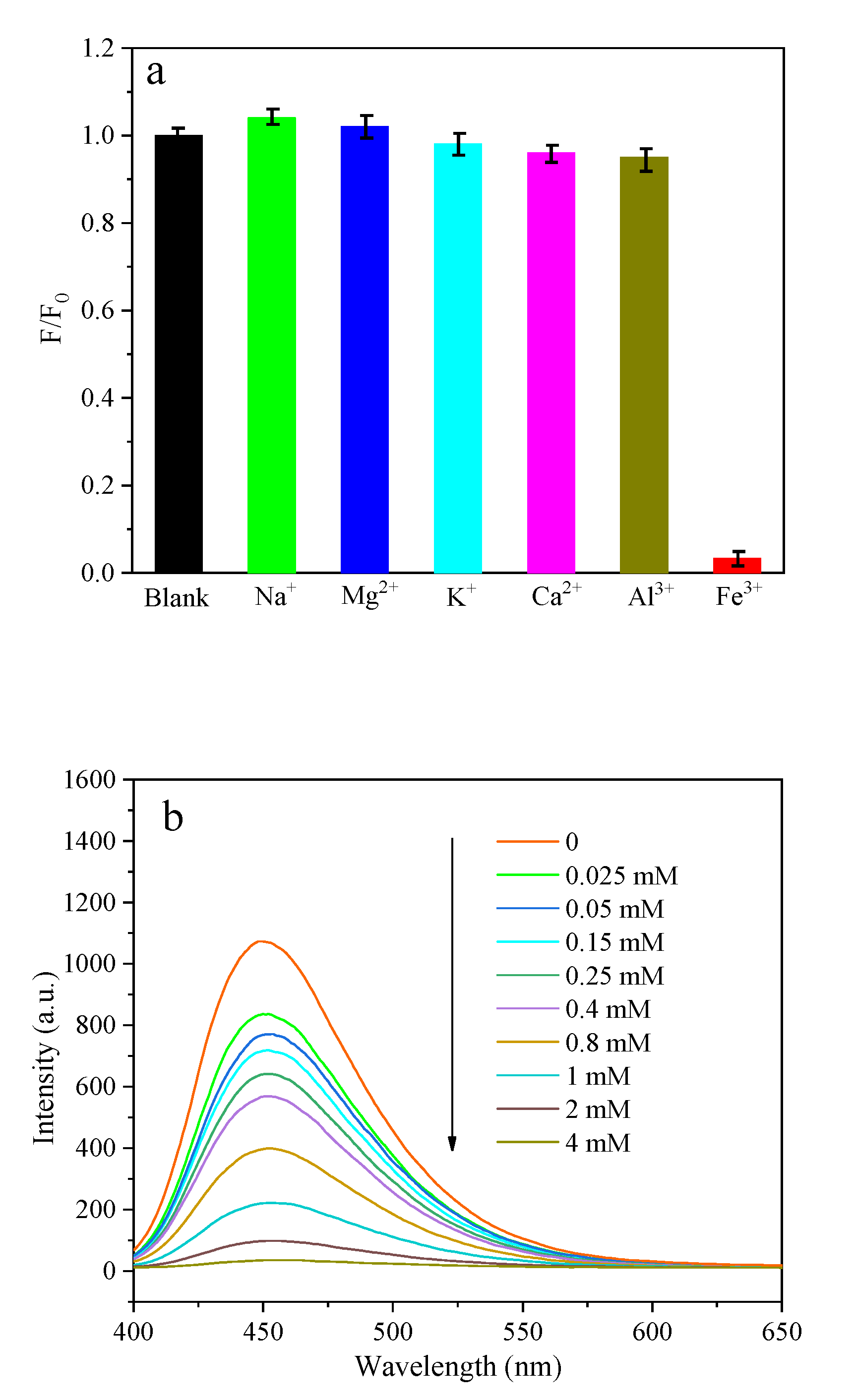
3.5. The Poly-N-CQDs as Fluorescence Probe for Fe3+ Ion Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hola, K.; Zhang, Y.; Wang, Y.; Giannelis, E.P.; Zboril, R.; Rogach, A.L. Carbon dots-Emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today 2014, 9, 590–603. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.; Aseer, K.R.; Perumal, S.; Karthik, N.; Lee, Y.R. Highly fluorescent nitrogen-doped carbon dots derived from Phyllanthus acidus utilized as a fluorescent probe for label-free selective detection of Fe3+ ions, live cell imaging and fluorescent ink. Biosens. Bioelectron. 2018, 99, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Y.; Shi, H.; Yan, Y.; Liu, E.; Hu, X.; Fan, J. A strong blue fluorescent nanoprobe for highly sensitive and selective detection of mercury (II) based on sulfur doped carbon quantum dots. Mater. Chem. Phys. 2019, 232, 145–151. [Google Scholar] [CrossRef]
- Ju, J.; Zhang, R.; Chen, W. Photochemical deposition of surface-clean silver nanoparticles on nitrogen-doped graphene quantum dots for sensitive colorimetric detection of glutathione. Sens. Actuators B Chem. 2016, 228, 66–73. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Y.; Cheng, H.; He, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-doped graphene quantum dots with oxygen-rich functional groups. J. Am. Chem. Soc. 2012, 134, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Y.; Guo, T.; Yang, T.; Chen, M.; Wang, J. Green preparation of carbon dots with papaya as carbon source for effective fluorescent sensing of Iron (III) and Escherichia coli. Biosens. Bioelectron. 2016, 85, 68–75. [Google Scholar] [CrossRef]
- Fan, Z.T.; Li, Y.C.; Li, X.H.; Fan, L.Z.; Zhou, S.X.; Fang, D.C.; Yang, S.H. Surrounding media sensitive photoluminescence of Boron-doped graphene quantum dots for highly fluorescent dyed crystals, chemical sensing and bioimaging. Carbon 2014, 70, 149–156. [Google Scholar] [CrossRef]
- Li, R.S.; Yuan, B.F.; Liu, J.H.; Liu, M.L.; Gao, P.F.; Li, Y.F.; Li, M.; Huan, C.Z. Boron and nitrogen co-doped single-layered graphene quantum dots: A high-affinity platform for visualizing the dynamic invasion of HIV DNA into living cells through fluorescence resonance energy transfer. J. Mater. Chem. B 2017, 5, 8719–8724. [Google Scholar] [CrossRef]
- Ding, H.; Wei, J.S.; Xiong, H.M. Nitrogen and sulfur co-doped carbon dots with strong blue luminescence. Nanoscale 2014, 6, 13817–13823. [Google Scholar] [CrossRef]
- Yang, P.X.; Su, J.; Guo, R.W.; Yao, F.L.; Yuan, C.D. B,N-Co-doped graphene quantum dots as fluorescence sensor for detection of Hg2+ and F− ions. Anal. Methods 2019, 11, 1879–1883. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, B.; Rao, Z.; Zhang, B.H.; Gong, J.R. Strong two-photon-induced fluorescence from photostable, biocompatible nitrogen-doped graphene quantum dots for cellular and deep-tissue imaging. Nano Lett. 2013, 13, 2436–2441. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cui, Y.; Li, T.; Diao, H.; Wei, S.; Li, L.; Chang, H.; Zhang, B.; Wei, W. Green and facile synthesis of highly photoluminescent nitrogen-doped carbon dots for sensors and cell Imaging. Chem. Lett. 2018, 47, 421–424. [Google Scholar] [CrossRef]
- Tavakoli, J.; Tang, Y.H. Hydrogel based sensors for biomedical applications: An updated review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Wang, M.X.; Sun, L.; Yang, J.H.; Zrinyi, M.; Chen, Y.M. Dual-physical cross-linked tough and photoluminescent hydrogels with good biocompatibility and antibacterial activity. Macromol. Rapid Commun. 2017, 38, 1600788. [Google Scholar] [CrossRef]
- Shao, J.; Yu, Q.; Wang, S.; Hu, Y.; Guo, Z.; Kang, K.; Ji, X. Poly(vinyl alcohol)-carbon nanodots fluorescent hydrogel with superior mechanical properties and sensitive to detection of iron (III) ions. Macromol. Mater. Eng. 2019, 304, 1900326. [Google Scholar] [CrossRef]
- Zhou, L.; He, B.Z.; Huang, J.C. Amphibious fluorescent carbon dots: One-step green synthesis and application for light-emitting polymer nanocomposites. Chem. Commun. 2013, 49, 8078–8080. [Google Scholar] [CrossRef]
- Zhang, P.; Li, W.C.; Zhai, X.Y.; Liu, C.J.; Dai, L.M.; Liu, W.G. A facile and versatile approach to biocompatible “fluorescent polymers” from polymerizable carbon nanodots. Chem. Commun. 2012, 48, 10431–10433. [Google Scholar] [CrossRef]
- Zuo, D.; Liang, N.; Xu, J.; Chen, D.; Zhang, H. UV protection from cotton fabrics finished with boron and nitrogen co-doped carbon dots. Cellulose 2019, 26, 4205–4212. [Google Scholar] [CrossRef]
- Wei, Z.; Ren, L.; Xiao, X.; Zhang, Q.; Huang, J.; Liu, R.; Zhao, S.; Xu, W. Biomimetic mineralization of nanoscale lanthanide metal-organic frameworks with thermo-sensitive polymer as organic ligands for solvent recognition and water detection. J. Solid State Chem. 2019, 277, 594–601. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Itzhaki, E.; Hadad, E.; Gedanken, A.; Margel, S. Microwave-synthesized polysaccharide-derived carbon dots as therapeutic cargoes and toughening agents for elastomeric gels. ACS Appl. Mater. Interfaces 2020, 12, 51940–51951. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Gao, Z.; Zhang, W.; Wei, W.; Chang, J.; Jiang, K. Fluorescent carbon dots as nanoprobe for determination of lidocaine hydrochloride. Sens. Actuators B: Chem. 2018, 262, 928–937. [Google Scholar]
- Li, X.; Wu, W.; Liu, W. Synthesis and properties of thermo-responsive guar gum/poly(N-isopropylacrylamide) interpenetrating polymer network hydrogels. Carbohydr. Polym. 2008, 71, 394–402. [Google Scholar] [CrossRef]
- Dang, D.K.; Sundaram, C.; Ngo, Y.-L.T.; Chung, J.S.; Kim, E.J.; Hur, S.H. One pot solid-state synthesis of highly fluorescent N and S co-doped carbon dots and its use as fluorescent probe for Ag+ detection in aqueous solution. Sens. Actuators B Chem. 2018, 255, 3284–3291. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Silva, R.; Girotto, E.M.; Rubira, A.F.; Muniz, E.C. Hydrogels based on PAAm network with PNIPAAm included: Hydrophilic–hydrophobic transition measured by the partition of Orange II and Methylene Blue in water. Polymer 2003, 44, 4213–4219. [Google Scholar] [CrossRef]
- Jiang, Y.N.; Yang, X.D.; Ma, C.; Wang, C.X.; Li, H.; Yang, B. Photoluminescent smart hydrogels with reversible and linear thermoresponses. Small 2010, 6, 2673–2677. [Google Scholar] [CrossRef] [Green Version]
- Barth, H.G. Modern Methods of Particle Size Analysis; John Willey & Sons: New York, NY, USA, 1884; pp. 135–150. [Google Scholar]
- Gao, J.; Wu, C. The “coil-to-globule” transition of poly(N-isopropylacrylamide) on the surface of a surfactant-free polystyrene nanoparticle. Macromolecules 1997, 30, 6873–6876. [Google Scholar] [CrossRef]
- Boxman, A. Particle Size Measurement for Control of Industrial Crystallizers. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 1992; pp. 25–27. [Google Scholar]
- Yi, Z.; Li, X.; Zhang, H.; Ji, X.; Sun, W.; Yu, Y.; Liu, Y.; Huang, J.; Sarshar, Z.; Sain, M. High quantum yield photoluminescent N-doped carbon dots for switch sensing and imaging. Talanta 2021, 222, 121663. [Google Scholar] [CrossRef]
- Zhou, M.; Zhou, A.; Gong, A.; Zhang, Y.; Li, Q. Synthesis of highly photoluminescent carbon dots via citric acid and Tris for iron(III) ions sensors and bioimaging. Talanta 2015, 143, 107–113. [Google Scholar] [CrossRef]
- Zhao, S.P.; Cao, M.J.; Li, L.Y.; Xu, W.L. Synthesis and properties of biodegradable thermo- and pH-sensitive poly[(N-isopropylacrylamide)-co-(methacrylic acid)] hydrogels. Polym. Degrad. Stab. 2010, 95, 719–724. [Google Scholar] [CrossRef]
- Kong, W.; Wu, H.; Ye, Z.; Li, R.; Xu, T. Optical properties of pH-sensitive carbon-dots with different modifications. Luminescence 2014, 148, 238–242. [Google Scholar] [CrossRef]
- Mohapatra, S.; Bera, M.K.; Das, R.K. Rapid “turn-on” detection of atrazine using highly luminescent N-doped carbon quantum dot. Sens. Actuators B Chem. 2018, 263, 459–468. [Google Scholar] [CrossRef]
- Lu, W.B.; Qin, X.Y.; Liu, S.; Chang, G.H.; Zhang, Y.W.; Luo, Y.L.; Asiri, A.M.; Alyoubi, A.O.; Sun, X.P. Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of Mercury(II) ions. Anal. Chem. 2012, 84, 5351–5357. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Wang, J.; Ren, J.; Qu, X. Carbon dots prepared by hydrothermal treatment of dopamine as an effective fluorescent sensing platform for the label-free detection of iron(III) ions and dopamine. Chem. Eur. J. 2013, 19, 7243–7249. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zhou, S.; Li, Y.; Li, X.; Fan, L.; Voelcker, N.H. Rhodamine-functionalized graphene quantum dots for detection of Fe3+ in cancer stem cells. ACS Appl. Mater. Interfaces 2015, 7, 23958–23966. [Google Scholar] [CrossRef]
- Duan, L.; Wang, P.; Ren, M.; Liao, F. Selective probes for ferric ion: A highly fluorescent nitrogen-doped carbon quantum dots. Inorg. Chem. Commun. 2018, 96, 111–115. [Google Scholar] [CrossRef]



 ) at 380 nm and the emission peak intensity (
) at 380 nm and the emission peak intensity (  ) at 451 nm as a function of heating-cooling cycles between 25 °C and 48 °C.
) at 451 nm as a function of heating-cooling cycles between 25 °C and 48 °C.
 ) at 380 nm and the emission peak intensity (
) at 380 nm and the emission peak intensity (  ) at 451 nm as a function of heating-cooling cycles between 25 °C and 48 °C.
) at 451 nm as a function of heating-cooling cycles between 25 °C and 48 °C.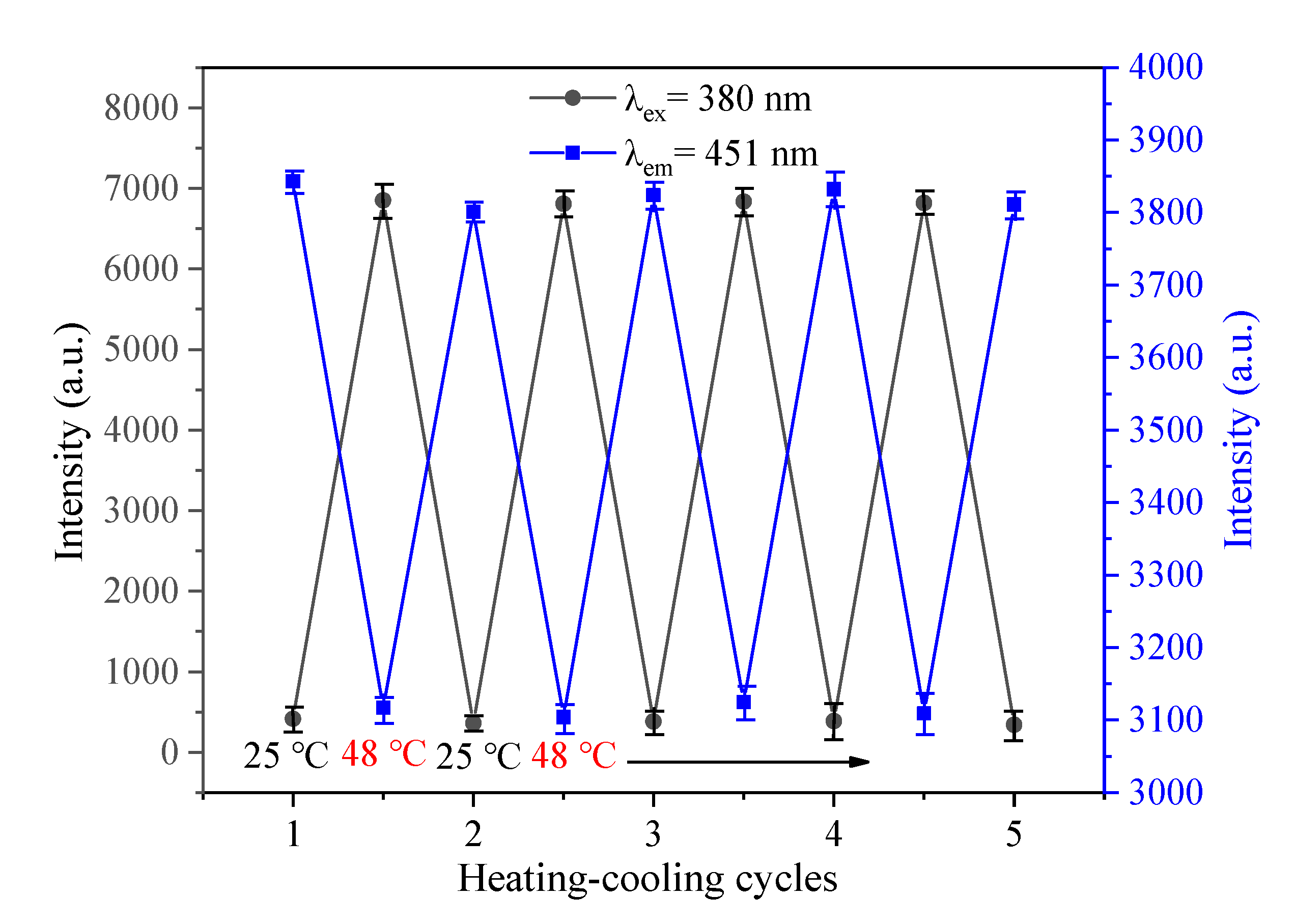



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Zhang, H.; Zhao, S. Stimulus-Responsiveness of Thermo-Sensitive Polymer Hybridized with N-Doped Carbon Quantum Dots and Its Applications in Solvent Recognition and Fe3+ Ion Detection. Polymers 2022, 14, 1970. https://doi.org/10.3390/polym14101970
Chen T, Zhang H, Zhao S. Stimulus-Responsiveness of Thermo-Sensitive Polymer Hybridized with N-Doped Carbon Quantum Dots and Its Applications in Solvent Recognition and Fe3+ Ion Detection. Polymers. 2022; 14(10):1970. https://doi.org/10.3390/polym14101970
Chicago/Turabian StyleChen, Tong, Hongwei Zhang, and Sanping Zhao. 2022. "Stimulus-Responsiveness of Thermo-Sensitive Polymer Hybridized with N-Doped Carbon Quantum Dots and Its Applications in Solvent Recognition and Fe3+ Ion Detection" Polymers 14, no. 10: 1970. https://doi.org/10.3390/polym14101970





